Abstract
Transgender individuals experience incongruence between their gender identity and birth-assigned sex. The resulting gender dysphoria (GD), which some gender-incongruent individuals experience, is theorized to be a consequence of atypical cerebral sexual differentiation, but support for this assertion is inconsistent. We recently found that GD is associated with disconnected networks involved in self-referential thinking and own body perception. Here, we investigate how these networks in trans men (assigned female at birth with male gender identity) are affected by testosterone. In 22 trans men, we obtained T1-weighted, diffusion-weighted, and resting-state functional magnetic resonance imaging scans before and after testosterone treatment, measuring cortical thickness (Cth), subcortical volumes, fractional anisotropy (FA), and functional connectivity. Nineteen cisgender controls (male and female) were also scanned twice. The medial prefrontal cortex (mPFC) was thicker in trans men than controls pretreatment, and remained unchanged posttreatment. Testosterone treatment resulted in increased Cth in the insular cortex, changes in cortico-cortical thickness covariation between mPFC and occipital cortex, increased FA in the fronto-occipital tract connecting these regions, and increased functional connectivity between mPFC and temporo-parietal junction, compared with controls. Concluding, in trans men testosterone treatment resulted in functional and structural changes in self-referential and own body perception areas.
Keywords: cortical thickness, diffusion tensor imaging, functional connectivity, testosterone, transgender
Introduction
Gender dysphoria (GD) has been theorized to be a consequence of different cerebral sexual differentiation (Swaab 2004). Brain imaging studies, while largely consistent with respect to structural and functional differences among cisgender male and female controls (cisgender, according to new nomenclature, refers to the population which is not transgender; Carabez et al. 2015; Bouman et al. 2016), have been much less consistent in demonstrating atypical or divergent sexual differentiation in the brains of those with GD compared with cisgender control groups. This especially applies to trans men (individuals assigned female at birth with male gender identity, formerly called female-to-male) for whom the available neuroimaging data are relatively scarce, and findings have been diverse and often not replicated (Manzouri et al. 2015; Smith et al. 2015; Kreukels and Guillamon 2016). Some studies, which investigated trans men before the start of androgen treatment, report a cerebral pattern congruent with the sex assigned at birth (female) (Zubiaurre-Elorza et al. 2013; Hoekzema et al. 2015), some found trans men to have similar structural and functional neural characteristics as cisgender males (Rametti et al. 2011a; Simon et al. 2013; Burke et al. 2016, 2014), and others found a pattern wherein trans men differed from both cis male and female control groups (Soleman et al. 2013, 2016; Zubiaurre-Elorza et al. 2013; Junger et al. 2014; Kranz et al. 2014; Manzouri et al. 2015).
A better understanding of the neurobiology of GD is aided by characterizations of the fundamental subjective experiences and basic symptoms associated with this condition. GD in DSM-5 (American Psychiatric Association 2013), or Transsexualism in ICD 10 (World Health Organization 1992), refers to a feeling of distress due to an incongruence between a person's experienced gender and the assigned sex at birth. Body dysphoria and body-related avoidance (e.g., not looking in the mirror or hiding one's body under baggy clothes) (Cohen-Kettenis and Pfäfflin 2010; American Psychiatric Association 2013) are key features of GD, emerging from a strong perception of incongruence between one's sense of self and one's body. The perception of one's own body is molded by a reciprocal interaction between sensory perceptions of one's physical appearance, experiences based on self-observation and reactions of others (Cash and Pruzinsky 2002), and own body image representation in the brain (Vocks et al. 2010a, 2010b).
We recently published a series of studies suggesting that cerebral networks involved in own body perception in the context of self (including the pregenual anterior cingulate cortex [pACC], temporo-parietal junction [TPJ], and fusiform body area) are different/singular in individuals with GD compared with cisgender persons (Savic and Arver 2011, 2014; Manzouri et al. 2015; Feusner et al. 2016b). Other studies in individuals with GD have also reported specific differences in brain morphology and functional brain networks between transgender individuals and cisgender controls, in regions playing a role in body representation, although these studies did not explicitly highlight involvement of these regions in own body perception in the context of self (Luders et al. 2012; Simon et al. 2013; Lin et al. 2014). Possible differences in the own-body and self-perception networks could explain the reported discomfort with the own body in individuals with GD, which leads to the request for cross-sex hormone treatment as well as gender confirming surgery. One theoretical possibility is that in individuals with GD the typical physical traits of their sex assigned at birth are not incorporated into their own body image representation, and that this is associated with specific functional and structural signatures in the brain. Indeed, our more recent studies (Manzouri et al. 2015; Feusner et al. 2016a, 2016b) support this model, as do data from other groups (Kranz et al. 2014; Hahn et al. 2015; Case et al. 2016). Together, these observations of differences in cerebral networks involved in the processing of the own body in the context of self go beyond the proposed model of sex-atypical sexual dimorphism of the brain.
In the quest for a better understanding of the neurobiology of GD, it is also important to investigate how cross-sex hormonal treatment affects the brain in individuals with GD. The primary goal of cross-sex hormone treatment is to align the body phenotype of an individual with GD with his/her experienced gender. Cross-sex hormone treatment induces virilization in individuals assigned female at birth (deepening of the voice, male pattern of body hair growth, cessation of menses) and feminization (breast development) of the body in individuals assigned male at birth (Gooren et al. 2015). Transgender individuals report improved feelings of congruence between their gender identity and their body, that is, remission of GD, with cross-sex hormone treatment (Smith et al. 2014), raising the important question as to whether cross-sex hormone treatment also alters the morphology and function of the brain.
However, only few studies thus far investigated the effects of cross-sex hormone treatment on the brain in a gender dysphoric population. Hulshoff Pol et al. (2006) reported that 4 months after testosterone treatment in trans men there was an increase in total brain volumes and no changes in hypothalamus volumes, compared with cis female controls (untreated) who had significant decreases in hypothalamic volumes with time. A group of trans women (individuals assigned male at birth with a female gender identity), on the other hand, showed a decrease in hypothalamus volumes after 4 months of estradiol treatment. Also, ventricle volumes increased in trans women with estrogen treatment (Hulshoff Pol et al. 2006), a finding that was later confirmed by Zubiaurre-Elorza et al. (2014). Zubiaurre-Elorza et al. (2014) found that testosterone treatment during at least 6 months led to increased cortical thickness (Cth) in left lingual, postcentral, supramarginal, inferior parietal, pericalcarine, right cuneus, rostral middle frontal, and postcentral regions, and increased right thalamus volume in trans men. In another study, trans men showed an increase in fractional anisotropy (FA) values (measured by diffusion tensor imaging [DTI]) in the right superior longitudinal, corticospinal, and the fronto-occipital tract after at least 7 months of testosterone treatment (Rametti et al. 2012). The same sample of trans men had been shown to have greater FA in the same tracts compared with cis female controls, but similar FA as cis male controls, even before the start of testosterone treatment (Rametti et al. 2011a, 2011b). Thus, trans men showed regional male-typical FA before any hormonal intervention, and testosterone treatment had an additional stimulating effect on the white matter microstructure in specific white matter tracts (Rametti et al. 2012). The latter 2 longitudinal studies lacked a control group scanned over the same time period but without intervention, which draws into question the specificity of changes detected in the transgender populations. Recently, Hahn et al. (2016) combined different metrics, that is, gray matter volume, white matter diffusivity, and resting-state functional magnetic resonance imaging (MRI), to investigate the effect of 4 weeks of testosterone treatment in 18 trans men compared with 16 cis female controls. Focusing on language-related areas, their results suggested increased white matter diffusivity and functional connectivity, but reduction of gray matter volume in Broca's and Wernicke's area after testosterone treatment (Hahn et al. 2016).
However, none of the previous studies were able to provide information about possible whole-brain coordinated cerebral changes in response to cross-sex hormone treatment, and their relevance for the subjective changes in self-congruence. In the current study, we therefore used a multimodal neuroimaging approach and a prospective longitudinal design to investigate in a group of 22 trans men changes in brain morphology, and functional and structural connections in neural networks after naturalistic testosterone treatment. We compared pretreatment MRI scans to follow-up scans at least 3 months after the start of testosterone treatment and measured Cth, subcortical volumes, white matter microstructure, and functional connectivity during rest. Additionally, to control for test–retest scanning and the passage of time we scanned 19 heterosexual cis male and female control participants.
The primary hypothesis was that testosterone treatment would increase functional and structural connectivity within self- and own-body perception networks in trans men. We also explored whether testosterone would have specific masculinizing effects on the brain, leading to sexually dimorphic changes, following the pattern of cerebral differences usually found between cisgender men and women. We expected thinning of the cortex in response to testosterone treatment based on our previous finding of a negative correlation between testosterone levels and thickness of the parietal cortex (Savic and Arver 2014), and based on developmental studies of puberty effects on Cth (Herting et al. 2015). Also, recently Hahn et al. (2016) showed reduction of gray matter volume in language areas with testosterone treatment (gray matter volume is a composite metric of Cth and surface area). In line with this tentative masculinization of the brain, we expected enlargement of the amygdala, putamen, and thalamus volumes, and possibly, also a decrease of the hippocampal and caudate volumes, which normally are larger in cis women (Filipek et al. 1994; Giedd et al. 1997; Goldstein et al. 2001; Savic and Arver 2014). Furthermore, we expected increases in FA values given previous reports of FA increases with testosterone (Herting et al. 2012) and higher FA values in cis men compared with cis women (Huster et al. 2009; Inano et al. 2011), and also a decrease in Cth, primarily in the parietal cortex (Lentini et al. 2013).
Materials and Methods
Participants
Twenty-two trans men (mean age in years = 23.0, standard deviation (SD) = 4.9) and 19 cisgender controls (mean age = 30.7, SD = 7.8; 12 females, 7 males) participated in the longitudinal study of possible treatment effects. To assess possible masculine/feminine baseline differences between trans men and controls, we also carried out group comparisons before treatment using an extended control group (the cross-sectional control group) of age-matched 22 heterosexual cis male and 22 heterosexual cis female participants (mean age 26 ± 5 and 28 ± 7; number of years of education 16 ± 2 and 16 ± 3, and mean Kinsey score 0.29 ± 0.46 and 0.45 ± 0.51 for the male and female control groups, respectively). These controls were selected from the larger samples previously described in our cross-sectional study (Manzouri et al. 2015). They had, like the longitudinal control group, more years of education than the group of trans men (F = 9.3, P < 0.001, df = 2).
The trans men were recruited at ANOVA, the integrated center for Transgender Medicine, Karolinska University Hospital (Stockholm, Sweden). During the inclusion period, first of January 2011 to the end of August 2015, there were 143 trans men (aged 18–45 years) who, after diagnostic evaluation, received the diagnosis of Transsexualism F64.0. Eighty-three were excluded due to psychiatric comorbidity (n = 58), ongoing cross-sex hormone therapy (n = 15), or due to unstable social situations that made it difficult for them to participate (n = 10). Out of the 60 invited 38 declined as they did not have time, or did not want to participate in research.
None of the trans men had received hormone treatment at the time of scan session 1, or gender confirmation surgery at the time of scan session one or 2. Individuals on current hormonal treatment, known chromosomal or hormonal disorder, or current psychiatric disorder (as confirmed by the Mini International Neuropsychiatric Interview [MINI]; Sheehan et al. 1998), including body dysmorphic disorder, neurological or other major medical disorder, or any current use of medications with psychotropic effects (antipsychotic or antiepileptic agents, lithium, benzodiazepines or opioid analgesics) were excluded. We also excluded individuals with known autism spectrum disorder (ASD) (diagnosed before being referred to the team) or participants who showed clinical signs of ASD when being assessed by the team. Exclusion criteria for the control group included GD, neurological or psychiatric disorders, substance use disorders, family history of psychiatric disorders, and ongoing medication.
Cisgender controls were excluded if they experienced any major diseases, life trauma, or initiated ongoing medication between the 2 scans. All female control participants had regular menstrual cycles and were investigated during the second week after their menstruation.
The study was approved by the ethical committee of the Karolinska Institute (application number: Dnr 2011/281-31/4) and each participant provided signed informed consent before entering the study.
Both longitudinal groups were scanned twice using MRI: trans men before starting testosterone treatment and again at least 3 months after institution of testosterone (average interscan interval 14.4 ± 6 months, range 6–25.2 months), and controls before and after a period of time without intervention (average interscan interval 34.8 ± 6 months, range 27.6–38.4). In the trans men, treatment duration between scan sessions, and hence actual total duration of exposure to testosterone, was on average 11.3 ± 7.6 months, range 3–31 months.
Sexual orientation was assessed using the self-report Kinsey scale (Kinsey et al. 1948), a 7-point scale ranging from 0 (heterosexual, which was exclusively gynephilic [in cis males], i.e., sexually attracted to females; exclusively androphilic [in cis females], i.e., sexually attracted to males) to 6 (homosexual, exclusively androphilic [in cis males]; exclusively gynephilic [in cis females]). The trans men were informed before answering the questions that the Kinsey scale was constructed for cisgender individuals and asked to interpret “homosexual” as gynephilic and “heterosexual” as androphilic. All participants were tested for handedness according to Oldfield (1971).
Body Perception Test
To explore behavioral responses to the perception of the own body, we carried out a “body perception test,” in which participants, outside the scanner, viewed photographs of their bodies morphed by 20% increments toward either cis male or cis female bodies (for details of the procedure, see Feusner et al. 2016a), and were asked to respond “to what degree is this picture you?”. This allowed us to obtain ratings that index own body identification with images of their body that are morphed to appear more masculine or more feminine.
During each experimental trial, the 62 morphed or unmorphed images depicting the participant's body were randomly presented on the computer screen for either 0.5 or 2 s (short vs. long exposure times). Participants were instructed to respond as quickly as possible after each image to rate the picture based on the degree to which they felt it represented them. The specific question posed was “to what degree is this picture you?” Participants were instructed to press computer keys 1–4 (1 corresponding to 0–25% me, 2 to 25–50% me, 3 to 50–75% me, and 4 to 75–100% me). Next, we calculated an “own body perception index” by multiplying each degree (1–4) of “self” rated for each morph with the degree of each morph. Positive values indicated morphs to their sex assigned at birth (0.20, 0.40, 0.60, 0.80, and 1.00) and negative values for morphs to the sex congruent with their gender identity, and opposite assigned sex at birth (−0.20, −0.40, −0.60, −0.80, and −1.00). These weighted values were averaged for each participant across ratings for all 62 images and then divided by the number of rated images, providing an average index of self-perception for each participant weighted by how close or far from the actual self-photograph the image was morphed, and in which direction. Seventeen of the twenty-two trans men performed the body perception test at both time points (this part of the experiment was added after MRI data collection had begun). The data of the body perception test were not compared with the cross-sectional controls, as in our previous studies we did not detect any sex differences in the body perception index, and the comparisons between trans men and controls did not show any specific gender interaction (Feusner et al. 2016a, 2016b).
Hormone Assessments and Testosterone Treatment
In trans men, hormone levels in serum were assessed by routine clinical checkups, and we used the assessment closest in time to the MR sessions for the purpose of this study. No blood samples were collected in cisgender controls. Naturalistic testosterone treatment, per clinic protocol, consisted of intramuscular injections of Nebido (Testosterone undecanoat Bayer) 1000 mg every 12 weeks. See Table 1 for hormone levels of trans men, before and after testosterone treatment.
Table 1.
Subject characteristics
| Trans men pretreatment | Trans men during treatment | Controls first visit | Controls second visit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range | |
| Age in years | 22 | 23.0 (4.9) | 18–34 | 22 | 24.2 (5.0) | 19–35 | 19 | 30.7 (7.8) | 20–44 | 19 | 33.6 (7.6) | 23–46 |
| Years of education | 22 | 13.0 (2.0) | 9–17 | 18 | 16.4 (3.4) | 13–24 | ||||||
| Handednessa | 20 | 89.6 (20.1) | 12.5–100 | 19 | 86.9 (20.0) | 17.7–100 | ||||||
| Sexual orientationb | 22 | 4.7 (1.5) | 1–6 | 20 | 4.2 (1.5) | 1–6 | 19 | 0.4 (0.5) | 0–1.5 | |||
| Body perception index, short trials | 17 | −3.0 (28.6) | −48.4–61.4 | 17 | −9.4 (17.0) | −50.4–17.3 | ||||||
| Body perception index, long trials | 13 | −1.6 (25.5) | −42.2–42.5 | 13 | −15.1 (21.2) | −54.8–23.3 | ||||||
| Estradiol levels (in pmol/L) | 21 | 477.4 (456.5) | 125.0–1680.0 | 8 | 165.0 (32.2) | 150.0–243.0 | ||||||
| Testosterone levels (in nmol/L) | 22 | 1.8 (0.7) | 1.2–3.5 | 21 | 22.4 (13.1) | 1.8–51.0 | ||||||
| SHBG levels (in nmol/L) | 22 | 45.1 (19.1) | 10.0–90.0 | 18 | 26.1 (9.7) | 9.9–49.0 | ||||||
| Free androgen indexc (in nmol/L) | 22 | 5.2 (3.7) | 2.0–17 | 18 | 94.8 (55.8) | 4.2–242.9 | ||||||
aHandedness was assessed according to Oldfield (1971).
bSexual orientation was assessed using the Kinsey scale (Kinsey et al. 1948).
cThe Free Androgen Index is the total testosterone level divided by the sex hormone binding globulin (SHBG) level × 100.
Data Acquisition
MRI data were acquired on a 3-Tesla MRI scanner (Discovery 3 T GE-MR750, General Electric) equipped with a 32-channel or 8-channel phased array receiving coil. 3D T1-weighted Spoiled Gradient Echo pulse sequence (SPGR) images were acquired with 1 mm3 isotropic voxel size (time echo [TE] = 3.1 ms, time repetition [TR] = 7.9 ms, time to inversion [TI] = 450 ms, field of view = 24 cm, 176 axial slices, flip angle 12°). Resting-state functional MRI (fMRI) was performed with a gradient echo pulse sequence using a voxel size of 2.25 × 2.25 × 3 mm (TE = 30 ms, TR = 2500 ms, FoV = 28.8 cm, 45 bottom up interleaved axial slices, 3 mm thickness, flip angle 90°). During the fMRI session, which lasted 8 min, participants were instructed to close their eyes, not to try to solve any special task but just “let the mind wander,” and to try not to fall asleep. In addition, multislice diffusion-weighted imaging was performed using an echo planar imaging sequence with 1 × 1 mm in-plane resolution (TE = 83 ms, TR = 8000 ms, FoV = 24 cm, 60 interleaved axial slices, thickness = 2.9 mm, 60 diffusion gradient directions [b = 1000], flip angle 90°). For all sequences, we used a 32-channel phased array receiving coil, except for the T1 sequence for which we used an 8-channel coil because it provided better demarcations between white and gray matter in the occipital cortex for the purposes of the Freesurfer analyses.
Cth and Subcortical Volume Analyses
Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis suite, version 5.1 (Fischl and Dale 2000) (www.surfer.nmr.mgh.harvard.edu) to derive Cth, subcortical volumes, and total intracranial volume (ICV). The T1-weighted images were processed using the Freesurfer Longitudinal Stream, in which an unbiased within-subject template space and average image (Reuter and Fischl 2011) are created using robust, inverse consistent registration (Reuter et al. 2010). The resulting surface models were visually inspected for accuracy and manually edited for all participants.
Group differences and the percentage of change (pc1) in Cth from time 1 to time 2 [(Cth2 − Cth1/interscan interval)/Cth1] were evaluated with the QDEC toolbox of Freesurfer. Bilateral maps were analyzed at a vertex-wise level using a general linear model approach, after registration to the Montreal Neurological Institute (MNI) standard space and smoothing with a Gaussian kernel of 10 mm full-width at half-maximum (FWHM). To control for multiple comparisons, cluster correction was done using Monte Carlo simulation with 5000 iterations (vertex-wise threshold of P < 0.05). Demeaned age and number of years of education were used as nuisance covariates for the pretreatment (session 1) between-group comparison.
Subcortical segmentations generated with FreeSurfer (Fischl et al. 2002, 2004) were used to calculate the volumes of 5 subcortical brain structures: amygdala, hippocampus, caudate nucleus, putamen, and thalamus. These were chosen because they typically show sex differences in volume (Savic and Arver 2011, 2014; Lentini et al. 2013). Furthermore, these structures also express high androgen receptor densities (Clark et al. 1988; Simerly et al. 1990) and could thus be affected by the testosterone treatment. In addition, we assessed total ICV. Using SPSS Statistics 21 (SPSS Inc.), we performed multivariate ANOVA of regional volumes corrected for ICV and age, to compare trans men to both cross-sectional and longitudinal cis controls at session 1. To investigate the effects of the testosterone treatment on changes in subcortical volumes, we performed repeated-measures multivariate ANOVA of regional volumes with session as within-subject factor and group as between-subject factor. Time difference (in years) between scan sessions was used as covariate. No Bonferroni correction was employed as there is specific evidence from previous studies of sex differences that testosterone could, theoretically, affect each of these structures (see Introduction).
Fractional Anisotropy
Diffusion images were analyzed and corrected for (motion) artifacts and eddy current distortions using DTIPrep (Oguz et al. 2014). Using DTIfit, part of the FMRIB's Diffusion Toolbox implemented in FSL v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/), images were realigned to one of the nonweighted images using affine registration, nonbrain tissue removed using BET (part of FSL), and finally a tensor model was fit to the diffusion data, defining the eigenvalues of the tensor for each voxel to calculate individual FA maps. Voxel-wise statistical analyses were performed using Tract-Based-Spatial Statistics (TBSS). Participants’ FA maps, from both sessions, were registered to the FMRIB58_FA template, and then transformed to MNI152 space. The normalized individual FA maps were averaged to create a group-wise mean FA white matter skeleton. A threshold of 0.3 was applied to reduce partial volume effects. “Time-difference” FA images were calculated for each participant according to this formula: (session 2 − session 1/interscan interval in years), that is, the rate of change in FA from session 1 to session 2, to control for differences in time between the 2 MR sessions. Finally, these individual aligned time-difference FA images were projected onto the mean FA skeleton for subsequent voxel-wise statistical analyses. We tested for pretreatment differences between trans men and cis controls, separately in relation to the cross-sectional and the longitudinal control group, including age and number of years of education as covariates. We also tested within-group changes between sessions, and group by session interactions. Using Randomize (part of FSL), permutation-based nonparametric testing (5000 permutations) was performed, by applying the Threshold-Free Cluster Enhancement option. Results were considered significant at PFWE < 0.01 (family-wise error corrected) and a minimal cluster size of k = 100. Anatomical locations were identified using the JHU White Matter Tractography atlas and JHU ICBM-DTI-81 white matter labels (Mori et al. 2005; Wakana et al. 2007; Hua et al. 2008).
Seed Region Functional Connectivity Analysis
Spatial preprocessing and statistical analysis of functional images were performed using SPM8 (Welcome Department of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk/spm/). The functional images were slice-time corrected, realigned, and registered to structural T1 SPGR images for each participant. After segmenting the individual T1 SPGR images into gray matter, white matter, and cerebrospinal fluid, the gray matter images were used to determine the normalization parameters for the standard MNI gray matter template. At this point, the spatial parameters were applied to the slice-timed and realigned functional volumes that were resampled to 1.5 × 1.5 × 1.5 mm voxels and smoothed with a 6-mm FWHM kernel. Each voxel's time series was corrected for noise using the SPM standard 128-s high-pass filter combined with AR auto correlation correction modeling. In addition, we employed voxel-wise multidimensional regression analysis in a standardized manner to remove artifacts resulting from motion and changes in ventricle and white matter signals (Verhagen et al. 2006), by adding 18 movement regressors; 6 parameters obtained from rigid-body head motion correction (SPM 8 statistical package), and their squares and cubes. For all participants, the average fMRI time course within the seed region was used as the regressor of interest. Individual time series in each seed region was extracted using MarsBar toolbox (http://marsbar.sourceforge.net/). The seed region in time course for each participant was then regressed voxel-wise against the participant's fMRI time course using the entire brain as the search space. This approach reveals the strength of functional connectivity with respect to the seed region. In correspondence with our previous study (Manzouri et al. 2015), the seed regions used were the left and right TPJ, and left and right pACC. The rationale for using these regions is that they have been found to be involved in own body perception (TPJ) (Blanke et al. 2002; Hodzic et al. 2009; Vocks et al. 2010a, 2010b; Blanke 2012) and in self-referential processing (pACC) (Northoff et al. 2006; Northoff 2016). All seeds were defined as spheres with 5-mm radii. The spheres’ center MNI coordinates for the TPJ were −51 −60 25 (left) and 51 −60 25 (right), and for the pACC −6 45 0 (left) and 6 45 0 (right).
Using SPM, first, pretreatment (session 1) group differences were tested by means of independent t-tests for each seed region, comparing cis controls with trans men, and using age as a covariate. In order to investigate the effects of the testosterone treatment, we then conducted paired t-test analyses for each seed region within the trans men group for time one versus time 2. Results are reported at a cluster-level threshold of PFWE < 0.05 and a minimal cluster size of k = 50.
Functional MRI data were available for 22 trans men and 19 cis controls. We did not carry out separate pretreatment comparisons between trans men and the cross-sectional control groups, because these data have already been reported in one of our earlier studies, and showed no specific gender interaction (Manzouri et al. 2015). There were technical problems during image acquisition in one trans man and one male control. Furthermore, there was a change of MRI head-coil before the follow-up fMRI data collection of the cis controls, and these data sets had to be discarded for the longitudinal aspects of the functional connectivity analyses, which were, therefore, restricted to 21 trans men. No coil effects were detected on the DTI data. This was tested by a post hoc TBSS comparison between 2 groups of age- and sex-matched persons investigated before (21 females, mean age = 23.3, SD = 5.0) and after (20 females, mean age = 24.7, SD = 6.2) change of the coil which showed no significant difference with PFWE < 0.05, F = 0.9, df = 1.
Results
Demographics, Body Perception Test
Data are presented in Table 1. The control group was significantly older (t(39) = 3.9, P = 0.001) and had more years of education (t(38) = 3.8, P = 0.001), but did not differ from the trans men with regard to handedness (t(37) = 0.4, P = 0.667). Testosterone levels in trans men were in the range of cis females before treatment, and within the cis male range during testosterone treatment. Sexual orientation differed significantly between the groups (t(39) = 12.4, P < 0.001); trans men rated themselves on average as gynephilic. Nineteen trans men were exclusively gynephilic (homosexual according to sex assigned at birth) whereas 3 were attracted to men or both sexes (nonhomosexual according to sex assigned at birth). Cis female controls rated themselves as exclusively androphilic and cis male controls as exclusively gynephilic. All except 2 trans men reported tomboyish behavior before the age of 7 and GD as adults. Two reported that they started to show tomboyish behavior at the ages of 12 and 15, respectively.
Complete data for both sessions on the body perception test were available for 17 trans men. Four of these had a high proportion of nonresponses on the long exposure trials (2 s), so a reliable body perception index could not be calculated for them. Paired t-tests revealed no significant change in body perception indices (long trials: one-tailed t(12) = 1.4, P = 0.093; short trials: one-tailed t(16) = 0.9, P = 0.191), although from before to after testosterone treatment the mean body perception scores became more in line with their experienced gender (see Table 1 and Fig. 1).
Figure 1.
Scores on the body perception test of 17 trans men as a function of task condition (short, long) and treatment condition. Before T and during T relates to testosterone treatment.
Analyses of Testosterone-Related Changes in Cth
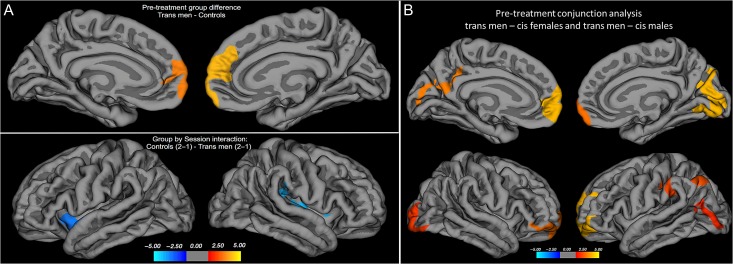
Before treatment, the cortex in trans men was significantly thicker than in the longitudinal control group in the left middle frontal and bilateral superior frontal gyri (see Table 2B and Fig. 2A). An additional repeated-measures analysis, using the extracted mean values of Cth for these 2 frontal clusters (left: x y z = −10 62 −4, 8.2 cm2; right: x y z = 9 59 9, 11.6 cm2) of both groups and both sessions as inputs, with age and time between scans as nuisance covariates, revealed that this group difference in Cth remained significant during testosterone treatment in trans men (significant main effects of group, left: F37,1 = 9.4, P = 0.004; right: F37,1 = 12.4, P = 0.001). No main effects of time or time by group interactions for these regional mean values of Cth were found. Group comparisons at baseline and including the 44 cross-sectional controls confirmed our previous finding of a generally thicker cortex in cis female versus male controls, with exception of the left superior temporal cortex, which was thicker in cis male controls (see Table 2B). It also showed that cerebral cortex in the mesial prefrontal and precuneus regions was thicker in trans men in relation to both cis male and cis female controls, confirmed by conjunctional analysis of common clusters between (trans men–cis female controls) and (trans men–cis male controls), using P < 0.01 Monte Carlo correction for multiple comparisons (see Fig. 2B). These findings were in agreement with our previous publication (Manzouri et al. 2015) and also generally with the clusters detected when comparing our trans men population with the more restricted, gender mixed longitudinal control group (see Fig. 2A, Table 2B, Supplementary Table 8, and also Methodological considerations section).
Table 2.
Group differences and group by session interaction effects for vertex-wise Cth comparisons
| Region | Max. −log10(P) | Cluster size, cm2 | Talairach coordinates | P value |
|---|---|---|---|---|
| A: cross-sectional comparisons | ||||
| Session 1: 22 cis female–22 cis male controls | ||||
| L superior temporal | −4.6 | 11.9 | −53 1–7 | <0.01 |
| L superior frontal | −3.0 | 13.2 | −21 21 34 | <0.01 |
| B: longitudinal comparisons | ||||
| Session 1: Trans men–controls | ||||
| L rostral middle frontal | 4.6 | 7.9 | −39 27 22 | <0.01 |
| L caudal middle frontal | 4.2 | 4.9 | −41 16 44 | <0.01 |
| L superior frontal | 3.3 | 8.2 | −10 62–4 | <0.01 |
| R superior frontal | 4.4 | 11.6 | 9 59 9 | <0.01 |
| Group by session interaction: controls (session 2–session 1) > Trans men (session 2–session 1) | ||||
| L superior temporal | −2.5 | 14.7 | −50 –3 −9 | <0.05 |
| R supramarginal | −2.7 | 17.2 | 43 −35 16 | <0.05 |
Part A: cross-sectional, pretreatment comparisons of 22 cis female and 22 cis male controls; Part B: longitudinal comparisons of 22 trans men and 19 male and female cis controls; negative −log10(P) values indicate more changes in trans men than controls; Talairach coordinates indicate location of maximum difference; L, left; R, right.
Figure 2.
(A) Pretreatment group difference in Cth (upper panel) and group by session interaction effect (lower panel) showing increased Cth in trans men during testosterone treatment as compared with before, in comparison with cis controls (longitudinal sample). The contrasts were calculated at P < 0.05, corrected for multiple comparisons (Monte Carlo permutations). (B) Conjunction analysis of pretreatment group differences in Cth in 22 trans men–22 cis female controls and 22 trans men–cis 22 male controls (cross-sectional sample). The contrasts were calculated at P < 0.01, corrected for multiple comparisons (Monte Carlo permutations). The projection of cerebral hemispheres (MR images of the Freesurfer atlas) is standardized. Scale is logarithmic and shows –log10(P), with cool colors indicating negative contrasts, warm colors positive contrasts.
There was a significant whole-brain interaction effect between group and time. Compared with the longitudinal cis control group, trans men displayed a significantly greater change in Cth between scans 1 and 2 in areas covering the bilateral insula and superior temporal gyri (see Table 2B and Fig. 2A). Within-group analyses confirmed that this difference was constituted by a significant increase of Cth in the right insula, left superior temporal gyrus in trans men, in addition to an increase in right lateral occipital cortex, and right superior frontal cortex (P < 0.01, corrected for multiple comparisons using Monte Carlo simulations), which was not significant in group comparisons (see Supplementary Table 3a). No significant between-scan changes were detected in cis controls compared with trans men. However, within-group comparisons in cis controls suggested significant changes in Cth, mainly in the superior frontal, inferior, and superior temporal areas (see Supplementary Table 3b).
Testosterone-Related Changes in Subcortical Volumes
The ICV at baseline and in comparison with the cross-sectional control groups (to account for possible sex interaction) showed significantly larger values in male controls compared with both trans men and cis female controls without any difference between the 2 latter groups (F = 31,2, P < 0.001, df = 1, one-way ANOVA with Scheffe's post hoc test; P < 0.001); the respective mean values in cm3 were 1646 ± 99 cis male controls, 14 124 ± 110 cis female controls, 14 910 ± 126 trans men. An overall group difference was also detected in the left amygdala (F = 3.6, P = 0.33), right caudate nucleus (F = 3.1, P = 0.43), and right hippocampus (F = 4.6, P = 0.01), df = 2 for all comparisons. The right hippocampus was relatively larger in trans men, as well as in female controls compared with male controls (P = 0.04 and P = 0.027 respectively, Scheffe's post hoc test). No other difference between trans men and the cross-sectional control groups was detected. As reported previously, the relative right caudate volume was significantly larger in cis female compared with cis male controls (P = 0.024). Mean subcortical volumes as a function of group and session in the longitudinal groups are presented in Table 3. Before testosterone institution (session 1), there were no significant group differences. Also, there were no significant testosterone-related changes in subcortical volumes in trans men compared with cis controls, when controlling for ICV and time between scans. ICV did not change significantly between scans in any of the groups.
Table 3.
Subcortical volumes as a function of group and session
| Trans men | Controls | ||||
|---|---|---|---|---|---|
| Session | Mean (cm3) | SD | Mean (cm3) | SD | |
| L caudate | 1 | 4.0 | 0.5 | 3.9 | 0.3 |
| 2 | 3.8 | 0.4 | 3.6 | 0.4 | |
| R caudate | 1 | 4.0 | 0.4 | 3.9 | 0.3 |
| 2 | 3.9 | 0.4 | 3.7 | 0.4 | |
| L putamen | 1 | 5.1 | 0.4 | 5.0 | 0.5 |
| 2 | 4.8 | 0.4 | 4.9 | 0.7 | |
| R putamen | 1 | 4.9 | 0.4 | 4.7 | 0.6 |
| 2 | 4.7 | 0.3 | 4.7 | 0.6 | |
| L thalamus | 1 | 6.8 | 0.8 | 7.0 | 0.6 |
| 2 | 7.0 | 0.7 | 6.4 | 0.7 | |
| R thalamus | 1 | 6.9 | 0.6 | 7.0 | 0.7 |
| 2 | 6.9 | 0.6 | 6.4 | 0.7 | |
| L hippocampus | 1 | 4.0 | 0.5 | 4.0 | 0.6 |
| 2 | 4.0 | 0.4 | 4.2 | 0.4 | |
| R hippocampus | 1 | 4.1 | 0.4 | 4.2 | 0.3 |
| 2 | 4.2 | 0.4 | 4.2 | 0.4 | |
| ICV | 1 | 1413.9 | 126.7 | 1490.9 | 168.4 |
| 2 | 1437.9 | 121.0 | 1474.5 | 193.8 | |
L, left; R, right.
Testosterone-Related Changes in FA
Pretreatment comparisons between trans men and the longitudinal cis controls, controlling for age and number of years of education, revealed no significant differences in FA (at PFWE < 0.05). Also, compared with the larger cross-sectional samples of 22 cis male and 22 cis female participants, trans men showed no significant (P ≤ 0.05) differences in FA. Increasing the statistical threshold to P ≤ 0.150 showed that trans men had higher FA values compared with cis female controls in the left superior longitudinal fasciculus and left forceps minor, and trans men relative to cis male controls had lower FA values in the right corticospinal tract (see Table 4). Among the cisgender controls, men showed significantly (P ≤ 0.05) higher FA than women in several white matter tracts (see Table 4).
Table 4.
Baseline group comparisons of FA values
| Region | Cluster size, k | PFWE: .115, .134, .108 | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Trans men–cis female controls | |||||
| L SLF | 1413 | 0.885 | −30 | −32 | 7 |
| L Forceps minor | 265 | 0.866 | −4 | 29 | 0 |
| Cis males–Trans men | |||||
| R CST | 565 | 0.892 | 11 | −27 | −27 |
| Cluster size, k | t-value | MNI coordinates | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Cis male–cis female controls | t | ||||
| L anterior thalamic radiation | 569 | 5.4 | −19 | −6 | 17 |
| L cingulum (cingulate gyrus) | 199 | 4.6 | −17 | 27 | 29 |
| R forceps minor | 125 | 4.3 | 25 | 40 | 20 |
| L forceps minor | 124 | 4.7 | −21 | 47 | −3 |
Comparisons of 22 trans men–22 cis female and 22 male controls are displayed at PFWE < 0.15, and a minimal cluster size of k ≥ 100 voxels; comparisons between 22 cis male and 22 cis female controls are displayed at PFWE < 0.05, cluster thresholded at t = 2.3, and a minimal cluster size of k ≥ 100 voxels.
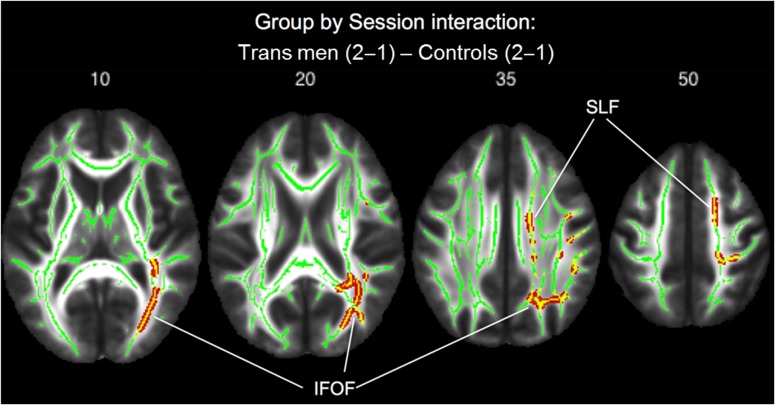
Group comparisons with regard to the rate of FA change over time (group by time interaction effect) revealed significant increases in FA in trans men compared with cis controls, in a cluster located in the right posterior inferior fronto-occipital fasciculus (k = 4718, x y z = 34 −62 −2, PFWE < 0.01), and in addition in 2 clusters of the right superior longitudinal fasciculus (k = 510, x y z = 37 −11 27; k = 138, x y z = 45 −40 35, both PFWE < 0.01) (see Fig. 3). Within-group comparisons confirmed a significant increase in FA after testosterone treatment in trans men in the posterior part of the right inferior fronto-occipital fasciculus (k = 2432, x y z = 24 −76 14, PFWE < 0.01). There were no significant FA changes over time in cis controls. Thus, in trans men, we found right hemispheric increases in FA after testosterone treatment, most pronounced in the posterior portion of the fronto-occipital fasciculus, a white matter tract connecting occipito-parietal with frontal brain regions (Sarubbo et al. 2013).
Figure 3.
Group by session interaction effect of FA values showing significant (PFWE < 0.01) increases in FA (indicated with red–yellow color) with testosterone treatment in trans men in comparison with cis controls. Transverse sections, numbers indicate MNI z-coordinates.
Testosterone Effects on Seed Region Functional Connectivity
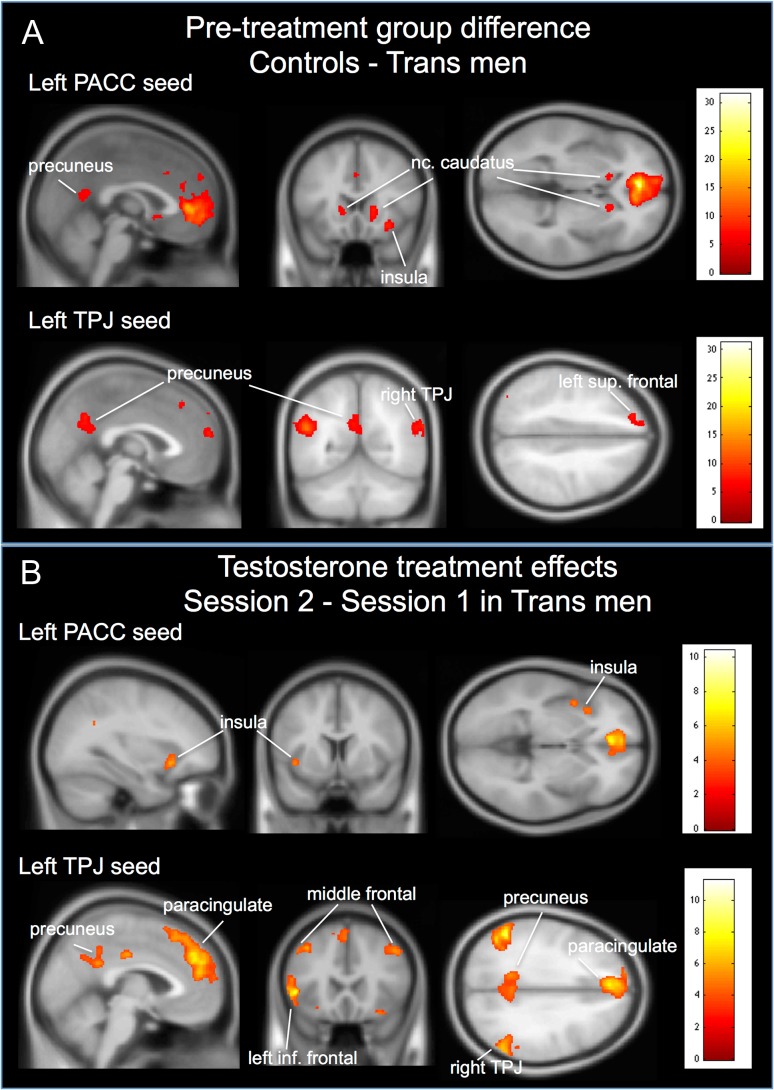
Pretreatment (session 1) group comparisons of functional connectivity revealed significantly (PFWE < 0.001) “weaker” connectivity between the pACC seeds and the right insular cortex, bilateral caudate nucleus, and left precuneus (see Table 5 and Fig. 4A) in trans men. Also for the TPJ seeds, functional connectivity was significantly weaker (PFWE < 0.001) in trans men compared with cis controls with the left precuneus, the contralateral TPJ areas, and several medial frontal and superior frontal areas (see Table 5 and Fig. 4A). There were no areas that showed significantly stronger functional connectivity with any of the seed regions in trans men compared with cis controls.
Table 5.
Group differences in resting-state functional connectivity of selected seed regions between trans men and controls before start of testosterone treatment
| Region | Session 1: Controls > Trans men | Session 1: Trans men > Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak level Z | k | x | y | z | PFWE | Peak level Z | k | x | y | z | PFWE | |
| pACC seed | ||||||||||||
| L pACC | ||||||||||||
| R insular cortex | 6.0 | 170 | 30 | 18 | −12 | <0.001 | n.s. | |||||
| R nc. caudatus | 5.9 | 181 | 16 | 18 | 3 | <0.001 | ||||||
| L precuneus | 5.6 | 554 | −3 | −54 | 16 | <0.001 | ||||||
| L nc. caudatus | 5.5 | 80 | −14 | 18 | 1 | <0.001 | ||||||
| R ant. midtemporal gyrus | 5.3 | 79 | 57 | 0 | −21 | <0.001 | ||||||
| L ant. paracingulate gyrus | 5.2 | 53 | −2 | 23 | 36 | 0.001 | ||||||
| R ant. hypothalamus | 5.0 | 51 | 2 | 10 | −6 | 0.001 | ||||||
| R pACC | ||||||||||||
| R nc. caudatus | 5.5 | 91 | 16 | 20 | –6 | <0.001 | n.s. | |||||
| L ant. cingulate gyrus | 5.4 | 66 | −9 | 24 | 28 | <0.001 | ||||||
| R orbito-frontal cortex | 5.2 | 119 | 40 | 22 | −12 | <0.001 | ||||||
| TPJ seed | ||||||||||||
| L TPJ | ||||||||||||
| L medial frontal gyrus | 6.0 | 164 | −2 | 58 | 18 | <0.001 | n.s. | |||||
| L precuneus | 6.0 | 692 | −4 | –60 | 31 | <0.001 | ||||||
| R TPJ | 5.9 | 497 | 63 | −54 | 22 | <0.001 | ||||||
| R ant. midtemporal gyrus | 5.9 | 87 | 60 | –6 | −26 | <0.001 | ||||||
| L post. midtemporal gyrus | 5.7 | 63 | −57 | −33 | −14 | <0.001 | ||||||
| L frontal pole | 5.6 | 169 | −18 | 42 | 40 | <0.001 | ||||||
| L superior frontal gyrus | 5.5 | 89 | −3 | 33 | 46 | <0.001 | ||||||
| L post. midfrontal gyrus | 5.5 | 56 | −57 | −19 | −17 | 0.001 | ||||||
| L frontal pole | 5.3 | 55 | −15 | 66 | 13 | 0.001 | ||||||
| R TPJ | ||||||||||||
| L TPJ | 7.4 | 2871 | −45 | –75 | 27 | <0.001 | ||||||
| L precuneus | 5.9 | 1070 | −2 | –63 | 24 | <0.001 | ||||||
| R paracingulate gyrus | 5.7 | 101 | 8 | 50 | 12 | <0.001 | ||||||
| R medial frontal gyrus | 5.4 | 116 | 4 | 50 | 39 | <0.001 | ||||||
| R superior frontal gyrus | 5.3 | 53 | 21 | 29 | 46 | 0.001 | ||||||
| L insular cortex | 4.3 | 133 | −33 | 8 | 15 | 0.093 | ||||||
| R insular cortex | 3.8 | 72 | 33 | 12 | 13 | 0.207 | ||||||
x y z = coordinates in MNI space; k = number of voxels; R, right, L, left; results are reported at a cluster-level threshold of P < 0.05, family-wise error (FWE) corrected and a minimal cluster size of 50 voxels result in italic are based on a threshold of P< 0.001, uncorrected and a minimal cluster size of 50 voxels; auto-correlations with the seed region are not listed as results.
Figure 4.
(A) Pretreatment group difference in functional connectivity for the left seed regions pACC and TPJ. (B) Paired t-test results of 22 trans men showing increases in functional connectivity with treatment for the left pACC and TPJ. The figure illustrates that areas with weaker functional connections, notably in the networks processing own body perception in the context of self, show significantly increased connectivity with testosterone treatment. Color bars indicate t-statistic.
Within-group paired t-test comparisons in the trans men revealed a significant (PFWE < 0.001) increase in functional connectivity (session 2 > session 1) for the TPJ seeds with the left paracingulate gyrus, the left inferior frontal gyrus, left precuneus, and the contralateral TPJ areas (Table 6 and Fig. 4B). Decrease in functional connectivity (session 1 > session 2) with testosterone treatment was significant only for the right TPJ seed with a cluster in the left inferior frontal gyrus (Table 6 and Fig. 4B). Thus, among the trans men we found significant changes with treatment in functional connectivity between parietal and frontal regions involved in own body identification in the context of self (see Introduction), which before treatment showed lower functional connections in comparison with cis controls. Follow-up fMRI data were not available for cis controls.
Table 6.
Testosterone treatment effects on resting-state functional connectivity of selected seed regions in trans men
| Region | Session 1 > session 2 (decreased connectivity with treatment) | Session 2 > session 1 (increased connectivity with treatment) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak level Z | k | x | y | z | PFWE | Peak level Z | k | x | y | z | PFWE | |
| pACC seed | ||||||||||||
| L pACC | ||||||||||||
| L insular cortex | 4.3 | 288 | −32 | 20 | −9 | 0.043 | ||||||
| R pACC | ||||||||||||
| L angular gyrus | 4.1 | 290 | −44 | –60 | 16 | 0.043 | ||||||
| R paracingulate gyrus | 4.1 | 294 | 12 | 33 | 24 | 0.040 | ||||||
| TPJ seed | ||||||||||||
| L TPJ | ||||||||||||
| L paracingulate gyrus | 6.3 | 7499 | −6 | 44 | 36 | <0.001 | ||||||
| L inf. frontal gyrus | 5.5 | 868 | −48 | 23 | 1 | <0.001 | ||||||
| R TPJ | 5.4 | 1871 | 52 | −55 | 25 | <0.001 | ||||||
| L precuneus | 4.8 | 1176 | −14 | –49 | 27 | <0.001 | ||||||
| L midtemporal gyrus | 4.5 | 696 | −52 | −25 | −14 | <0.001 | ||||||
| R midtemporal gyrus | 4.5 | 627 | 63 | −28 | −11 | <0.001 | ||||||
| L midfrontal gyrus | 4.4 | 694 | −38 | 21 | 43 | <0.001 | ||||||
| R midfrontal gyrus | 4.4 | 448 | 46 | 20 | 40 | 0.004 | ||||||
| R TPJ | ||||||||||||
| L insular cortex | 4.0 | 364 | −40 | 2 | 3 | 0.016 | ||||||
| L inf. frontal gyrus | 4.7 | 977 | −33 | 30 | 4 | <0.001 | ||||||
| L precuneus | 4.6 | 2035 | −3 | −55 | 22 | <0.001 | ||||||
| L TPJ | 4.4 | 980 | −52 | −66 | 31 | <0.001 | ||||||
x y z = coordinates in MNI space; k = number of voxels; R = right, L = left; results are reported at a cluster-level threshold of P < 0.05, family-wise error (FWE) corrected and a minimal cluster size of 50 voxels; auto-correlations with the seed region are not listed as results.
Post Hoc Cortico-Cortical Covariation Analyses
In order to investigate whether the observed cortical and connectivity changes (particularly the changes in FA) were coincident, which could reflect physiological interdependence, we carried out post hoc cortico-cortical covariation analyses in the 2 longitudinal groups. The underlying rationale was that cerebral functional networks have an intrinsically cohesive modular structure, where the modules are composed of functionally as well as anatomically related brain regions, and these networks can be identified by maps of structural covariance (He et al. 2008). Given that changes in FA were detected in tracts connecting occipital, parietal and frontal cortex, and given that our hypothesis was that testosterone would have effect in networks mediating own body perception in the context of self, it was of particular interest to investigate whether cortico-cortical covariation patterns from the pACC were specifically affected by testosterone treatment. For this purpose, we selected 2 seed regions: the left and right mesial prefrontal clusters (left: 8.2 cm2, x y z = −10 62 −4; right: 11.6 cm2, x y z = 9 59 9), covering the pACC, where trans men showed thicker cortex compared with cis controls before treatment (see Table 2A and Fig. 2A). Using demeaned age as nuisance covariate, we investigated differences in cortico-cortical covariation patterns for these 2 seed regions between groups, both before and after testosterone institution (with a threshold of P < 0.05, corrected for multiple comparisons using Monte Carlo simulations).
“Before treatment, for the left mesial frontal seed region,” we found a significant group difference in cortico-cortical covariation such that the covariation with the left TPJ and right fusiform gyrus was significantly greater in trans men than in the cis control group. This could suggest that Cth in the TPJ and the fusiform gyrus was more coordinated with the observed frontal changes in the trans men in relation to cis controls. “For the right mesial frontal seed region,” trans men had greater positive correlations than cis controls with Cth in the right middle temporal gyrus (see Table 7 and Supplementary Fig. 5). “During treatment” (session 2), trans men showed a stronger cortico-cortical covariation than cis controls (session 2) for Cth of the “left frontal seed region” and the left lateral occipital cortex, and the bilateral fusiform gyri. Also for the “right frontal seed region,” trans men had stronger positive covariations than cis controls with the right lateral occipital Cth (see Table 7 and Supplementary Fig. 5). Cis controls did not demonstrate any significantly stronger correlations than trans men. Thus, the cortico-cortical covariations between the left mesial frontal region and the left TPJ, which initially were increased in trans men, no longer differed between groups after testosterone treatment. Additionally, the covariation with the lateral occipital and fusiform regions increased in trans men.
Table 7.
Group differences in cortico-cortical thickness covariation patterns before and after institution of testosterone treatment
| Region | Session 1: pretreatment trans men > controls | Session 2: during treatment trans men > controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max. −log10(P) | Cluster size cm2 | x | y | z | Max. −log10 (P) | Cluster size cm2 | x | y | z | |
| Left mesial frontal seed | ||||||||||
| L TPJ | 3.2 | 13.4 | −44 | −49 | 11 | |||||
| R fusiform gyrus | 2.5 | 12.0 | 40 | −56 | −9 | |||||
| Right mesial frontal seed | ||||||||||
| R midtemporal gyrus | 4.3 | 10.7 | 58 | −24 | −14 | |||||
| Left mesial frontal seed | ||||||||||
| L fusiform gyrus | 3.4 | 12.1 | −40 | –59 | −13 | |||||
| L lateral occipital cortex | 2.1 | 16.4 | −12 | –90 | 19 | |||||
| R fusiform gyrus | 3.5 | 20.7 | 29 | –71 | −5 | |||||
| Right mesial frontal seed | ||||||||||
| R lateral occipital cortex | 3.0 | 13.7 | 26 | –90 | −6 | |||||
The seed regions were defined based on the resulting clusters from the group comparison for Cth in Trans men > Controls at session 1, using a threshold of P< 0.01, corrected for multiple comparisons using Monte Carlo simulations (see Table 2B and Fig. 2A); x y z = coordinates in Talairach space; R, right, L, left.
We also carried out post hoc correlation analyses among the group of trans men to evaluate whether possible testosterone-induced changes in Cth in the left superior temporal gyrus and insular cortex correlated with changes in testosterone levels or the body perception index (short and long durations). We found a significant (P = 0.05) correlation between the percentage of change per unit of time of Cth in the left insular cortex and change in body perception index of the long trials (see Supplementary Fig. 6). There was no significant correlation between changes in testosterone levels and any of the other neural measures.
Discussion
In this multimodal neuroimaging study, we provide converging evidence for cerebral changes associated with testosterone treatment in a group of trans men. The longitudinal design of investigating trans men before and after institution of testosterone treatment, and including a cis control group scanned on 2 occasions without treatment, allowed for investigation of specific effects of testosterone on functional and structural brain networks. This report extends previous lines of research in GD in its goal to distinguish specific masculinizing effects of testosterone on the brain, and selective effects on distinct neural networks involved in own-body perception.
The results corroborated those from our previous cross-sectional study (Manzouri et al. 2015) in that, prior to treatment, the mesial prefrontal cortex was thicker in the trans men than in cisgender controls. Furthermore, there was an increased covariation in Cth between frontal areas and the TPJ and the fusiform gyrus, suggesting coordinated effects, possibly as a result of common influences. With testosterone treatment, Cth increased bilaterally in the insular and superior temporal cortices in trans men compared with cis controls. In trans men, considering within-group changes only, we also found thickening in lateral occipital cortex. In this respect, these observations accord with a previous report by Zubiaurre-Elorza et al. (2014), although we did not detect any specific thickening of the left temporo-parietal cortex. This could partly be due to differences in methodology. Zubiaurre-Elorza et al. did not employ vertex-vise analyses, but compared regional mean values of Cth. Furthermore, they did not include a control group. Consequently, their study did not account for nonspecific changes that could occur over time, and the results therefore may not have been specifically associated with testosterone treatment. Contrary to our expectations, frontal lobe cortex remained thicker than in cis controls in our group of trans men during testosterone treatment. However, the cortico-cortical connectivity from these same frontal regions showed dynamic changes, with stronger covariation between pACC and Cth in the fusiform gyrus and the lateral occipital cortex in trans men compared with controls posttreatment. Congruent with these testosterone-induced cortico-cortical covariation changes, the present group of trans men also displayed a significant increase in FA values in the posterior portion of the fronto-occipital tract. The fronto-occipital tract connects the extrastriate lateral occipital and frontal cortices (Sarubbo et al. 2013). Its fibers pass from the occipital and temporal lobes conveying visual information, which could include early visual information related to own body perception from the extrastriate body area (part of the lateral occipital cortex) (David et al. 2007; Myers and Sowden 2008). Together, these observations suggest coordinated, testosterone-induced, “structural connectivity” changes between frontal, occipital, and temporal regions in trans men.
In line with these structural findings, we also detected significant alterations in “functional connectivity” associated with testosterone treatment in areas implicated in body perception and self-referential processing. Using the same seed regions (pACC, TPJ) as in our previous cross-sectional study (Manzouri et al. 2015), trans men, prior to treatment, showed weaker connectivity than cis controls with the precuneus, anterior (para-)cingulate cortex, insular cortex, and caudate nucleus. With testosterone treatment, these areas were found to “reconnect.” They thus showed increased functional connectivity with testosterone treatment (session 2 > session 1). In particular the TPJ, a region which is considered to be involved in own body identification (Blanke et al. 2002; Hodzic et al. 2009; Vocks et al. 2010a, 2010b; Blanke 2012), showed significant increases (session 2 > session 1) in connectivity with the bilateral paracingulate gyrus, left precuneus, and left inferior frontal gyrus. In line with our findings, a randomized placebo controlled study in women with anorexia nervosa receiving low-dose testosterone treatment over a 3-week period found testosterone-associated increases in brain metabolism in very similar regions (Miller et al. 2004). The posterior cingulate gyrus, pACC, right caudate nucleus, and a cluster in the right parietal lobe, close to the TPJ, showed treatment-related increases in metabolism. These findings are of particular interest, since they suggest similar effects of testosterone on the brain, but in another condition that is similarly characterized by feelings of distress about the own body and body-related avoidance. Interestingly, a recent magnetoencephalography study showed reduced activation in the TPJ area of trans men compared with cis women in response to tactile stimulation of the breast, a body part that is particularly affected by feelings of incongruence and dysphoria (Case et al. 2016).
Since the aforementioned areas are part of the own body perception network (Northoff et al. 2006; Hodzic et al. 2009; Northoff 2016), as are the areas in which we detected changes in Cth and the cortico-cortical covariations, one may argue that testosterone had direct effects on the brain, particularly in regions involved in integration of own body perception in the context of self. The present findings may, thus, represent neural correlates to trans men's subjective improvement in congruence between perception of one's own body and self (i.e., gender identity), due to testosterone treatment. The hypothesis that we put forth is that several major hubs in the own body processing network in relation to self may differ in trans men, as suggested by several of our previous studies (Manzouri et al. 2015; Feusner et al. 2016a, 2016b).
An alternative, although not mutually exclusive, explanation is that mere androgenization of the body would lead to increased integration of self-own body perception and improved feeling of own body-self-congruence, leading to increased synaptic and white matter connections. Both explanatory models accord with the present findings in FA, functional connectivity, and cortico-cortical covariations. They also accord with the detection of increased thickness of the insular cortex. The insula, in particular its anterior part on the right, has been reported to be pivotal for the generation of a mental image of one's physical state (Churchland 2002), for self-referential emotions (Damasio et al. 2000), and interoception (Craig 2002, 2009), providing the basis for self-awareness and thereby also contributing to self-congruence. Indeed, the present behavioral results of the body perception test showed that trans men scored more in accordance with their experienced gender during testosterone treatment, as compared to before. Also, the subsample of trans men that received both MRI and body perception testing before and after treatment showed significant positive correlations between improved perception of self (indexed by increase in body perception index) and increase in Cth in the left insula (see Supplementary Fig. 6), suggesting that improved self-own body congruence and changes in Cth were coordinated and related to testosterone treatment.
Given that both the occipital and insular cortices express androgen receptors (Clark et al. 1988; Simerly et al. 1990; Kolb and Stewart 1991; Goldstein et al. 2001; Lepore et al. 2008) it is important to discuss also a possibility that the presently detected increases in Cth could simply reflect trophic testosterone receptor-mediated effects (see also Lentini et al. 2013). This seems, however, unlikely, albeit not exclusive, as such effects would be expected also in certain subcortical volumes expressing androgen receptors, for example, the amygdala and thalamus (Filipek et al. 1994; Giedd et al. 1997; Goldstein et al. 2001), which was not found. Neither do the present observations provide support for specific masculinizing effects on the brain by testosterone, usually characterized by a cortical thinning in the parietal lobe, volume increase of the amygdala, volume decrease of the hippocampus, and a more general increase of white matter volume (Lenroot et al. 2007; Perrin et al. 2008, 2009; Lentini et al. 2013; Savic and Arver 2014), and FA values (Schmithorst et al. 2008; Huster et al. 2009; Inano et al. 2011). The latter statement deserves a special comment, considering that in the majority of neuroimaging studies on effects of cross-sex hormone therapy in individuals with GD the results were discussed assuming that sexual dimorphism of the human brain is influenced by sex hormones, that the degree of dimorphism should be less pronounced or even reversed (“sex-atypical”) in individuals with GD, and that cross-sex hormone treatment would enhance the sexual dimorphism in the direction of the sex the individual with GD identifies with. Also, these studies failed to consistently show, for example, masculinization of brain structures in trans men. For example, the reported finding of increased Cth in the parietal cortex of trans men after testosterone treatment (Zubiaurre-Elorza et al. 2014) is rather contrary to the normal synaptic pruning effects of testosterone (Zehr et al. 2006; Bramen et al. 2012; Herting et al. 2015), which would lead to thinning rather than thickening of the cortex in this region.
Testosterone effects on the brain with the perspective on sexual dimorphism are, on the other hand, not easy to predict because the available data are scarce. In addition, they are mainly based on studies of adolescents, which are not directly applicable to possible testosterone-triggered processes in the adult brain. Moreover, they could differ between cisgender and transgender individuals. The present findings add to the current literature by providing converging evidence from functional and structural imaging performed on the same set of individuals that testosterone has effects on one's own body perception and self-referential networks.
Interestingly, the observed functional connectivity changes were not accompanied by reductions in Cth in the areas of the frontal lobe, where Cth remained significantly increased in trans men compared with cis controls. This could either indicate that these changes are inherent and immutable to trans men, or, that a tentative modulation of Cth in the frontal lobe regions requires longer time. Effects of cross-sex hormone treatments on the brain have usually been investigated after a minimal exposure time of 3–7 months (Burke et al. 2016; Rametti et al. 2012; Zubiaurre-Elorza et al. 2014; present study). Future longitudinal studies of cross-sex hormone effects should therefore incorporate several time points and a longer temporal perspective.
Methodological Considerations
The behavioral data from the body perception test need to be interpreted with caution, given the relatively small sample size available.
The longitudinal groups differed significantly in terms of age, number of years of education, and sexual orientation, and we did not specifically assess intelligence levels. We accounted for age and years of education by adding these as covariates to the baseline group comparisons of FA and Cth. The groups were not matched for sexual orientation, as the major objective of the present study was to investigate effects of testosterone treatment. Possible interaction between sexual orientation and GD with respect to brain is specifically addressed in a separate study by our group.
Of note also is that our findings on functional connectivity changes with testosterone treatment did not account for any possible changes in time in the cis controls, because follow-up (session 2) resting-state functional data were available only for the trans men. Thus, the present results of the functional data may not be as specific with respect to testosterone effects as the morphological and diffusion data.
Furthermore, the longitudinal control group consisted of both males and females, and each set was too small individually to conduct meaningful direct sex by group comparisons. Therefore, we also conducted analyses of the baseline data (for FA, Cth, and structural volumes), with a larger control group of 22 male and 22 female cis control participants, and specifically matched for mean age. As before with the smaller and mixed-sex control group, we found that trans men did not differ significantly in FA from either the cis male or cis female controls. At a more lenient threshold (P < 0.150) trans men showed higher FA compared with cis females, but lower FA than cis males, thus showing the previously described “in-between pattern” of cerebral sexual differentiation of transgender compared with cisgender individuals (Rametti et al. 2011b; Kranz et al. 2015). Also in terms of Cth, our overall findings did not substantially change, with trans men showing thicker cortex compared with both male and female controls particularly in the midfrontal regions (see Supplementary Table 8). Data from the cross-sectional control groups were not used for tests of functional connectivity from TPJ, and measures of the body perception index, as our previous studies showed no sex difference in these aspects (Manzouri et al. 2015; Feusner et al. 2016a, 2016b).
The groups differed with respect to the interscan interval, which was higher in controls. To avoid this bias and in accordance with FreeSurfer guidelines for longitudinal studies of Cth, we tested group differences using percentage of change of Cth/year as input. Furthermore, the observed increase in Cth in the transgender population was regional rather than general. This argues against a bias due to different interscan intervals on the present results.
Testosterone levels were only measured in trans men and not in the cis controls. However, there is no reason to assume that these healthy control participants suffered from testosterone (or any other hormonal) deficiency or aberration. Also, information about menstrual cycle phase was not assessed in all the trans men, but the majority (15) were, like the cis women, scanned during the second week of their menstrual cycle. Finally, the size of study groups was relatively small, but large enough to allow statistical comparisons according to a recent report on power analysis with the MRI methods applied (Liem et al. 2015).
Conclusion
To conclude, on the basis of the present results and the available literature, we provide evidence to support the hypothesis that testosterone treatment in trans men has major effects on brain areas involved in the modulation of own body perception in the context of self. These effects are structural as well as functional. However, they do not reverse the frontal lobe cortical thickening, which could be interpreted possibly as a cause rather than an effect of GD, although definitively this needs attention in future studies. The detected effects were not in favor of a direct “masculinization” of the brain. However, it is possible that at least some of the testosterone-associated changes were secondary to a masculinization of the body, better congruence between perception of self and the own body, and a “reconnection” of the pathways processing these functions. It is also possible that there is interplay and facilitation between the 2 mechanisms. This would indicate that testosterone effects on the brain can be modulated by “behavioral activation,” meaning that cognitive or behavioral changes (e.g., feeling more comfortable with the own body, being more self-confident) trigger specific changes in structural and functional brain systems, in addition to, and in interplay with, the rather more diffuse effects of testosterone itself. This possible mechanism requires further inquiry. Another important issue requiring future studies is whether similar changes would be found after estrogen treatment in trans women, which would imply a common GD-associated phenomenon.
Supplementary Material
Notes
We thank Jasenko Dervisic and Ludwig Honk for their help in recruitment and assessment of participants, as well as for their help in preparing the photos used in the body perception test. Conflict of Interest: None declared.
Supplementary Material
Supplementary material are available at Cerebral Cortex online.
Funding
Swedish Research Council (I.S.-B., DNA 2007-3107, and DNA: 2015-02716); FORTE (I.S.-B.); AFA (I.S.-B.).
References
- American Psychiatric Association 2013. Diagnostic and statistical manual of mental disorders. 6th ed. (DSM-5) Arlington (VA): American Psychiatric Association. [Google Scholar]
- Blanke O. 2012. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci. 13:556–571. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. 2002. Stimulating illusory own-body perceptions. Nature. 419:269–270. [DOI] [PubMed] [Google Scholar]
- Bouman WP, Schwend AS, Motmans J, Smiley A, Safer JD, Deutsch MB, Adams NJ, Winter S. 2016. Language and trans health. Int J Transgenderism. 1–6. http://dx.doi.org/10.1080/15532739.2016.1262127. [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER. 2012. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 7:e33850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Cohen-Kettenis PT, Veltman DJ, Klink DT, Bakker J. 2014. Hypothalamic response to the chemo-signal androstadienone in gender dysphoric children and adolescents. Front Endocrinol (Lausanne). 5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Kreukels BPC, Cohen-Kettenis PT, Veltman DJ, Klink DT, Bakker J. 2016. Male-typical visuo-spatial functioning in gynephilic girls with Gender Dysphoria – organizational and activational effects of testosterone. J Psychiatry Neurosci. 41(6):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabez R, Pellegrini M, Mankovitz A, Eliason M, Scott M. 2015. Does your organization use gender inclusive forms? Nurses’ confusion about trans* terminology. J Clin Nurs. 24:3306–3317. [DOI] [PubMed] [Google Scholar]
- Case LK, Brang D, Landazuri R, Viswanathan P, Ramachandran VS. 2016. Altered white matter and sensory response to bodily sensation in female-to-male transgender individuals. Arch Sex Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TF, Pruzinsky T.. 2002. Body image: a handbook of theory, research, and clinical practice. New York: Guilford Press. [Google Scholar]
- Churchland PS. 2002. Self-representation in nervous systems. Science. 296:308–310. [DOI] [PubMed] [Google Scholar]
- Clark AS, MacLusky NJ, Goldman-Rakic PS. 1988. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 123:932–940. [DOI] [PubMed] [Google Scholar]
- Cohen-Kettenis PT, Pfäfflin F. 2010. The DSM diagnostic criteria for gender identity disorder in adolescents and adults. Arch Sex Behav. 39:499–513. [DOI] [PubMed] [Google Scholar]
- Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig ADB. 2009. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. 2000. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 3:1049–1056. [DOI] [PubMed] [Google Scholar]
- David N, Cohen MX, Newen A, Bewernick BH, Shah NJ, Fink GR, Vogeley K. 2007. The extrastriate cortex distinguishes between the consequences of one's own and others’ behavior. Neuroimage. 36:1004–1014. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Dervisic J, Kosidou K, Dhejne C, Bookheimer S, Savic I. 2016. a. Female-to-male transsexual individuals demonstrate different own body identification. Arch Sex Behav. 45:525–536. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Lidström A, Moody TD, Dhejne C, Bookheimer SY, Savic I. 2016. b. Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging Behav. doi:10.1007/s11682-016-9578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS. 1994. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 4:344–360. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Segonne F, Quinn BT, Dale AM. 2004. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 23(Suppl 1):S69–S84. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. 1997. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 21:1185–1201. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT. 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 11:490–497. [DOI] [PubMed] [Google Scholar]
- Gooren LJ, Kreukels B, Lapauw B, Giltay EJ. 2015. (Patho)physiology of cross-sex hormone administration to transsexual people: the potential impact of male-female genetic differences. Andrologia. 47:5–19. [DOI] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Küblböck M, Kaufmann U, Ganger S, Hummer A, Seiger R, Spies M, Winkler D, Kasper S, et al. 2015. Structural connectivity networks of transgender people. Cereb Cortex. 25:3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Sladky R, Kaufmann U, Ganger S, Hummer A, Seiger R, Spies M, Vanicek T, Winkler D, et al. 2016. Testosterone affects language areas of the adult human brain. Hum Brain Mapp. 37:1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. 2008. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci. 28:4756–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. 2015. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 10:e0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. 2012. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 22:1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic A, Muckli L, Singer W, Stirn A. 2009. Cortical responses to self and others. Hum Brain Mapp. 30:951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Schagen SEE, Kreukels BPC, Veltman DJ, Cohen-Kettenis PT, Delemarre-van de Waal H, Bakker J. 2015. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology. 55:59–71. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S. 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol EH, Cohen-Kettenis PT, Van Haren NEM, Peper JS, Brans RGH, Cahn W, Schnack HG, Gooren LJG, Kahn RS. 2006. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. Eur J Endocrinol. 155:S107–S114. [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W. 2009. Hemispheric and gender related differences in the midcingulum bundle: a DTI study. Hum Brain Mapp. 30:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. 2011. Effects of age and gender on white matter integrity. Am J Neuroradiol. 32:2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger J, Habel U, Bröhr S, Neulen J, Neuschaefer-Rube C, Birkholz P, Kohler C, Schneider F, Derntl B, Pauly K. 2014. More than just two sexes: the neural correlates of voice gender perception in gender dysphoria. PLoS One. 9:e111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey A, Pomeroy W, Martin C. 1948. Sexual behavior in the human male. Philadelphia: W.B. Saunders Co. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Stewart J. 1991. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. J Neuroendocrinol. 3:95–99. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Küblböck M, Hummer A, Ganger S, Seiger R, Winkler D, Swaab DF, Windischberger C, et al. 2014. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci. 34:15466–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Wadsak W, Kaufmann U, Savli M, Baldinger P, Gryglewski G, Haeusler D, Spies M, Mitterhauser M, Kasper S, et al. 2015. High-dose testosterone treatment increases serotonin transporter binding in transgender people. Biol Psychiatry. 78:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreukels BPC, Guillamon A. 2016. Neuroimaging studies in people with gender incongruence. Int Rev Psychiatry. 28(1):120–128. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini E, Kasahara M, Arver S, Savic I. 2013. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb Cortex. 23:2322–2336. [DOI] [PubMed] [Google Scholar]
- Lepore G, Gadau S, Mura A, Arru B, Mulliri G, Zedda M, Farina V. 2008. Androgen receptor immunoreactivity in rat occipital cortex after callosotomy. Eur J Histochem. 52:163–168. [DOI] [PubMed] [Google Scholar]
- Liem F, Merillat S, Bezzola L, Hirsiger S, Philipp M, Madhyastha T, Jancke L. 2015. Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. Neuroimage. 108:95–109. [DOI] [PubMed] [Google Scholar]
- Lin C-S, Ku H-L, Chao H-T, Tu P-C, Li C-T, Cheng C-M, Su T-P, Lee Y-C, Hsieh J-C. 2014. Neural network of body representation differs between transsexuals and cissexuals. PLoS One. 9:e85914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Sánchez FJ, Tosun D, Shattuck DW, Gaser C, Vilain E, Toga AW. 2012. Increased cortical thickness in male-to-female transsexualism. J Behav Brain Sci. 2:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri A, Kosidou K, Savic I. 2015. Anatomical and functional findings in female-to-male transsexuals: testing a new hypothesis. Cereb Cortex. pii: bhv278. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Miller KK, Deckersbach T, Rauch SL, Fischman AJ, Grieco KA, Herzog DB, Klibanski A. 2004. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Res. 132:197–207. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Zijl PCM, van, Nagae-Poetscher LM. 2005. MRI atlas of human white matter. Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- Myers A, Sowden PT. 2008. Your hand or mine? The extrastriate body area. Neuroimage. 42:1669–1677. [DOI] [PubMed] [Google Scholar]
- Northoff G. 2016. Is the self a higher-order or fundamental function of the brain? The “basis model of self-specificity” and its encoding by the brain's spontaneous activity. Cogn Neurosci. 7(1–4):203–222. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. 2006. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. Neuroimage. 31:440–457. [DOI] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, Johnson HJ, Styner M. 2014. DTIPrep: quality control of diffusion-weighted images. Front Neuroinform. 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve P-Y, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. 2008. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 28:9519–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. 2009. Sex differences in the growth of white matter during adolescence. Neuroimage. 45:1055–1066. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gomez-Gil E, Junque C, Segovia S, Gomez A, Guillamon A. 2011. a. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J Psychiatr Res. 45:199–204. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gomez-Gil E, Junque C, Segovia S, Gomez A, Guillamon A. 2011. b. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J Psychiatr Res. 45:199–204. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gómez-Gil E, Junque C, Zubiaurre-Elorza L, Segovia S, Gomez A, Karadi K, Guillamon A. 2012. Effects of androgenization on the white matter microstructure of female-to-male transsexuals. A diffusion tensor imaging study. Psychoneuroendocrinology. 37:1261–1269. [DOI] [PubMed] [Google Scholar]
- Reuter M, Fischl B. 2011. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. 2010. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. 2013. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 218:21–37. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2011. Sex dimorphism of the brain in male-to-female transsexuals. Cereb Cortex. 21:2525–2533. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2014. Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb Cortex. 24:3246–3257. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. 2008. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 29:696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59(Suppl 20):22-33-57. [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. 1990. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 294:76–95. [DOI] [PubMed] [Google Scholar]
- Simon L, Kozák LR, Simon V, Czobor P, Unoka Z, Szabó A, Csukly G. 2013. Regional grey matter structure differences between transsexuals and healthy controls-a voxel based morphometry study. PLoS One. 8:e83947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Junger J, Derntl B, Habel U. 2015. The transsexual brain – A review of findings on the neural basis of transsexualism. Neurosci Biobehav Rev. 59:251–266. [DOI] [PubMed] [Google Scholar]
- Smith KP, Madison CM, Milne NM. 2014. Gonadal suppressive and cross-sex hormone therapy for gender dysphoria in adolescents and adults. Pharmacotherapy. 34:1282–1297. [DOI] [PubMed] [Google Scholar]
- Soleman RS, Schagen SEE, Veltman DJ, Kreukels BPC, Cohen-Kettenis PT, Lambalk CB, Wouters F, Delemarre-van de Waal HA. 2013. Sex differences in verbal fluency during adolescence: a functional magnetic resonance imaging study in gender dysphoric and control boys and girls. J Sex Med. 10:1969–1977. [DOI] [PubMed] [Google Scholar]
- Soleman RS, Staphorsius AS, Cohen-Kettenis PT, Lambalk CB, Veltman DJ, van Trotsenburg MAA, Hompes PGA, Drent ML, de Ronde WP, Kreukels BPC. 2016. Oestrogens are not related to emotional processing: a study of regional brain activity in female-to-male transsexuals under gonadal suppression. Cereb Cortex. 26(2):510–516. [DOI] [PubMed] [Google Scholar]
- Swaab DF. 2004. Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol. 19:301–312. [DOI] [PubMed] [Google Scholar]
- Verhagen L, Grol M, Dijkerman H, Toni I. 2006. Studying visually guided reach to grasp movements in an MR-environment. Neuroimage. 31:S45. [Google Scholar]
- Vocks S, Busch M, Grönemeyer D, Schulte D, Herpertz S, Suchan B. 2010. a. Neural correlates of viewing photographs of one's own body and another woman's body in anorexia and bulimia nervosa: an fMRI study. J Psychiatry Neurosci. 35:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S, Busch M, Schulte D, Grönermeyer D, Herpertz S, Suchan B. 2010. b. Effects of body image therapy on the activation of the extrastriate body area in anorexia nervosa: an fMRI study. Psychiatry Res. 183:114–118. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, et al. 2007. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 1992. Chapter V Mental and behavioural disorders In: International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Geneva: World Health Organization; p. F00–F09. [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. 2006. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 66:578–590. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gómez-Gil E, Guillamon A. 2014. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J Sex Med. 11:1248–1261. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gómez-Gil E, Segovia S, Carrillo B, Rametti G, Guillamon A. 2013. Cortical thickness in untreated transsexuals. Cereb Cortex. 23:2855–2862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.