ABSTRACT
Background
Data on saturated fatty acids (SFAs) in relation to metabolic function and glucose homeostasis remain controversial. Such data are lacking among pregnant women.
Objective
We prospectively investigated objectively measured individual and subclasses of plasma phospholipid SFAs throughout pregnancy in relation to cardiometabolic markers and gestational diabetes mellitus (GDM) risk.
Design
Within the National Institute of Child Health and Human Development Fetal Growth Studies–Singleton Cohort of 2802 singleton pregnancies, 107 GDM cases were ascertained via medical record review and matched to 214 non-GDM controls on age, race/ethnicity, and gestational week (GW) at blood collection. Individual plasma phospholipid SFA concentrations were repeatedly measured throughout pregnancy at GWs 10–14, 15–26, 23–31, and 33–39 and also grouped into subclasses of even- or odd-chain SFAs.
Results
From GW 10, even-chain SFA concentrations were significantly higher among women who later developed GDM, whereas odd-chain SFAs were significantly lower among GDM cases compared with controls. At GWs 10–14, the SFA palmitic acid (16:0) was positively associated with impaired insulin resistance and cardiometabolic markers and the risk of GDM [adjusted OR comparing the highest with the lowest quartile (aORQ4-Q1): 4.76; 95% CI: 1.72, 13.10; P-trend = 0.001]. In contrast, odd-chain SFAs were inversely related to the previously mentioned markers and GDM risk [aORQ4-Q1 for pentadecanoic acid (15:0): 0.32; 95% CI: 0.11, 0.92; P-trend = 0.025; for heptadecanoic acid (17:0): 0.20; 95% CI: 0.07, 0.58; P-trend = 0.003]. Women with high (median or greater) even-chain SFA concentrations and low (less than median) odd-chain SFAs had a 9.43-fold (95%: CI 3.26-, 27.30-fold) increased risk compared with women with low even-chain and high odd-chain SFA concentrations. Similar results were observed at GWs 15–26.
Conclusions
The study provided one of the first lines of evidence suggesting that circulating concentrations of SFAs varying by SFA chain length, as early as GWs 10–14, were significantly and differentially associated with subsequent risk of GDM. Our findings highlight the importance of assessing objectively measured, individual, and subclasses of SFAs to investigate their distinct biological and pathophysiologic roles in glucose homeostasis and cardiometabolic outcomes. This study was registered at www.clinicaltrials.gov as NCT00912132.
Keywords: chain length, etiology, gestational diabetes, objective measurement, saturated fatty acids
INTRODUCTION
With an increase in prevalence of >35% during the past decades in the United States and similarly increasing prevalences in other countries worldwide (1, 2), gestational diabetes mellitus (GDM) has become the most common pregnancy metabolic disorder, representing a growing, urgent public health concern. Furthermore, the hyperglycemic intrauterine environment exemplified in GDM-complicated pregnancies may be fueling the epidemic of adverse cardiometabolic outcomes, including type 2 diabetes and obesity among offspring, forming a vicious intergenerational cycle (3). Thus, research efforts aimed to improve our understanding of the etiology of GDM and identify potentially modifiable risk factors are of great clinical and public health significance.
Emerging evidence suggests that SFAs may be implicated in metabolic dysregulation and impaired glucose homeostasis (4). Research on SFAs has been marked by long-standing controversy with respect to their role in cardiometabolic outcomes. Epidemiologic evidence has been inconsistent, with positive (5, 6), inverse (7, 8), or null associations of total dietary SFAs with cardiometabolic outcomes (9–12), thus calling for a further examination of their effect on health. Notably, dietary assessment via participant report is inevitably subject to measurement errors and has mostly focused on total dietary, not individual, SFAs (10, 11). Furthermore, given the heterogeneous origins of plasma phospholipid SFAs, the objective measurement of individual circulating SFAs is essential to advance our understanding of their potentially distinct pathophysiologic roles. Specifically, concentrations of circulating even-chain SFAs [myristic acid (14:0), palmitic acid (16:0), and stearic acid (18:0)] are functions of both exogenous (via dietary intake) and endogenous (via de novo lipogenesis) origins (13, 14), whereas odd-chain SFAs [pentadecanoic acid (15:0) and heptadecanoic acid (17:0)] may mainly reflect dietary intakes of dairy fat (15), although emerging evidence suggests that endogenous sources cannot be ruled out (16, 17). Notably, plasma phospholipid fatty acid composition reflects that of cellular membranes, which is an important determinant of the membrane structure, property, and functions and may, in turn, influence hormone binding, intracellular signaling, enzyme activity, and thus the action of insulin (18). In particular, increased concentrations of membrane phospholipid SFAs decrease the number of insulin receptors and insulin-binding activities (19, 20). Despite the varied origins and potential distinct roles of individual SFAs on glucose homeostasis (15, 21), data are lacking on the respective roles of individual and subclasses of plasma phospholipid SFAs in relation to subsequent GDM risk. Furthermore, despite the dynamic physiologic alterations during pregnancy, variation in the phospholipid SFA profile throughout pregnancy is as-yet understudied. To address these critical knowledge gaps, we prospectively 1) investigated the associations of individual and subclasses of circulating SFAs varying by chain length with a comprehensive panel of glucose metabolism and cardiovascular markers and the risk of subsequent GDM and 2) profiled the longitudinal trends of plasma phospholipid SFAs throughout pregnancy.
METHODS
Study population and design
This study was conducted in participants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies–Singleton Cohort, a multiracial prospective study in low-risk, singleton pregnant women (22). Briefly, 2802 pregnant women (2334 nonobese and 468 obese) aged 18–40 y with a prepregnancy BMI (kg/m2) ranging from 19.0 to 45.0 and without pre-existing diseases (i.e., hypertension, diabetes, renal or autoimmune disease, psychiatric disorder, cancer, or HIV/AIDS) were recruited between gestational weeks (GWs) 8 and 13 at 12 clinical centers across the United States (2009–2013). The study was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all participants. This study was registered at www.clinicaltrials.gov as NCT00912132.
In the entire cohort, we identified 107 GDM cases via medical record review according to the Carpenter and Coustan criteria as recommended by the American College of Obstetrics and Gynecologists (23). Non-GDM controls (n = 214) were selected and individually matched 2:1 to cases on age (±2 y), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or Asian/Pacific Islander), and GW of blood sample collection (±2 wk) (Supplemental Figure 1) (24). During pregnancy, maternal blood specimens were longitudinally collected during 4 study visits (i.e., target weeks 8–13, 16–22, 24–29, and 34–37), at which fasting samples after an overnight fast of 8–14 h were collected at the second visit, for the purpose of an etiology study of GDM as a secondary outcome of interest in the National Institute of Child Health and Human Development Fetal Growth Studies–Singleton Cohort. Biospecimens were stored at −80°C until being thawed immediately before assay. By design, participants were randomly assigned within each time window of blood collection to maximize the opportunity of capturing weekly data across the entire cohort. Because some women arrived late for the scheduled visit, the actual GWs at blood collection ranged slightly beyond the original planned visit time window (i.e., 10–14, 15–26, 23–31, and 33–39 wk), the first 2 of which were before GDM diagnosis. Of note, ranges of certain visit windows appeared to overlap (i.e., 15–26 and 23–31 wk); however, those for each individual were in chronological order and did not overlap. For instance, a participant made a second and third study visit at GWs 26 and 30, respectively.
Biomarker assessment
From the 2 blood collections before the diagnosis of GDM (i.e., GWs 10–14 and 15–26), biomarkers were measured among all cases (n = 107) and controls (n = 214). From blood collections at the following 2 visits (i.e., GWs 23–31 and 33–39), biomarkers were measured among all cases and 1 of their randomly selected controls (n = 107). Plasma phospholipid SFAs were measured with the use of a Hewlett Packard 5890 gas chromatography system, as described previously (25). The plasma phospholipid fraction isolated from the gas chromatograph consists mostly of phosphatidylcholine (∼80%) and phosphatidylethanolamine. A small amount of sphingomyelin and lysolecithin (or lysophosphatidylcholine) may also be present, but their contribution to total fatty acids is negligible compared with phosphatidylcholine and phosphatidylethanolamine. The content of individual SFAs was expressed as a weight percentage of the total phospholipid fatty acids. Samples of matched case-control pairs were assayed in the same analytical run by the same technicians in a random order. Even- and odd-chain SFAs with relative concentrations >0.05% included myristic acid (14:0; CV: 9.0%), pentadecanoic acid (15:0; CV: 5.0%), palmitic acid (16:0; CV: 2.7%), heptadecanoic acid (17:0; CV: 5.7%), and stearic acid (18:0; CV: 7.2%). The individual SFAs were also grouped into subclasses of even-chain (sum of myristic, palmitic, and stearic) or odd-chain (sum of pentadecanoic and heptadecanoic) SFAs.
Plasma glucose and high-sensitivity C-reactive protein were measured by enzymatic assays with the use of the Roche Modular P Chemistry analyzer; total cholesterol, HDL cholesterol, and triglycerides were measured by the Roche COBAS 6000 Chemistry Analyzer; and insulin and C-peptide were measured by the Roche Elecsys 2010 Analyzer (Roche Diagnostics). LDL cholesterol was calculated by the Friedewald's formula: LDL cholesterol = total cholesterol − HDL cholesterol − triglycerides ÷ 5 (26). All values of plasma lipid concentrations were expressed in mg/dL. Plasma adiponectin was measured by using a quantitative sandwich enzyme immunoassay (Beckman Coulter, Inc.). Plasma leptin was measured by using the Mercodia Leptin ELISA (Mercodia AB). The interassay CVs of these biomarkers were all <6.0%. All of the assays were performed without knowledge of the case-control status.
Covariates
Data on maternal demographic, lifestyle, and clinical factors were obtained from structured questionnaires and extracted from medical records. Covariates were a priori selected including conventional risk factors for GDM: parity (nulliparous or multiparous), family history of diabetes (yes or no), and prepregnancy BMI (<25.0, 25.0–29.9, 30.0–34.9, or 35.0–44.9). Of note, in this low-risk population, nonobese women who smoked in the 6 mo preceding the index pregnancy were ineligible, and only 5 obese women reported smoking in the 6 mo before pregnancy. Thus, smoking was not included as a covariate. Given that maternal age (years) and GW of blood collection (weeks) were matched between cases and controls within a certain range, these 2 matching factors were also included as covariates to derive conservative risk estimates.
Statistical methods
Differences in participant characteristics and plasma phospholipid SFA concentrations at the 2 visits before GDM diagnosis between cases and controls were assessed by linear mixed models with associated likelihood ratio tests for continuous variables, and by binomial or multinomial logistic regression with generalized estimating equations for binary or multilevel categorical variables, accounting for matched case-control pairs. Longitudinal trends of SFAs throughout pregnancy were plotted according to gestational age intervals among women with and without GDM. Similarly, between-group comparisons of SFA concentrations at each gestational age interval were obtained by linear mixed models, accounting for matched case-control pairs.
Multivariable conditional logistic regression models adjusting for the previously mentioned covariates were fit to assess the associations of individual circulating SFAs at GWs 10–14 and 15–26 with subsequent GDM risk, respectively. To ensure that biomarker measurements preceded the diagnosis of GDM, we excluded 1 case subject at GWs 10–14 and 5 at 15–26 wk from the final analysis whose blood samples were collected after the diagnosis of GDM. We analyzed each SFA by categorizing each measurement into quartiles on the basis of the distribution among controls and also by treating each SFA as a continuous variable standardized by the SD of the measurements among controls. Tests of linear trend were conducted by using the median value for each quartile and fitting it as a continuous variable in the conditional logistic regression models. Similar analyses were conducted for SFA subclasses (i.e., even- and odd-chain SFAs). Furthermore, to investigate the potential synergistic effect of SFA subclasses, we assessed the risk estimates associated with joint categories of even- and odd-chain SFAs above or below the respective median.
To gain mechanistic insight into the underlying pathogenic processes involved in glucose homeostasis, we calculated partial Spearman's correlation coefficients of individual and subclasses of circulating SFAs at GWs 10–14 with glucose homeostasis (fasting plasma glucose, insulin, C-peptide, high-sensitivity C-reactive protein, and HOMA-IR) (27) and cardiometabolic markers (adiponectin, leptin, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) at the subsequent visit (GWs 15–26), after adjusting for covariates. Heat maps were also created to visualize and evaluate the correlations between individual SFAs and the previously mentioned biomarkers at GWs 10–14 and 15–26 among non-GDM controls, respectively. In addition, we conducted prediction or diagnostic analyses to explore the relative incremental predictive ability of individual SFAs with the use of receiver operating characteristic curves and C statistics (28), beyond conventional risk factors for GDM (i.e., age, parity, family history of diabetes, and prepregnancy BMI) and plasma glucose with the use of DeLong's test (29). To avoid model overfitting, leave-one-out cross-validation was performed to derive conservative estimates by successively leaving out each observation from the sample (n) one at a time and using the model fit based on the remaining observations (n − 1) to compute the predicted probability for the left-out observation. This process was repeated n times until all observations were validated (30).
To evaluate whether the associations of circulating SFAs with subsequent GDM risk were modified by major risk factors for GDM (prepregnancy obesity status, family history of diabetes, and race/ethnicity), we included cross-product (interaction) terms to assess the potential effect modification. All of the analyses were conducted with the use of SAS version 9.4 (SAS Institute).
RESULTS
Participant characteristics and plasma phospholipid SFA profiles before GDM diagnosis
Compared with non-GDM controls, women with GDM were more likely to have a family history of diabetes and have a higher prepregnancy BMI (Table 1). Among SFAs, the even-chain SFA palmitic acid was the most abundant form and contributed ∼27% to the total plasma phospholipid fatty acid concentration, followed by the SFA stearic acid (∼12%) at GWs 10–14, whereas the odd-chain SFAs contributed <1% (Table 2). Compared with non-GDM controls, concentrations of even-chain SFAs (sum and palmitic acid) were significantly higher, whereas odd-chain SFAs (sum, pentadecanoic acid, and heptadecanoic acid) were significantly lower among GDM cases at GWs 10–14 and 15–26, respectively.
TABLE 1.
Participant characteristics among women with GDM and their matched controls: the NICHD Fetal Growth Studies–Singleton Cohort1
| GDM cases | Non-GDM controls | ||
|---|---|---|---|
| (n = 107) | (n = 214) | P 2 | |
| Age, y | 30.5 ± 5.7 | 30.4 ± 5.4 | |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 25 (23.4) | 50 (23.4) | |
| Non-Hispanic black | 15 (14.0) | 30 (14.0) | |
| Hispanic | 41 (38.3) | 82 (38.3) | |
| Asian/Pacific Islander | 26 (24.3) | 52 (24.3) | |
| Education, n (%) | 0.18 | ||
| Less than high school | 17 (15.9) | 26 (12.1) | |
| High school graduate or equivalent | 15 (14.0) | 23 (10.7) | |
| More than high school | 75 (70.1) | 165 (77.1) | |
| Insurance, n (%) | 0.43 | ||
| Private or managed care | 68 (63.5) | 143 (66.8) | |
| Medicaid, self-pay, or other | 39 (36.5) | 71 (33.1) | |
| Marital status, n (%) | 0.12 | ||
| Never married | 11 (10.3) | 35 (16.4) | |
| Married/living with a partner | 92 (86.0) | 167 (78.0) | |
| Divorced/separated | 4 (3.7) | 12 (5.6) | |
| Nulliparous, n (%) | 48 (44.9) | 96 (44.9) | 1 |
| Family history of diabetes, n (%) | 40 (37.4) | 48 (22.4) | 0.003 |
| Prepregnancy BMI, kg/m2 | 28.2 ± 6.4 | 25.6 ± 5.3 | <0.001 |
| Prepregnancy BMI categories (kg/m2), n (%) | <0.001 | ||
| <25.0 | 37 (34.6) | 123 (58.0) | |
| 25.0–29.9 | 35 (32.7) | 56 (26.4) | |
| 30.0–34.9 | 20 (18.7) | 17 (8.0) | |
| 35.0–44.9 | 15 (14.0) | 16 (7.6) | |
| Smoking 6 mo preconception, n (%) | 4 (3.7) | 1 (0.5) | 0.06 |
| Alcoholic beverage consumption 3 mo preconception, n (%) | 61 (57.0) | 137 (64.0) | 0.22 |
1Values are means ± SDs unless otherwise specified. GDM, gestational diabetes mellitus; NICHD, National Institute of Child Health and Human Development.
2Obtained by linear mixed models with associated likelihood ratio tests for continuous variables and binomial/multinomial logistic regression with generalized estimating equations for binary/multilevel categorical variables (Wald tests), accounting for matched case-control pairs. P values are not shown for matching variables, age, and race/ethnicity.
TABLE 2.
Distribution of plasma phospholipid SFAs among GDM cases and non-GDM controls1
| GDM cases (n = 107), % | Non-GDM controls (n = 214), % | P 2 | |
|---|---|---|---|
| Gestational weeks 10–14 | |||
| Even-chain SFAs | |||
| Sum of even-chain SFAs3 | 40.6 (40.1–41.4) | 39.9 (39.2–40.8) | <0.0001 |
| Myristic acid (14:0) | 0.31 (0.26–0.36) | 0.30 (0.24–0.36) | 0.24 |
| Palmitic acid (16:0) | 27.9 (27.1–28.9) | 27.1 (26.0–28.3) | <0.0001 |
| Stearic acid (18:0) | 12.6 (11.8–13.4) | 12.6 (12.0–13.4) | 0.63 |
| Odd-chain SFAs | |||
| Sum of odd-chain SFAs3 | 0.62 (0.55–0.72) | 0.68 (0.61–0.75) | <0.0001 |
| Pentadecanoic acid (15:0) | 0.23 (0.19–0.27) | 0.24 (0.20–0.29) | 0.01 |
| Heptadecanoic acid (17:0) | 0.39 (0.35–0.45) | 0.44 (0.38–0.49) | <0.0001 |
| Gestational weeks 15–26 | |||
| Even-chain SFAs | |||
| Sum of even-chain SFAs3 | 41.1 (40.3–42.8) | 40.7 (39.8–41.9) | 0.04 |
| Myristic acid (14:0) | 0.32 (0.26–0.36) | 0.32 (0.27–0.37) | 0.18 |
| Palmitic acid (16:0) | 29.2 (28.3–30.2) | 28.6 (27.4–29.7) | 0.02 |
| Stearic acid (18:0) | 11.8 (11.4–12.7) | 12.0 (11.5–12.7) | 0.56 |
| Odd-chain SFAs | |||
| Sum of odd-chain SFAs3 | 0.62 (0.53–0.68) | 0.68 (0.60–0.77) | <0.0001 |
| Pentadecanoic acid (15:0) | 0.24 (0.19–0.27) | 0.25 (0.21–0.30) | 0.0001 |
| Heptadecanoic acid (17:0) | 0.37 (0.34–0.42) | 0.41 (0.37–0.46) | <0.0001 |
1Values are medians (25th–75th percentiles). GDM, gestational diabetes mellitus.
2 P values for differences between cases and controls were obtained by linear mixed models with associated likelihood ratio tests, accounting for matched case-control pairs (likelihood ratio tests).
3Sum of even-chain SFAs = sum of myristic acid (14:0), palmitic acid (16:0), and stearic acid (18:0); sum of odd-chain SFAs = sum of pentadecanoic acid (15:0) and heptadecanoic acid (17:0).
Longitudinal SFA profiles throughout pregnancy
Overall, concentrations of plasma phospholipid even-chain SFAs increased, whereas odd-chain SFAs decreased among both cases and controls as pregnancy progressed, particularly before GW 28 (Figure 1). Specifically, mean concentrations of even-chain SFAs were consistently higher among cases than controls at GWs 10–12, 13–15, and 24–27. Conversely, mean concentrations of odd-chain SFAs were significantly lower among GDM cases than controls from GWs 10–12 throughout GWs 24–27. After GWs 24–27, which was close to the conventional time of GDM screening and diagnosis (i.e., GWs 24–28), the significant differences in even- and odd-chain SFAs between cases and controls diminished and did not persist.
FIGURE 1.
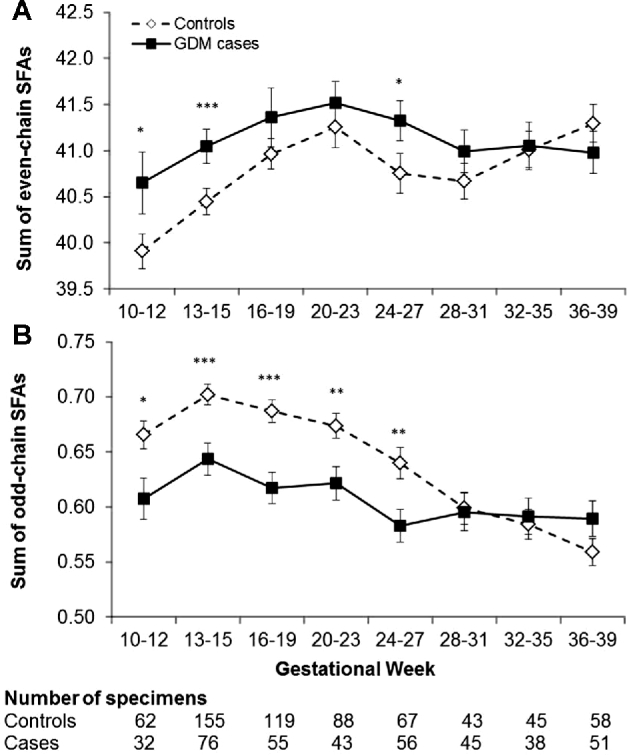
Longitudinal profiles of plasma even-chain and odd-chain SFAs throughout pregnancy according to gestational age intervals among women with and without GDM. Even-chain SFAs: sum of myristic acid (14:0), palmitic acid (16:0), and stearic acid (18:0); odd-chain SFAs: sum of pentadecanoic acid (15:0) and heptadecanoic acid (17:0). Values are mean percentages ± SEs. *P < 0.05, **P < 0.01, ***P < 0.001 for case-control comparisons obtained by linear mixed models with associated likelihood ratio tests accounting for matched case-control pairs at each gestational age interval. P-interaction between gestational weeks and GDM status was 0.042 for even-chain SFAs and <0.001 for odd-chain SFAs, obtained by likelihood ratio test. GDM, gestational diabetes mellitus.
Plasma phospholipid SFAs in relation to glucose metabolism and cardiometabolic biomarkers
Plasma phospholipid even-chain SFAs (particularly palmitic acid) at GWs 10–14 were positively correlated with subsequent fasting biomarkers implicated in impaired glucose homeostasis and cardiometabolic function (i.e., HOMA-IR, insulin, C-peptide, total cholesterol, LDL cholesterol, and triglycerides) and inversely correlated with adiponectin at GWs 15–26, after adjustment for covariates (Table 3). In contrast, opposite correlation patterns were observed for odd-chain SFAs (particularly heptadecanoic acid) at GWs 10–14 and the previously mentioned biomarkers at GWs 15–26. A similar trend was observed when examining cross-sectional correlations between individual SFAs and these clinical markers at GWs 10–14 and 15–26, respectively (Supplemental Figure 2).
TABLE 3.
Adjusted Spearman correlation coefficients of plasma phospholipid SFAs at gestational weeks 10–14 with subsequent fasting plasma biomarkers and indexes at gestational weeks 15–26 among non-GDM controls1
| HOMA-IR | Glucose | Insulin | C-peptide | hs-CRP | Adiponectin | Leptin | Total cholesterol | HDL-C | LDL-C | Triglycerides | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Even-chain SFAs | |||||||||||
| Sum of even-chain SFAs | 0.221** | 0.069 | 0.230*** | 0.170 8* |
0.237*** | −0.190** | 0.217** | 0.029 | −0.237*** | 0.085 | 0.176** |
| Myristic acid (14:0) | −0.031 | 0.007 | −0.022 | −0.009 | 0.066 | 0.123 | 0.044 | 0.098 | 0.066 | 0.078 | 0.027 |
| Palmitic acid (16:0) | 0.180** | 0.065 | 0.184** | 0.1591 | 0.108 | −0.193** | 0.109 | 0.202** | −0.123 | 0.215** | 0.263*** |
| Stearic acid (18:0) | −0.064 | −0.069 | −0.043 | −0.103 | 0.119 | 0.069 | 0.062 | −0.099 | −0.099 | −0.002 | −0.179** |
| Odd-chain SFAs | |||||||||||
| Sum of odd-chain SFAs | −0.088 | −0.046 | −0.071 | −0.139* | −0.066 | 0.151* | −0.081 | 0.037 | 0.221** | −0.001 | −0.144* |
| Pentadecanoic acid (15:0) | 0.033 | 0.086 | 0.039 | −0.016 | 0.002 | 0.087 | 0.017 | 0.153* | 0.196** | 0.092 | 0.034 |
| Heptadecanoic acid (17:0) | −0.187** | −0.162* | −0.163* | −0.213** | −0.086 | 0.160* | −0.161* | −0.063 | 0.164* | −0.066 | −0.238*** |
1 *P < 0.05, **P < 0.01, ***P < 0.001. P values were adjusted for age (years), gestational age at blood collection (weeks), parity (nulliparous or multiparous), family history of diabetes (yes or no), and prepregnancy BMI (kg/m2; <25.0, 25.0–29.9, 30.0–34.9, or 35.0–44.9). GDM, gestational diabetes mellitus; HDL-C, HDL cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, LDL cholesterol.
Plasma phospholipid SFAs in relation to subsequent GDM risk
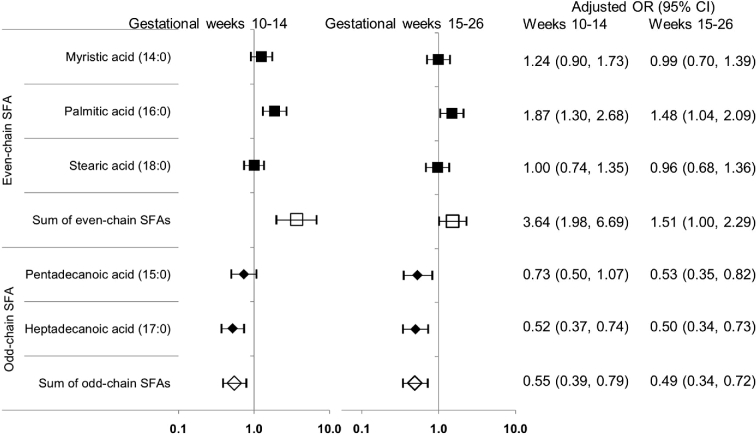
The sum of plasma phospholipid even-chain SFAs concentrations during early to midpregnancy was significantly and positively related to subsequent GDM risk, whereas the sum of odd-chain SFAs was significantly and inversely related to GDM risk (Table 4). Among even-chain SFAs, comparing the highest with the lowest quartile, palmitic acid at GWs 10–14 and 15–26 was significantly associated with a 4.76-fold (95% CI: 1.72-, 13.10-fold; P-trend = 0.001) and 6.38-fold (95% CI: 2.06-, 19.80-fold; P-trend = 0.007) increased risk of GDM, respectively (multivariable model). Conversely, among odd-chain SFAs, comparing the highest with the lowest quartile at the 2 gestational periods, heptadecanoic acid was significantly associated with an 80% [adjusted OR (aOR): 0.20; 95% CI: 0.07, 0.58; P-trend = 0.003] and 86% (aOR: 0.14; 95% CI: 0.04, 0.43; P-trend < 0.001) lower risk of GDM, respectively. Similar inverse associations were observed for pentadecanoic acid at both gestational periods. Furthermore, results were similar when individual or subclasses of SFAs were parameterized continuously, with a 3.64-fold (95% CI: 1.98-, 6.69-fold) increased risk of GDM per a 1-SD increase in the sum of even-chain SFAs and a 45% (aOR: 0.55; 95% CI: 0.39, 0.79) reduced risk per 1-SD increase in the sum of odd-chain SFAs at GWs 10–14 (Figure 2). Similar results for continuous SFAs were observed at GWs 15–26. In addition, the observed positive associations of even-chain SFAs and inverse associations of odd-chain SFAs with GDM risk persisted across strata of conventional risk factors for GDM, including prepregnancy obesity, family history of diabetes, and race/ethnicity.
TABLE 4.
ORs (95% CIs) for subsequent risk of GDM according to quartiles of plasma phospholipid SFAs at gestational weeks 10–14 and 15–261
| Gestational weeks 10–14, % | Gestational weeks 15–26, % | |||
|---|---|---|---|---|
| Crude | Multivariable2 | Crude | Multivariable2 | |
| Even-chain SFAs | ||||
| Sum of even-chain SFAs3 | ||||
| Q2 vs. Q1 (reference) | 1.63 (0.51, 5.17) | 1.86 (0.52, 6.62) | 1.90 (0.79, 4.55) | 2.03 (0.69, 5.95) |
| Q3 vs. Q1 | 8.26 (2.92, 23.30) | 10.7 (3.20, 35.60) | 3.69 (1.59, 8.56) | 3.56 (1.29, 9.84) |
| Q4 vs. Q1 | 13.7 (4.19, 45.00) | 13.0 (3.41, 49.50) | 5.97 (2.06, 17.30) | 6.56 (1.84, 23.40) |
| P-trend4 | <0.0001 | <0.0001 | 0.001 | 0.002 |
| Myristic acid (14:0) | ||||
| Q2 vs. Q1 | 1.54 (0.73, 3.24) | 1.74 (0.77, 3.92) | 0.73 (0.35, 1.54) | 0.80 (0.33, 1.93) |
| Q3 vs. Q1 | 2.18 (1.02, 4.67) | 2.32 (1.01, 5.31) | 0.68 (0.30, 1.56) | 1.62 (0.72, 3.62) |
| Q4 vs. Q1 | 1.67 (0.71, 3.92) | 2.40 (0.93, 6.16) | 1.14 (0.55, 2.39) | 1.25 (0.48, 3.27) |
| P-trend4 | 0.166 | 0.056 | 0.542 | 0.353 |
| Palmitic acid (16:0) | ||||
| Q2 vs. Q1 | 1.84 (0.69, 4.88) | 1.35 (0.47, 3.90) | 3.45 (1.45, 8.22) | 5.12 (1.79, 14.60) |
| Q3 vs. Q1 | 6.38 (2.43, 16.70) | 5.25 (1.91, 14.40) | 3.47 (1.44, 8.36) | 3.98 (1.41, 11.20) |
| Q4 vs. Q1 | 6.22 (2.36, 16.40) | 4.76 (1.72, 13.10) | 4.74 (1.82, 12.30) | 6.38 (2.06, 19.80) |
| P-trend4 | <0.001 | 0.001 | 0.004 | 0.007 |
| Stearic acid (18:0) | ||||
| Q2 vs. Q1 | 0.52 (0.27, 1.02) | 0.55 (0.26, 1.19) | 0.94 (0.47, 1.87) | 0.80 (0.37, 1.73) |
| Q3 vs. Q1 | 0.67 (0.34, 1.31) | 0.81 (0.38, 1.73) | 0.58 (0.28, 1.23) | 0.63 (0.27, 1.49) |
| Q4 vs. Q1 | 0.71 (0.35, 1.44) | 0.84 (0.37, 1.91) | 1.05 (0.48, 2.29) | 1.03 (0.43, 2.48) |
| P-trend4 | 0.403 | 0.812 | 0.908 | 0.980 |
| Odd-chain SFAs | ||||
| Sum of odd-chain SFAs3 | ||||
| Q2 vs. Q1 | 0.29 (0.14, 0.62) | 0.31 (0.14, 0.72) | 0.46 (0.23, 0.91) | 0.48 (0.22, 1.07) |
| Q3 vs. Q1 | 0.29 (0.14, 0.62) | 0.30 (0.13, 0.68) | 0.37 (0.18, 0.77) | 0.41 (0.18, 0.93) |
| Q4 vs. Q1 | 0.22 (0.10, 0.53) | 0.32 (0.12, 0.83) | 0.10 (0.03, 0.29) | 0.11 (0.03, 0.42) |
| P-trend4 | <0.0001 | 0.005 | <0.0001 | <0.0001 |
| Pentadecanoic acid (15:0) | ||||
| Q2 vs. Q1 | 0.79 (0.39, 1.59) | 0.70 (0.33, 1.49) | 0.58 (0.26, 1.27) | 0.53 (0.22, 1.30) |
| Q3 vs. Q1 | 0.49 (0.23, 1.05) | 0.47 (0.20, 1.10) | 0.44 (0.19, 0.99) | 0.44 (0.18, 1.12) |
| Q4 vs. Q1 | 0.28 (0.10, 0.74) | 0.32 (0.11, 0.92) | 0.16 (0.06, 0.48) | 0.17 (0.05, 0.62) |
| P-trend4 | 0.007 | 0.025 | 0.001 | 0.009 |
| Heptadecanoic acid (17:0) | ||||
| Q2 vs. Q1 | 0.59 (0.32, 1.10) | 0.66 (0.34, 1.29) | 0.41 (0.21, 0.81) | 0.44 (0.20, 0.99) |
| Q3 vs. Q1 | 0.39 (0.20, 0.77) | 0.51 (0.24, 1.07) | 0.40 (0.20, 0.82) | 0.31 (0.13, 0.74) |
| Q4 vs. Q1 | 0.20 (0.08, 0.47) | 0.20 (0.07, 0.58) | 0.14 (0.05, 0.36) | 0.14 (0.04, 0.43) |
| P-trend4 | <0.001 | 0.003 | <0.001 | <0.001 |
1GDM, gestational diabetes mellitus; Q, quartile.
2Adjusted for age (years), gestational age at blood collection (weeks), parity (nulliparous or multiparous), family history of diabetes (yes or no), and prepregnancy BMI (kg/m2; <25.0, 25.0–29.9, 30.0–34.9, or 35.0–44.9).
3Sum of even-chain SFAs = sum of myristic acid (14:0), palmitic acid (16:0), and stearic acid (18:0); sum of odd-chain SFAs = sum of pentadecanoic acid (15:0) and heptadecanoic acid (17:0).
4Obtained by using the median value for each quartile and fitting it as a continuous variable in the conditional logistic regression models.
FIGURE 2.
Adjusted ORs (95% CIs) of subsequent GDM risk per a 1-SD increase in plasma phospholipid SFAs (%) at gestational weeks 10–14 and 15–26. The risk estimates were adjusted for age (years), gestational age at blood collection (weeks), parity (nulliparous or multiparous), family history of diabetes (yes or no), and prepregnancy BMI (kg/m2; <25.0, 25.0–29.9, 30.0–34.9, or 35.0–44.9). GDM, gestational diabetes mellitus.
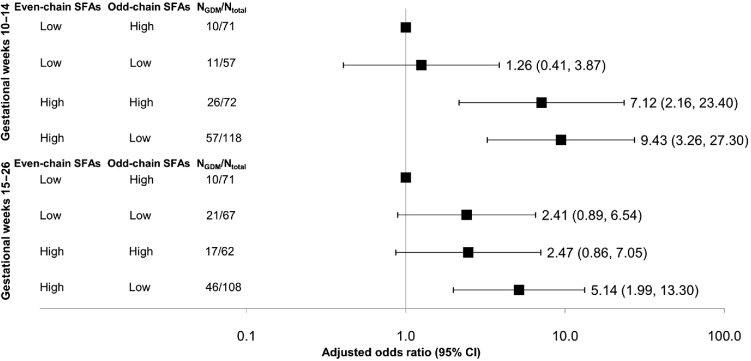
Furthermore, the combination of high concentrations (median or greater) of even-chain SFAs and low concentrations (less than median) of odd-chain SFAs showed a synergistic effect. Compared with women with low even-chain and high odd-chain SFAs, those with high even-chain and low odd-chain SFAs were at a 9.43-fold (95% CI: 3.26-, 27.30-fold) and 5.14-fold (95% CI: 1.99-, 13.30-fold) increased risk of GDM at GWs 10–14 and 15–26, respectively (Figure 3).
FIGURE 3.
Adjusted ORs (95% CIs) of subsequent GDM risk in association with plasma phospholipid even-chain and odd-chain phospholipid SFAs (%) at gestational weeks 10–14 and 15–26. The point estimates were adjusted for age (years), gestational age at blood collection (weeks), parity (nulliparous or multiparous), family history of diabetes (yes or no), and prepregnancy BMI (kg/m2; <25.0, 25.0–29.9, 30.0–34.9, or 35.0–44.9). GDM, gestational diabetes mellitus; High, concentrations above the median; Low, concentrations below the median.
Incremental predictive value of plasma phospholipid SFAs for GDM risk
We further explored the relative incremental predictive ability of individual SFAs in risk prediction of subsequent GDM in addition to conventional risk factors (age, family history of diabetes, and prepregnancy BMI) and plasma glucose concentrations. At GWs 10–14 (Supplemental Figure 3), plasma glucose did not significantly improve predictive value beyond conventional risk factors (P = 0.09), whereas the plasma phospholipid even-chain SFA palmitic acid showed a significant incremental predictive value in addition to conventional risk factors (P = 0.005) and, more notably, conventional risk factors plus plasma glucose (P = 0.034). Similar results were observed for the plasma phospholipid odd-chain SFA heptadecanoic acid at GWs 10–14 and 15–26, respectively. Heptadecanoic acid, in addition to conventional risk factors and fasting plasma glucose at GWs 15–26, significantly improved C statistic values from 0.70 to 0.74 (P = 0.024).
DISCUSSION
In this prospective study with longitudinal measurement of plasma phospholipid SFAs throughout pregnancy, we observed that both individual and subclasses of SFAs, starting from as early as 10 wk of gestation, were significantly associated with glucose metabolism and cardiometabolic markers in pregnancy and subsequent GDM risk. Of note, the associations differed by chain length; concentrations of even-chain SFAs (particularly the most abundant form, palmitic acid) were significantly and positively associated with GDM risk, whereas odd-chain SFAs (pentadecanoic acid and heptadecanoic acid) were inversely related to GDM risk. Moreover, high concentrations of even-chain SFAs and low concentrations of odd-chain SFAs at GWs 10–14 and 15–26, respectively, may synergistically exacerbate subsequent GDM risk.
Prospective data on individual plasma phospholipid SFAs during pregnancy and subsequent GDM risk are lacking, whereas limited previous data have focused on total dietary SFAs with inconsistent findings (31, 32). Despite the lack of previous data on plasma phospholipid SFAs and GDM risk, our findings are in line with some, although not all, emerging data on type 2 diabetes. For instance, among nonpregnant individuals, positive associations have been observed for the plasma phospholipid even-chain SFA palmitic acid and type 2 diabetes (4, 33, 34), whereas null associations for the plasma phospholipid myristic acid (34) and erythrocyte-membrane stearic acid (35) have also been reported. Conversely, the plasma phospholipid odd-chain SFAs pentadecanoic acid (4, 36) and heptadecanoic acid (4, 34) have been linked to a significantly lower risk of type 2 diabetes.
The differential associations with GDM risk between even- and odd-chain SFAs as observed in our study may also shed light on inconsistent findings from previous studies. Notably, plasma phospholipid even-chain SFAs, the abundant forms of circulating SFAs, are a function of both exogenous (i.e., dietary intake of foods such as butter, palm oil, and red meat rich in the typical Western diet) and endogenous (i.e., de novo lipogenesis stimulated by excessive carbohydrate intake) sources (13, 14). On the other hand, despite the relatively low concentrations in plasma phospholipids, odd-chain SFAs are thought to mainly exogenously originate from dairy fat and have been linked to a lower risk of cardiometabolic consequences (36), although the endogenous sources cannot be ruled out and need to be further examined (16, 17). In addition, future studies examining the associations between dietary intakes of dairy products and the risk of gestational diabetes are warranted. Furthermore, as shown here, high even-chain and low odd-chain SFA concentrations may exert a synergistic effect in association with increased risk of subsequent GDM, suggesting the potential significance of both even- and odd-chain SFA profiles in glucose homeostasis. Thus, endogenous biosynthetic pathways, coupled with opposing effects of individual plasma phospholipid SFAs and joint effects of SFA subclasses, collectively add complexity to the methodology and interpretation of SFA profiles in relation to disease risk. Taken together, these data highlight the importance of assessing objectively measured individual and subclasses of plasma phospholipid SFAs to investigate their biological and pathophysiologic roles in cardiometabolic outcomes.
Although the exact metabolic pathways whereby circulating SFAs are involved in glucose homeostasis remain to be elucidated, our findings are biologically plausible. Specifically, the major even-chain SFA palmitic acid may induce insulin resistance via proteasomal degradation of key insulin-signaling molecules (37), proinflammatory signaling via activation of Toll-like receptor 4 (38), and lipoapoptosis in β cells via enhanced endoplasmic reticulum stress (39). In contrast, limited experimental data on odd-chain SFAs suggest that pentadecanoic acid and heptadecanoic acid may exert their protective effects on glucose homeostasis via inhibition of hepatic oxidation (40). Consistently, our findings of chain-length specific correlations of individual SFAs with glucose metabolism and cardiometabolic markers supported the experimental data and may provide mechanistic insight. Specifically, even-chain SFAs (particularly palmitic acid) were positively correlated with HOMA-IR and markers of hyperlipidemia (i.e., total cholesterol, LDL cholesterol, and triglycerides) and inversely correlated with adiponectin, which, in turn, were linked to impaired glucose and insulin homeostasis and cardiometabolic disorders (41), whereas the plasma phospholipid SFAs myristic acid or stearic acid were not significantly correlated with any of these markers or indexes, with the exception of stearic acid with triglycerides. Opposite patterns of correlations were observed for odd-chain SFAs (particularly heptadecanoic acid) and the previously mentioned markers.
Also, notably, our data are uniquely suited to profile the longitudinal changes of SFAs throughout pregnancy and showed that even-chain SFAs were gradually enriched, whereas odd-chain SFAs were progressively depleted, during pregnancy among both GDM cases and controls. As shown in a longitudinal analysis, serum even-chain SFAs (particularly palmitic acid) were significantly associated with a decreasing trend of plasma adiponectin across trimesters (42). The exact mechanisms for the gestational alterations of these SFAs remain to be shown; however, it is presumably plausible that the increasing adipose deposition throughout pregnancy and associated physiologic changes may be important factors underlying the observed alterations (43). Interestingly, the significant differences in plasma phospholipid even- and odd-chain SFAs between GDM cases and controls were only present before GWs 24–27, which may suggest the potential responsiveness or modifiable potentials of these circulating markers after the diagnosis and treatment of GDM.
Our study has some notable strengths. The prospective and longitudinal data collection allowed examination of the temporal associations of SFAs during early to midpregnancy with subsequent GDM risk. Furthermore, we had the unique ability to profile the longitudinal physiologic trends of plasma phospholipid SFAs throughout pregnancy, which showed significant, differential temporal variations of SFA subclasses according to chain length among women with and without GDM. Most notably, the objective measurement of plasma phospholipid SFA concentrations enabled the assessment of GDM risk with individual and subclasses of circulating SFAs. Indeed, objective measurement is particularly important for the even-chain SFAs myristic acid, palmitic acid, and stearic acid in terms of showing their pathophysiologic roles, because their circulating concentrations are a function of both exogenous and endogenous sources (13, 15). In addition, in the present study, we measured a comprehensive panel of metabolic and cardiovascular markers simultaneously with circulating SFA profiles. Examining their associations with SFAs provided further mechanistic insight into the association of SFAs with GDM risk.
Some potential limitations of our study merit discussion. The contents of individual SFAs were measured and expressed as relative (i.e., weight percentage), not absolute, concentrations of total phospholipid fatty acids. Nonetheless, this approach has been validated and widely adopted in epidemiologic studies and tends to facilitate a better interpretation of metabolic associations than absolute measurements (15). Also noteworthy, given that concentrations of circulating even-chain SFAs are influenced by both dietary intakes and de novo lipogenesis, we were unable to distinguish the impact of each source or their interplay in relation to GDM risk. However, in the present study, our main focus is the investigation of plasma phospholipid SFAs, as end products and integrated profiles of both exogenous and endogenous processes, in relation to glucose metabolism and cardiometabolic markers and subsequent GDM risk. Therefore, our findings may shed light on previous inferences about dietary SFAs in relation to GDM and other cardiometabolic outcomes, which should be interpreted with caution if de novo lipogenesis stimulated by excessive carbohydrate intake has not been adequately accounted for. In this study, we focused on the plasma phospholipid fraction only and cannot determine whether the same observations may exist in other types of lipid molecules. In addition, we did not assess the associations of specific fractions of plasma phospholipid SFAs (e.g., phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, etc.) with the risk of GDM, which should be examined in future studies. Finally, some wide CIs of the risk estimates may be due to the relatively modest sample size, which, however, may have resulted in our reported risk estimates to be less significant. To date, our study is one of the largest prospective and longitudinal studies of plasma phospholipid SFAs throughout pregnancy in relation to GDM risk; future studies with a larger sample size are warranted to validate our findings.
In conclusion, we observed a significantly increased risk of GDM in association with higher concentrations of even-chain SFAs and lower concentrations of odd-chain SFAs as early as GWs 10–14, ∼10–18 wk before GDM is conventionally screened for or diagnosed. Furthermore, high concentrations of even-chain SFAs and low concentrations of odd-chain SFAs may exert synergistic effects in association with subsequent GDM risk. These findings among pregnant women highlight the need to recognize the differential associations between individual or subclasses of SFAs and subsequent GDM risk. Our findings provide impetus for future investigation that targets circulating individual SFAs to improve our understanding of their distinct nutritional, metabolic, and physiologic roles in cardiometabolic outcomes. Efforts to guide GDM prevention as well as preventive strategies for other cardiometabolic disorders could be potentially strengthened by considering individual SFA metabolism and the distinct cardiometabolic effects that vary by SFA chain length.
Supplementary Material
Acknowledgements
We thank all participating clinical centers in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies–Singleton Cohort, including Christina Care Health Systems; University of California, Irvine; Long Beach Memorial Medical Center; Northwestern University; Medical University of South Carolina; Columbia University; New York Hospital Queens; St. Peters’ University Hospital; University of Alabama at Birmingham; Women and Infants Hospital of Rhode Island; Fountain Valley Regional Hospital and Medical Center; and Tufts University. We also thank the C-TASC Corporation, which provided data coordination, and the Department of Laboratory Medicine and Pathology, University of Minnesota, which provided laboratory resources essential for biochemical analysis.
The authors’ responsibilities were as follows—YZ and CZ: conceived the study concept and are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; YZ: analyzed the data and drafted the manuscript; CZ: obtained funding, designed and oversaw the study, and revised the manuscript; MYT: led laboratory testing, contributed to data interpretation, and revised the manuscript; QS, SNH, SR, PM, and AF: contributed to data interpretation and revision of the manuscript; PSA: contributed to data analysis, interpretation, and revision of the manuscript; and all authors: contributed to the interpretation of the results and revision of the manuscript for important intellectual content, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and American Recovery and Reinvestment Act funding via contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z. YZ is also supported in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant number 3K12HD052163.
Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: aOR, adjusted OR; GDM, gestational diabetes mellitus; GW, gestational week.
REFERENCES
- 1. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2):S141–6. [DOI] [PubMed] [Google Scholar]
- 2. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011;60(7):1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW et al. . Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ 1996;313(7049):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu J, Eilat-Adar S, Loria C, Goldbourt U, Howard BV, Fabsitz RR, Zephier EM, Mattil C, Lee ET. Dietary fat intake and risk of coronary heart disease: the Strong Heart Study. Am J Clin Nutr 2006;84(4):894–902. [DOI] [PubMed] [Google Scholar]
- 7. Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA 1997;278(24):2145–50. [PubMed] [Google Scholar]
- 8. Mozaffarian D, Rimm EB, Herrington DM. Dietary fats, carbohydrate, and progression of coronary atherosclerosis in postmenopausal women. Am J Clin Nutr 2004;80(5):1175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG et al. . Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160(6):398–406. [DOI] [PubMed] [Google Scholar]
- 10. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J et al. . Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010;91(3):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harcombe Z, Baker JS, Cooper SM, Davies B, Sculthorpe N, DiNicolantonio JJ, Grace F. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Heart 2015;2(1):e000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest 1996;97(9):2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr 1996;16:523–57. [DOI] [PubMed] [Google Scholar]
- 15. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47(5):348–80. [DOI] [PubMed] [Google Scholar]
- 16. Ratnayake WM. Concerns about the use of 15:0, 17:0, and trans-16:1n–7 as biomarkers of dairy fat intake in recent observational studies that suggest beneficial effects of dairy food on incidence of diabetes and stroke. Am J Clin Nutr 2015;101(5):1102–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfeuffer M, Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr 2016;7(4):730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993;328(4):238–44. [DOI] [PubMed] [Google Scholar]
- 19. Ginsberg BH, Jabour J, Spector AA. Effect of alterations in membrane lipid unsaturation on the properties of the insulin receptor of Ehrlich ascites cells. Biochim Biophys Acta 1982;690(2):157–64. [DOI] [PubMed] [Google Scholar]
- 20. Grunfeld C, Baird KL, Kahn CR. Maintenance of 3T3-L1 cells in culture media containing saturated fatty acids decreases insulin binding and insulin action. Biochem Biophys Res Commun 1981;103(1):219–26. [DOI] [PubMed] [Google Scholar]
- 21. Hara T, Kimura I, Inoue D, Ichimura A, Hirasawa A. Free fatty acid receptors and their role in regulation of energy metabolism. Rev Physiol Biochem Pharmacol 2013;164:77–116. [DOI] [PubMed] [Google Scholar]
- 22. Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D'Alton ME, Skupski D et al. . Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol 2015;213(4):449.e1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics Practice Bulletin No. 137: gestational diabetes mellitus. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol 2013;122(2 Part 1):406–16. [DOI] [PubMed] [Google Scholar]
- 24. Zhu Y, Mendola P, Albert PS, Bao W, Hinkle SN, Tsai MY, Zhang C. Insulin-like growth factor axis and gestational diabetes: a longitudinal study in a multiracial cohort. Diabetes 2016;65(11):3495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem 2006;52(12):2265–72. [DOI] [PubMed] [Google Scholar]
- 26. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502. [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 28. Pepe MS, Fan J, Seymour CW. Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol 2013;20(7):863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–45. [PubMed] [Google Scholar]
- 30. Stone M. Cross-validatory choice and assessment of statistical predictions. J R Stat Soc Series B Methodol 1974;36(2):111–47. [Google Scholar]
- 31. Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr 2012;95(2):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbieiri P, Nunes JC, Torres AG, Nishimura RY, Zuccolotto DC, Crivellenti LC, Franco LJ, Sartorelli DS. Indices of dietary fat quality during midpregnancy is associated with gestational diabetes. Nutrition 2015. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78(1):91–8. [DOI] [PubMed] [Google Scholar]
- 34. Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort. Am J Clin Nutr 2010;92(5):1214–22. [DOI] [PubMed] [Google Scholar]
- 35. Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. Am J Clin Nutr 2011;93(1):127–42. [DOI] [PubMed] [Google Scholar]
- 36. Santaren ID, Watkins SM, Liese AD, Wagenknecht LE, Rewers MJ, Haffner SM, Lorenzo C, Hanley AJ. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr 2014;100(6):1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ishii M, Maeda A, Tani S, Akagawa M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch Biochem Biophys 2015;566:26–35. [DOI] [PubMed] [Google Scholar]
- 38. Jin J, Zhang X, Lu Z, Perry DM, Li Y, Russo SB, Cowart LA, Hannun YA, Huang Y. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am J Physiol Endocrinol Metab 2013;305(7):E853–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 2006;47(12):2726–37. [DOI] [PubMed] [Google Scholar]
- 40. Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (c15:0) and heptadecanoic acid (c17:0) in health and disease. Molecules 2015;20(2):2425–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116(7):1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lepsch J, Farias DR, Vaz Jdos S, de Jesus Pereira Pinto T, da Silva Lima N, Freitas Vilela AA, Cunha M, Factor-Litvak P, Kac G. Serum saturated fatty acid decreases plasma adiponectin and increases leptin throughout pregnancy independently of BMI. Nutrition 2016;32(7–8):740–7. [DOI] [PubMed] [Google Scholar]
- 43. Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med (Berl) 2002;80(11):696–702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.