Abstract
Aims
Maternal alcohol drinking adversely affects pregnancy outcome. Although fetal health and development are highly dependent upon the integrity of placentation, little is known about placental morphology following gestational exposure to alcohol in humans.
Methods
In this clinical study, subjects were recruited between 2010 and 2013 from an outpatient substance abuse and mental health treatment center for women. Data collected included maternal age, substance abuse history, comorbidities, pregnancy outcomes, gestational age at delivery, birth weight and placental weight. Placental histopathological lesions associated with uteroplacental malperfusion (UPM) were scored under code. Villous cytotrophoblasts reflecting another measure of UPM were immunostained with E-cadherin and quantified using stereology. Inter-group comparisons were made using univariate or multivariate analysis.
Results
Of the 92 women enrolled in the study, 61 (66%) had histories of alcohol use during the pregnancy while the remaining 31 served as controls. The alcohol-exposed group significantly differed from controls based on (a) higher rates of smoking and polydrug use during pregnancy, (b) lower mean gestational age and birth weight of their infants, (c) elevated UPM scores, (d) increased densities of villous cytotrophoblastic cells and (e) more extensive chorangiosis in placenta. Logistic regression analysis demonstrated alcohol to be the only significant predictor of these outcomes and responses.
Conclusions
Gestational alcohol exposure is significantly associated with UPM, which could account for the higher rates of preterm delivery and reduced mean birth weights. Since chorangiosis reflects altered placental oxygen dynamics, the greater abundance of this abnormality could represent a compensatory response to UPM.
Short Summary
In this clinical study, we assessed the effects of maternal alcohol drinking on placental morphology. Applying a cumulative scoring system, we determined that gestational alcohol exposure is significantly associated with uteroplacental malperfusion, which could account for the higher rates of preterm delivery and reduced mean birth weights.
Introduction
In the USA, each month, ~3.3 million women are at risk for an alcohol-exposed pregnancy (Green et al., 2016). While the high rate of unintended pregnancies (~50%) (Finer and Zolna, 2014) with inadvertent early gestational exposures is a contributing factor, persistent alcohol drinking during pregnancy remains problematic, despite public health warnings and endpoint labeling. Centers for Disease Control and Prevention data from 2006 to 2010 indicate that 7.6% of women consume alcohol during pregnancy, and 1.4% binge drink (Marchetta et al., 2012). Consequently, fetal alcohol syndrome (FAS) remains the leading preventable cause of birth defects and neurodevelopmental disorders in the USA (May et al., 2014). The reported prevalence of FAS in the USA ranges from 6 to 9 per 1000 children (May et al., 2014). The overall problem is far more serious given the fact that in addition to FAS, the most severe form of fetal alcohol effect-associated diseases, there is an entire spectrum of related birth defects and neurobehavioral disabilities whose occurrence and severity are modulated by timing, dose and duration of the alcohol exposure during pregnancy. The entire phenotypic continuum is termed Fetal Alcohol Spectrum Disorders (FASD); the prevalence of FASD in USA approaches 33.5 per 1000 live births (Roozen et al., 2016). Besides alcohol intake, other factors contributing to the pathogenesis of FASD include poor nutritional status, genetic polymorphisms, maternal health status such as body mass index and possibly smoking and illicit drug exposures.
The placenta is vital to the maintenance of pregnancy and well-being of the fetus. Though it acts like a partial barrier between the mother and fetus, alcohol readily crosses the placenta and accumulates in the fetus at levels proportionate to maternal blood alcohol concentrations (Idanpaan-Heikkila et al., 1972). Alcohol is also detectable in amniotic fluid where the levels can approach 50% of the concentration in maternal blood (Idanpaan-Heikkila et al., 1972). Besides its teratogenic effects, alcohol increases the risk of adverse pregnancy outcomes such as stillbirth (Kaminski et al., 1978; Marbury et al., 1983), preterm delivery (PTD) and small for gestational age infants (Patra et al., 2011), and placenta-associated syndromes such as abruption, placenta previa and preeclampsia (Salihu et al., 2011).
In vivo and in vitro experiments demonstrated that prenatal alcohol exposures have broad adverse effects on placental development and function (Burd et al., 2007) including impairment of placental nutrient transport (Fisher et al., 1981, 1982), and placental and umbilical cord vasoconstriction (Altura et al., 1982; Erskine and Ritchie, 1986). In a rat model, we showed that chronic gestational exposure to alcohol impairs placentation via its adverse effects on insulin and insulin-like growth factor signaling in relation to trophoblast motility and invasiveness (Gundogan et al., 2008, 2013). Alcohol disrupts the physiological transformation of maternal spiral arteries and inhibits trophoblastic cell motility in a dose-dependent fashion (Gundogan et al., 2008, 2010, 2015). In addition, gestational alcohol exposure alters the branching morphogenesis in the labyrinth layer of rat placenta where the maternal–fetal exchange takes place (Gundogan et al., 2015). Experimental studies demonstrated that placental consequences of ethanol exposure include disruption of placental vasculogenesis, reduced effectiveness of nutrient/waste exchange, increased placental oxidative stress (Kay et al., 2000), DNA damage, lipid peroxidation and mitochondrial dysfunction (Gundogan et al., 2010), impaired trophoblast survival and altered trophoblast subtype-specific gene expression (Gundogan et al., 2013; Kalisch-Smith et al., 2016). Furthermore, mechanistic studies demonstrated that ethanol and its metabolite acetaldehyde negatively affects first trimester human placental villous tissue growth and trophoblast migration and also reduces the transport of taurine, which is vital for developmental neurogenesis (Lui et al., 2014).
Despite the ongoing studies, how gestational alcohol exposure mechanistically impacts human placental morphology and function is not fully understood. Human placental studies have not clearly linked those pathological changes to alterations in placental weight (Kaminski et al., 1978; Niemela et al., 1991; Wang et al., 2014). However, data from experimental models suggest that impaired placentation and attendant uteroplacental malperfusion (UPM) are major factors driving gestational alcohol effects on the fetus (Gundogan et al., 2008, 2015). In the present study, we characterized the nature and extent of placental pathology, including indices of UPM in relation to pregnancy outcomes in human subjects with known histories of gestational alcohol misuse.
Materials and Methods
Clinical data
Human subjects were recruited from an outpatient substance abuse and mental health treatment center specialized in helping pregnant women and women with small children. The subjects were identified by retrospective review of charts from 2001 to 2011 and prospective enrollment from the clinic between 2012 and 2013. Inclusion criteria were availability of placenta for examination and reported history of alcohol use or abstinence during the pregnancy. Exclusion criteria were multiple gestation pregnancies, miscarried pregnancies (pregnancy loss prior to 20 weeks gestation) and fetal or infant chromosome abnormalities. Drinking levels were determined based on NIAAA criteria (Dufour, 1999) as follows: light = 3 or fewer drinks per week; moderate = 4–7 drinks per week; heavy = greater than 1 drink per day. In addition, we obtained self-reported data about tobacco and illicit drug use during pregnancy and health status of the neonate including admission to neonatal intensive care unit (NICU). The Institutional Review Board of Women and Infants Hospital approved this study protocol.
Placental examination
Fresh placentas were examined using a standard protocol (Langston et al., 1997). Reference values determined if placentas were small or large for gestational age (Pinar et al., 1996). Routine paraffin blocks were made from representative samples of placental membranes, umbilical cord from fetal and placental ends and four random full-thickness sections of parenchyma. In addition, macroscopic lesions were sampled and submitted separately. All tissues were fixed in 10% neutral buffered formalin, and embedded in paraffin. Histological sections were stained with hemotoxylin and eosin (H&E) and examined under code. In addition, adjacent sections were used for immunohistochemical staining (see below).
Diagnosis of UPM (also known as maternal vascular malperfusion) relies on compilation of macroscopic and microscopic placental findings that have been indicative of impaired placentation (Redline et al., 2004; Khong et al., 2016). Since standardized criteria for grading the placental hypoxic lesions have not yet been established, to assess degrees of UPM, we developed a cumulative scoring system (Ravishankar et al., 2015). We assigned 1 point per UPM-associated placental abnormality following well-established diagnostic criteria (Langston et al., 1997; Redline et al., 2004; Khong et al., 2016) based on the presence of (a) placental hypoplasia (placental weight < 10th percentile for the gestational age); (b) decidual vasculopathy (Khong, 1991); (c) increased syncytial knots (1 point for <30% and 2 points for ≥30% of placental involvement) (Loukeris et al., 2010); (d) distal villous hypoplasia (Parks, 2015); (e) increased perivillous fibrin deposition (Parks, 2015); (f) villous agglutination (Parks, 2015); (g) infarcts (Parks, 2015); (h) islands of extravillous cytotrophoblastic cells (Stanek, 2012) and (i) implantation-site trophoblastic giant cells (Stanek, 2012). Placental histopathological scoring was performed under code; scores ranged from 0 to 10.
In addition, diabetes-associated findings such as delayed villous maturity (Higgins et al., 2011) and chorangiosis (Altshuler, 1984; Evers et al., 2003) were recorded based on established diagnostic criteria because alcohol exposures induce placental insulin resistance (Gundogan et al., 2008). To complement the chorangiosis assessment, we used stereology (see below) to determine the numerical density of vasculo-syncytial membranes that are primary site of fetomaternal exchange. Vasculo-syncytial membrane is formed when syncytiotrophoblast surrounding the terminal villi make a close contact with capillaries and the barrier separating the maternal and fetal circulations is reduced to 1–2 μm (Burton and Tham, 1992).
Immunohistochemistry
Clinical conditions inducing chronic hypoxia and UPM lead to increased populations of villous cytotrophoblastic cells (Arnholdt et al., 1991). Experimentally, hypoxia inhibits the cytotrophoblastic fusion and differentiation to syncytiotrophoblast (Alsat et al., 1996). E-cadherin immunoreactivity distinguishes villous cytotrophoblasts from stromal cells and syncytiotrophoblasts (Brown et al., 2005; Longtine et al., 2012). In the present study, we quantified E-cadherin-immunoreactive villous cytotrophoblasts as an additional measure of UPM.
Immunohistochemical staining was performed on all tissue samples simultaneously using a DakoAutostainer (Dako, Carpenteria, CA). Histological sections of placental parenchyma (4 μm thick) were deparaffinized, rehydrated and subjected to antigen retrieval treatment using a Dako PT Link module with Tris/ethylenediaminetetraacetic acid buffer (pH = 9). After rinsing in EnVisionTM FLEX wash buffer and treating with 3% hydrogen peroxide for 5 min to quench endogenous peroxidase activity, the sections were incubated for 20 min at room temperature with monoclonal E-cadherin antibody (clone NHC-38). Antibody binding was detected with EnVisionTM FLEX/horseradish peroxidase (HRP) reaction (dextran backbone coupled to a large number of HRP molecules and secondary antibody molecules) and diamino benzidinetetrahydrochloride. The sections were counterstained lightly with hematoxylin and preserved under cover glass. Sections of a breast fibroadenoma served as positive controls, and primary antibody omissioned served as a negative control.
Stereology
E-cadherin-positive cytotrophoblasts and vasculo-syncytial membranes were quantified using unbiased stereology (Gundogan et al., 2011). Numerical density calculation was performed with the aid of an Olympus BX60 light microscope (Olympus America Inc., Center Valley, PA) with attached MS-2000 XYZ Inverted Stage (Applied Scientific Instrumentation, Eugene, OR), and Stereologer software (Stereology Resource Center, Inc., Chester, MD). Unbiased counting frames were applied under software control. Cells of interest were counted at high power magnification (40×) using optical dissector method and normalized to the volume, which was based on Cavalieri point grid method. Same method was applied to determine the numerical density of vasculo-syncytial membranes.
Statistical analysis
Descriptive analyses were performed to calculate medians, proportions and means with standard errors (SEM). Inter-group comparisons were made using the Mann–Whitney, Fisher's exact or Student's t tests (GraphPad Prism 6, San Diego, CA). Logistic regression analysis was performed using the R version 3.2.0 generalized linear model function followed by stepwise selection to determine associations between alcohol, smoking, drugs or gestational age with placental histomorphology. Significant P values (<0.05) are indicated in the tables and graphs.
Materials
The E-cadherin monoclonal antibody (clone NHC-38) was purchased from Dako (Dako, Carpenteria, CA). All immunohistochemical staining reactions were performed using EnVisionTM FLEX reagents and DakoAutostainer (Dako, Carpenteria, CA).
RESULTS
Maternal factors
The study included 44 retrospectively identified and 48 prospectively enrolled women, among whom 61 (66%) had histories of alcohol use during the pregnancy and 31 did not (controls). The pregnancies were all singleton; the outcomes included 84 live born infants, 4 stillbirths and 4 miscarriages. The four women with miscarriages were all from the alcohol-user group; they were excluded from the study. Three of the four stillbirths were also from the alcohol group.
Comparative analysis of maternal clinical data revealed that the alcohol-exposed group was significantly older (P = 0.02), and had higher rates of smoking (P = 0.005) and poly-substance abuses (P = 0.002), whereas the rates of any drug (illicit) use, diabetes mellitus, hypertension, abruption and delivery by cesarean section did not significantly differ for the two groups (Table 1). Using criteria set by the NIAAA (Dufour, 1999; Rethinking drinking, 2015), within the alcohol-exposed group, the majority were heavy drinkers (53%), 7% were moderate drinkers and 23% were light drinkers, and in 10 subjects (17%), the amounts of alcohol consumed during pregnancy were unknown. With regard to the durations of alcohol exposure during pregnancy, data were available for 55 of the 57 alcohol using subjects, among whom 20 (36.4%) reported drinking throughout pregnancy, 18 (32.7%) drank only during the first trimester and 11 (20%) drank in both the first and second trimesters. Only small percentages restricted their alcohol consumption to the second (n = 1; 1.8%), third (n = 2; 3.6%), first and third (n = 2; 3.6%) or second and third (n = 1; 1.8%) trimesters.
Table 1.
Maternal and neonatal characteristics
| Outcome variables | Maternal alcohol use | P | |
|---|---|---|---|
| No (31) | Yes (57) | ||
| Maternal | |||
| Maternal age (years) | 26 (17–37) | 31 (20–44) | 0.02a |
| Tobacco | 13 (42%) | 42 (74%) | 0.0053b |
| Illicit drug use | 18 (58%) | 42 (74%) | NSb |
| Poly-substance use | 4 (13%) | 26 (46%) | 0.0021b |
| Diabetes mellitus | 4 (13%) | 6 (10%) | NSb |
| Hypertension | 1 (3%) | 1 (2%) | NSb |
| Abruption | 1 (3%) | 3 (5%) | NSb |
| Cesarean section delivery | 11 (36%) | 27 (47%) | NSb |
| Neonatal | |||
| Gestational age (weeks) | 38.2 ± 0.4 | 36.6 ± 0.5 | 0.01a |
| PTD (%) | 5 (16%) | 20 (35%) | 0.08b |
| Birth weight (g) | 3167 ± 115.9 | 2691 ± 103.7 | 0.006a |
| IUGR (<10th %ile) | 5 (16%) | 14 (25%) | NSb |
| Macrosomia (>97th %ile) | 2 (6.5%) | 2 (3.5%) | NSb |
| Placenta-to-birth weight ratio | 6.76 ± 0.26 | 6.43 ± 0.23 | NSb |
| Gender (M:F) | 1:0.9 | 1:1.07 | NSb |
| Apgar score | |||
| 1 min | 7.6 ± 0.3 | 7.2 ± 0.2 | NSa |
| 5 min | 9 ± 0 | 8.7 ± 0.1 | NSa |
| Admission to NICU | 6 (20%) | 25 (47%) | 0.03b |
NS (P > 0.05).
aTwo-tailed t test.
bFisher's exact test.
Values represent median (range), mean ± SEM, proportion (%) of (N) patients or ratios.
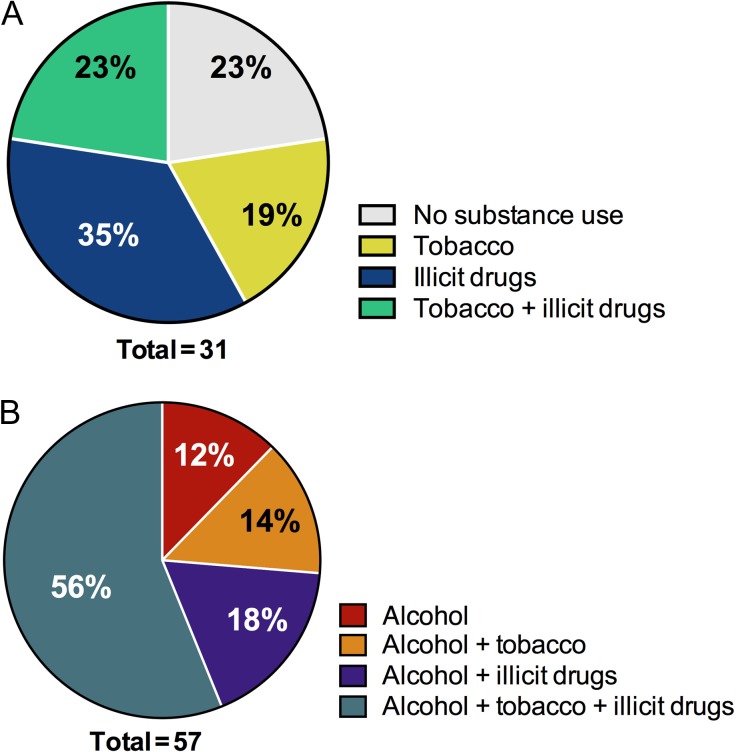
With regard to tobacco and illicit drug use during pregnancy, in the control group, 23% had no exposures, 19% smoked, 35% used at least one illicit drug (marijuana-44%, cocaine-22%, opiates-22% or poly-substance, including heroin-12%) and 23% smoked as well as used one or more illicit drugs (Fig. 1A). In the alcohol-exposed group, only 12% just consumed alcohol, while 14% also smoked, 18% also used one or more illicit drugs and 56% were triple exposed to alcohol, tobacco smoke and illicit drugs during pregnancy (Fig. 1B). In the alcohol group, although the most common illicit drug used during pregnancy was cocaine (69%), 56% reported poly-substance abuse including cocaine, marijuana, heroin, opiates and prescription analgesics. Fisher's exact test analysis demonstrated these rates of poly-substance and tobacco use to differ significantly between the alcohol and control groups (Table 1).
Fig. 1.
Maternal substance use characteristics. Based on self-reported alcohol use history during pregnancy, the patients were placed in either (A) control or (B) alcohol group. The patients were further inquired about tobacco and illicit drug use habits during the pregnancy. Rates of tobacco, illicit drug use, double or triple exposures are demonstrated in parts of whole graphs for each group.
Neonatal outcomes
The mean gestational age and birth weight were significantly lower in the alcohol-exposed group (P = 0.01 and P = 0.006, respectively) (Table 1). Similarly, the rate of PTD (<37 weeks) was higher in the alcohol-exposed group (35% vs. 16% in controls). The range of gestational ages was larger in alcohol-exposed group (range; median [95%CI]: 24–41; 38 [35.6–37.6]) than in control (31–41; 39 [37.4–38.9]). Despite similar mean 1-min and 5-min Apgar scores, infants who were gestationally exposed to alcohol had significantly higher rates of admission to the NICU (47%) relative to controls (20%, P = 0.03). However, when controlled for gestational age, NICU admission rate was comparable in cases and controls. There were no significant inter-group differences with respect to rates of intrauterine growth restriction (IUGR), macrosomia, infant gender ratios or mean ratio of placenta weight to birth weight.
Placental pathology
Gestational exposure to alcohol resulted in a trend for reduced placental weight (P = 0.06). However, macroscopic examinations revealed no significant differences in frequencies in which placentas were determined to be small (<10th percentile) or large (>90th percentile) for gestational age (Table 2). In contrast, standardized histopathological assessments revealed significantly higher mean UPM scores (P = 0.0001), higher rates of chorangiosis (P = 0.035) and lower frequencies of distal villous immaturity (P = 0.04) in the alcohol-exposed placentas. Histogram distributions of cumulative UPM scores demonstrated low and high peaks with leftward skewing of results from control placentas vs. a nearly Gaussian distribution of spectrum of scores varying from low to high in alcohol-exposed placentas (Supplementary Fig. S1). Similar to chorangiosis, the number of vasculo-syncytial membranes was significantly higher in alcohol group (4.3 ± 0.3) relative to controls (2.1 ± 0.6, P = 0.002). Sub-analyses of gestational age matched cases and controls revealed significant differences in UPM scores (control 1.346 ± 0.3592 vs. alcohol 3.541 ± 0.3479, P < 0.0001), E-cadherin staining (control 1.3 ± 0.1 vs. alcohol 1.8 ± 0.1, P = 0.01) and chorangiosis (control 7.7% vs. alcohol 29.7%, P = 0.03) similar to full cohort. However, the rate of distal villous immaturity was comparable in gestational age matched cases and controls.
Table 2.
Placental characteristics
| Gestational alcohol exposure | P | ||
|---|---|---|---|
| No (31) | Yes (57) | ||
| Placental weight | 483.6 ± 24.8 | 426.1 ± 18.1 | 0.06a |
| Small for gestational age (<10th %ile) | 8 (25.8%) | 22 (38.6%) | NSb |
| Large for gestational age (>90th %ile) | 6 (19.4%) | 7 (12.3%) | NSb |
| UPM score | 1.7 ± 0.4 | 3.6 ± 0.3 | 0.0001c |
| Distal villous immaturity | 7 (22.6%) | 4 (7%) | 0.04b |
| Chorangiosis | 3 (9.7%) | 17 (29.8) | 0.035b |
NS (P > 0.05).
aUnpaired t test.
bFisher's exact test.
cMann–Whitney test.
Values represent mean ± SEM or proportion (%) of (N) patients.
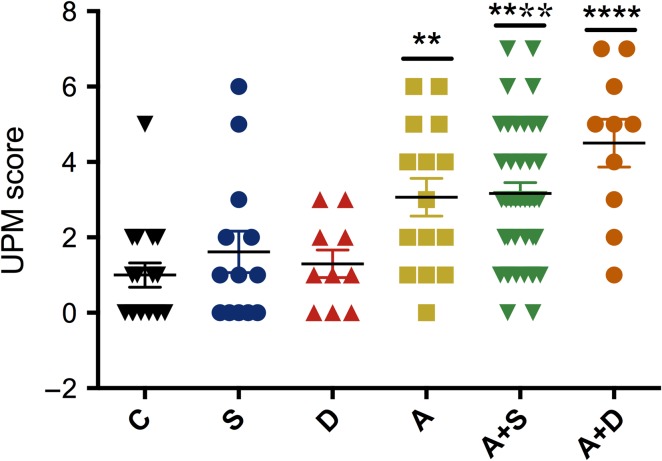
To differentiate alcohol effects on UPM from smoking and drug use-induced changes, we further segregated the full cohort into control (C, N = 17), smoking (S, N = 13), drug use (D, N = 10), alcohol (A, N = 15), alcohol with smoking (A + S, N = 42) and alcohol with drug use (A + D, N = 10) (Fig. 2). While the UPM scores in smokers and drug users were similar to control, the scores were significantly elevated by alcohol (P = 0.0014), alcohol with smoking (P < 0.0001) and alcohol with drug use (P < 0.0001). However, there was no significant difference among these three groups (A, A + S and A + D).
Fig. 2.
Individual effects of alcohol, smoking and drug use on UPM score. Full cohort was separated into five groups: control (no exposure, C, N = 17), smoking (S, N = 13), drug use (D, N = 10), alcohol (A, N = 15), alcohol with smoking (A + S, N = 42) and alcohol with drug use (A + D, N = 10). H&E sections of placentas were scored for the presence of placental hypoplasia (placental weight <10th %ile for the gestational age) and eight histologic findings that have been associated with maternal vascular malperfusion (**P < 0.01, ****P < 0.0001).
Multivariate regression was used to evaluate effects of alcohol dose on UPM score, chorangiosis and distal villous immaturity. Alcohol dose demonstrated an effect and heavy alcohol use was most significantly correlated with higher UPM scores (P < 0.001). Alcohol use had a trend effect on the presence of chorangiosis, whereas triple exposure to alcohol, tobacco and illicit drugs was a highly significant predictor of chorangiosis (P = 0.001). In contrast, gestational age, smoking and illicit drug use during pregnancy were not independently correlated with UPM score, chorangiosis or distal villous immaturity.
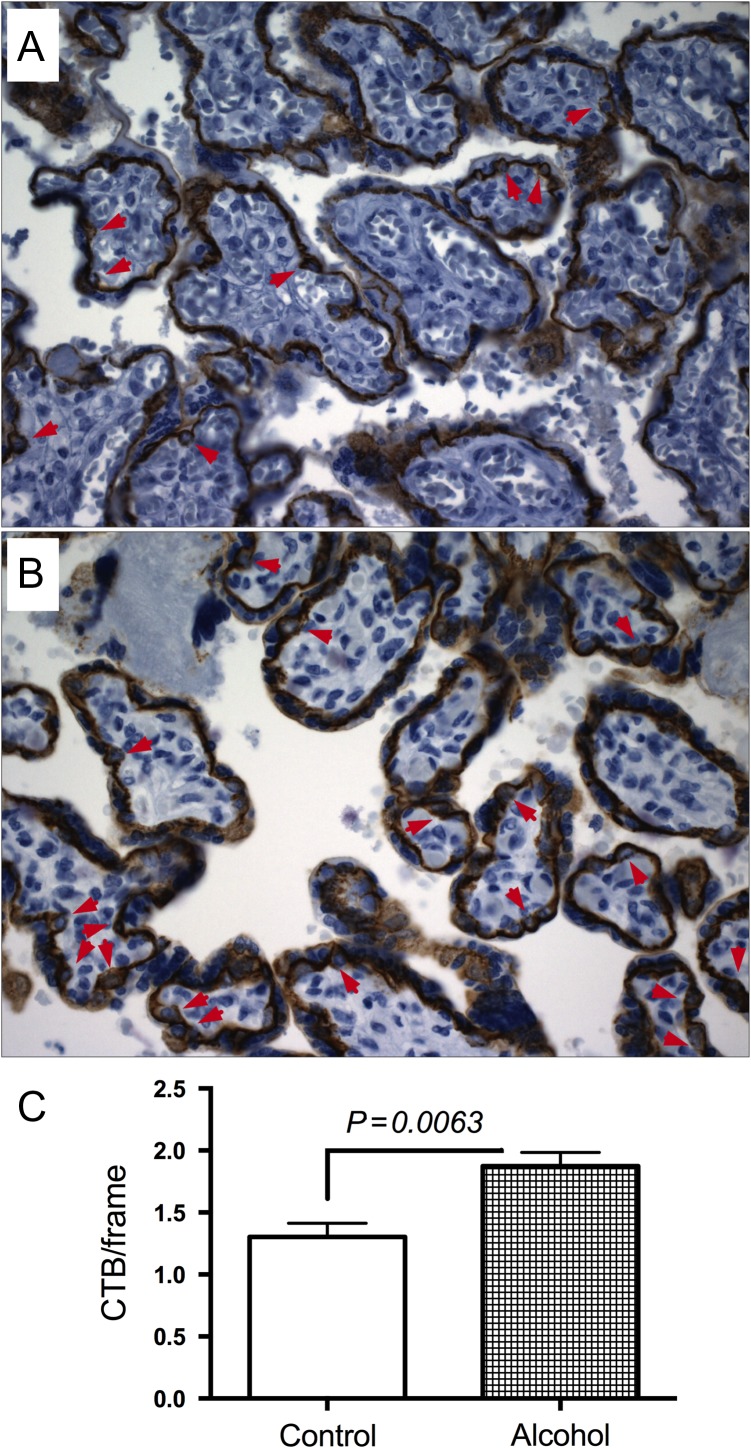
Immunohistochemical studies demonstrated E-cadherin immunoreactivity in villous cytotrophoblasts of both control (Fig. 3A) and alcohol-exposed (Fig. 3B) placentas, but significantly greater mean numbers of cytotrophoblasts in the alcohol-exposed group (P = 0.0063) (Fig. 3C).
Fig. 3.
Effect of gestational exposure to alcohol on cytotrophoblasts. E-cadherin immunostained sections demonstrate the distribution of villous cytotrophoblasts (arrows) in control (A, N = 31) and alcohol-exposed (B, N = 57) placentas. Villous cytotrophoblasts were quantified using unbiased stereology (C).
Discussion
This retrospective/prospective human study was designed to test the hypothesis that gestational alcohol exposures induce UPM, which correlates with fetal growth restriction, PTD and stillbirth (Stanek, 2013). All subjects were enrolled or recruited from a high-risk pregnancy clinic, and 61% were found to have consumed alcohol during pregnancy. In the alcohol-exposed group, PTD and impaired fetal growth were significantly increased. In addition, all four of the miscarriages (cases excluded from the study) were from the alcohol-user group. Therefore, the findings in this study correspond with results of experimental model demonstrating that gestational exposure to alcohol causes fetal loss and restricts fetal growth (Gundogan et al., 2008). Although the rates of illicit drug and tobacco use were also significantly higher in the alcohol use group, these co-factor exposures were not significantly correlated with pregnancy outcomes, and instead alcohol was the only significant predictive variable. Despite the limitation that the alcohol, tobacco and drug exposure histories were all obtained by self-report and that the exposure levels and durations may not have been fully accurate, subject categorization was considered reliable due to extensive involvement and consultation with social services.
Corresponding with previous experimental animal model studies (Gundogan et al., 2008, 2015), the findings herein demonstrate that prenatal alcohol exposure in humans also impairs fetal growth and adversely affects pregnancy outcome. In these regards, we observed significantly reduced birth weights and gestational ages and increased rates of prematurity (PTD). In addition, three of four stillbirth deliveries occurred in the alcohol-user group. Multivariate linear regression analysis demonstrated that gestational alcohol exposure was the main factor that significantly contributed to these pregnancy outcomes, although tobacco use (which occurred more frequently in the alcohol-user group) had a trend effect. A larger and better-powered study is needed to resolve potential contributions of smoking as an independent or co-factor exposure modulating risk for PTD, impaired fetal growth and early postnatal admission to the NICU. Altogether, these findings raise concern because prematurity and low birth weight resulting from an unhealthy fetal environment caused by prenatal alcohol exposure can have long-term effects on neurodevelopment. Moreover, alterations in fetal programming can negatively impact health in adulthood (Godfrey and Barker, 2001).
One of the main goals of this study was to determine the degree to which gestational alcohol exposure impairs placentation leading to significant UPM (Ravishankar et al., 2015) with associated adverse effects on pregnancy outcomes. The higher overall mean UPM score in alcohol-exposed placentas suggests that gestational exposure to alcohol significantly altered maternal vascular transformation and placental perfusion, as reported previously in a rodent model (Gundogan et al., 2008, 2015). Although our results appear to be discordant with recent findings by Carter et al. (2016), the differences could be explained by the study designs. Instead of using a point-based scoring system, the diagnosis of maternal vascular malperfusion was rendered by consensus and based on overall impressions, an approach that has just moderate degrees of reproducibility (Redline et al., 2004). In contrast, the systematic scoring system captures the full spectrum of UPM-related pathologies, potentially enabling identification of just a few critical factors that predict pregnancy outcomes.
Despite its many adverse effects including fetal growth restriction, smoking has been shown to reduce the risk of preeclampsia and gestational hypertension (England and Zhang, 2007). Considering that placental lesions associated with preeclampsia result from UPM (Khong et al., 1986; Pijnenborg et al., 1991), one might expect improved UPM scores with smoking in alcohol-exposed pregnancies. However, the UPM scores were comparable in cases with or without smoking history.
The findings of higher rates of chorangiosis and greater numbers of cytotrophoblastic cells in alcohol-exposed placentas correspond with the higher UPM scores. The association of chorangiosis with elevated UPM scores could represent a compensatory response to altered placental oxygen tension caused by impaired maternal vascular perfusion (Stanek, 2013; Parks, 2015). The greater numbers of cytotrophoblasts suggest that differentiation into syncytiotrophoblastic cells is altered. This phenomenon has been reported in association with placental hypoxia (Arnholdt et al., 1991; Alsat et al., 1996) as occurs in UPM.
The higher frequencies of distal villous immaturity and trend toward placentomegaly in the control group are not readily explained, as the rates of diabetes mellitus were similar in the control and alcohol-user groups. One potentially contributing factor for these findings is the high rate of marijuana use among controls since marijuana use has been correlated with higher placental weights (Carter et al., 2016). Alternatively, prenatal alcohol exposure may have accelerated villous maturity. In conclusion, this study demonstrates that in humans, gestational alcohol exposures cause UPM and alter cytotrophoblastic cell differentiation. These effects are linked to adverse pregnancy outcomes including PTD. The findings suggest that alcohol exposure is necessary and sufficient to cause these impairments. In light of facts that the subjects enrolled in this study were determined to be at high risk for substance abuse and poor pregnancy outcomes, and they were of low socioeconomic status, additional and alternative aggressive and creative strategies are needed to effectively convey preventive concepts and develop treatments to help support UPM when gestational alcohol exposure risks and rates are high.
Supplementary Material
Acknowledgements
We are grateful to Kursat Emre Gundogan for assistance with the statistical analysis and to Terry Pasquariello for performing the immunohistochemistry.
Supplementary material
Supplementary material is available at Alcohol and Alcoholism online.
Funding
The work was supported by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health [grant numbers AA-11431, AA-12908 to S.D.M.] and presented at the Society for Pediatric Pathology Annual Meeting in March 2014.
Conflict of Interest Statement
None declared.
References
- Alsat E, Wyplosz P, Malassine A, et al. (1996) Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol 168:346–53. [DOI] [PubMed] [Google Scholar]
- Altshuler G. (1984) Chorangiosis: an important placental sign of neonatal morbidity and mortality. Arch Pathol Lab Med 108:71–4. [PubMed] [Google Scholar]
- Altura BM, Altura BT, Carella A, et al. (1982) Alcohol produces spasms of human umbilical blood vessels: relationship to fetal alcohol syndrome (FAS). Eur J Pharmacol 86:311–2. [DOI] [PubMed] [Google Scholar]
- Arnholdt H, Meisel F, Fandrey K, et al. (1991) Proliferation of villous trophoblast of the human placenta in normal and abnormal pregnancies. Virchows Arch B Cell Pathol Incl Mol Pathol 60:365–72. [DOI] [PubMed] [Google Scholar]
- Brown LM, Lacey HA, Baker PN, et al. (2005) E-cadherin in the assessment of aberrant placental cytotrophoblast turnover in pregnancies complicated by pre-eclampsia. Histochem Cell Biol 124:499–506. [DOI] [PubMed] [Google Scholar]
- Burd L, Roberts D, Olson M, et al. (2007) Ethanol and the placenta: a review. J Matern Fetal Neonatal Med 20:361–75. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Tham SW (1992) Formation of vasculo-syncytial membranes in the human placenta. J Dev Physiol 18:43–7. [PubMed] [Google Scholar]
- Carter RC, Wainwright H, Molteno CD, et al. (2016) Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res 40:753–64. [DOI] [PubMed] [Google Scholar]
- Dufour MC. (1999) What is moderate drinking? Defining ‘drinks’ and drinking levels. Alcohol Res Health 23:5–14. [PMC free article] [PubMed] [Google Scholar]
- England L, Zhang J (2007) Smoking and risk of preeclampsia: a systematic review. Front Biosci 12:2471–83. [DOI] [PubMed] [Google Scholar]
- Erskine RL, Ritchie JW (1986) The effect of maternal consumption of alcohol on human umbilical artery blood flow. Am J Obstet Gynecol 154:318–21. [DOI] [PubMed] [Google Scholar]
- Evers IM, Nikkels PG, Sikkema JM, et al. (2003) Placental pathology in women with type 1 diabetes and in a control group with normal and large-for-gestational-age infants. Placenta 24:819–25. [DOI] [PubMed] [Google Scholar]
- Finer LB, Zolna MR (2014) Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 104:S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SE, Atkinson M, Burnap JK, et al. (1982) Ethanol-associated selective fetal malnutrition: a contributing factor in the fetal alcohol syndrome. Alcohol Clin Exp Res 6:197–201. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Atkinson M, Van Thiel DH, et al. (1981) Selective fetal malnutrition: the effect of ethanol and acetaldehyde upon in vitro uptake of alpha amino isobutyric acid by human placenta. Life Sci 29:1283–8. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ (2001) Fetal programming and adult health. Public Health Nutr 4:611–24. [DOI] [PubMed] [Google Scholar]
- Green PP, Mcknight-Eily LR, Tan CH, et al. (2016) Vital signs: alcohol-exposed pregnancies - United States, 2011–2013. Morb Mortal Wkly Rep 65:91–7. [DOI] [PubMed] [Google Scholar]
- Gundogan F, Bedoya A, Gilligan J, et al. (2011) siRNA inhibition of aspartyl-asparaginyl beta-hydroxylase expression impairs cell motility, Notch signaling, and fetal growth. Pathol Res Pract 207:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Longato L, et al. (2008) Impaired placentation in fetal alcohol syndrome. Placenta 29:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Mark P, et al. (2010) Ethanol-induced oxidative stress and mitochondrial dysfunction in rat placenta: relevance to pregnancy loss. Alcohol Clin Exp Res 34:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F, Gilligan J, Qi W, et al. (2015) Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta 36:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F, Gilligan J, Ooi JH, et al. (2013) Dual mechanisms of ethanol-impaired placentation: experimental model. J Clin Exp Pathol 3:142. [Google Scholar]
- Higgins M, Felle P, Mooney EE, et al. (2011) Stereology of the placenta in type 1 and type 2 diabetes. Placenta 32:564–9. [DOI] [PubMed] [Google Scholar]
- Idanpaan-Heikkila J, Jouppila P, Akerblom HK, et al. (1972) Elimination and metabolic effects of ethanol in mother, fetus, and newborn infant. Am J Obstet Gynecol 112:387–93. [DOI] [PubMed] [Google Scholar]
- Kalisch-Smith JI, Outhwaite JE, Simmons DG, et al. (2016) Alcohol exposure impairs trophoblast survival and alters subtype-specific gene expression in vitro. Placenta 46:87–91. [DOI] [PubMed] [Google Scholar]
- Kaminski M, Rumeau C, Schwartz D (1978) Alcohol consumption in pregnant women and the outcome of pregnancy. Alcohol Clin Exp Res 2:155–63. [DOI] [PubMed] [Google Scholar]
- Kay HH, Grindle KM, Magness RR (2000) Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol 182:682–8. [DOI] [PubMed] [Google Scholar]
- Khong TY. (1991) Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants, and diabetes mellitus. Arch Pathol Lab Med 115:722–5. [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, et al. (1986) Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93:1049–59. [DOI] [PubMed] [Google Scholar]
- Khong TY, Mooney EE, Ariel I, et al. (2016) Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 140:698–713. [DOI] [PubMed] [Google Scholar]
- Langston C, Kaplan C, Macpherson T, et al. (1997) Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med 121:449–76. [PubMed] [Google Scholar]
- Longtine MS, Chen B, Odibo AO, et al. (2012) Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta 33:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukeris K, Sela R, Baergen RN (2010) Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age. Pediatr Dev Pathol 13:305–9. [DOI] [PubMed] [Google Scholar]
- Lui S, Jones RL, Robinson NJ, et al. (2014) Detrimental effects of ethanol and its metabolite acetaldehyde, on first trimester human placental cell turnover and function. PLoS One 9:e87328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbury MC, Linn S, Monson R, et al. (1983) The association of alcohol consumption with outcome of pregnancy. Am J Public Health 73:1165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetta CM, Denny CH, Floyd RL, et al. (2012) Alcohol use and binge drinking among women of childbearing age - United States - 2006–2010. MMWR Morb Mortal Wkly Rep 61:534–8. [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, et al. (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela O, Halmesmaki E, Ylikorkala O (1991) Hemoglobin-acetaldehyde adducts are elevated in women carrying alcohol-damaged fetuses. Alcohol Clin Exp Res 15:1007–10. [DOI] [PubMed] [Google Scholar]
- Parks WT. (2015) Placental hypoxia: the lesions of maternal malperfusion. Semin Perinatol 39:9–19. [DOI] [PubMed] [Google Scholar]
- Patra J, Bakker R, Irving H, et al. (2011) Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG 118:1411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Anthony J, Davey DA, et al. (1991) Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98:648–55. [DOI] [PubMed] [Google Scholar]
- Pinar H, Sung CJ, Oyer CE, et al. (1996) Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med 16:901–7. [DOI] [PubMed] [Google Scholar]
- Ravishankar S, Bourjeily G, Lambert-Messerlian G, et al. (2015) Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol 18:380–6. [DOI] [PubMed] [Google Scholar]
- Redline RW, Boyd T, Campbell V, et al. (2004) Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 7:237–49. [DOI] [PubMed] [Google Scholar]
- Rethinking drinking Alcohol and your health. 2015. NIH Publication No: 15-3770.
- Roozen S, Peters GJ, Kok G, et al. (2016) Worldwide prevalence of fetal alcohol spectrum disorders: a systematic literature review including meta-analysis. Alcohol Clin Exp Res 40:18–32. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Kornosky JL, Lynch O, et al. (2011) Impact of prenatal alcohol consumption on placenta-associated syndromes. Alcohol 45:73–9. [DOI] [PubMed] [Google Scholar]
- Stanek J. (2012) Utility of diagnosing various histological patterns of diffuse chronic hypoxic placental injury. Pediatr Dev Pathol 15:13–23. [DOI] [PubMed] [Google Scholar]
- Stanek J. (2013) Hypoxic patterns of placental injury: a review. Arch Pathol Lab Med 137:706–20. [DOI] [PubMed] [Google Scholar]
- Wang N, Tikellis G, Sun C, et al. (2014) The effect of maternal prenatal smoking and alcohol consumption on the placenta-to-birth weight ratio. Placenta 35:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.