Abstract
Manganese (Mn) is an essential trace metal. It is also a component of welding fume. Chronic inhalation of manganese from welding fume has been associated with decreased neurological function. Currently, there is not a universally recognized biomarker for Mn exposure; however, hair and toenails have shown promise. In a cohort of 45 male welders and 35 age-matched factory control subjects, we assessed the sensitivity and specificity of toenail Mn to distinguish occupationally exposed subjects from unexposed controls. Further we examined the exposure time window that best correlates with the proposed biomarker, and investigated if non-occupational exposure factors impacted toenail Mn concentrations. Toenail clippings were analyzed for Mn using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Exposure to respirable Mn-containing particles (<4 µm) was estimated using an exposure model that combines personal air monitoring, work history information, and dietary intake to estimate an individual’s exposure to Mn from inhalation of welding fume. We assessed the group differences in toenail concentrations using a Student’s t-test between welders and control subjects and performed a receiver operating characteristic (ROC) curve analysis to identify a threshold in toenail concentration that has the highest sensitivity and specificity in distinguishing welders from control subjects. Additionally, we performed mixed-model regressions to investigate the association between different exposure windows and toenail Mn concentrations. We observed that toenail Mn concentrations were significantly elevated among welders compared to control subjects (6.87 ± 2.56 versus 2.70 ± 1.70 µg g−1; P < 0.001). Our results show that using a toenail Mn concentration of 4.14 µg g−1 as cutoff allows for discriminating between controls and welders with 91% specificity and 94% sensitivity [area under curve (AUC) = 0.98]. Additionally, we found that a threshold of 4.66 µg g−1 toenail Mn concentration enables a 90% sensitive and 90% specific discrimination (AUC = 0.96) between subjects with average exposure above or below the American Conference of Governmental Industrial Hygienist (ACGIH) Threshold Limit Value (TLV) of 0.02 mg m−3 during the exposure window of 7–12 months prior to the nail being clipped. Investigating which exposure window was best reflected by toenail Mn reproduced the result from another study of toenail Mn being significantly (P < 0.001) associated with exposure 7–12 months prior to the nail being clipped. Lastly, we found that dietary intake, body mass index, age, smoking status, and ethnicity had no significant effect on toenail Mn concentrations. Our results suggest that toenail Mn is a sensitive, specific, and easy-to-acquire biomarker of Mn exposure, which is feasible to be used in an industrial welder population.
Keywords: biomarker, exposure assessment, manganese, toenails, welding
Introduction
Manganese (Mn) is an essential trace metal that is necessary for normal functioning of a variety of physiological processes. Exposure to Mn occurs through two main routes of entry: inhalation and ingestion. The occupational and environmental sources of Mn exposure can be numerous: constituents of ingested foods, multivitamins, water, and beverages, as well as inhaled airborne particulates from occupational sources (welding, ferroalloy production, iron or steel manufacturing etc.). The Mn that enters the body through ingestion is tightly regulated by the liver and excreted via biliary excretion (Fitsanakis et al., 2007). Inhalation exposure is less tightly regulated and dependent upon particle size for deposition location and efficiency, and subsequent absorption of Mn particles (Lam et al., 1978). Larger Mn particles, greater than 10 µm, deposit with higher efficiencies in the upper respiratory tract; however, they can be removed by mucociliary clearance and are swallowed into the gastrointestinal tract where the absorption of Mn is tightly regulated. Smaller, respirable Mn particles, <4 µm in aerodynamic diameter, can be deposited and absorbed with relatively high efficiencies in the alveolar regions of the lungs. Thus, the particles of the greatest concern are in the fine and ultra-fine respirable size fractions (Carvalho et al., 2011).
In the brain, Mn is an important co-factor for many enzymes, including the anti-oxidant enzyme, superoxide dismutase, as well as enzymes involved in neurotransmitter synthesis and metabolism (Takeda, 2003). Several studies have shown that chronic low-level exposure to airborne Mn can significantly impair the functionality of the central nervous system affecting motor and cognitive abilities (Bowler et al., 2006; Guilarte et al., 2006; Schneider et al., 2006; Roels et al, 2012; Meyer-Baron et al., 2013). Once clinical parkinsonian signs of Mn intoxication are established, they may become progressive and irreversible as they reflect damage to neurological structures (Zheng et al., 2011). Therefore, establishing a valid and sensitive biomarker of Mn exposure that reliably estimates the Mn body burden is an issue of importance.
A biomarker of exposure is defined as any measureable biological parameter that indicates the levels of exposure to a given toxic substance over time. Ideally, a biomarker of Mn exposure should be measurable, clearly distinguish between exposed and unexposed individuals, correlate with exposure for a specific span of time, and be sensitive over a range of exposure levels in various industries.
Blood, urine, and less frequently nail and hair manganese, have been suggested as biomarkers of exposure. Since the liver actively sequesters Mn from the blood circulation to keep systemic Mn concentrations within homeostatic range and tends to lower blood Mn concentrations relatively quickly, Mn in whole blood or plasma seems to be typically useful in relatively short time frames (hours to days) (Bernard, 1995; Laohaudomchok et al., 2011; Hoet et al., 2012). Findings from several studies suggest that the utility of blood as a biomarker is only valid for a group comparisons (Järvisalo et al., 1992; Bader et al., 1999; Apostoli et al., 2000; Smith et al., 2007). A recent study by Baker et al. (2015) was able to observe a significant association between blood Mn and the preceding 90-day exposure as well as cumulative exposure, indicating blood Mn concentrations may be dependent on exposure intensity, variability, and duration. A study among occupationally exposed adults found a complex and limited relationship between exposure [as measured by total air Mn level and cumulative exposure indices (CEIs)] and blood Mn levels that may depend upon exposure attributes and the latency of blood measurements relative to exposure (Smith et al., 2007). However, a study by Hoet et al. (2012) has shown that when blood and plasma are taken immediately after the shift, the Mn levels in the plasma are significantly correlated with the airborne Mn concentration. As the kidney is an organ which retains Mn and is poor at excreting Mn into the urine, urinary Mn concentrations are not useful as a biomarker of exposure (Järvisalo et al., 1992; Bader et al., 1999; Apostoli et al., 2000; Smith et al., 2007; Laohaudomchok et al., 2011).
Hair and especially nails, due to their slow growth rate, are thought to cover longer exposure periods (several months to a year) and have shown promise as potential biomarkers (Garland et al., 1993; Menezes-Filho et al., 2009; Laohaudomchok et al, 2011; Eastman et al., 2013; Grashow et al., 2014; Reiss et al., 2015). Human toenails, fingernails, and hair are body tissues that consist of keratins, which are fibrous proteins that contain disulfide bridges, which are thought to chelate metals present during their formation (Raab et al., 2005; Slotnick and Nriagu, 2006). The high sulfur concentration in the nail provides many binding sites for excreted metals. It should be pointed out that the toxicokinetics of the incorporation of Mn into the hair or nail matrices are not yet fully elucidated.
A study, Laohaudomchok et al. (2011), which looked at the utility of blood, urine, and toenails as biomarkers of Mn exposure from welding fume, found that only toenail Mn has promise as a biomarker. The study was conducted on 46 welders, many of whom were apprentices, from a local boilermaker union with <3 years of experience. These welders had high variability in exposures due to a seasonal work pattern. In addition, the levels of exposure to respirable Mn concentrations was very low (0.0002–0.0137 mg m−3 (median = 0.0013 mg m−3). The study was able to show that recent exposure to respirable manganese on an individual level was not correlated with blood and urine Mn levels. However, toenails were significantly correlated with an individual’s exposure 7–12 months prior to the toenail being clipped. This study was an important step in identifying toenails as biomarker of exposure, reflecting an exposure window of 7–12 months prior to the nail being clipped, but the study did not compare welder toenail Mn concentrations to that of non-exposed subjects.
A more recent study conducted by Grashow et al. (2014), investigated the use of toenail metal as a biomarker for Mn, lead, cadmium, nickel, and arsenic from occupational welding fume exposure. This study recruited subjects from the same cohort of welders as Laohaudomchok et al., 2011. They found that Mn was associated with welding hours 7–9 months prior to clipping. However, neither air monitoring or exposure models were utilized to estimate each individual’s CEI, but instead the cumulative welding hours for the past year were divided into quarters. While this study reproduced a similar finding to Laohaudomchok, and narrowed the exposure window to 7–9 months prior to the nail being clipped, it did not compare toenail Mn concentrations to a matched control population either; and only used the exposure metric of welding hours. The use of welding hours to assess exposure is commonly found in the literature, since it can be easier to obtain. However, in order to use this metric, several assumptions on exposure information must be made. For example, it must be known or assumed that all the welders in the sample population have the same work environment (ventilation/air flow from fans, welding type, steel types, amount of space, and arc times), or else welding hours would represent a very poor estimate for the individual exposure. A change in any one of these could greatly change the exposure.
While the Laohaudomchok et al. (2011) and Grashow et al. (2014) studies were great steps in assessing the use of toenail Mn as a biomarker of exposure, the results from the two studies come from the same population of boilermakers and are still awaiting to be reproduced in a different cohort of professional welders. Additionally, to date, there have been no investigations into the sensitivity and specificity of toenail Mn.
In this study, we aim to fill these gaps by studying a cohort of full-time career male welders, and a matched control group of shift workers from the same factory, making use of personal air sampling for a robust exposure assessment. The objectives of this study are: (i) to investigate the sensitivity and specificity of toenail Mn concentrations in distinguishing occupationally exposed welders from matched control subjects; (ii) to assess if the exposure window of 7–12 months prior to the toenail being clipped found by two prior studies on the same population can be reproduced in a separate cohort; and (iii) to determine if toenail Mn levels can be used to differentiate subjects exposed on average, based on CEI, above and below the American Conference of Governmental Industrial Hygienist (ACGIH) Threshold Limit Value (TLV) of 0.02 mg m−3 at high sensitivity and specificity.
Materials and methods
Study population
The study was approved by the Institutional Review Board of Purdue University. All individuals recruited for the study were informed of the study objectives before obtaining written consent for their participation. Forty-five male welders were recruited from a semi-trailer manufacturer from 17 different departments located at two different plants. The exact number of welders recruited per department is listed in Table S1 in the online supplementary material (available at the Annals of Work Exposures and Health). The welders had been employed full-time at the company for at least the past 3 years. Many of the welders had been employed as welders for at least 10 years (mean = 12.75 years, range = 2–36 years). Welders predominately (82–84%) welded on mild steel (MS) (50K—3–6% Mn/weight.), stainless steel (SS) (15–17%) (50K—4–8% Mn/weight), and galvanized steel (GS) (<1%) (60K—3–7% Mn/weight). The welders also primarily (95%) used Gas Metal Arc Welding—Metal Inert Gas (GMAW-MIG), but also to a lesser extent (5%) Gas Tungsten Arc Welding—Tungsten Inert Gas (GTAW-TIG). The welders did not have access to local exhaust ventilation, but instead relied on fans and general dilution ventilation from open loading doors.
Additionally, 35 male age-matched employees from seven departments, not exposed to welding fumes, were recruited from the same semi-trailer manufacturer. These subjects were non-management shift workers performing maintenance, working in assembly lines, or transporting materials around the plants. Further details on welding and control populations can be found in Table S1 in the online supplementary material (available at the Annals of Work Exposures and Health).
Exposure assessment
Exposure via the two major routes of entry, inhalation, and ingestion, was assessed for different exposure windows. Personal air monitoring was utilized for the assessment of each subject’s Mn exposure. In addition, work history and food frequency questionnaires were administered in a personal interview. Data from the work history questionnaire and personal air monitoring were combined in an exposure model to estimate the individual’s inhalation exposure as detailed below.
Questionnaires
All participants completed an interview that utilized questionnaires to assess work history, medical history, diet history, and lifestyle habits. The work history section of the questionnaire was developed to gather information related to occupational Mn exposure for each subject from welding fume. The detailed work history included the subject’s current employment (CE), separated by different departments (work stations) at the plant that the subject had previously worked in, past employments (PE), and exposure away from work either for a hobby or side job welding (‘Off the job’: OJ) back to age 18. Subjects were asked about the time in years (T) they worked in each welding department for their current employer and past employers. They were asked to estimate the following factors: the percentage of time the welding arc was on or ‘arc time’; the percentage of time they spent welding either of the two base metals, MS and SS; the frequency and types of respirators they utilized; the amount and types of space they welded in; and the types of ventilation they utilized. A summary of these variables for our cohort is given in Table S2 in the online supplementary material (available at the Annals of Work Exposures and Health). This information together with the personal air sampling results were then utilized in the exposure model to determine the CEI in units of mg m−3 year for each participant for different exposure windows as detailed below.
In order to account for the dietary sources of Mn, a food frequency questionnaire was used. The frequent food questionnaire (FFQ) from the Laohaudomchok et al. (2011) study was modified to include more Mn rich foods. Each participant was asked to consider a typical serving size for each of the foods (indicated by the FFQ) and fill in the frequency that best reflected their average consumption of each food in the last year (9 levels from never to 6+ times a day). The amount of Mn intake from foods (mg day−1) was calculated for each subject based on the United States Department of Agriculture (USDA) nutrient tables (USDA, 2015). Additionally, Mn from multivitamins and supplements were included in the daily intake of Mn. A copy of both the food frequency questionnaire and the work history questionnaire can be found in the online supplementary material in sections S4 and S5 (available at the Annals of Work Exposures and Health).
Personal air monitoring
Personal samples of respirable air-Mn particles were collected over the duration of the work shift (8 hours). We used SKC 25 mm aluminum cyclones with an aerodynamic diameter cut point of 4.0 µm, in line with SKC Airchek 52 personal sampling pumps drawing 2.5 l min−1 of air. The cyclone was fitted with a cassette holding a 25 mm Mixed Cellulous Ester Filter (MCEF) with a pore size of 0.8 µm weight-matched to 50 µg. The cyclone was placed inside the welding helmet. For the control subjects, the cyclone was placed on the shoulder in the personal breathing zone (PBZ). For an illustration on the placement of the samplers, please see Figure S3 in the online supplementary material (available at the Annals of Work Exposures and Health).
Over the study period from 2013–2016, 119 samples (89 from study subjects and 30 additional samples) were acquired with an average of six samples per department. These departmental averages were used in our exposure model since an average of several measurements better reflects the mean exposure of an individual within a department over a certain time window than a one-time measurement. Detailed information on specific department air levels can be found in Table S1 of the online supplementary material (available at the Annals of Work Exposures and Health).
Early during the study, one department (Frack Tank) which we had recruited subjects from, was shut down prior to conducting air sampling in that particular department. This department had previously been an area of very high exposure (0.4–0.8 mg m−3), as welders would weld the inside of a tank without ventilation. The company was able to provide recently (2010–2013) collected personal air sampling measurements taken outside the welding helmet from this department using NIOSH method 7300. However, the samples were for total Mn instead of respirable Mn. After comparing the company data to our own data for other departments, it was observed that on average our respirable Mn measurements were 90% of the company’s total Mn measurements. Therefore, for this one department, we used a value of 90% of the average total Mn concentration reported by the company as the departmental average airborne concentration in our exposure model.
Exposure model
An exposure model was developed to estimate each participant’s CEI as an estimate of the cumulative respirable airborne Mn exposure from welding for their lifetime (back to age 18), or for more recent time periods or ‘exposure windows’ (past 3 months and 7–12 months prior to the nail being clipped). These windows of exposure were selected for the following reasons: (i) past 3 months is a recent exposure which should not be reflected by current nail tissue and (ii) 7–12 months is the window of exposure that has been shown to have the strongest degree of correlation to toenail Mn concentrations in two previous studies (Laohaudomchok et al., 2011; Grashow et al., 2014), and is consistent with the biological growth of the nail tissue (Yaemsiri, 2010). Cumulative exposure was selected as it has not been previously studied and it was unknown if cumulative exposure would have a significant impact on toenail Mn levels. Previous studies have only investigated cumulative exposure by using metrics such as years of welding, whereas this study is calculating the CEI for each individual which is assumed to be a more accurate estimate of each individual’s exposure.
The CEI calculated by the exposure model is a summation of respirable Mn exposures from welding at the subject’s current employer (CEICE), past employers (CEIPE), and any ‘Off the job’ welding (CEIOJ) for a specific window of time (w) in mg m−3 year as seen in equation 1.
| (1) |
The CEICE is equal to the sum of the exposures for each department where the individual has worked during the exposure window (w), as shown below in equation 2. The cumulative exposure for each department is calculated by multiplying the time spent in a specific department in units of years, by the departmental average airborne concentration in units of mg m−3. Lastly, the product is multiplied by unit-less weighting factors designed to account for changes in individual relative welding arc time (Tarc), respirator use (RF), welding type (WT), base metal types (BM), ventilation (VF), and workspace (WS).
| (2) |
| (3) |
| (4) |
Since we had access to the facility of the current employer, the weighting factors WT, BM, VF, and WS were intrinsically characterized by our air sampling and thus were not used to calculate the CEI of the current employer. Only the weighting factors for the relative arc time (Tarc) and respirator use (RF) were utilized for the current employer, since these can vary between individuals in the same department. Specifically, the respirator factor (RF) is obtained by dividing the percentage of the time that a subject reported to wear his respirator during the specified exposure window by the assigned protection factor (APF) for the respirator type specified by the National Institute for Occupational Safety and Health (NIOSH; NIOSH, 2004). However, a study by Nicas and Neuhaus (2004) has shown that the APFs for half-masks do vary greatly and suggests that the APF for half-mask respirators be reduced from 10 down to 5. Following this suggestion and due to the potential for the respirator typically not fitting and sealing perfectly to the user, we used an APF of 5 instead of 10 for the type of half-mask respirators used by the current employer, as was done by the Laohaudomchok study.
To better estimate exposures outside of the current employer, such as those for past employers or OJ welding utilized in the cumulative exposure model, we developed additional weighting factors based on the Hobson et al. (2011) study. Hobson et al. examined 66 welding studies to determine how Mn concentration changed with respect to changes in welding types, ventilation, base metal types, and workspace. A general comparison of PE and OJ fume levels to the current job enabled us to use the current airborne concentration as a reference for exposure, which then was adjusted using weighting factors determined from the work history questionnaire. When calculating the CEI for the past employers (CEIPE) and OJ welding (CEIOJ) for exposure window (w) we thus used equations 7 and 8 respectively.
| (6) |
| (7) |
| (8) |
TEMP is equal to the time in years at each employer. Tarc, AirMn, and RF are relative arc time, the current departmental average of airborne Mn concentration, and the respirator factor described above. Weighting factors developed by the Hobson et al. (2011) study were used for WT, BM, EF, VF, and are listed in Table 1.
Table 1.
Summary of exposure model weighting factors.
| Exposure model variable | Weighting factor |
|---|---|
| Welding type (WT) | |
| Gas metal arc welding (GMAW-MIG) | 1.00 |
| Flux core arc welding (FCAW) | 0.13 |
| Shielded metal arc welding (SMAW) | 0.42 |
| Gas tungsten arc welding (GTAW-TIG) | 0.00 |
| Base metal (BM) | |
| Mild steel (MS) | 1.00 |
| Stainless steel (SS) | 0.33 |
| Ventilation (VT) | |
| Local exhaust ventilation (LEV) | 0.33 |
| General exhaust ventilation (GEV) w/fans | 1.00 |
| None | 2.20 |
| Enclosure factor—welding space (EF) | |
| Open space | 1.00 |
| Enclosed space | 2.00 |
| Confined space | 5.85 |
| Respirator factor (RF)—NIOSH APF/2 | |
| ½ Mask respirator, N95 | 5 |
| Power air purifying respirator (PAPR) | 25 |
Determination of Mn in toenails and air
Toenail clippings from all toes were collected and placed in small envelopes. The toenail clippings were prepared and analyzed as described by Kile et al. (2007). Briefly, external contamination was removed from the nails by sonicating the samples in a 1% Triton X-100 surfactant solution for 20 minutes. The toenails were then rinsed five times with Distilled De-Ionized (DDI) water. Further, the toenails were dried, weighed, and digested using microwave nitric acid digestion. The samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) by the Purdue University’s Campus-wide Mass Spectrometry Center (PUCMSC), which is equipped with a sector-field ELEMENT-2 mass spectrometer (ThermoFinnigan, Bremen, Germany). Quality control measures were routinely taken in the lab to ensure the accuracy and precision of the sample results. Toenail Mn concentrations are in units of µg g−1.
Filters from the air samples were stored in a temperature and humidity controlled area prior to analysis. The filter samples were weighed and recorded prior to digestion. The digestion and analysis protocol that we used was a slight variation of NIOSH method 7300 for elemental analysis using microwave assisted nitric acid digestion, with all other procedures remaining the same. Following digestion, the samples were analyzed for Mn at the PUCMSC. Air samples were then calculated by volume of air collected to determine the concentration in units of mg m−3.
Data analysis
All data were checked for normal distribution. If it was not normally distributed, the data was log10-transformed for statistical analyses. Student’s t-tests were used to assess the group differences in toenail Mn concentrations between welders and control subjects and results are reported as mean ± standard deviation. A receiver operating characteristic (ROC) curve analysis was performed to assess the sensitivity and specificity of toenail Mn in distinguishing welders from controls as well as subjects with average exposures above and below the ACGIH TLV of 0.02 mg m−3. An annual average exposure at the TLV of 0.02 mg m−3 corresponds to a CEI 7–12 months prior, calculated over 6 months, of 0.01 mg m−3 year. We thus used the CEI over the past 7–12 months values of each subject for classification into the two groups. Classification of individuals into these groups may be of interest in the field, where exact exposure histories of individuals may not be known. Due to the multicollinearity between CEI windows, a linear mixed regression analysis was used to determine the relationship between toenail Mn and CEI during specific time windows in the presence of potential confounders (age, smoking status, race, body mass index (BMI), and dietary intake of Mn were used as fixed effects). All statistical analyses were performed with Statistical Analysis System (SAS Institute Inc., NC) version 9.4. Statistical significance was set at α < 0.05.
Results
A summary of both exposure and demographic data (age, race, airborne Mn exposure, toenail Mn concentration, etc.) for welders and control subjects can be seen in Table 2. While airborne Mn concentrations were normally distributed, toenail and CEI data were skewed and not normally distributed. Therefore, for statistical analyses only, a log10-transformation was utilized and data was checked to show a normal distribution after the transformation. However, data presented in the text and tables are the raw, untransformed data values.
Table 2.
Cohort demographics and exposure summary.
| Welders | Controls | |||
|---|---|---|---|---|
| Total number (N) | 45 | 35 | ||
| Age | ||||
| Range | 21–61 | 18–61 | ||
| Mean (SD) | 40.7 (10.4) | 39.3 (11.3) | ||
| Race | ||||
| White, N (%) | 35 (78%) | 27 (77%) | ||
| Non-White, N (%) | 10 (22%) | 8 (23%) | ||
| Smoking habit | ||||
| Current smoker, N (%) | 14 (31%) | 8 (23%) | ||
| Non-smoker, N (%) | 31 (69%) | 27 (77%) | ||
| Dietary Mn intake | ||||
| Mean (SD) | 2.95 (2.89) | 2.32 (1.96) | ||
| Body mass index (BMI) | ||||
| Mean (SD) | 29.99 (4.03) | 29.09 (4.39) | ||
| Toenail Mn (µg g−1) | ||||
| Range | 3.53–15.56 | 0.24–4.82 | ||
| Mean (SD) | 6.87 (2.56)*** | 2.70 (1.17) | ||
| Years welding | ||||
| Range | 2–36 | 0 | ||
| Mean (SD) | 12.75 (8.66)*** | 0 | ||
| Respirable airborne Mn (mg m−3) | ||||
| Range | 0.008–0.477 | 0.000–0.028 | ||
| Mean (SD) | 0.129 (0.079)*** | 0.004 (0.006) | ||
| CEI past 3 months (mg m−3 year) | ||||
| Mean (SD) | 0.042 (0.036)*** | 0.001 (0.001) | ||
| CEI 7–12 months (mg m−3 year) | ||||
| Mean (SD) | 0.079 (0.072)*** | 0.002 (0.003) | ||
| CEI cumulative (mg m−3 year) | ||||
| Mean (SD) | 1.421 (1.234)*** | 0.073 (0.125) | ||
SD = standard deviation; ***P value <0.001 from t-test.
Comparison of groups
Welders versus control subjects
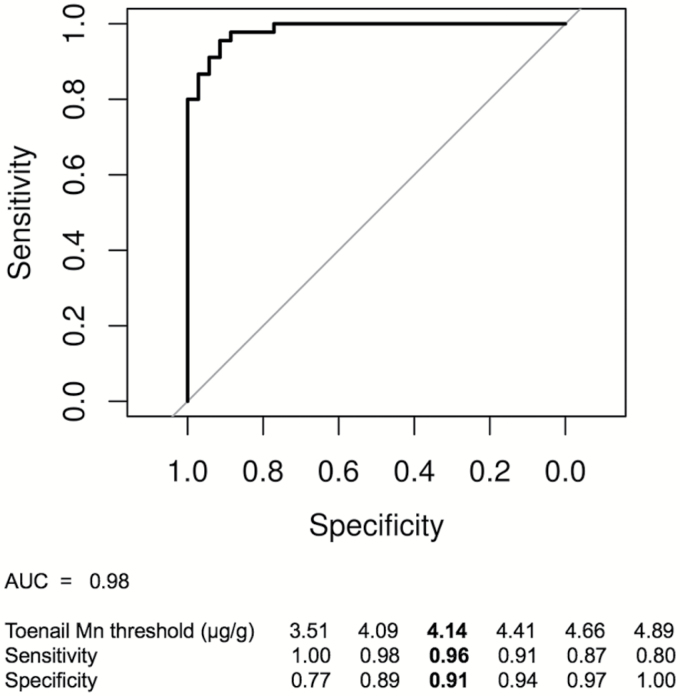
The average of respirable airborne Mn concentrations was observed to be significantly increased for welders compared to control subjects (0.129 ± 0.079 versus 0.004 ± 0.006 mg m−3; P < 0.001). To better illustrate the difference in toenail concentration between welders and control subjects, toenail Mn concentrations are shown in the raw and untransformed state in Fig. 1; however, statistical analysis was performed on the log10-transformed toenail Mn concentrations. We observed that toenail Mn were significantly elevated among welders compared to control subjects (6.87 ± 2.56 versus 2.70 ± 1.70 µg g−1; P < 0.001). The ROC curve analysis shown in Fig. 2 demonstrates that toenail Mn best discriminates between controls and welders at a toenail Mn concentration of 4.14 µg g−1 (area under curve, AUC = 0.98), correctly classifying subjects with a specificity of 91% and a sensitivity of 94%.
Figure 1.
Comparison of toenail Mn in welders versus controls. Boxplot comparison of the untransformed group toenail Mn concentrations. The whiskers show the range of the data, and top and bottom of each box represents the 1st and 3rd quartiles, respectively. The middle line represents the median, and the diamond represents the mean. Student’s t-test was used to compare the log transformed toenail Mn concentrations. ***P < 0.001.
Figure 2.
ROC curve analysis for toenail Mn to distinguish welders versus controls. Toenail Mn best discriminates between controls and welders at a toenail Mn concentration of 4.14 μg g−1 , correctly classifying subjects with a specificity of 91% and a sensitivity of 94%.
Comparison of subjects above and below ACGIH TLV
Our exploration in determining if toenail Mn levels could be utilized to distinguish subjects exposed on average, based on CEI, above and below the ACGIH TLV of 0.02 mg m−1 for the exposure window of 7–12 months prior to the toenails being clipped, confirmed that the average toenail Mn concentrations (6.98 ± 2.62 versus 2.92 ± 1.36 µg g−1) for subjects exposed above and below the TLV were significantly different (P < 0.001). Further investigation revealed that all but three welders had average exposures above the current ACGIH TLV, as well as one control subject working in the metal fabrication department. The ROC curve analysis indicated that a toenail Mn concentration of 4.66 µg g−1 was the best threshold to discriminate between subjects with average exposures above and below the TLV in the exposure window of 7–12 months prior to the nail being clipped (AUC = 0.96), with high specificity (90%) and sensitivity (90%) as shown in Fig. 3.
Figure 3.
ROC curve analysis of the sensitivity and specificity of distinguishing subjects with average exposure above versus below the ACGIH TLV of 0.02 mg m−3 7–12 months prior to the nail being clipped.
Exposure windows analysis
Exposure window reflected by toenail Mn
We performed linear regressions of log10 transformed toenail Mn against each of the log10 transformed CEI exposure windows (past 3 months, 7–12 months, and cumulative), as shown in Fig. 4. We observed that the exposure window with highest adjusted R-square value (adj. R2 = 0.42) and best fit was the cumulative exposure 7–12 months prior to the nail being clipped. However, all exposure windows were significantly correlated with toenail Mn concentration. After performing Spearman correlations between the exposure windows, shown in Table 3, it was observed that exposure windows are significantly correlated with each other. Therefore, using mixed-effect models, we further evaluated the association of log10 transformed CEI windows and log10 transformed toenail Mn concentrations, after adjusting for age, race, BMI, diet, and smoking habits. The results confirm that the toenail Mn levels were mainly associated with exposure 7–12 months prior to the toenail being clipped (P < 0.001), as shown in Table 4. Non-occupational variables such as age, race, BMI, smoking status, and dietary intake did not contribute significantly to toenail Mn levels.
Figure 4.
Regression plots of log CEI exposure windows versus log toenail Mn concentration. (A) CEI past three months; (B) CEI past 7-12 months; (C) CEI cumulative exposure.
Table 3.
Results of Spearman correlation analysis of log CEI exposure windows amongst each other.
| Spearman correlation table of log CEI windows | ||||||
|---|---|---|---|---|---|---|
| Log past 3 months | Log 7–12 months | Log cumulative | ||||
| Log past 3 months | 1 | ρ = 0.75*** | ρ = 0.51*** | |||
| Log 7–12 months | 1 | ρ = 0.60*** | ||||
| Log cumulative | 1 | |||||
***P < 0.001.
Table 4.
Linear mixed regression analysis of toenail Mn levels.
| Linear mixed regression model analysis | ||||||
|---|---|---|---|---|---|---|
| Variable (X) | Parameter estimate (β) | 95% CI (lower, upper bounds) | P value | |||
| Log CEI past 3 months (mg m−3 year) | −0.070 | (−0.215, 0.076) | 0.29 | |||
| Log CEI 7 to 12 months (mg m −3 year) | 0.284 | (0.123, 0.445) | <0.001*** | |||
| Log CEI cumulative exposure (mg m−3 year) | 0.020 | (−0.083, 0.122) | 0.36 | |||
| Race (white/non-white) | −0.040 | (−0.258, 0.179) | 0.96 | |||
| Age | 0.002 | (−0.007, 0.012) | 0.91 | |||
| Smoking status | 0.070 | (−0.121, 0.259) | 0.37 | |||
| Body mass index (BMI) | −0.009 | (−0.031, 0.013) | 0.59 | |||
| Dietary intake of Mn (mg day−1) | 0.010 | (−0.019, 0.038) | 0.86 | |||
***P < 0.001.
Discussion
The results from this study support the interpretation of toenail Mn as being a sensitive and specific biomarker of Mn exposure in career welders. We observed that welders had significantly elevated Mn levels as compared to matched control subjects. The ROC curve analysis identified that using a toenail Mn level threshold of 4.14 µg g−1 is able to distinguish welders and control subjects in our cohort with a high degree of sensitivity and specificity. To our knowledge, this study is the first to show (i) that toenail Mn concentrations are significantly elevated in male welders compared to non-exposed matched control subjects and (ii) the sensitivity and specificity of toenail Mn as a marker of Mn exposure.
Three different exposure windows (past 3 months, 7–12 months prior to the nail being clipped, and the lifetime cumulative exposure) were tested to determine which exposure window is best reflected by toenail Mn levels in career welders. Our result that toenail Mn levels are most significantly associated with exposure 7–12 months prior to the nail being clipped is in agreement with the results of the Grashow et al. (2014) and Laohaudomchok et al. (2011) studies. Since an exposure time window of 7–12 months prior to the nail being clipped is also consistent with the growth rate of nail tissue (Yaemsiri et al., 2010), confidence in toenail Mn levels being able to predict exposure 7–12 months ago is high.
Lastly, our results suggest that toenail Mn levels can distinguish between subjects who are exposed on average above and below the ACGIH TLV of 0.02 mg m−3. The results of the ROC curve analysis indicate that a threshold for toenail Mn concentrations set at 4.66 µg g−1 yields the best specificity and sensitivity to discern subjects with exposures greater than or equal to the TLV averaged over the exposure window 7–12 months prior to clipping the nails. It needs to be noted that this ROC curve analysis simply assumes an average exposure at the TLV over the time window reflected by toenail concentrations. More research studies will be needed to confirm this threshold, especially on populations with exposures near the TLV.
Compared to blood and urine, toenails provide a measure of longer term exposure, which may be useful in assessing the effects of Mn exposure in the context of chronic Mn-associated disease processes. Toenails also have practical advantages over blood and urine in their relative ease of collection, transport, and storage; however, blood and urine Mn have the advantage of not being very susceptible to exogenous contamination providing the samples are collected according to state-of-the-art procedures. Previous work by Bainter (2014), in which nail tissue was directly exposed to welding fume and subsequently cleaned and analyzed using the method by Kile et al. (2007), found that 97–99% of the contamination were removed, indicating that the analyzed Mn portion in our study was not surface contamination but true toenail Mn inside the nail matrix. Utilizing toenails instead of blood and urine, however, may present its own limitations. For example, the different toes may have slightly different growth rates, and growth rates may vary across individuals. Additionally, subjects need to be told to allow their toenails to grow for at least 2 weeks to ensure collecting enough toenail tissue. Additionally, it is unclear how utilizing foot products may affect tissue concentrations.
There are several limitations to the accuracy of the exposures estimated by the model used. Each individual’s respirator protection factor may be different due to how well the respirator seals to the individuals’ face and the accuracy of the reported frequency of use. Additionally, the weighting factors for variables such as welding type, base metal, and enclosure factor may not be fully representative of this study population, as they were derived from the Hobson et al. (2011) study, which compared 66 other studies to see how these factors may affect air Mn concentration. Additional uncertainty could arise from the arc time and past working condition factors as they were based on self-reported estimates. Because our welder subjects have all worked at the current employer for at least the past 3 years, it is likely that the exposure model is more accurate for the shorter time windows (i.e. past year and shorter). Finally, since the variability of the exposure in a career welder cohort is relatively small, e.g. compared to the seasonal workers analyzed in other studies, Mn exposure for the three different time windows explored in our study is strongly correlated with each other. This does not allow to truly set apart one exposure window as the sole cause for the toenail Mn levels. Rather our conclusions were based on the best regression results and the result from the mixed-effect model.
Conclusions
Our results indicate that toenail Mn may serve as a highly sensitive and specific biomarker of occupational exposure to Mn. Measurements of toenail Mn are sensitive enough to distinguish exposed from unexposed subjects, as well as to distinguish exposure above or below the TLV. Finally, our study confirmed that toenail Mn levels best reflect exposure to Mn 7–12 months prior to the toenail being clipped. The consistency of this result with two prior studies and with the growth rate of nail tissue strongly supports toenail Mn as a biomarker of exposure to Mn in humans.
Supplementary Material
Supplementary data are available at the Annals of Work Exposures and Health online.
Conflict of Interest Statement
The authors declare no conflict of interest relating to the material presented in this Article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.
Supplementary Material
Acknowledgements
This research was supported by grants NIEHS R01 ES020529 and CDC/NIOSH T03 OH008615. The authors thank David Smigiel for useful discussions and advice. We would also like to thank Dr. Karl Wood and Arlene Roth for help with the ICP-MS analysis of our samples.
References
- Apostoli P, Lucchini R, Alessio L (2000)Are current biomarkers suitable for the assessment of manganese exposure in individual workers?Am J Ind Med; 37: 283–90. [DOI] [PubMed] [Google Scholar]
- Bader M, Dietz MC, Ihrig A et al. (1999)Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occup Environ Health; 72: 521–7. [DOI] [PubMed] [Google Scholar]
- Bainter J. (2014)Efficacy of cleaning method for removal of exogenous welding fume contamination from nail tissue prior to use as a biomarker for welding fume manganese exposure MS Thesis. West Lafayette, IN: Purdue University. [Google Scholar]
- Baker MG, Stover B, Simpson CD et al. (2015)Using exposure windows to explore an elusive biomarker: blood manganese. Int Arch Occup Environ Health; 89: 679–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard AM. (1995)Biokinetics and stability aspects of biomarkers: recommendations for application in population studies. Toxicology; 101: 65–71. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E et al. (2006)Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology; 27: 315–26. [DOI] [PubMed] [Google Scholar]
- Carvalho TC, Peters JI, Williams RO 3rd (2011)Influence of particle size on regional lung deposition–what evidence is there?Int J Pharm; 406: 1–10. [DOI] [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C et al. (2013)Hair as a biomarker of environmental manganese exposure. Environ Sci Technol; 47: 1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Piccola G, Marreilha dos Santos AP et al. (2007)Putative proteins involved in manganese transport across the blood-brain barrier. Hum Exp Toxicol; 26: 295–302. [DOI] [PubMed] [Google Scholar]
- Garland M, Morris JS, Rosner BA et al. (1993)Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev; 2: 493–7. [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC et al. (2014)Toenail metal concentration as a biomarker of occupational welding fume exposure. J Occup Environ Hyg; 11: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL et al. (2006)Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol; 202: 381–90. [DOI] [PubMed] [Google Scholar]
- Hobson A, Seixas N, Sterling D et al. (2011)Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg; 55: 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet P, Vanmarcke E, Geens T et al. (2012)Manganese in plasma: a promising biomarker of exposure to Mn in welders. A pilot study. Toxicol Lett; 213: 69–74. [DOI] [PubMed] [Google Scholar]
- Järvisalo J, Olkinuora M, Kiilunen M et al. (1992)Urinary and blood manganese in occupationally nonexposed populations and in manual metal arc welders of mild steel. Int Arch Occup Environ Health; 63: 495–501. [DOI] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV et al. (2007)Association between total ingested arsenic and toenail arsenic concentrations. J Environ Sci Health A Tox Hazard Subst Environ Eng; 42: 1827–34. [DOI] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF et al. (2011)Toenail, blood, and urine as biomarkers of manganese exposure. J Occup Environ Med; 53: 506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HF, Hewitt PJ, Hicks R (1978)A study of pulmonary deposition, and the elimination of some constituent metals from welding fume in laboratory animals. Ann Occup Hyg; 21: 363–73. [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, Paes CR, Pontes AM et al. (2009)High levels of hair manganese in children living in the vicinity of a ferro-manganese alloy production plant. Neurotoxicology; 30: 1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Baron M, Schäper M, Knapp G et al. (2013)The neurobehavioral impact of manganese: results and challenges obtained by a meta-analysis of individual participant data. Neurotoxicology; 36: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (2004)NIOSH Respirator Selection Logic. Cincinnati, OH: DHHS – NIOSH. Publication No. 2005–100. [Google Scholar]
- Nicas M, Neuhaus J (2004)Variability in respiratory protection and the assigned protection factor. J Occup Environ Hyg; 1: 99–109. [DOI] [PubMed] [Google Scholar]
- Raab A, Feldmann J (2005)Arsenic speciation in hair extracts. Anal Bioanal Chem; 381: 332–8. [DOI] [PubMed] [Google Scholar]
- Reiss B, Simpson CD, Baker MG et al. (2015)Hair manganese as an exposure biomarker among welders. Ann Occup Hyg; 60: 139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels HA, Bowler RM, Kim Y et al. (2012)Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology; 33: 872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ et al. (2006)Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res; 1118: 222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick MJ, Nriagu JO (2006)Validity of human nails as a biomarker of arsenic and selenium exposure: a review. Environ Res; 102: 125–39. [DOI] [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R et al. (2007)Biomarkers of Mn exposure in humans. Am J Ind Med; 50: 801–11. [DOI] [PubMed] [Google Scholar]
- Takeda A. (2003)Manganese action in brain function. Brain Res Brain Res Rev; 41: 79–87. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture.(2015)Nutrient Content. Retrieved from USDA Nutrient Data Laboratory Available at http://fnic.nal.usda.gov/food-composition/usda-nutrient-data-laboratory. Accessed 15 June 2015.
- Yaemsiri S, Hou N, Slining MM et al. (2010)Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol; 24: 420–3. [DOI] [PubMed] [Google Scholar]
- Zheng W, Fu SX, Dydak U et al. (2011)Biomarkers of manganese intoxication. Neurotoxicology; 32: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.