Individuals residing in areas of high neighborhood socioeconomic status (nSES) demonstrated lower rates of infection and sepsis relative to those in low-nSES neighborhoods. Individual income and physical function mediate the association, highlighting pathways through which nSES could impact infection risk.
Keywords: sepsis, risk factor, mediation, socioeconomic status
Abstract
Background
Prior studies suggest disparities in sepsis risk and outcomes based on place of residence. We sought to examine the association between neighborhood socioeconomic status (nSES) and hospitalization for infection and sepsis.
Methods
We conducted a prospective cohort study using data from 30239 participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. nSES was defined using a score derived from census data and classified into quartiles. Infection and sepsis hospitalizations were identified over the period 2003–2012. We fit Cox proportional hazards models, reporting hazard ratios (HRs) with 95% confidence intervals (CIs) and examining mediation by participant characteristics.
Results
Over a median follow-up of 6.5 years, there were 3054 hospitalizations for serious infection. Infection incidence was lower for participants in the highest nSES quartile compared with the lowest quartile (11.7 vs 15.6 per 1000 person-years). After adjustment for demographics, comorbidities, and functional status, infection hazards were also lower for the highest quartile (HR, 0.84 [95% CI, .73–.97]), with a linear trend (P = .011). However, there was no association between nSES and sepsis at presentation among those hospitalized with infection. Physical weakness, income, and diabetes had modest mediating effects on the association of nSES with infection.
Conclusions
Our study shows that differential infection risk may explain nSES disparities in sepsis incidence, as higher nSES is associated with lower infection hospitalization rates, but there is no association with sepsis among those hospitalized. Mediation analysis showed that nSES may influence infection hospitalization risk at least partially through physical weakness, individual income, and comorbid diabetes.
Sepsis is life-threatening organ dysfunction resulting from a dysregulated host response to infection. Sepsis places an immense burden on the US healthcare system, resulting in 1.5 million hospitalizations and 850000 emergency department visits annually [1, 2]. While numerous studies have addressed the acute care of sepsis patients, relatively little is known about long-term risk factors for infection and sepsis-related organ dysfunction [3]. However, prior efforts have noted geographic and racial differences in sepsis mortality, as well as differences in sepsis hospitalization by insurance type [4–7].
Place of residence is an important predictor of health, above and beyond the effects of individual lifestyle factors and genetics [8–13]. Neighborhood socioeconomic status (nSES) may be associated with hospitalization for infection and sepsis through several potential mechanisms. For instance, individuals in low-nSES areas may delay seeking appropriate ambulatory care for infections, resulting in an increased risk of hospitalization. Increased levels of chronic, low-grade inflammation could also lead individuals living in low-SES neighborhoods to experience more severe infections. This is supported by studies examining the associations of inflammatory biomarkers with sepsis risk [14, 15]. Factors related to low-nSES residence, such as increased psychosocial stress, environmental exposures, and limited access to preventive care, may then create a proinflammatory state that facilitates the development of severe infection or sepsis-related organ dysfunction.
Despite a wealth of studies examining the relationship between neighborhood socioeconomic characteristics and health outcomes, there is a paucity of data pertaining to infection and sepsis. Hence, we sought to examine the associations of nSES with risk of future hospitalization for infection and sepsis using a national cohort of community-dwelling adults in the United States.
METHODS
Study Design
We performed a retrospective study using data prospectively collected as part of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. REGARDS is one of the largest ongoing national cohorts in the United States, comprised of 30239 white and black community-dwelling adults aged ≥45 years; the study design, objectives, and sampling strategy have been previously described in detail [16]. In brief, the study oversampled black individuals and those living in the southeastern United States, with the overall cohort being 45% male, 42% black, and 69% aged >60 years.
The REGARDS study enrolled participants between 2003 and 2007, obtaining baseline data for each participant using phone interview and an in-person evaluation. REGARDS contacted study participants at 6-month intervals by telephone, identifying the date, location, and attributed reason for all hospitalizations. The REGARDS-Sepsis ancillary study collected additional data on hospitalization for infection. The REGARDS study was approved by the institutional review boards of participating institutions, and all participants provided verbal consent before the telephone interview and written informed consent before the in-home study visit.
Outcome Measures
We identified hospital admissions and emergency department visits attributed to serious infection [17]. Trained abstractors reviewed relevant medical records to confirm the presence of infection on initial hospital presentation and its relevance as a reason for admission (Supplementary Appendix 1). Discordances were adjudicated among abstractors, with additional physician review as needed. Initial review of 1349 hospital records indicated excellent interrater agreement for presence of serious infection (κ = 0.92). For the current analysis, we analyzed events occurring from participant enrollment through 31 December 2012.
In accordance with international consensus definitions, we identified sepsis upon the first infection event using the worst physiologic and laboratory measurements observed during the initial 28 hours [18]. We identified hospitalizations for infection with ≥2 systemic inflammatory response syndrome (SIRS) criteria, including heart rate >90 beats/minute, fever or low body temperature, tachypnea (>20 breaths/minute) or partial pressure of carbon dioxide (pCO2) <32 mm Hg, and leukocytosis (white blood cells >12000/µL or <4000 cells/µL or >10% band forms) [19]. We also defined sepsis as infection with ≥2 sepsis-related organ failure assessment (SOFA) score points according to established criteria [19]. Last, we identified infection events with ≥2 “quick” SOFA (qSOFA) criteria, including altered mentation (Glasgow coma score <14 or nonalert per the Alert, Voice, Pain, Unresponsive scale), systolic blood pressure ≤100 mm Hg, or respiratory rate ≥22 breaths/minute [19].
Using clinical data available in patient charts, we also reported infection type and organ dysfunction severity in the form of the SOFA score, length of stay and admission destination among those admitted, in-hospital mortality, and discharge to a nursing, assisted care, or rehabilitation facility.
Primary Exposure and Participant Characteristics
We determined nSES using block group–level data from the 2000 census. The nSES summary score included the following components: (1) median household income; (2) median home value; (3) percentage of households receiving interest, dividend, or rental fees; (4) percentage of adults completing high school or (5) college; and (6) percentage of working individuals in managerial, executive, or professional occupations [8]. For each factor, we calculated Z scores as the offset from the mean divided by the standard deviation, with respect to all block groups in the sample [8]. We calculated the summary score by summing across all 6 standardized components, with lower values corresponding to lower nSES and higher values representing higher nSES. We then grouped composite nSES summary scores into quartiles [8]. Our analyses were limited to those with complete neighborhood characteristic information. Supplementary Figure 1 presents an example nSES score calculation.
Comprehensive data on participant characteristics were obtained at baseline enrollment; detailed variable definitions are provided in Supplementary Table 1. In brief, demographics included age, gender, self-reported annual household income and education, and geographic region. Health behaviors included tobacco and alcohol use [20]. Chronic medical conditions included chronic lung disease, diabetes, hypertension, myocardial infarction, stroke, and body mass index [21]. Baseline biomarkers included serum creatinine and high-sensitivity C-reactive protein (hs-CRP) [22]. Functional status measures included the 12-Item Short-Form Health Survey (SF-12) physical composite score (PCS) and self-reported physical activity/exhaustion [23–25].
Statistical Analysis
We examined differences in nSES score components across nSES quartiles, reporting medians, with 25th and 75th percentiles. We compared demographic, health behaviors, and clinical characteristics across nSES quartiles using Pearson χ2 tests of association for categorical variables or analysis of variance for continuous variables.
We reported 10-year infection incidence per 1000 person-years with 95% confidence intervals (CIs) across nSES categories. To assess the association of nSES with risk of infection, we fit Cox proportional hazards models, reporting hazard ratios (HRs) and 95% CIs. Models were sequentially adjusted for participant demographics, health behaviors, chronic medical conditions, biomarkers, and measures of functional status. Participants were censored on death, loss to follow-up, or 31 December 2012. The lowest nSES quartile was the reference group. For the full model, we reported adjusted failure curves with all covariates set to reference values. We also performed tests of linear trend over nSES groupings. In sensitivity analyses, we included sepsis subtypes as outcomes in Cox proportional hazards models. The proportional hazards assumption was verified by examining Schoenfeld residuals and testing interactions with the logarithm of time.
Following sequential analyses of the association between nSES and infection, we examined the relative mediating effect of several factors (individual income, physical weakness, exhaustion, low physical activity, obesity, and diabetes). We fit a Cox proportional hazards model including age, sex, race, education, geographic region, chronic lung disease, hypertension, stroke, myocardial infarction, smoking, alcohol use, hs-CRP, and chronic kidney disease, then added each mediator to the model separately. We reported natural direct (association not through the mediator) and indirect (association through the mediator) effects on the hazard ratio scale, with mediation quantified as the percentage of the total effect mediated on the log hazard scale [26]. Confidence intervals for natural direct and indirect effects were constructed using the delta method. Supplementary Figure 2 presents the conceptual framework for mediation analysis.
Among those with infection, we reported percentages for infection type, sepsis type, intensive care unit admission, SOFA category, in-hospital death, and discharge to a nursing facility, performing χ2 tests to examine differences by nSES. We also reported median length of stay and examined differences using Kruskal-Wallis tests. In a sensitivity analysis, we fit logistic regression models and compared adjusted odds of sepsis among those with infection.
All analyses were performed using Stata version 13.1 software (StataCorp, College Station, Texas) and SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
Among 30239 participants enrolled over 2003–2007, follow-up was available for 29683 (98.2%). Geocode and nSES measure data at the block group level were available for 26604 participants (89.6%). Summary scores varied widely across the cohort, with a median nSES score of –0.92 (25th–75th percentile, –3.90 to 3.08; minimum–maximum, –11.79 to 28.95). The individual components of the nSES score differed substantially across nSES quartiles (Table 1). Participant characteristics differed by nSES quartile, with low-nSES participants being disproportionately black, female, smokers, nonusers of alcohol, from the “Stroke Belt,” and having low income and educational attainment (Table 2). Low-nSES participants were also more likely to have comorbidities, abnormal biomarker levels, and reduced functional status.
Table 1. .
Census Block Group Socioeconomic Measures
| Block Group nSES Measure | nSES Quartile, Median (25th–75th Percentile) | |||
|---|---|---|---|---|
| Q1 [Lowest] nSES Score (–11.79 to –3.90) (n = 6651) |
Q2 nSES Score (–3.89 to –0.93) (n = 6654) |
Q3 nSES Score (–0.92 to 3.08) (n = 6648) |
Q4 [Highest] nSES Score (3.09–28.95) (n = 6651) |
|
| Median household income, $ | 21955 (17298–26445) | 31855 (27283–36645) | 42022 (36259–48261) | 62143 (51759–75509) |
| Median home value, $ | 49300 (39400–59900) | 69500 (58100–83200) | 94600 (81300–116200) | 162800 (129400–224400) |
| Households with interest, dividend, or rental income, % | 10.1 (6.3–14.3) | 19.3 (14.2–24.4) | 30.1 (23.1–37.7) | 50.3 (40.8–59.9) |
| Adult residents completed high school, % | 61.2 (55.2–66.4) | 73.9 (69.4–78.4) | 83.7 (79.5–87.3) | 93.0 (89.8–95.9) |
| Adult residents completed college, % | 10.3 (7.2–13.5) | 18.0 (14.5–21.9) | 28.5 (23.9–33.7) | 49.4 (40.7–59.5) |
| Employed residents with executive, managerial, or professional occupations, % | 15.7 (11.7–20.1) | 23.1 (19.3–27.2) | 31.6 (27.4–36.4) | 47.7 (41.1–55.7) |
Total of 26604 Reasons for Geographic and Racial Differences in Stroke (REGARDS) participants. Block group–level measures obtained from economic census data.
Abbreviation: nSES, neighborhood socioeconomic status.
Table 2.
Characteristics of Participants by Neighborhood Socioeconomic Status Score
| Participant Characteristic | nSES Quartile | P Valuea | |||
|---|---|---|---|---|---|
| Q1 [Lowest] nSES Score (–11.79 to –3.90) (n = 6651) |
Q2 nSES Score (–3.89 to –0.93) (n = 6654) |
Q3 nSES Score (–0.92 to 3.08) (n = 6648) |
Q4 [Highest] nSES Score (3.09–28.95) (n = 6651) |
||
| Demographics | |||||
| Age, y, mean (SD) | 64.8 (9.5) | 64.8 (9.4) | 64.9 (9.4) | 65.2 (9.4) | .09 |
| Race, % | <.001 | ||||
| Black | 71.3 | 47.3 | 33.8 | 15.7 | |
| White | 28.7 | 52.7 | 66.2 | 84.4 | |
| Gender, % | <.001 | ||||
| Male | 38.6 | 43.1 | 46.8 | 51.4 | |
| Female | 61.4 | 56.9 | 53.2 | 48.6 | |
| Education, % | <.001 | ||||
| Less than high school | 25.5 | 14.7 | 7.4 | 2.5 | |
| High school graduate or more | 74.6 | 85.3 | 92.6 | 97.6 | |
| Missing, No. (%) | 10 (0.2) | 4 (0.1) | 5 (0.1) | 4 (0.1) | |
| Income, % | <.001 | ||||
| <$20000 | 34.6 | 20.8 | 11.7 | 5.0 | |
| ≥$20000 | 51.8 | 66.8 | 76.7 | 83.2 | |
| Not reported | 13.6 | 12.4 | 11.6 | 11.9 | |
| Geographic region, % | <.001 | ||||
| Non–Stroke Belt/Buckle | 35.2 | 39.1 | 49.4 | 57.3 | |
| Stroke Belt | 40.8 | 38.3 | 32.7 | 25.8 | |
| Stroke Buckle | 24.0 | 22.6 | 17.9 | 16.9 | |
| Health behaviors | |||||
| Tobacco use, % | <.001 | ||||
| Current | 19.5 | 16.2 | 13.2 | 8.8 | |
| Past | 36.7 | 40.0 | 41.7 | 43.1 | |
| Never | 43.8 | 43.7 | 45.1 | 48.1 | |
| Missing, No. (%) | 32 (0.5) | 29 (0.4) | 20 (0.3) | 25 (0.4) | |
| Alcohol use, % | <.001 | ||||
| Heavy | 2.6 | 3.4 | 4.2 | 6.0 | |
| Moderate | 21.6 | 27.3 | 35.3 | 49.4 | |
| None | 75.8 | 69.4 | 60.5 | 44.6 | |
| Missing, No. (%) | 129 (1.9) | 143 (2.2) | 127 (1.9) | 110 (1.7) | |
| Chronic medical conditions, % | |||||
| Chronic lung disease | 9.3 | 9.5 | 9.1 | 9.3 | .89 |
| Diabetes | 33.5 | 28.1 | 21.9 | 16.2 | <.001 |
| Hypertension | 68.8 | 62.8 | 57.2 | 48.4 | <.001 |
| Myocardial infarction | 13.9 | 13.3 | 12.1 | 10.9 | <.001 |
| Stroke | 9.0 | 7.1 | 5.8 | 4.1 | <.001 |
| BMI category, % | <.001 | ||||
| Underweight/normal | 20.6 | 22.1 | 25.3 | 31.1 | |
| Overweight | 33.3 | 35.4 | 38.0 | 40.8 | |
| Obese | 46.2 | 42.5 | 36.7 | 28.1 | |
| Missing, No. (%) | 81 (1.2) | 43 (0.7) | 32 (0.5) | 38 (0.6) | |
| Biomarkers, % | |||||
| eGFR <60 mg/min/1.73 m2 | 12.5 | 11.6 | 10.4 | 9.4 | <.001 |
| hs-CRP >3.0 mg/dL | 45.4 | 41.1 | 36.0 | 29.7 | <.001 |
| Functional status | |||||
| Weakness (SF-12 PCS <75), % | 40.0 | 33.9 | 26.5 | 19.3 | <.001 |
| Missing, No. (%) | 135 (2.0) | 123 (1.9) | 91 (1.4) | 59 (0.9) | |
| Participant-reported exhaustion, % | 18.2 | 16.0 | 12.2 | 9.5 | <.001 |
| Missing, No. (%) | 21 (0.3) | 27 (0.4) | 27 (0.4) | 19 (0.3) | |
| Low physical activity, % | 39.0 | 36.1 | 34.7 | 28.1 | <.001 |
| Missing, No. (%) | 142 (2.1) | 99 (1.5) | 74 (1.1) | 84 (1.3) | |
Total of 26604 Reasons for Geographic and Racial Differences in Stroke (REGARDS) participants. nSES composite score ranges provided for nSES quartiles.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; nSES, neighborhood socioeconomic status; PCS, physical composite score; SD, standard deviation; SF-12, 12-Item Short-Form Health Survey.
a P values from analysis of variance for continuous variables (age) and Pearson χ2 tests of association for categorical variables.
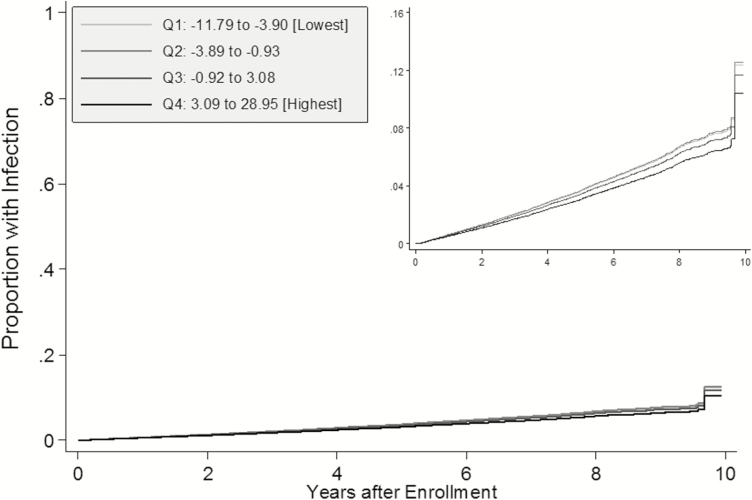
From 5 February 2003 through 31 December 2012, there were 3054 hospitalizations for serious infection among 2325 individuals. Median follow-up time was 3.7 years for participants experiencing a first infection event and 6.6 years for censored participants. Infection incidence was highest for participants in the lowest nSES quartile and lowest for the highest quartile (Table 3). After adjustment for participant characteristics, infection hazards were 0.84-fold lower for the highest vs lowest quartiles. There was a significant linear trend, but differences in infection hazard were limited to the highest and lowest quartiles, as shown in adjusted failure curves (Figure 1). In analyses of sepsis, we observed similar patterns of incidence and adjusted measures of association for SIRS and SOFA criteria as compared with those for infection (Supplementary Table 2). However, after adjustment, the associations for SOFA criteria were not statistically significant at the .05 level and there was no association for qSOFA.
Table 3.
Relative Infection Hazard by Neighborhood Socioeconomic Status Score
| nSES Score Quartile | Infections, No. (%) |
Infection Incidence per 1000 PY (95% CI) |
Crude | Add Race | Add Other Demographicsa |
Add Behaviorsb + Chronic Conditionsc + Biomarkersd |
Add Functional Statuse |
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
|||
| Q1: –11.79 to –3.90 [Lowest] | 605 (9.1) | 15.6 (14.4–16.9) | Ref | Ref | Ref | Ref | Ref |
| Q2: –3.89 to –0.93 | 630 (9.5) | 15.7 (14.5–17.0) | 1.00 (.90–1.12) | 0.90 (.81–1.01) | 0.96 (.86–1.08) | 1.00 (.89–1.12) | 1.01 (.90–1.14) |
| Q3: –0.92 to 3.08 | 585 (8.8) | 14.0 (12.9–15.2) | 0.89 (.80–1.00) | 0.76 (.68–.86) | 0.85 (.75–.96) | 0.93 (.82–1.06) | 0.94 (.83–1.07) |
| Q4: 3.09–28.95 [Highest] | 505 (7.6) | 11.7 (10.7–12.8) | 0.74 (.66–0.84) | 0.59 (.52–.68) | 0.68 (.60–.78) | 0.81 (.70–.93) | 0.84 (.73–.97) |
| Trend P value | <.001 | <.001 | <.001 | .002 | .011 |
Total of 26604 Reasons for Geographic and Racial Differences in Stroke (REGARDS) participants. HRs estimated using Cox proportional hazards regression. P values represent tests for linear trends across nSES score quartiles.
Abbreviations: CI, confidence interval; HR, hazard ratio; nSES, neighborhood socioeconomic status; PY, person-years.
aAge, gender, region, income, and education.
bSmoking status and alcohol use.
cBody mass index category, chronic lung disease, diabetes, hypertension, myocardial infarction, and stroke.
dHigh-sensitivity C-reactive protein and estimated glomerular filtration rate.
eWeakness, exhaustion, and low physical activity.
Figure 1.
Adjusted failure curves for time to infection by neighborhood socioeconomic status (nSES) score. Total of 26604 Reasons for Geographic and Racial Differences in Stroke (REGARDS) participants. Q1–Q4 represent quartiles of nSES score in the REGARDS cohort. All failure functions estimated among white, male, nonsmoking, and non-alcohol-consuming participants residing in the non–Stroke Belt region, with no history of comorbidities, normal biomarker levels, and no functional status impairments (all binary variables set to zero and all categorical variables set to the reference groups).
Of potential mediators, we found that physical weakness, participant income, and comorbid diabetes had modest indirect effects (mediating at least 10% of the association between nSES and infection), suggesting that nSES may impact infection risk through associations with physical health, reported income levels, or risk of diabetes (Table 4).
Table 4.
Mediation of the Association Between Neighborhood Socioeconomic Status Score and Infection
| Characteristic | Natural Indirect Effecta | Natural Direct Effectb | Percentage Mediated (Log Hazard Scale) |
||
|---|---|---|---|---|---|
| HR | (95% CI)c | HR | (95% CI)c | ||
| Adjusted (Q4 [highest] vs Q1 [lowest])d | … | … | 0.76 | (.65–.90) | … |
| Potential mediators | |||||
| Weakness (SF-12 PCS <75) | 0.94 | (.92–.96) | 0.81 | (.69–.95) | 21.5 |
| Individual income | 0.96 | (.92–.99) | 0.80 | (.68–.94) | 16.5 |
| Diabetes | 0.97 | (.96–.98) | 0.78 | (.67–.92) | 10.7 |
| Exhaustion | 0.98 | (.97–.99) | 0.78 | (.67–.92) | 8.5 |
| BMI (obese vs nonobese) | 0.98 | (.97–1.00) | 0.78 | (.66–.91) | 6.1 |
| Low physical activity | 0.99 | (.98–1.00) | 0.77 | (.66–.91) | 4.5 |
Total of 12463 Reasons for Geographic and Racial Differences in Stroke (REGARDS) participants in lowest or highest quartiles of nSES score with complete covariate data. Characteristics added as the only additional covariate relative to the adjusted model.
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; nSES, neighborhood socioeconomic status; PCS, physical composite score; SF-12, 12-Item Short-Form Health Survey.
aNatural indirect effect represents the component of the exposure effect that is mediated, reported on the hazard ratio scale.
bNatural direct effect represents the component of the exposure effect not mediated, reported on the hazard ratio scale.
cConfidence intervals estimated using the delta method.
dInitial hazard ratio comparing lowest to highest quartile of nSES score (lowest as referent) from Cox proportional hazards model adjusted for age, sex, race, education, geographic region, chronic lung disease, hypertension, stroke, myocardial infarction, smoking, alcohol use, C-reactive protein, and estimated glomerular filtration rate (chronic kidney disease).
Among first infections, infection type was similar across nSES quartiles, with respiratory infections being most common (Table 5). In addition, more than half of the participants met ≥2 SIRS criteria, with lower proportions for SOFA and qSOFA criteria. There were no associations between nSES and the likelihood of sepsis at presentation. Median length of stay was longer for participants in the lowest nSES quartile, but measures of event severity did not differ. In a sensitivity analysis using logistic regression to model the odds of sepsis, we did not observe an association between nSES and sepsis (Supplementary Table 3).
Table 5.
First Infection Hospitalization Characteristics by Neighborhood Socioeconomic Status Score
| Participant Characteristic | nSES Quartile | P Valuea | |||
|---|---|---|---|---|---|
| Q1 [Lowest] nSES Score (–11.79 to –3.90) (n = 605) |
Q2 nSES Score (–3.89 to –0.93) (n = 630) |
Q3 nSES Score (–0.92 to 3.08) (n = 585) |
Q4 [Highest] nSES Score (3.09–28.95) (n = 505) |
||
| Primary infection type, % | .65 | ||||
| Respiratory | 41.0 | 40.2 | 39.3 | 40.4 | |
| Urinary/kidney | 18.0 | 19.8 | 19.2 | 16.8 | |
| Abdominal | 16.2 | 17.9 | 20.2 | 20.0 | |
| Skin/soft tissue | 13.7 | 11.9 | 12.8 | 13.5 | |
| Sepsis | 5.3 | 4.9 | 2.7 | 4.8 | |
| Other | 5.8 | 5.2 | 5.8 | 4.6 | |
| ≥2 SIRSb criteria, % | 54.1 | 54.1 | 53.7 | 49.7 | .41 |
| ≥2 SOFAc points, % | 38.7 | 37.8 | 35.6 | 34.1 | .37 |
| ≥2 qSOFAd points, % | 12.6 | 11.4 | 9.9 | 12.3 | .49 |
| SOFA score category, % | .54 | ||||
| 0 | 36.2 | 37.1 | 40.0 | 40.4 | |
| 1 | 25.1 | 25.1 | 24.4 | 25.5 | |
| 2 | 14.6 | 17.5 | 15.2 | 13.3 | |
| 3–4 | 15.4 | 13.8 | 14.2 | 15.1 | |
| 5 | 8.8 | 6.5 | 6.2 | 5.7 | |
| Admitted length of stay e, d, median (25th–75th percentile) | 5 (3–8) | 4 (3–7) | 5 (3–7) | 4 (3–7) | .03 |
| Admitted to ICU vs floore, % | 11.0 | 11.2 | 6.8 | 10.3 | .08 |
| In-hospital death, % | 7.4 | 6.5 | 5.0 | 5.5 | .30 |
| Discharged to nursing home, % | 9.3 | 8.7 | 6.7 | 9.1 | .35 |
Total of 2325 participants hospitalized with a first infection event.
Abbreviations: ICU, intensive care unit; nSES, neighborhood socioeconomic status; qSOFA, “quick” sepsis-related organ failure assessment; SIRS, systemic inflammatory response syndrome; SOFA, sepsis-related organ failure assessment.
a P values from Kruskal-Wallis tests of equal distribution for continuous variables (length of stay) and Pearson χ2 tests of association for categorical variables.
bSepsis-SIRS defined as infection event meeting ≥2 SIRS criteria.
cSepsis-SOFA defined as infection event with ≥2 SOFA points.
dSepsis-qSOFA defined as infection event meeting ≥2 qSOFA criteria.
eIncludes only participants admitted as an inpatient with inpatient records available.
DISCUSSION
We examined the association of nSES with 10-year risk of hospitalization for infection and sepsis in the REGARDS study, one of the largest contemporary cohorts of community-dwelling adults in the United States. Compared to low nSES, participants residing in high-nSES neighborhoods demonstrated lower rates of infection, but no difference in odds of sepsis at presentation. This suggests that the association between nSES and sepsis may be explained by differences in infection risk. Our study also demonstrates that physical function, individual income, and comorbid diabetes mediate the association, highlighting potential pathways through which nSES could impact infection risk.
Prior studies have examined associations between markers of SES and sepsis using administrative databases [4, 27–29]. O’Brien et al studied the association between insurance type and admission for sepsis as well as sepsis outcomes [4]. Among discharges of individuals aged ≥18 years in the 2003 Nationwide Inpatient Sample, those with Medicaid or Medicare experienced higher risk-adjusted odds of sepsis compared to discharged patients with private insurance. Mendu et al examined critical care discharges from 2 hospitals in the northeastern United States, finding that census tract poverty was significantly associated with bloodstream infection [28]. In addition, using data from nonfederal hospitals in South Carolina, Goodwin et al found that residence in a medically underserved area was associated with increased incidence of sepsis admission and higher mortality [29]. However, the authors did not find similar associations with ZIP code–level SES measures.
The current study expands on prior efforts in that we were able to examine outcomes prospectively and isolate the effects of nSES on infection risk and odds of sepsis. In contrast to previous studies, our results indicate that residing in a low nSES neighborhood is associated with an increased risk hospitalization for infection, but not event severity among those hospitalized. Although our prospective design may explain this discordance, several alternative explanations are possible. To define “neighborhood,” we used census block groups, which represent a smaller and more granular geographic unit than previously studied. We also used a comprehensive summary score incorporating multiple domains to define nSES, as opposed to focusing our analyses on a single factor. In addition, we identified hospitalizations for serious infection and sepsis via record review, while many prior studies have employed discharge diagnosis code algorithms. Thus, the observed differences could be explained by the infection population in the current study being lower acuity as compared with sepsis patients identified using discharge diagnoses.
Our findings could help to inform approaches for reducing the national burden of sepsis. Specifically, our results suggest that strategies for sepsis prevention may need to target infection risk prior to a sepsis event, as opposed to focusing exclusively on improvements in the provision of acute care. We have also identified several potential mechanisms that could be incorporated into focused efforts to ameliorate SES disparities in sepsis. The mediating role of individual income suggests that factors associated with living in poverty, such as reduced food availability, lack of transportation, and social isolation, could be targeted for intervention [30]. Mediation of the association by physical weakness suggests that mobility, neighborhood walkability, blight, and physical safety could also be important factors to consider in efforts to reduce sepsis disparities. In addition, reducing diabetes prevalence may also serve as an important component of efforts to prevent infection. Further research must develop and evaluate effective community-based interventions for reducing infection and sepsis risk among populations with limited resources, such as individuals living in low-nSES neighborhoods.
Our study has notable strengths, including substantial heterogeneity and variety in neighborhoods, comprehensive baseline participant data, and nearly 10 years of follow-up. However, our results must be interpreted in light of several important limitations. Although outcomes were defined via a rigorous abstraction and medical record review process, due to the fact that events were participant reported, it is possible that hospitalizations were missed. In addition, for the analysis of qSOFA criteria, we did not identify a large number of events, which could explain the change in magnitude of association after adjustment for participant characteristics. To define nSES, we used a widely published summary score calculated at the block group level. Although census data are available for larger geographic units, we believed that the block group provided the most granular impression of participant nSES. It is possible that participants could have moved or neighborhood characteristics could have changed over follow-up, leading to potential misclassification of nSES. However, we limited our analyses to nSES as defined at baseline to characterize the exposure at the time of enrollment in REGARDS. There is the potential for unmeasured confounding due to factors not included in our models as well as the potential that other important mediators were missed (eg, measures of realized access to care). These factors could attenuate the observed association or play important mediating roles. However, we made use of a wide range of data collected at baseline enrollment among REGARDS participants in all models. Generalizability of our findings could also be limited by specific characteristics of the study population: Participants were enrolled in a long-term cohort study, nearly 70% of the population was >60 years old, and only 6% of the population lacked health insurance at the time of enrollment.
We examined the association of nSES with risk of infection and sepsis over a 10-year period using data from a national cohort of community-dwelling adults. Compared to low nSES, participants residing in high-nSES neighborhoods had an increased risk of hospitalization for infection, even after adjustment for participant characteristics. However, we found no association between nSES and odds of sepsis at presentation. Physical weakness, individual income, and comorbid diabetes mediated the association of nSES and infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author contributions. J. P. D. and H. E. W. conceived of the study. H. E. W. and M. M. S. organized and oversaw data collection. J. P. D. and S. L. conducted the analysis and all authors contributed to review of results. J. P. D. produced an initial draft of the manuscript and all authors contributed to its editorial review and revision. J. P. D. and H. E. W. assume responsibility for the work as a whole.
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: http://www.regardsstudy.org and http://www.regardssepsis.org.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Finanical support. This study was supported by the National Institute for Nursing Research (award number R01-NR012726) and the National Center for Research Resources (award number UL1-RR025777), as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (cooperative agreement U01-NS041588). J. P. D. was also supported by the Agency for Healthcare Research and Quality (grant number T32-HS013852) and the National Institute of General Medical Sciences (grant number F31-GM122180).
Potential conflicts of interest. E. B. L. reports research support from Amgen, consulting from Novartis, and service on advisory boards for Amgen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McDermott KW, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005–2014: statistical brief 225. Healthcare Cost and Utilization Project (HCUP) Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.jsp. Accessed 24 September 2017.
- 2. Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med 2017; 45:1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang HE, Donnelly JP, Griffin R et al. . Derivation of novel risk prediction scores for community-acquired sepsis and severe sepsis. Crit Care Med 2016; 44:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Brien JM Jr, Lu B, Ali NA, Levine DA, Aberegg SK, Lemeshow S. Insurance type and sepsis-associated hospitalizations and sepsis-associated mortality among US adults: a retrospective cohort study. Crit Care 2011; 15:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med 2008; 177:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang HE, Devereaux RS, Yealy DM, Safford MM, Howard G. National variation in United States sepsis mortality: a descriptive study. Int J Health Geogr 2010; 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore JX, Donnelly JP, Griffin R, Howard G, Safford MM, Wang HE. Defining sepsis mortality clusters in the United States. Crit Care Med 2016; 44:1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diez Roux AV, Merkin SS, Arnett D et al. . Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001; 345:99–106. [DOI] [PubMed] [Google Scholar]
- 9. Unger E, Diez-Roux AV, Lloyd-Jones DM et al. . Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014; 7:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray ET, Diez Roux AV, Carnethon M, Lutsey PL, Ni H, O’Meara ES. Trajectories of neighborhood poverty and associations with subclinical atherosclerosis and associated risk factors: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2010; 171:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens 2011; 24:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lisabeth LD, Diez Roux AV, Escobar JD, Smith MA, Morgenstern LB. Neighborhood environment and risk of ischemic stroke: the brain attack surveillance in Corpus Christi (BASIC) Project. Am J Epidemiol 2007; 165:279–87. [DOI] [PubMed] [Google Scholar]
- 13. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010; 1186:125–45. [DOI] [PubMed] [Google Scholar]
- 14. Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Inflammatory and endothelial activation biomarkers and risk of sepsis: a nested case-control study. J Crit Care 2013; 28:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang HE, Shapiro NI, Safford MM et al. . High-sensitivity C-reactive protein and risk of sepsis. PLoS One 2013; 8:e69232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howard VJ, Cushman M, Pulley L et al. . The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005; 25:135–43. [DOI] [PubMed] [Google Scholar]
- 17. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 18. Singer M, Deutschman CS, Seymour CW et al. . The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seymour CW, Liu VX, Iwashyna TJ et al. . Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician’s guide 2005. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. Accessed 13 February 2012.
- 21. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002; 162:2074–9. [DOI] [PubMed] [Google Scholar]
- 22. James MT, Hemmelgarn BR, Wiebe N et al. ; Alberta Kidney Disease Network Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 2010; 376:2096–103. [DOI] [PubMed] [Google Scholar]
- 23. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007; 18:2960–7. [DOI] [PubMed] [Google Scholar]
- 24. Johansen KL, Dalrymple LS, Delgado C et al. . Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis 2014; 64:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woods NF, LaCroix AZ, Gray SL et al. ; Women’s Health Initiative Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–30. [DOI] [PubMed] [Google Scholar]
- 26. Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology 2015; 26:e23–4. [DOI] [PubMed] [Google Scholar]
- 27. Schnegelsberg A, Mackenhauer J, Nibro HL, Dreyer P, Koch K, Kirkegaard H. Impact of socioeconomic status on mortality and unplanned readmission in septic intensive care unit patients. Acta Anaesthesiol Scand 2016; 60:465–75. [DOI] [PubMed] [Google Scholar]
- 28. Mendu ML, Zager S, Gibbons FK, Christopher KB. Relationship between neighborhood poverty rate and bloodstream infections in the critically ill. Crit Care Med 2012; 40:1427–36. [DOI] [PubMed] [Google Scholar]
- 29. Goodwin AJ, Nadig NR, McElligott JT, Simpson KN, Ford DW. Where you live matters: the impact of place of residence on severe sepsis incidence and mortality. Chest 2016; 150:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Devoe JE, Baez A, Angier H, Krois L, Edlund C, Carney PA. Insurance + access not equal to health care: typology of barriers to health care access for low-income families. Ann Fam Med 2007; 5:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.