Summary
Measurement of the aerosols generated during 7 healthcare procedures showed an increase in particle concentrations over baseline only during nebulized medication administration (NMA) and bronchoscopy with NMA. Recovered bacteria were common environmental organisms.
Keywords: aerosols, infection prevention, aerosol-generating procedures
Abstract
Background
Questions remain about the degree to which aerosols are generated during routine patient care activities and whether such aerosols could transmit viable pathogens to healthcare personnel (HCP). The objective of this study was to measure aerosol production during multiple patient care activities and to examine the samples for bacterial pathogens.
Methods
Five aerosol characterization instruments were used to measure aerosols during 7 patient care activities: patient bathing, changing bed linens, pouring and flushing liquid waste, bronchoscopy, noninvasive ventilation, and nebulized medication administration (NMA). Each procedure was sampled 5 times. An SKC BioSampler was used for pathogen recovery. Bacterial cultures were performed on the sampling solution. Patients on contact precautions for drug-resistant organisms were selected for most activity sampling. Any patient undergoing bronchoscopy was eligible.
Results
Of 35 sampling episodes, only 2 procedures showed a significant increase in particle concentrations over baseline: NMA and bronchoscopy with NMA. Bronchoscopy without NMA and noninvasive ventilation did not generate significant aerosols. Of 78 cultures from the impinger samples, 6 of 28 baseline samples (21.4%) and 14 of 50 procedure samples (28.0%) were positive.
Conclusions
In this study, significant aerosol generation was only observed during NMA, both alone and during bronchoscopy. Minimal viable bacteria were recovered, mostly common environmental organisms. Although more research is needed, these data suggest that some of the procedures considered to be aerosol-generating may pose little infection risk to HCP.
The majority of pathogens are spread person to person under normal circumstances through contact or droplet transmission, with a small number known to be transmitted by small particle aerosols. For pathogens spread by contact or droplet, additional respiratory protection with a respirator is not considered necessary to protect healthcare personnel (HCP) from exposure [1]. However, concerns have been raised that some infections usually spread by contact or droplet routes could also be transmitted through aerosols generated during certain medical procedures. These concerns have been heightened during outbreaks of emerging infections such as Ebola, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and pandemic influenza. Some infection prevention guidelines therefore recommend that HCP use additional respiratory protection (eg, a fitted particulate respirator) when performing “aerosol-generating procedures” to protect themselves from exposure to infectious agents [1–8].
Concerns about disease transmission to HCP during aerosol-generating procedures were raised during the 2003 SARS outbreak [9], when there were multiple reports of disease transmission to HCP who were wearing appropriate personal protective equipment [10–12]. Aerosol transmission during medical procedures was the suspected source of infection, based largely on observational and anecdotal evidence [11, 13, 14]. Air sampling conducted in the rooms of SARS patients at a hospital in Toronto provided experimental confirmation of the possibility of airborne transmission of SARS, but did not correlate this with the performance of medical procedures [15].
Aerosol-generating procedures have also been suspected as a source of HCP infection in other outbreaks, such as 2009 H1N1 influenza [2, 4, 16], seasonal influenza [3, 17, 18], and MERS [19, 20]. Some have also raised concerns that filoviruses, including Ebola, may be transmitted through aerosols, though this remains controversial [21]. Other infections that may occasionally be transmitted via aerosols include norovirus [8, 22] and methicillin resistant Staphylococcus aureus [22].
Routine healthcare procedures most often identified as potentially “aerosol-generating” include intubation and extubation, cardiopulmonary resuscitation, bronchoscopy, noninvasive ventilation, tracheotomy, sputum induction, airway suctioning, manual ventilation, and administering oxygen or nebulized medication [2, 4, 8, 13, 22–24]. For most of these procedures, evidence for the generation of infectious aerosols is based mostly on case reports and anecdotal evidence rather than on epidemiological studies or environmental air sampling. A 2009 review by Davies et al concluded that, although there are a number of procedures considered to be aerosol-generating, few have sufficient evidence to confirm that they actually do produce aerosols [23].
In absence of clear evidence, questions remain about the degree to which aerosols are generated during “aerosol-generating” medical procedures, the size and concentration of medically aerosolized particles, and whether such aerosols could transmit viable pathogens to HCP or to other patients [1, 7, 25]. Uncertainty about which procedures are associated with increased risk makes it difficult for hospitals to develop effective preventive measures [7, 23, 25]. The objective of this investigation was to characterize any aerosols generated during several common medical procedures, and to determine whether bacterial pathogens could be isolated from these aerosols.
Methods
Sampling Strategy
Aerosol production was measured during 7 routine patient care activities: changing bed linens; patient bathing; pouring liquids into a hopper; flushing liquid waste; noninvasive ventilation using bilevel positive airway pressure; nebulized medication administration (NMA); and bronchoscopy with and without NMA, including both intubation (laryngeal mask) and extubation during the procedure. Prior to sampling, both the patient and the HCP performing the procedure were informed about the aerosol sampling and asked to provide verbal assent. For patients who were unconscious or sedated, a family member or surrogate was asked to provide assent, if they were present.
Each type of procedure was sampled 5 separate times. All samples were collected in patient and procedure rooms at a large tertiary care medical center. Most samples were collected in the medical intensive care unit (ICU), although some NMA samples were collected in the cystic fibrosis ward. These rooms all had routine air handling. Bronchoscopy samples were collected in both the interventional pulmonology suite (routine air handling) and the ICU bronchoscopy suite (negative pressure ventilation). During some of the bronchoscopies, nebulized medication was administered to the patient before and after the procedure.
Subjects
For all procedures except bronchoscopy, patients were selected from among inpatients on contact precautions for drug-resistant organisms, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), multidrug-resistant gram-negative organisms (MDROs), and Clostridium difficile. Bronchoscopy patients were not prescreened for colonization or infection with specific organisms.
Aerosol Sampling
Whenever possible, baseline samples were collected in the room before the procedure began. It was not possible to collect baseline samples for mechanical ventilation or noninvasive ventilation, which were continuous. For pouring/flushing of patient waste in a hopper, a single baseline sample was collected for 2 procedure samples (1 pouring, 1 flushing). For bronchoscopies, 1 baseline and 2 procedure samples were collected (1 including intubation and 1 including extubation).
During each procedure, 5 real-time aerosol characterization instruments were used to detect and characterize any generated aerosols. These included a P-Trak Ultrafine Particle Counter (TSI Inc), which measures particle number concentration (number/cm3); a SidePak AM510 personal aerosol mobility spectrometer (TSI Inc), which measures particle mass concentration (mg/cm3); a portable aerosol mobility spectrometer (PAMS, Kanomax Inc), which measures the particle number size distribution of sub-micrometer aerosols (14–862 nm); an aerodynamic particle sizer (APS) spectrometer (TSI Inc), which measures the particle number size distribution of larger aerosols (0.5–20 µm); and a nanoparticle surface area monitor (NSAM,TSI Inc), which measures lung-deposited surface area, providing an estimate of the total surface area of particles that would deposit in the alveolar regions of the human lung (µm2/cm3). All samples were collected using 2 sets of conductive silicone tubing that were hung at a single point 3 feet from the patient’s head at approximately HCP head level. One set of tubing was connected to the impinger inlet and the other was connected to the real-time aerosol sampling instruments. The tubing was inspected prior to sampling to ensure that it had no sharp bends or kinks. All instruments were calibrated prior to each use to ensure accurate measurements.
Testing of Biological Samples
To determine whether the aerosols generated during the various procedures included any potentially infectious particles, a BioSampler (SKC Inc) was used to collect samples for bacterial pathogen recovery. The sterile BioSampler was filled with 15 mL of phosphate-buffered saline with Tween-80. Tubing attached to the impinger inlet was hung alongside the collection tubing for the aerosol characterization instruments. After sampling, the collection liquid was decanted and centrifuged, and the pellet resuspended. A Gram stain and culture was performed, and the sample was inoculated on several culture plates: 5% sheep’s blood agar (Hardy Diagnostics); Spectra MRSA agar (Remel); chromID VRE (bioMérieux); CCMB-TAL broth for Clostridium difficile detection (Anaerobe Systems); and a 6.5% sodium chloride broth (Hardy), which was incubated for 18–26 hours and then plated to the blood, MRSA, and VRE agars. Bacterial colonies were identified using the VITEK MS matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS) system [26–29]. Following each procedure, the collection tubing was rinsed with ethanol and the BioSamplers were rinsed and autoclaved to reduce the potential for cross-contamination.
Data Analysis
Averages and standard deviations were calculated for all aerosol characterization data (particle counts, mass, size, lung-deposited surface area) for each procedure and associated baseline (when available), so the contribution of each procedure to overall measured particle concentrations could be compared.
The study protocol was reviewed by the Washington University Human Research Protection Office, which determined that it did not require institutional review board oversight because no personally identifiable information was collected.
RESULTS
A total of 35 procedures were sampled (5 samples for each of 7 types of procedure) over a 4-month period from June through October 2015.
Particle Concentration
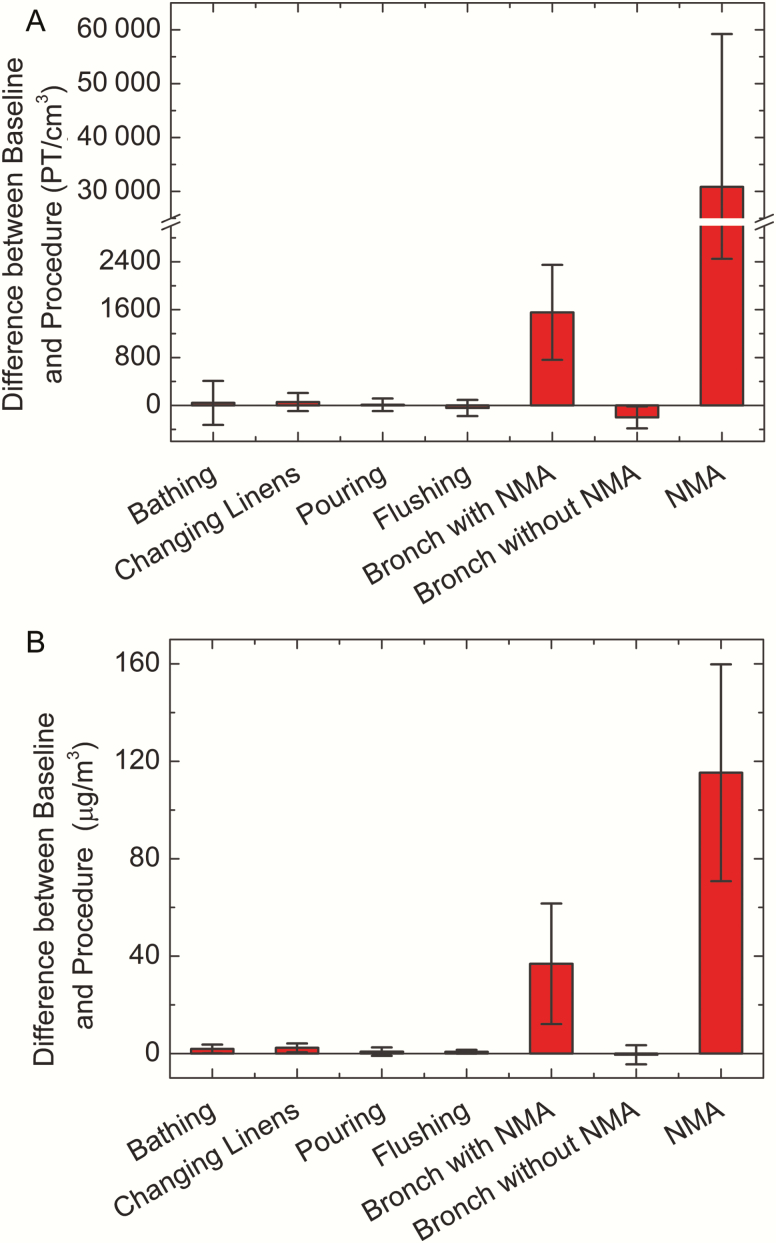
Differences between baseline and procedure particle number and mass for the different types of procedure samples are presented in Figure 1. Data from the mechanical ventilation and noninvasive ventilation samples are not included in this figure because no baseline samples were collected for these procedures. No significant differences between the baseline and procedure measurements were observed for bathing, changing linens, pouring liquids into the hopper, flushing the hopper, and bronchoscopy without NMA. However, there was an increase in particle concentrations during NMA and during bronchoscopy procedures that started and ended with NMA. Bronchoscopy with NMA was associated with up to a 30000 number/cm3 increase in particle counts and a 1.5 mg/m3 increase in particle mass, while NMA alone was associated with up to a 70000 number/cm3 increase in particle count and a 0.8 mg/m3 increase in particle mass. However, as indicated by the error bars in Figure 1, there was a high amount of variation in particle concentration among the NMA samples.
Figure 1.
Change from preprocedure baseline in particle number (A) and mass (B) concentrations during the sampled procedures. Mechanical ventilation and noninvasive ventilation are not included in this figure because no baseline samples could be collected for these procedures. Error bars = standard deviation. Abbreviations: Bronch, bronchoscopy; NMA, nebulized medication administration; PT, particle.
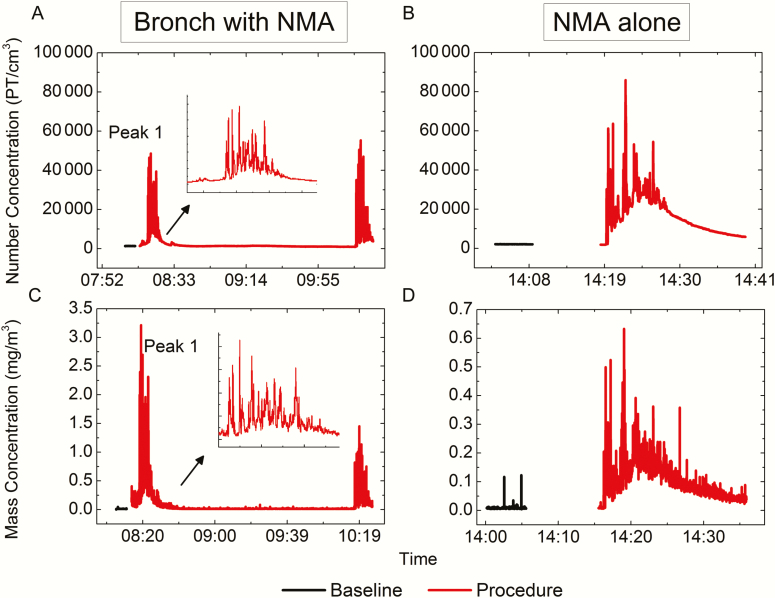
Figure 2 presents particle number and mass concentration time-series data comparing baseline and procedure samples collected during bronchoscopy with NMA (Figure 2A and 2C) and NMA alone (Figure 2B and 2D). For samples collected during bronchoscopy with NMA, 2 narrow concentration peaks are observed, which correspond with the nebulized medication administration before and after the procedure. The samples collected during NMA alone demonstrate wider concentration peaks (when adjusted for the different time scales), as the nebulizer was running throughout the entire procedure, and higher particle counts but lower mass concentrations, indicative of smaller particle sizes. For the NMA alone procedures, baseline aerosol concentration levels were not reestablished until 10–20 minutes after the procedure had ended.
Figure 2.
Particle (PT) number and mass concentrations for bronchoscopy (Bronch) with nebulized medication administration (NMA) (A and C) and for NMA alone (B and D). Please note the different y-axis scales for the 2 mass concentration graphs (C and D). The inset shows an enlarged view of the first peak of the bronchoscopy with NMA graph (A and C) to make the time scale comparable to the NMA alone graphs (B and D).
Particle Size Distribution
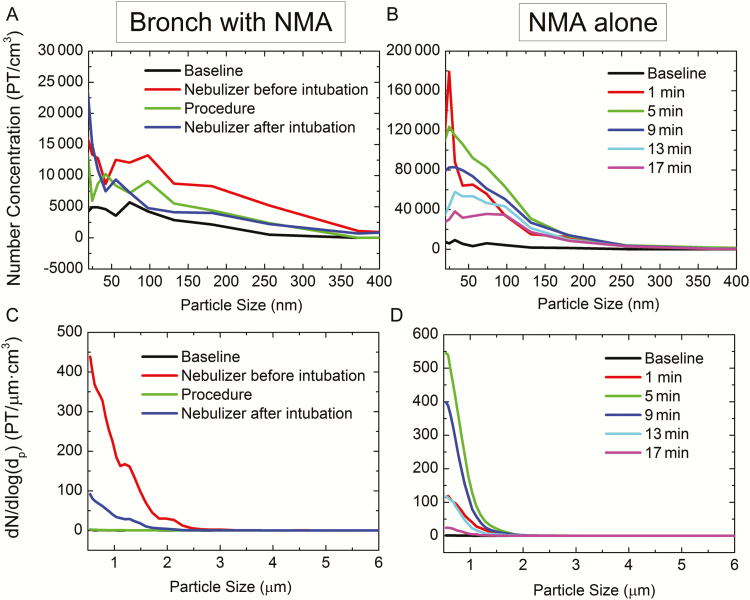
Particle number size distribution data (Figure 3) indicate that the particles generated during bronchoscopy with NMA (Figure 3A and 3C) were generally larger (geometric mean diameter [GMD], 44 nm; standard deviation [SD], 2.75) than those generated during NMA alone (Figure 3B and 3D) (GMD, 33 nm; SD, 2.61). This is consistent with the results presented in Figure 2, which showed higher mass concentrations for bronchoscopy with NMA, as larger particles contribute more to mass concentration than do smaller particles. Because the nebulized particles are composed mainly of water, their sizes are largely affected by the nebulizing conditions (such as pressure and air flow rate) and time allowed for evaporation after they are emitted from the nebulizer [30]. It is therefore possible that the different particle sizes observed during the 2 types of procedures may be due to different air flow patterns in the rooms where the procedures were performed (some bronchoscopies were performed in negative pressure ventilation rooms, unlike those used for NMA). The particle size observations may also be influenced by different locations of the patient relative to air intake/outlet in the rooms, different NMA administration techniques (mask vs mouth piece), and whether albuterol was coadministered with another medication. Particle size distribution data for the other procedures evaluated in this study showed that pouring and flushing liquid waste in the hopper did produce a few aerosolized particles of approximately 1 μm (<0.5 number/cm3); however, this peak was no longer discernible after 20 seconds, as particles most likely drifted, settled, or were carried away by convection. Changing linens also produced small amounts of particles of around 40 nm in size. Bathing patients produced a low concentration of 0.5- to 1.5-μm particles, possibly caused by the evaporation of chlorhexidine gluconate in the soap used for bathing.
Figure 3.
Particle (PT) number size distribution curves for bronchoscopy (Bronch) with nebulized medication administration (NMA) as measured by portable aerosol mobility spectrometer (PAMS) (A); NMA alone as measured by PAMS (B); bronchoscopy with NMA as measured by aerodynamic particle sizer spectrometer (APS) (C); and NMA alone as measured by APS (D). Please note the different y-axis scales for the 2 number concentration graphs (A and B).
Lung-Deposited Surface Area
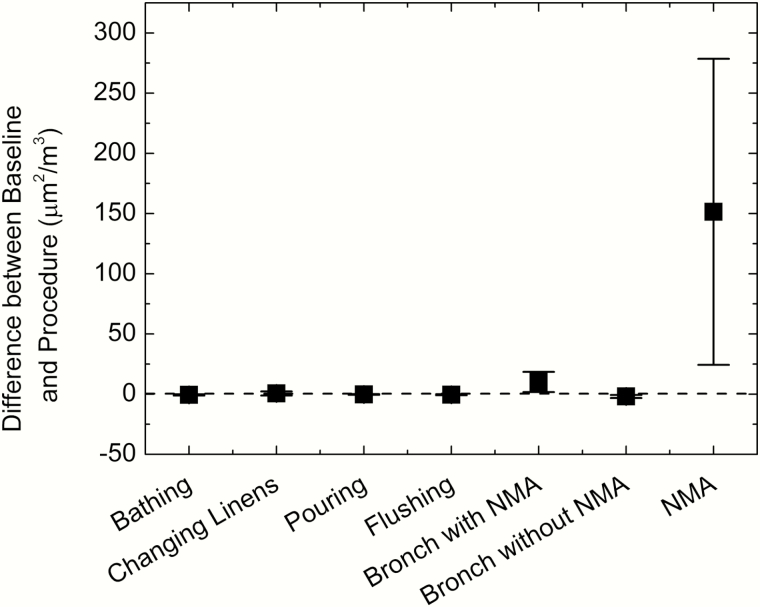
Figure 4 shows the difference between the average baseline and procedure measurements of the particle surface area that would deposit in the alveoli of the lung if inhaled. Bronchoscopy with NMA showed only a small elevation from baseline, whereas concentrations during NMA alone were much higher. No substantial elevation from baseline was observed during any of the other patient care activities that were sampled.
Figure 4.
Change from preprocedure baseline in lung-deposited surface area concentrations (alveolar region) during the sampled procedures. Mechanical ventilation and noninvasive ventilation are not included in this figure because no baseline samples could be collected for these procedures. Error bars = standard deviation. Abbreviations: Bronch, bronchoscopy; NMA, nebulized medication administration.
Microbiology
Of 78 baseline and procedure BioSampler collection liquid samples that were cultured, bacteria were isolated from 6 of the 28 baseline samples (21.4%), compared with 14 of 50 procedure samples (28.0%). In all cases, the bacterial burden was low (rare growth on solid medium or growth only upon broth enrichment). The most common culture result was mixed gram-positive flora, with the most frequently isolated organisms being coagulase-negative Staphylococcus species (n = 12) and Micrococcus species (n = 6). Other organisms identified included viridans group Streptococcus, Bacillus species, Paenibacillus species, Corynebacterium species, and a non-meningitidis species of Neisseria. Twenty-five samples were collected during procedures involving patients who were on contact precautions for drug-resistant organisms (18 patients with VRE; 3 with C. difficile; 8 with MRSA; and 5 with MDROs). None of the drug-resistant organisms were recovered from any of these samples.
DISCUSSION
The protection of HCP from disease transmission during potentially aerosol-generating procedures is a priority. Effective recommendations require a clear understanding of the physical characteristics of any aerosols produced during these procedures and whether they carry viable pathogens that could pose an infection risk. In this study, multiple air sampling instruments were used to collect detailed real-time measurements of the aerosols generated during 7 common medical procedures, including several that are generally considered to be “aerosol-generating.” Microbiological analysis was used to determine the presence and viability of any bacterial organisms in these aerosols.
Significant aerosol generation was only observed during 2 types of procedures: NMA and bronchoscopy with NMA. The NMA findings are not surprising because nebulized medications are designed to be administered in aerosol form. Changing bed linens, patient bathing, pouring liquids into a hopper, flushing liquid waste, noninvasive ventilation, and bronchoscopy without NMA were not associated with significantly greater aerosols than at baseline. In addition, minimal amounts of viable bacteria were recovered during the sampled procedures, and what was recovered represented mainly common environmental or skin contaminants. These comprehensive aerosol assessment results, while from only a small number of sampled procedures, are reassuring about the potential risk to HCP.
Other studies have indicated that the risks posed by potentially aerosol-generating procedures may be overestimated [31–33]. Two reviews evaluating evidence for whether noninvasive ventilation should be considered a high-risk procedure found little epidemiologic data to support the theory that noninvasive ventilation increases occupational exposure [9, 34]. A 2013 review of evidence for whether flushing toilets is associated with infectious disease transmission found that no studies have clearly demonstrated toilet plume–related disease transmission [35]. Although bronchoscopy is frequently cited as a possible aerosol-generating procedure, a 2012 systematic review found no evidence of a significant association between bronchoscopy and increased risk of SARS transmission to HCP [24].
The most consistent clinical evidence for the transmission of infections via aerosols generated during medical procedures is during patient intubation [7, 24]. Although no increase in aerosol production over baseline was observed during patient intubations in this study, most captured intubations were laryngeal mask intubations on sedated patients for the purpose of bronchoscopy and may not be representative of emergent or less controlled settings.
Only NMA and bronchoscopy with NMA were found to generate a significant increase in particle concentrations (number, mass, and lung-deposited surface area) over baseline levels. The high particle concentrations are likely related to the use of a nebulizer during these procedures, and the particles are most likely aerosolized medication that escaped from the nebulizer device. This conclusion is supported by the results of a previous study, which evaluated droplet dispersion during nebulizer treatment and found an aerosol output profile consistent with nebulizer characteristics, rather than with dissemination of droplets from patients [36]. The extent of particle generation during NMA is probably related to the type of nebulizer used, treatment length, and patient characteristics, as a high amount of variability in particle concentration was observed during the different NMA sampling episodes. Although there was no significant bacterial pathogen recovery during NMA, the high concentrations of small aerosolized particles (median of 1 μm) could potentially affect HCP who administer the treatments.
Limitations of this study include small sample numbers (5 samples for each procedure), lack of clinical data, having only 1 sampling location for each sample, noncontinuous air sampling, and lack of viral pathogen recovery. In addition, the study focused on only 7 of the many medical procedures that may be considered “aerosol-generating.” The SKC BioSamplers used to capture aerosolized particles in this study also have limited sampling efficiency for particles <1 µm or ≥9 µm in diameter, though most bacterial particles are expected to fall within the 1- to 9-µm range [37].
Strengths of this study include the use of multiple real-time aerosol measurement instruments, use of culture to determine the presence of viable microbes as a metric to assess the infection risk posed by medically generated aerosols, and sampling during 7 types of medical procedures in a real-world healthcare setting.
Studies documenting the frequency and type of aerosols generated during common medical procedures in healthcare settings provide critical information needed to inform infection prevention strategies and guidelines. Evidence-based guidelines are necessary to help protect HCP from infection, especially in outbreak situations. Current guidelines for HCP participating in suspected aerosol-generating procedures have had to rely on minimal or low-quality evidence [6, 7]. Though additional research is needed, the results of this study suggest that some of the procedures that are widely considered to be high risk for the generation of infectious aerosols may actually pose little infection risk to HCP.
While this study has provided some information on aerosol-generating procedures that could potentially be used to inform infection prevention protocols, further research is needed to confirm these findings. Additional studies are also needed to describe aerosol generation during other procedures suspected to be aerosol-generating, to investigate whether viruses can be isolated from medically generated aerosols, and to examine the impact of patient clinical characteristics on aerosol production and pathogen recovery. Such studies would provide a more solid base of evidence on which to base infection prevention guidelines and would provide information that could be used to develop methods that reduce aerosol generation during medical procedures, thereby reducing the risk of environmental contamination and infection transmission.
Notes
Acknowledgments. The study team thanks the healthcare personnel and patients who participated in sampling.
Financial support. This work was supported by a US Centers for Disease Control and Prevention Epicenters Program Ebola Supplement Grant (grant number 3U54CK000162-05S1).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Coia JE, Ritchie L, Adisesh A, et al. ; Healthcare Infection Society Working Group on Respiratory and Facial Protection Guidance on the use of respiratory and facial protection equipment. J Hosp Infect 2013; 85:170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Interim guidance on infection control measures for 2009 H1N1 influenza in healthcare settings, including protection of healthcare personnel Available at: http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed 10 August 2016. [PubMed]
- 3. Centers for Disease Control and Prevention. Prevention strategies for seasonal influenza in healthcare settings. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed 10 June 2015.
- 4. World Health Organization. Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses. Available at: http://www.who.int/csr/resources/publications/cp150_2009_1612_ipc_interim_guidance_h1n1.pdf?ua=1. Accessed 8 August 2016.
- 5. Chughtai AA, Seale H, MacIntyre CR. Availability, consistency and evidence-base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: a global analysis. BMC Res Notes 2013; 6:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gralton J, McLaws ML. Protecting healthcare workers from pandemic influenza: N95 or surgical masks? Crit Care Med 2010; 38:657–67. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Infection prevention and control of epidemic-and pandemic-prone acute respiratory infections in health care: WHO guidelines. Geneva, Switzerland: WHO, 2014. [PubMed] [Google Scholar]
- 8. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35:S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCracken J. Should noninvasive ventilation be considered a high-risk procedure during an epidemic? CMAJ 2009; 181:663–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christian MD, Loutfy M, McDonald LC, et al. ; SARS Investigation Team Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis 2004; 10:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fowler RA, Lapinsky SE, Hallett D, et al. ; Toronto SARS Critical Care Group Critically ill patients with severe acute respiratory syndrome. JAMA 2003; 290:367–73. [DOI] [PubMed] [Google Scholar]
- 12. Ofner M, Lem M, Sarwal S, Vearncombe M, Simor A. Cluster of severe acute respiratory syndrome cases among protected health care workers—Toronto, April 2003. Can Commun Dis Rep 2003; 29:93–7. [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Infection control precautions for aerosol-generating procedures on patients who have suspected severe acute respiratory syndrome (SARS). Atlanta, GA: CDC, 2013. [Google Scholar]
- 14. Hui DS. Severe acute respiratory syndrome (SARS): lessons learnt in Hong Kong. J Thorac Dis 2013; 5suppl 2:S122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis 2005; 191:1472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuster SP, Coleman BL, Raboud J, et al. Risk factors for influenza among health care workers during 2009 pandemic, Toronto, Ontario, Canada. Emerg Infect Dis 2013; 19:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface 2009; 6suppl 6:S783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindsley WG, Blachere FM, Davis KA, et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis 2010; 50:693–8. [DOI] [PubMed] [Google Scholar]
- 19. Al-Dorzi HM, Alsolamy S, Arabi YM. Critically ill patients with Middle East respiratory syndrome coronavirus infection. Crit Care 2016; 20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Tawfiq JA, Perl TM. Middle East respiratory syndrome coronavirus in healthcare settings. Curr Opin Infect Dis 2015; 28:392–6. [DOI] [PubMed] [Google Scholar]
- 21. Mekibib B, Arien KK. Aerosol transmission of filoviruses. Viruses 2016; 8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eames I, Tang JW, Li Y, Wilson P. Airborne transmission of disease in hospitals. J R Soc Interface 2009; 6suppl 6:S697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies A, Thompson G, Walker J, Bennett A. A review of the risks and disease transmission associated with aerosol generating medical procedures. J Infect Prev 2009; 10:122–6. [Google Scholar]
- 24. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gamage B, Moore D, Copes R, Yassi A, Bryce E; BC Interdisciplinary Respiratory Protection Study Group Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. Am J Infect Control 2005; 33:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Branda JA, Rychert J, Burnham CA, et al. Multicenter validation of the VITEK MS v2.0 MALDI-TOF mass spectrometry system for the identification of fastidious gram-negative bacteria. Diagn Microbiol Infect Dis 2014; 78:129–31. [DOI] [PubMed] [Google Scholar]
- 27. Manji R, Bythrow M, Branda JA, et al. Multi-center evaluation of the VITEK MS system for mass spectrometric identification of non-Enterobacteriaceae gram-negative bacilli. Eur J Clin Microbiol Infect Dis 2014; 33:337–46. [DOI] [PubMed] [Google Scholar]
- 28. Richter SS, Sercia L, Branda JA, et al. Identification of Enterobacteriaceae by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using the VITEK MS system. Eur J Clin Microbiol Infect Dis 2013; 32: 1571–8. [DOI] [PubMed] [Google Scholar]
- 29. McElvania TeKippe E, Burnham CA. Evaluation of the bruker biotyper and VITEK MS MALDI-TOF MS systems for the identification of unusual and/or difficult-to-identify microorganisms isolated from clinical specimens. Eur J Clin Microbiol Infect Dis 2014; 33:2163–71. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Li J, Leavey A, O’Neil C, Babcock HM, Biswas P. Comparative study on the size distributions, respiratory deposition, and transport of particles generated from commonly used medical nebulizers. J Aerosol Med Pulm Drug Deliv 2016; 30:132–40. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Seale H, Yang P, et al. Factors associated with the transmission of pandemic (H1N1) 2009 among hospital healthcare workers in Beijing, China. Influenza Other Respir Viruses 2013; 7:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seto WH. Airborne transmission and precautions: facts and myths. J Hosp Infect 2015; 89:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson KA, Pappachan JV, Bennett AM, et al. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic—the risk of aerosol generation during medical procedures. PLoS One 2013; 8:e56278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esquinas AM, Egbert Pravinkumar S, Scala R, et al. ; International NIV Network Noninvasive mechanical ventilation in high-risk pulmonary infections: a clinical review. Eur Respir Rev 2014; 23:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson DL, Mead KR, Lynch RA, Hirst DV. Lifting the lid on toilet plume aerosol: a literature review with suggestions for future research. Am J Infect Control 2013; 41:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simonds AK, Hanak A, Chatwin M, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess 2010; 14:131–72. [DOI] [PubMed] [Google Scholar]
- 37. Kesavan J, Schepers D, McFarland AR. Sampling and retention efficiencies of batch-type liquid-based bioaerosol samplers. Aerosol Sci Tech 2010; 44:817–29. [Google Scholar]