Abstract
Babesiosis treatment failures with standard therapy have been reported, but the molecular mechanisms are not well understood. We describe the emergence of atovaquone and azithromycin resistance associated with mutations in the binding regions of the target proteins of both drugs during treatment of an immunosuppressed patient with relapsing babesiosis.
Keywords: Babesia microti, drug resistance, atovaquone, azithromycin, tick-borne diseases
Babesia microti is an intraerythrocytic tick-borne protozoan that can lead to organ failure and death in immunocompromised patients [1]. Infectious Diseases Society of America (IDSA) guidelines recommend atovaquone (ATV) and azithromycin (AZ) for mild/moderate babesiosis and clindamycin and quinine for severe disease [2]. For immunocompromised patients, treatment failures with ATV and AZ have been reported, and experts recommend treatment for ≥6 weeks [1]. The molecular basis for treatment failure is poorly characterized but is likely to be related to mutations in the binding regions of both drugs’ molecular targets [3, 4]. ATV targets the parasite mitochondrial electron transport chain by binding to the cytochrome b protein (CYTb) [5], while AZ inhibits protein translation in a specialized organelle (the apicoplast) [6]. We describe a case of babesiosis in an immunocompromised patient with ATV/AZ treatment failure associated with mutations in the ATV-binding region of CYTb, encoded by the cytb gene, and the AZ-binding region of ribosomal protein L4 (RPL4), encoded by the rpl4 gene.
CASE REPORT
The patient was an 81-year-old man with chronic lymphocytic leukemia, diagnosed in 2008 and treated at that time with bendamustine and rituximab, resulting in remission. In February 2015, he developed new-onset fatigue associated with hemolytic anemia and thrombocytopenia. The initial diagnosis was presumptive autoimmune hemolytic anemia, which was treated with prednisone, intravenous immunoglobulin, and weekly rituximab for 2 months without a clinical response. Between February and July 2015, the patient’s hemoglobin level reached its nadir at 6.6 g/dL, with undetectable haptoglobin level, and he was transfused 8 units of red blood cells. In June 2015, he was referred to our institution, where a peripheral blood smear was positive for intraerythrocytic inclusions consistent with Babesia and was positive for B. microti DNA by polymerase chain reaction (PCR). Parasitemia was 0.3% and B. microti immunoglobulin (Ig) G and IgM results were negative.
Although the patient did not live in a Babesia-endemic area, the prior summer he reported outdoor activity in the Hudson Valley, a Babesia-endemic region of New York State, but he did not recall a tick bite. Treatment was initiated with AZ (500 mg/d) and ATV (750 mg twice daily), with resolution of symptoms and prompt clearance of parasites, as seen on a blood smear 1 week after the start of treatment. The patient completed 6 weeks of ATV/AZ treatment, including >4 weeks after parasite clearance and had a total of 4 negative follow-up blood smears before discontinuing treatment, although B. microti DNA was detected after 6 weeks of treatment. One month later, the patient experienced worsening hemolytic anemia, with Babesia parasitemia of 0.3% on a peripheral blood smear. Treatment with ATV and AZ was reinitiated; however, over the next several weeks, the patient had increasing parasitemia (up to 1%) and hemolytic anemia requiring transfusion (Figure 1A).
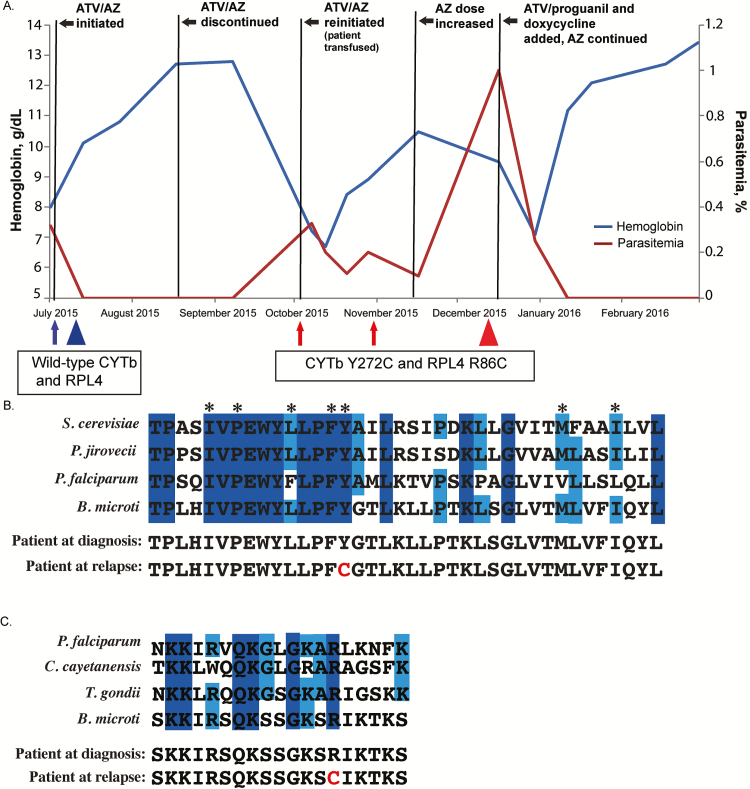
Figure 1.
A, Case patient’s clinical and treatment course, including hemoglobin values and parasitemia. Five residual samples were collected at different times during the course of infection. Arrows and triangles indicate when samples were obtained; arrows denote samples from which only cytb was sequenced; triangles, samples from which cytb and rpl4 were sequenced. Blue represents the presence of wild-type cytb and rpl4 alleles at initial diagnosis; red, the presumed resistant alleles after relapse following a 6-week course of atovaquone/azithromycin (ATV/AZ). B, Amino acid residue sequence conservation of the ATV-binding pocket. Alignment of Babesia microti and model organisms’ cytochrome b (CYTb) protein sequences: Saccharomyces cerevisiae, Pneumocystis jirovecii, and Plasmodium falciparum. Residues comprise the QO site. Dark- and light-blue shading indicate residues that are 100% or 75% conserved, respectively. Asterisks indicate residues previously described as harboring important side-chain interactions with ATV. Amino acid alignment of the case patient’s predicted CYTb sequences before and after relapse are shown, with the amino acid change at position 272 noted in red . C, Amino acid alignment of putative AZ-binding pocket of ribosomal protein L4 (RPL4), comparing B. microti and model organisms, P. falciparum, Cyclospora cayetanensis, and Toxoplasma gondii. Alignment demonstrates the patient’s predicted RPL4 sequence before and after treatment. Dark- and light-blue shading indicate residues that are 100% or 75% conserved, respectively. The amino acid change corresponding to presumed resistance is highlighted in red . All sequences were obtained from the EuPathDB database (eupathdb.org).
Dosages for ATV and AZ were increased 1750 and 1000 mg/d, respectively, and proguanil (400 mg/d) and doxycycline (100 mg twice daily) were added to the regimen. The haptoglobin level normalized at 60 days and B. microti PCR results were negative at 102 days after the start of the 4-drug regimen. Treatment with this regimen continued for 2 months, then doxycycline was discontinued, and treatment with ATV/proguanil and AZ was maintained for 6 months to achieve clinical cure, confirmed by repeated negative peripheral blood smears and B. microti PCR. In January 2017, 1.5 years from diagnosis, the patient remained without blood smear or PCR evidence of B. microti infection or B. microti IgG and IgM. We investigated the molecular basis of treatment failure.
METHODS
Residual samples were collected as part of an ongoing institutional review board–approved babesiosis registry at New York–Presbyterian Hospital/Weill Cornell Medical Center between July 2014 and July 2016. In addition to the case patient’s samples, 5 control B. microti samples from 5 patients without relapse were analyzed. B. microti genomic DNA was isolated from whole blood or material scraped from slides using standard methods (Supplementary Material) [7]. Nucleic acid amplification and direct Sanger DNA sequencing of the cytb and rpl4 genes were performed (Supplementary Material). Sequences were aligned against the B. microti reference genome for cytb or downloaded from published sources for rpl4 [7]. DNA and protein alignments were constructed.
RESULTS
Sequences of the cytb and rpl4 genes obtained from peripheral blood samples taken at different time points during the course of infection revealed the development of point mutations in both genes. Wild-type cytb and rpl4 were observed in peripheral blood samples taken at the time of diagnosis. However, after the initial 6-week ATV/AZ treatment course, resistant haplotypes emerged (Figure 1A). Specifically, in samples from October 2015, an adenine-to-guanine transition at position 815 in cytb was noted that was also present in samples from November and December 2015. This mutation correlates to an amino acid substitution from tyrosine (Y) to cysteine (C) at position 272 in CYTb (Figure 1B). Analysis of the rpl4 gene from a recrudescent sample in December 2015 revealed a substitution from arginine (R) to cysteine (C) at position 86 in RPL4 (Figure 1C). The sequences of PCR products derived from our control samples all lacked these cytb and rpl4 mutations. Control and case samples all harbored 4 identical silent single-nucleotide polymorphisms in cytb compared with the B. microti reference genome, potentially reflecting geographic strain variation.
Alignment of the B. microti CYTb ubiquinol-binding pocket (QO domain) with that of other organisms supports the role of the identified Y272C mutation in rendering the parasite less susceptible to ATV (Figure 1B). This mutation, and other mutations in this highly conserved QO domain of CYTb, have been associated with decreased susceptibility to ATV in Plasmodium species [5, 8] Toxoplasma gondii [9], and Pneumocystis jirovecii [10]. Similarly, mutations adjacent to R86 in a highly conserved region of RPL4 have been implicated in AZ resistance in Plasmodium and some bacterial species [6, 11].
DISCUSSION
We report a case of relapsing babesiosis associated with molecular evidence of both ATV and AZ resistance that arose de novo during the patient’s prolonged treatment course. One previous report also found evidence for cytb and rpl4 mutations as potential determinants of ATV/AZ resistance in B. microti. Lemieux and colleagues [4] performed whole-genome sequencing of 42 B. microti isolates and identified several different variants associated with relapsing disease. In that study, all 5 relapsing cases harbored various amino acid substitutions in the ATV-binding region of CYTb and 2 of 5 relapsing cases had mutations in the AZ-binding region of RPL4. The Y272C mutation we identified was not detected in that study but has been described in isolates of Plasmodium species associated with reduced susceptibility to ATV [8]. The RPL4 R86C mutation we observed was also identified by Lemieux et al [4] in a patient with relapsing babesiosis [4]. Our results corroborate the findings that mutations in CYTb and/or RPL4 are important resistance determinants and associated with relapse. Moreover, through testing of samples at multiple time points in our patient’s treatment course, we provide additional insight into the timing of ATV and AZ resistance.
The presence of wild-type cytb and rpl4 sequences in our patient’s pretreatment samples and all control samples from patients with nonrelapsing babesiosis suggests that drug exposure, rather than primary acquisition of a resistant genotype, led to drug resistance mutations and clinical treatment failure. This finding is supported by a hamster model in which ATV-resistant B. microti parasites emerged during ATV therapy; when the parasites were transferred to a new hamster, ATV treatment was ineffective [12]. Our patient’s chronic lymphocytic leukemia combined with rituximab, a monoclonal antibody inhibitor of B cells, probably facilitated the development of resistance by hampering immunologic clearance.
The deleterious effects of rituximab on B. microti clearance are well described, including a report of persistent infection for >2 years in a patient with rheumatoid arthritis who was seronegative for B. microti antibodies for 14 months after diagnosis, which resembles the prolonged seronegativity documented in our patient [1, 13]. Thus, we hypothesize that profound humoral immunodeficiency led to incomplete parasite clearance and, in the presence of ongoing antimicrobial pressure, induced point mutations in the cytb and rpl4 genes, resulting in amino acid changes in the ATZ and AZ target protein–binding sites.
Our case underscores challenges in determining optimal drug regimens and dosing in the management of immunocompromised patients with babesiosis. Prolonged use of clindamycin and quinine is precluded by high rates of toxicity [14]. Higher doses of AZ (600–1000 mg/d) have been suggested for immunocompromised patients [14], but it is unclear whether such dosing would prevent the development of resistance. Reports of adding proguanil and/or doxycycline to standard regimens in relapsing babesiosis have demonstrated cure, but evidence is limited to case series [14]. Additional studies are needed to better define optimal treatment regimens for ATV/AZ failures and identify agents with enhanced anti-Babesia activity, particularly as the population receiving immunosuppressants increases and the geographic range of B. microti expands.
Determining treatment duration in immunocompromised patients with babesiosis is also challenging. IDSA guidelines recommend treatment for ≥6 weeks including ≥2 weeks after blood smears are negative [2]. The use of PCR to guide duration of treatment is not recommended, because DNA may be detected for months to years in asymptomatic individuals and is of unclear clinical significance [15]. Although our patient was treated in accordance with current IDSA guidelines and had prompt clearance of parasites from peripheral blood smears, B. microti DNA was detected after the first 6-week ATV/AZ treatment course before relapse. Similarly, his anemia resolved with the initial 6-week ATV/AZ course, but his haptoglobin level remained undetectable and only normalized shortly before B. microti DNA was nondetectable by PCR. Our experience suggests that monitoring Babesia DNA and haptoglobin levels after parasites are no longer detected on peripheral blood smears may be useful to guide treatment duration in immunocompromised patients with babesiosis. Furthermore, lack of an effective immune response, as indicated by persistently negative serological findings, could signal incomplete parasite clearance and increased risk of relapse.
In summary, we report a case of relapsing babesiosis in an immunocompromised patient receiving rituximab, in which ATV and AZ resistance emerged during treatment and was associated with mutations in the ATV-binding region of CYTb and the AZ-binding region of RPL4. Future application of DNA sequencing to identify B. microti resistance mutations associated with relapse may assist in the management of this challenging infection in immunocompromised patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. L. A. K. is the William Randolph Hearst Clinical Scholar. This investigation was supported in part by grant U54RR024385 of the Weill Cornell Clinical and Translational Science Center (L. A. K.). The funding agency had no role in the design, conduct, analysis or reporting of the study.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Krause PJ, Gewurz BE, Hill D et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–6. [DOI] [PubMed] [Google Scholar]
- 2. Wormser GP, Dattwyler RJ, Shapiro ED et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1089–134. [DOI] [PubMed] [Google Scholar]
- 3. Wormser GP, Prasad A, Neuhaus E et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010; 50:381–6. [DOI] [PubMed] [Google Scholar]
- 4. Lemieux JE, Tran AD, Freimark L et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol 2016; 1:16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birth D, Kao WC, Hunte C. Structural analysis of atovaquone-inhibited cytochrome bc1 complex reveals the molecular basis of antimalarial drug action. Nat Commun 2014; 5:4029. [DOI] [PubMed] [Google Scholar]
- 6. Sidhu AB, Sun Q, Nkrumah LJ, Dunne MW, Sacchettini JC, Fidock DA. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J Biol Chem 2007; 282:2494–504. [DOI] [PubMed] [Google Scholar]
- 7. Garg A, Stein A, Zhao W et al. Sequence and annotation of the apicoplast genome of the human pathogen Babesia microti. PLoS One 2014; 9:e107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 2000; 44:2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McFadden DC, Tomavo S, Berry EA, Boothroyd JC. Characterization of cytochrome b from Toxoplasma gondii and Qo domain mutations as a mechanism of atovaquone-resistance. Mol Biochem Parasitol 2000; 108:1–12. [DOI] [PubMed] [Google Scholar]
- 10. Walker DJ, Wakefield AE, Dohn MN et al. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis 1998; 178:1767–75. [DOI] [PubMed] [Google Scholar]
- 11. Pihlajamäki M, Kataja J, Seppälä H et al. Ribosomal mutations in Streptococcus pneumoniae clinical isolates. Antimicrob Agents Chemother 2002; 46: 654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wittner M, Lederman J, Tanowitz HB, Rosenbaum GS, Weiss LM. Atovaquone in the treatment of Babesia microti infections in hamsters. Am J Trop Med Hyg 1996; 55:219–22. [DOI] [PubMed] [Google Scholar]
- 13. Raffalli J, Wormser GP. Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn Microbiol Infect Dis 2016; 85:231–2. [DOI] [PubMed] [Google Scholar]
- 14. Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 2016; 315:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krause PJ, Spielman A, Telford SR 3rd et al. Persistent parasitemia after acute babesiosis. N Engl J Med 1998; 339:160–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.