In San Francisco new human immunodeficiency virus diagnoses declined and care indicators improved during the “Getting to Zero” era. Linkage to care, antiretroviral therapy (ART) initiation and viral suppression increased, and time to viral suppression, ART, and AIDS diagnosis improved.
Keywords: HIV, Engagement in HIV Care, HIV Surveillance, HIV Care Continuum, Getting to Zero
Abstract
Background
San Francisco has launched interventions to reduce new human immunodeficiency virus (HIV) infections and HIV-associated morbidity and mortality during the San Francisco “Getting to Zero” era. We measured recent changes in HIV care indicators to assess the success of these interventions.
Methods
San Francisco residents with newly diagnosed HIV infection, diagnosed from 2009 to 2014, were included. We measured temporal changes from HIV diagnosis to (1) linkage to care in within ≤3 months, (2) initiation of antiretroviral therapy (ART) within ≤12 months, (3) viral suppression within ≤12 months, (4) development of AIDS within ≤3 months, (5) death within ≤12 months, and (6) retention in care 6–12 months after linkage. Kaplan-Meier analyses stratified by year of HIV diagnosis measured time from diagnosis to linkage, ART initiation, viral suppression, AIDS, and death.
Results
Overall, the number of new diagnoses declined from 473 in 2009 to 329 in 2014. The proportion of new diagnoses among men (P = .005), Latinos and Asian/Pacific Islanders (P = .02), and men who have sex with men (P = .003) increased. ART initiation and viral suppression ≤12 months after diagnosis increased (P < .001), while the proportion with AIDS diagnosed ≤3 months after HIV diagnosis declined (P < .001). Time to ART initiation and time to viral suppression were significantly shorter in more recent years of diagnosis (P < .001). Time from HIV to AIDS diagnosis was significantly longer in more recent years (P < .001). Retention in care did not significantly change.
Conclusions
In San Francisco new HIV diagnoses have declined and HIV care indicators have improved during the Getting to Zero era. Continued success requires attention to vulnerable populations and monitoring to adjust programmatic priorities.
(See the Major Article by Scott et al on pages 1019–26.)
Over the last 7 years, San Francisco has launched a series of interventions aimed at reducing new human immunodeficiency virus (HIV) infections and HIV-associated morbidity and mortality rates. These initiatives included the San Francisco Department of Public Health (SFDPH) recommendation for universal antiretroviral therapy (ART) irrespective of CD4+ lymphocyte count (CD4 cell count) in 2010 [1], increased coverage of and targeted HIV testing beginning in 2011 (Tracey Packer, SFDPH, personal communication), same-day initiation of ART at HIV diagnosis in 2012 [2], and scale-up of HIV preexposure prophylaxis (PrEP) to prevent HIV acquisition for high risk HIV-negative adults beginning in 2013 [3, 4]. In addition, the SFDPH Linkage, Integration, Navigation, and Comprehensive Services (LINCS) Program [5] that provides relinkage and reengagement into medical care and wraparound services to patients who have fallen out of care was launched in 2011.
After World AIDS Day (1 December) in 2013, these initiatives were formally coalesced into San Francisco’s “Getting to Zero SF” effort, led by a multisector consortium of public health, academic, and community-based organizations. The group’s goals, modeled after the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets [6], are zero new HIV infections, zero HIV-associated deaths, and zero HIV stigma and discrimination [7].
We examined trends from 2009, before implementation of the local initiatives, through 2014 in 6 HIV care indicators among persons with newly diagnosed HIV: linkage to care, retention in care, initiation of ART, timely viral suppression, time from HIV diagnosis to AIDS diagnosis, and time from HIV diagnosis to death. We selected these indicators because of strong evidence that HIV-related medical care, including ART, improves the health outcomes of persons living with HIV [8], and reduces HIV-associated morbidity and mortality rates [9, 10], in large part owing to rapid achievement of and maintenance of HIV viral suppression [11, 12]. In addition to the health benefits of ART, engagement in HIV care increases opportunities to prevent future HIV transmission by reducing HIV viral load [13], because persons with undetectable HIV in their bloodstream [11, 12] carry a decreased risk of transmitting HIV [8]. We used the population-based SFDPH HIV surveillance case registry to measure trends in these 6 HIV care indicators during the current Getting to Zero era.
MATERIALS AND METHODS
Study Population
The California Health and Safety Regulations mandates that persons with a diagnosis of HIV infection or AIDS be reported to their local health department by both the diagnosing medical provider and laboratory. By law, all HIV-positive antibody, HIV viral load tests, and CD4 cell count results must also be reported by name. In San Francisco, HIV case reporting is evaluated annually and was found to be 99% complete in 2014 [14]. Laboratory tests results for all persons reported with HIV were collected from ongoing laboratory reports of CD4 cell count and HIV viral load tests and from medical record reviews at the time of initial case report and through prospective medical record reviews every 18–24 months by surveillance staff. Laboratory reporting is evaluated annually through sensitivity studies at high volume laboratories and was found to be >99% complete between 2014 and June 2016 [15].
Information collected on persons reported with HIV, at initial case report and through medical record reviews, included sociodemographic and risk characteristics, date of initial and subsequent ART prescription, name of ART prescribed, AIDS-related opportunistic illnesses, development of an AIDS-defining condition, change of address, and vital status. Information on date of death was obtained through computer linkages with the National Death Index, complete through 2014, and with state and local vital statistics registries, complete through 2015. San Francisco residents with HIV or AIDS diagnosed from 1 January 2009 through 31 December 2014 were included in this analysis. Follow-up information was current through 31 December 2015.
Measures
Date of HIV diagnosis was defined as the earliest of any of the following: first confirmed positive HIV antibody test, detectable HIV viral load, or physician diagnosis of HIV/AIDS. Date of entry into HIV care was defined as the earliest date of either a CD4 cell count or HIV viral load test. Retention in care was defined as having a subsequent CD4 cell count or HIV viral load test within 6–12 months after the initial CD4 cell count or viral load test. Viral suppression was defined as a viral load <200 copies/mL. The proportion of persons who were linked to care ≤3 months after diagnosis, were retained in care 6–12 months after linkage, started ART ≤12 months after diagnosis, were virally suppressed ≤12 months after diagnosis, developed AIDS ≤3 months after diagnosis, or died ≤12 months after diagnosis were calculated.
Sex was categorized as female, male, and transgender female (no transgender men were reported with HIV). Race was categorized as African American, Hispanic, other (including multirace), or white. Age at diagnosis was categorized into 10-year age groups. HIV risk was categorized as men who have sex with men (MSM), persons who inject drugs (PWID), MSM-PWID, heterosexual, or other. We defined an individual as living in a low-income neighborhood if he or she lived in a census tract where >20% of persons aged ≥18 years had median annual household income below the US poverty level [16]. Persons were characterized as homeless if their medical record stated they were homeless or not housed at time of HIV diagnosis or if their address at diagnosis was a known homeless shelter or a free postal address not connected to a residence. Residence information is collected at time of HIV diagnosis and updated through routine medical chart reviews and laboratory reports. For persons without any available follow-up information, LexisNexis is used to update the current address.
Statistical Analysis
We compared the distribution of individual characteristics by year of HIV or AIDS diagnosis (whichever was first) using contingency tables, and P values were calculated using χ2 tests. Temporal trends for the HIV care indicators, by year of HIV diagnosis, were analyzed using the 1-sided Cochran-Armitage test for trend. Kaplan-Meier time to event analyses, stratified by year of HIV diagnosis, assessed time from diagnosis to (1) linkage to HIV care, (2) ART initiation, (3) HIV viral suppression, (4) AIDS diagnosis (for those with HIV previously diagnosed), and 5) death. Visual checks of the proportional hazard assumption were done 3 ways: by plotting survival, log survival, and log logs. The log-rank (for consistent hazards) or Wilcoxon statistic (for hazards that changed over time) measured significant differences across the year of diagnosis strata.
In the analysis of the time from diagnosis to HIV care, for persons not in care, data were censored at the date when they were known to have moved outside San Francisco, the date of death, or 31 December 2015 (the end of complete HIV surveillance follow-up time), whichever came first. In the time to ART initiation analyses, persons not known to have started ART were censored at the date of their last medical record review, the date of death, or 31 December 2015, whichever came first. For the time to HIV viral suppression, those not virally suppressed were censored at the date of their last viral load test, the date of death, or 31 December 2015, whichever came first. For the analysis of time from HIV diagnosis to development of AIDS, data were censored for those without AIDS at the date of death or on 31 December 2015, whichever came first. In the time to death analyses, data were censored for those not known to have died by 31 December 2015. All analyses were performed using SAS software (version 9; SAS Institute).
RESULTS
The total number of new HIV diagnoses in San Francisco declined from 473 in 2009 to 307 in 2014 (P = .005). Overall, 93% of new diagnoses occurred in men, the vast majority of whom were MSM, including MSM-PWID (Table 1). The absolute number of new HIV diagnoses declined or remained stable by age, sex, race/ethnicity, and risk group. However, the proportion of new diagnoses in the following groups increased: men (P = .005), Asian/Pacific Islanders and Latinos (P = .02), and MSM (P = .003) (Table 1). The median CD4 cell count increased from 417/mm3 in 2009 to 437/mm3 in 2014 (P = .50). The proportion of persons with newly diagnosed HIV living in low-income neighborhoods and the proportion who were homeless at diagnosis remained stable over time.
Table 1.
Demographic and Risk Characteristics of Persons With Newly Diagnosed Human Immunodeficiency Virus/AIDS by Year, 2009–2014, San Franciscoa
| Characteristic at Diagnosis | Persons With Newly Diagnosed HIV/AIDS by Year of Diagnosis, No. (%) | P Value | |||||
|---|---|---|---|---|---|---|---|
| 2009 (n = 473) |
2010 (n = 451) |
2011 (n = 422) |
2012 (n = 456) |
2013 (n = 399) |
2014 (n = 329) | ||
| Sex | .005b | ||||||
| Male | 417 (88.2) | 399 (88.5) | 367 (87.0) | 424 (93.0) | 362 (91.0) | 307 (93.3) | |
| Female | 31 (6.6) | 37 (8.2) | 42 (10.0) | 25 (5.5) | 27 (6.8) | 14 (4.3) | |
| Transgender female | 25 (5.3) | 15 (3.3) | 13 (3.1) | 7 (1.5) | 10 (2.5) | 8 (2.4) | |
| Race/ethnicity | .02b | ||||||
| African American | 71 (15.0) | 62 (13.8) | 66 (15.6) | 47 (10.3) | 52 (13.0) | 35 (10.6) | |
| Asian/Pacific Islander | 39 (8.3) | 39 (8.7) | 32 (7.6) | 46 (10.1) | 51 (12.8) | 42 (12.8) | |
| Latino | 112 (23.7) | 105 (23.3) | 78 (18.5) | 114 (25.0) | 100 (25.1) | 93 (28.3) | |
| White | 230 (48.6) | 217 (48.1) | 228 (54.0) | 231 (50.7) | 178 (44.6) | 142 (43.2) | |
| Other/unknown | 21 (4.4) | 28 (6.2) | 18 (4.3) | 18 (4.0) | 18 (4.5) | 17 (5.2) | |
| Age, y | .29 | ||||||
| 15–24 | 50 (10.6) | 54 (12.0) | 40 (9.5) | 56 (12.3) | 51 (12.8) | 35 (10.6) | |
| 25–34 | 138 (29.2) | 131 (29.1) | 127 (30.1) | 154 (33.8) | 147 (36.8) | 114 (34.7) | |
| 35–44 | 144 (30.4) | 136 (30.2) | 119 (28.2) | 123 (27.0) | 103 (25.8) | 83 (25.2) | |
| 45–55 | 103 (21.8) | 93 (20.6) | 100 (23.7) | 98 (21.5) | 65 (16.3) | 71 (21.6) | |
| ≥55 | 38 (8.0) | 37 (8.2) | 36 (8.5) | 25 (5.5) | 33 (8.3) | 26 (7.9) | |
| HIV risk factors | .003b | ||||||
| MSM | 330 (69.8) | 289 (64.1) | 301 (71.3) | 354 (77.6) | 301 (75.4) | 249 (75.7) | |
| PWID | 26 (5.5) | 37 (8.2) | 27 (6.4) | 17 (3.7) | 24 (6.0) | 19 (5.8) | |
| MSM-PWID | 77 (16.3) | 69 (15.3) | 53 (12.6) | 44 (9.7) | 43 (10.8) | 37 (11.3) | |
| Heterosexual | 26 (5.5) | 35 (7.8) | 31 (7.4) | 29 (6.4) | 22 (5.5) | 11 (3.3) | |
| Other/unknown | 14 (3.0) | 21 (4.7) | 10 (2.4) | 12 (2.6) | 9 (2.3) | 13 (4.0) | |
| CD4 cell count, median, cells/mm3 | 417 | 411 | 438 | 419 | 442 | 437 | .50 |
| Homeless | 52 (12.6) | 55 (13.0) | 38 (10.2) | 41 (9.5) | 31 (8.2) | 35 (11.2) | .20 |
| Residence in low-income neighborhood | 137 (30.1) | 144 (33.6) | 112 (27.7) | 119 (26.7) | 104 (27.0) | 95 (29.3) | .23 |
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; PWID, persons who inject drugs.
aData represent No. (%) unless otherwise specified.
bSignificant at P < .05.
The proportion of persons linked to care ≤3 months after HIV diagnosis increased from 86% in 2009 to 92% in 2014 (P = .04) (Table 2), The proportion of persons retained in care 6–12 months after linkage, approximately 70%, did not change significantly over time. The proportion virally suppressed ≤12 months after HIV diagnosis increased, as did the proportion of persons who started ART ≤12 months after diagnosis both (P < .001).
Table 2.
Human Immunodeficiency Virus (HIV) Care Indicators Among Persons With Newly Diagnosed HIV or AIDS by Year, 2009–2014, San Francisco
| Indicator | Persons With Newly Diagnosed HIV or AIDS by Year of Diagnosis, % | P Value for Trend Testa | |||||
|---|---|---|---|---|---|---|---|
| 2009 (n = 473) | 2010 (n = 451) |
2011 (n = 422) |
2012 (n = 456) | 2013 (n = 399) | 2014 (n = 329) |
||
| Linked to care ≤3 mo after diagnosis | 85.8 | 84.3 | 85.8 | 87.7 | 83.5 | 91.8 | .04b |
| Retained in care 6–12 mo after linkage | 70.2 | 65.9 | 71.1 | 69.8 | 67.2 | 72.6 | .26 |
| Starting ART ≤12 mo after diagnosis | 63.2 | 72.9 | 78.6 | 85.6 | 84.6 | 90.7 | <.001b |
| Virally suppressed ≤12 mo after diagnosisc | 49.2 | 59.5 | 63.2 | 72.6 | 73.2 | 82.3 | <.001b |
| Development of AIDS ≤3 mo after diagnosis | 26.9 | 26.6 | 23.9 | 21.1 | 18.3 | 16.4 | <.001b |
| Death ≤12 mo after diagnosis | 3.0 | 2.9 | 2.6 | 0.9 | 3.8 | 1.2 | .12 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
a P values based on 1-sided Cochran-Armitage trend test.
bSignificant at P < .05.
cViral suppression is defined as a viral load <200/copies/mL.
In recent years, the proportion of persons in whom AIDS developed ≤3 months after HIV diagnosis (late diagnoses) declined from 27% in 2009 to 16% in 2014 (P < .001). In addition, the proportion of persons who died ≤12 months after HIV diagnosis remained below 4% each year but did not change significantly (P = .12) (Table 2).
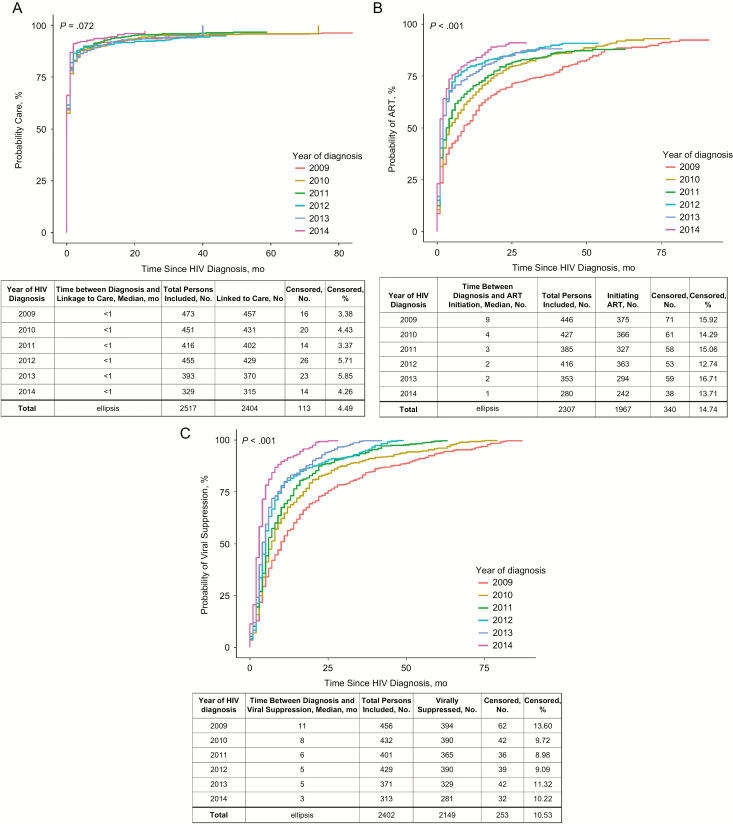
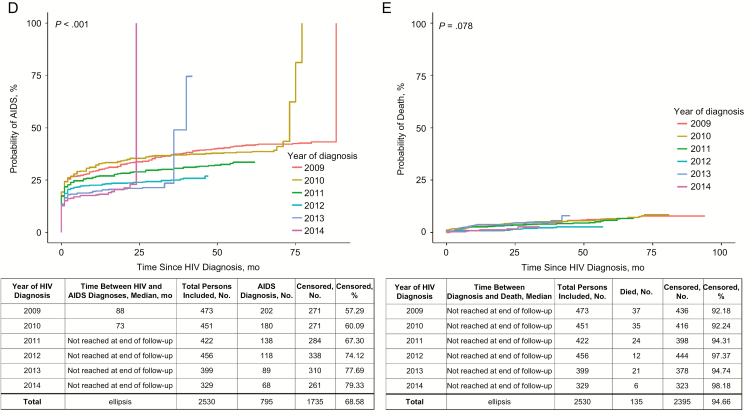
We found no significant change in time from diagnosis to linkage to care (P = .07); the median time was <1 month for all years (Figure 1A). The median time from diagnosis to ART initiation decreased over time (P < .001), from 9 months in 2009 to 1 month in 2014 (Figure 1B). The time from diagnosis to viral suppression was significantly reduced (P < .001), from a median of 11 months in 2009 to 3 months in 2014 (Figure 1C). There was also a significant increase in the time from HIV diagnosis to the development of AIDS (P < .001; Figure 1D). The median survival time from HIV diagnosis to death could not be calculated for any years because 50% of the population had not died as of the end of the observation period (Figure 1E).
Figure 1.
A, Time from human immunodeficiency virus (HIV) diagnosis to linkage to care (n = 2517). B, Time from HIV diagnosis to antiretroviral therapy (ART) initiation (n = 2307). C, Time from HIV diagnosis to HIV viral suppression (n = 2402). D, Time from HIV diagnosis to AIDS diagnosis (n = 2530). E, Time from HIV diagnosis to death (n = 2530).
DISCUSSION
Our findings document a substantial decrease in new HIV diagnoses and improvement in the HIV care indicators from 2009 through 2014, suggesting success toward achieving the Getting to Zero goals. The increases we observed in the proportion of persons with newly diagnosed HIV who were linked to care, started ART, achieved viral suppression, and remained AIDS free demonstrate the positive impact of local interventions designed to link persons to HIV care and provide ART as soon as possible.
Our findings of declining HIV diagnoses are similar to overall trends in the United States [17], and improvements in HIV care indicators have also been found in other regions in the United States although there are variations by region and demographic characteristics, including race [17–22]. Of note, King County in Washington State reports being the first large urban area in the United States to achieve the 90-90-90 targets [23]. Other jurisdictions worldwide have implemented initiatives similar to Getting to Zero SF, (eg, the “Ending the Epidemic” initiative in New York [24]), and have committed to ending the AIDS epidemic by 2030, by signing the 2014 Paris Declaration [25].
Although declines in new HIV diagnoses may not reflect decreases in HIV incidence, we believe that, to a large extent, the observed decline in new diagnoses probably reflects similar trends in new infections. Between 2004 and 2011 in San Francisco, a significant decline in the proportion of MSM with undiagnosed infection (from 21.7% in 2004 to 7.5% in 2011) and significant increases in the proportion of MSM who self-reported testing for HIV in the previous 6 months was documented [26].
Similar to the proportion in MSM, the proportion of undiagnosed HIV infection overall in San Francisco is estimated to be relatively low (7% in 2014) [14]. We also found significant declines in the proportion of persons with HIV diagnosed late in the course of disease. Improvements in timely linkage, treatment, and viral suppression may also be partially responsible for the decline in new diagnoses, because studies have shown that early treatment [27] and durable viral suppression [28–30] are associated with a substantially reduced risk of HIV transmission. Although we report on viral suppression among persons with newly diagnosed HIV, it is important to note that HIV transmission is influenced by the entire population of persons living with HIV in a community. In 2013, 72% of the 10682 persons reported with HIV and residing in San Francisco were virally suppressed [14].
Improvements in early and more frequent HIV testing have probably contributed to the decrease in the development of AIDS shortly after HIV diagnosis during this time period. Although the current study did not find a significant change in the number of persons who died ≤12 months after their HIV diagnosis, this was probably because of the very small number of deaths per year among persons with newly diagnosed HIV and possibly because of diagnosis at an earlier stage of disease.
The declines in new HIV diagnoses may also be due at least in part to PrEP use that has been found to substantially decrease the risk of HIV acquisition in recent studies [31, 32]. Several studies in San Francisco have found that knowledge, interest, and self-reported use of PrEP are high [33–35]. This may be particularly relevant for the declines in new diagnoses among MSM. Although reductions in risk behavior could account for decreases in new infections, this does not seem to be true for MSM in San Francisco. During the study period, there was an increase in the proportion of MSM, with or without HIV, who received a diagnosis of male rectal gonorrhea and early syphilis, as well as in self-reported condomless sex [14].
Despite improvements in HIV prevention and treatment, not all indictors showed improvement. In particular, retention in HIV care after initial linkage, a key indicator for sustained improvement along the HIV care continuum, did not improve. Existing programs focusing on retention in care will need to be expanded, with increased emphasis on ongoing wraparound services, particularly among vulnerable populations and persons with comorbid conditions and/or sociobehavioral issues affecting their ability to prioritize and maintain HIV care. In addition, creative and innovative approaches will be needed for the long term. New approaches to ensure that everyone living with HIV has access to HIV care will be necessary, especially given that the Affordable Care Act, which has enabled many low-income and previously uninsured persons to access HIV treatment, care and PrEP, is currently undergoing challenge and may be repealed and replaced. Although currently proposed changes and their impact on persons living with or at risk for HIV are unknown, it seems likely that many such persons will find it harder to access and maintain appropriate HIV care and prevention [36].
In addition, not all populations in San Francisco are benefitting equally. HIV diagnosis rates are exceedingly high in African American and Latino men compared with white men (140 and 83 vs 52 per 100000 population, respectively, in 2015) [14]. The high rate of HIV among African Americans in San Francisco parallels national rates in which African Americans are by far the most affected racial or ethnic group [17]. Although HIV diagnosis rates are relatively low in women in San Francisco, as seen nationally, African American women have the highest rate compared with women of all other races (31 per 100000 population compared with a 8 in Latina and 5 in white women in 2015), and their rate is approximately 60% of the rates in white men [14].
Persons defined as homeless under our strict surveillance-based definition accounted for 11% of new HIV diagnoses in San Francisco in 2014, double the maximum target proposed under the National HIV/AIDS Strategy [37]. San Francisco has experienced a steady increase in persons who lack stable housing in recent years [38] and has the second-highest rate of homelessness in the nation. Analyses of surveillance data in San Francisco have found that persons who were homeless at HIV diagnosis were less likely to be receiving HIV treatment, retained in care, or virally suppressed [14]. Moreover, despite efforts to increase and target HIV testing among those most at risk for HIV, as well as the advances in the HIV care, 16% of persons with newly diagnosed HIV develop an AIDS-defining condition within 3 months after diagnosis, and persons with HIV continue to die of AIDS complications.
Limitations of our analysis include the inherent inability of surveillance data to prove causality related to any of our citywide interventions and observed outcomes. In addition, surveillance data included only persons with diagnosed HIV infection and thus do not include those whose HIV is not yet diagnosed. The time to linkage to care and the time to retention in care were estimated by laboratory reports of CD4 cell counts and/or HIV viral load, which are proxies for true HIV care. Nonetheless, few persons enter HIV care and do not have these laboratory tests performed. Our analyses were restricted to San Francisco residents at the time of HIV diagnosis, and thus subsequent clinical care may be underestimated for persons who have since moved, although we have attempted to account for this through censoring, as described in Materials and Methods. Finally, the follow-up period was short, because we intentionally focused on newly diagnosed HIV infections to assess the impact of recently implemented San Francisco initiatives. Thus, our ability to determine the time from HIV diagnosis to AIDS or to death was limited.
It is also important, however, to consider the strengths and utility of HIV surveillance data in evaluating trends. Surveillance data are collected continuously using standardized methods, are population based, and are routinely evaluated for the completeness of case ascertainment, timeliness, and accuracy. Because of consistent and standardized surveillance methods, our analyses allow for accurate comparisons over time.
Our findings of a decline in new HIV diagnoses to the lowest level ever and treatment coverage and viral suppression at all-time highs point to the success of citywide HIV prevention and treatment programs now formalized under the Getting to Zero consortium. To ensure continued progress and to address the ongoing challenges, the consortium has prioritized expanding programs aimed at increasing uptake of PrEP, providing ART on diagnosis, and identifying new approaches to retention and reengagement in care. In fact, the consortium has set bold targets of a 90% reduction in new HIV diagnoses and HIV-associated deaths from 2013 levels by 2020. This would require that new diagnoses decrease from 399 in 2013 to 40 in 2020, and HIV-associated deaths from 261 in 2013 [14] to 26 in 2020. Reaching these goals will require additional innovation, programs focused on vulnerable populations, and monitoring of progress though high-quality surveillance data to further adjust programmatic priorities.
Notes
Acknowledgments. We thank Jason Zhe-Xu for assistance producing the Kaplan-Meier curves. We also thank all the Getting to Zero Consortium members for their efforts making Getting to Zero possible and successful in San Francisco.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC-RFA-PS13-1302: National HIV Surveillance System, grant 5U62PS004022-04).
Potential conflicts of interest. D. H. has received funding for projects outside this work from the National Institutes of Health NIH, the Bill & Melinda Gates Foundation for research, and medications from Gilead for a NIH funded study in Uganda and Kenya. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Russell S. City endorses new policy for treatment of HIV. New York Times. 3 April 2010. [Google Scholar]
- 2. Pilcher CD, Ospina-Norvell C, Dasgupta A et al. . The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr 2017; 74:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration. FDA approves first drug for reducing the risk of sexually acquired HIV infection. 2012. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm. Accessed 9 April 2017. [Google Scholar]
- 4. Grant RM, Anderson PL, McMahan V et al. ; iPrEx study team Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. San Francisco Department of Public Health (2014) Linkage, Integration, Navigation, and Comprehensive Services (LINCS) program. Available: http://www.sfhiv.org/our-services/linkage-integration-navigation-and-comprehensive-services-lincs/. Accessed 21 March 2017. [Google Scholar]
- 6. United Nations Joint Programme on HIV/AIDS (UNAIDS). 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014. Available at: https://www.unaids.org/sites/default/media_asset/90-90-90_en_0.pdf. Accessed 9 April 2017. [Google Scholar]
- 7. Havlir D, Buchbinder S.. Getting to Zero: San Francisco Consortium Working to Eliminate AIDS. San Francisco Medicine. 2017: 90(20–21). [Google Scholar]
- 8. Palella FJ Jr, Delaney KM, Moorman AC et al. ; HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 9. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC). Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep 2011; 60:1618–23. [PubMed] [Google Scholar]
- 11. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quinn TC, Wawer MJ, Sewankambo N et al. ; Rakai Project Study Group Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 13. Mugevero MJ. Elements of the HIV care continuum: improving engagement and retention in care. Top Antivir Med 2016; 24:115–9. [PMC free article] [PubMed] [Google Scholar]
- 14. San Francisco Department of Public Health. HIV epidemiology annual report 2015. Available at: https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2015-20160831.pdf. Accessed 9 April 2017. [Google Scholar]
- 15. San Francisco Department of Public Health. HIV epidemiology annual report 2016. Available at: https://www.sfdph.org/dph/comupg/oprograms/HIVepiSec/HIVepiSecReports.asp. Accessed 29 November 2017. [Google Scholar]
- 16. US Census Bureau. 2010–2014 American community survey. 2015. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed 29 November 2017. [Google Scholar]
- 17. Centers for Disease Control and Prevention. HIV surveillance report, 2015. Vol 27. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2016. Accessed 17 June 2017. [Google Scholar]
- 18. Rebeiro PF, Gange SJ, Horberg MA et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Geographic variations in retention in care among HIV-infected adults in the United States. PLoS One 2016; 11:e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson JA, Kinder A, Johnson AS et al. . Differences in selected HIV care continuum outcomes among people residing in rural, urban, and metropolitan areas—28 US jurisdictions. J Rural Health 2016; doi: 10.1111/jrh.12208. [DOI] [PubMed] [Google Scholar]
- 20. Toren KG, Buskin SE, Dombrowski JC, Cassels SL, Golden MR. Time from HIV diagnosis to viral load suppression: 2007–2013. Sex Transm Dis 2016; 43:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia Q, Lazar R, Bernard MA et al. . New York City achieves the UNAIDS 90-90-90 targets for HIV-infected whites but not Latinos/Hispanics and blacks. J Acquir Immune Defic Syndr 2016; 73:e59–62. [DOI] [PubMed] [Google Scholar]
- 22. Xia Q, Braunstein SL, Wiewel EW, Hadler JL, Torian LV. Persistent racial disparities in HIV infection in the USA: HIV prevalence matters. J Racial Ethn Health Disparities 2016; 4:87–93. [DOI] [PubMed] [Google Scholar]
- 23. HIV/AIDS Epidemiology Unit, Public Health, Seattle & King County and the Infectious Disease Assessment Unit, Washington State Department of Health. HIV/AIDS epidemiology report 2016. Volume 85 Available at: http://www.kingcounty.gov/depts/health/communicable-diseases/hiv-std/patients/epidemiology.aspx. Accessed 9 April 2017. [Google Scholar]
- 24. Daskalakis DC. If you can make it there: ending the HIV epidemic in New York. Conference on Retroviruses and Opportunistic Infections. February 2017. Seattle, Washington: Abstract 108. Available at: http://www.croiwebcasts.org/console/player/33560?mediaType=slideVideo&. Accessed 9 April 2017. [Google Scholar]
- 25. UNAIDS. Cities ending the AIDS epidemic. Fast-Track cities. 2016. Available at: www.unaids.org/en/resources/documents/2016/cities-report. Assessed 19 June 2017. [Google Scholar]
- 26. Raymond HF, Chen YH, Ick T et al. . A new trend in the HIV epidemic among men who have sex with men, San Francisco, 2004-2011. J Acquir Immune Defic Syndr 2013; 62:584–9. [DOI] [PubMed] [Google Scholar]
- 27. HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donnell D, Baeten JM, Kiarie J et al. ; Partners in Prevention HSV/HIV Transmission Study Team Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Del Romero J, Castilla J, Hernando V, Rodríguez C, García S. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ 2010; 340:c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012-2013. Clin Infect Dis 2016; 63:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCormack S, Dunn DT, Desai M et al. . Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molina JM, Capitant C, Spire B et al. ; ANRS IPERGAY Study Group On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 33. Scheer S. PrEP knowledge and use in San Francisco. Presented at: HIV Research for Prevention Conference (HIVR4P 2016); 17–21 October 2016; Chicago, IL. Abstract OA24.03. [Google Scholar]
- 34. Cohen SE, Vittinghoff E, Bacon O et al. . High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr 2015; 68:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcus JL, Hurley LB, Hare CB et al. . Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr 2016; 73:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. How repealing portions of the Affordable Care Act would affect health insurance coverage and premiums. Congressional Budget Office. January 2017. Available at: https://www.cbo.gov/sites/default/files/115th-congress-2017–2018/reports/52371-coverageandpremiums.pdf. Accessed 11 April 2017. [Google Scholar]
- 37. National HIV/AIDS Strategy for the United States: updated to 2020, July 2015. Available at: https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf. Accessed 9 April 2017. [Google Scholar]
- 38. San Francisco homeless point-in-time count & survey, 2015. Available at: https://sfgov.org/lhcb/sites/default/files/2015%20San%20Francisco%20Homeless%20Count%20%20Report.pdf. Assessed 11 April 2017. [Google Scholar]