Abstract
Premature decidual senescence is a contributing factor to preterm birth. Fatty acid amide hydrolase mutant females (Faah−/−) with higher endocannabinoid levels are also more susceptible to preterm birth upon lipopolysaccharide (LPS) challenge due to enhanced decidual senescence; this is associated with mitogen-activated protein kinase p38 activation. Previous studies have shown that mechanistic target of rapamycin complex 1 (mTORC1) contributes to decidual senescence and promotes the incidence of preterm birth. In this study, we sought to attenuate premature decidual aging in Faah−/− females by targeting mTORC1 and p38 signaling pathways. Because metformin is known to inhibit mTOR and p38 signaling pathways, Faah−/− females were treated with metformin. These mice had a significantly lower preterm birth incidence with a higher rate of live birth after an LPS challenge on day 16 of pregnancy; metformin treatment did not affect placentation or neonatal birth weight. These results were associated with decreased levels of p38, as well as pS6, a downstream mediator of mTORC1 activity, in day 16 Faah−/–decidual tissues. Since metformin treatment attenuates premature decidual senescence with limited side effects during pregnancy, careful use of this drug may be effective in ameliorating specific adverse pregnancy events.
Keywords: metformin, FAAH, endocannabinoids, preterm birth, decidual aging
Summary Sentence
Metformin treatment attenuates premature decidual senescence caused by higher endocannabinoid levels and susceptibility to inflammation-induced preterm birth in mice.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
N-arachidonoyl ethanolamine, also known as anandamide
- CB1
cannabinoid receptor 1, coded by Cnr1
- CB2
cannabinoid receptor 2, coded by Cnr2
- DIG
digoxigenin
- E2
estradiol-17β
- FAAH
fatty acid amide hydrolase
- LEA
N-linoleoylethanolamine
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- mTORC1
mechanistic target of rapamycin complex 1
- NAE
N-acylethanolamine
- OEA
N-oleoylethanolamine
- P4
progesterone
- PEA
N-palmitoylethanolamine
- PECAM
platelet/endothelial cell adhesion molecule 1
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- Prlr
prolactin receptor
- Ptgs2
prostaglandin-endoperoxide synthase 2
- S6
ribosomal protein S6
- SA-β-gal
senescence-associated-β-galactosidase
- SEA
N-stearoylethanolamine
- Tpbpa
trophoblast specific protein alpha
- WT
wild type
- γH2AX
phosphorylated histone-2AX
Introduction
Preterm birth is associated with low birth weight and immaturity of multiple organ systems, particularly the respiratory tree. In humans, preterm birth accounts for 12% of all births and is the leading cause of neonatal death. Preterm birth affects around 13 million babies worldwide, resulting in 1 million neonatal deaths per year [1]. Limited prevention and treatment of preterm birth is attributed to the varied etiology of this pregnancy disorder, including genetic factors, infection/inflammation, stress, decidual senescence, and environmental insults [2, 3].
Proliferation and differentiation of uterine stromal cells give rise to decidual cells; the process is termed decidualization. In mice, this process begins with the initiation of embryo implantation and peaks on day 8 of pregnancy followed by allantois-chorion fusion to initiate the process of placentation. With the progress of placentation, the maternal decidua in the mesometrial domain functions as an anchoring dock for the placenta and fetus, and as a buffer zone between the maternal myometrium and placental trophoblasts. The decidua regulates orderly invasion of trophoblasts, which remodel maternal blood vessels for the delivery of nutrition and oxygen from the mother to the fetus. While approaching parturition, maternal decidual cells undergo senescence and thinning of the decidual layer occurs. Our recent study showed that excessive exposure to elevated endocannabinoid signaling during the midgestational stage causes premature decidual senescence [4].
Research on cannabinoid signaling was greatly advanced by the discovery of several endogenous cannabis-like compounds and their target receptors in the early 1990s. N-arachidonoyl ethanolamine (known as anandamide, AEA) and 2-arachidonoyl glycerol (2-AG) are two most studied endocannabinoids [5–7]. Both compounds primarily target two G-protein coupled cannabinoid receptors: cannabinoid receptor 1 (CB1) encoded by Cnr1 [8, 9] and cannabinoid receptor 2 (CB2) encoded by Cnr2 [10]. Although AEA (anandamide) is known to be produced primarily from N-arachidonoyl phosphatidylethanolamine [11], complete suppression of AEA synthesis in vivo has not been successful due to the presence of multiple pathways for AEA synthesis [11–14]. AEA is degraded to ethanolamine and arachidonic acid by a membrane-bound fatty acid amide hydrolase (FAAH) [15, 16]. Although FAAH can hydrolyze a panel of endocannabinoids including 2-AG [17] and other bioactive lipids [18], studies in Faah−/− mice suggest that the magnitude and duration of AEA signaling is mainly regulated by FAAH [13, 19]. Activation of CB1 and CB2 exerts different biological effects in a cell-type specific manner. Coupling with Gi/o or Gq proteins, CB1 and CB2 can regulate Ca2+ channels [20, 21], inhibit adenylyl cyclase activity [9, 22], and stimulate mitogen-activated protein kinases (MAPKs) including ERK, JNK, and p38 [21, 23–25].
Several studies demonstrate that metformin inhibits both mechanistic target of rapamycin (mTOR) and p38 signaling pathways [26–28]. Metformin is the first line of medication for the treatment of type 2 diabetes and is also used to treat polycystic ovary syndrome (PCOS), and it has also been used during pregnancy to treat gestational diabetes mellitus [29]. In a double-blind, placebo-controlled trial, pregnant women without diabetes who had a body mass index (BMI) greater than 35 experienced reduced maternal weight gain and incidence of pre-eclampsia after Metformin treatment [30]. Both studies showed that metformin apparently has no adverse effects on neonatal outcomes.
Previously, we found that Faah−/− decidual cells show increased senescence-associated-β-galactosidase (SA-β-gal) staining on days 12 and 16 of pregnancy, suggesting that decidual cells undergo some premature senescence under elevated endocannabinoid signaling. In addition, Faah−/− mice are more prone to preterm birth in response to lipopolysaccharide (LPS) challenge [4]. Since metformin can inhibit p38 activation and mTOR signaling which influence decidual senescence, we speculated that metformin treatment would reduce the incidence of inflammation-induced preterm birth in Faah−/− mice. We administered metformin by oral gavage (1 mg kg−1 body weight) on days 8, 10, and 12 followed by an LPS injection (10 μg) on day 16 of pregnancy (Figure 1A). We found that Faah−/− females treated with metformin had a significantly lower preterm birth rate and a higher rate of live birth as compared to vehicle-treated Faah−/− after LPS challenge. Indeed, Metformin administration decelerated the decidual ageing process in Faah−/− females which was accompanied by reduced p38 and mTOR signaling.
Figure 1.
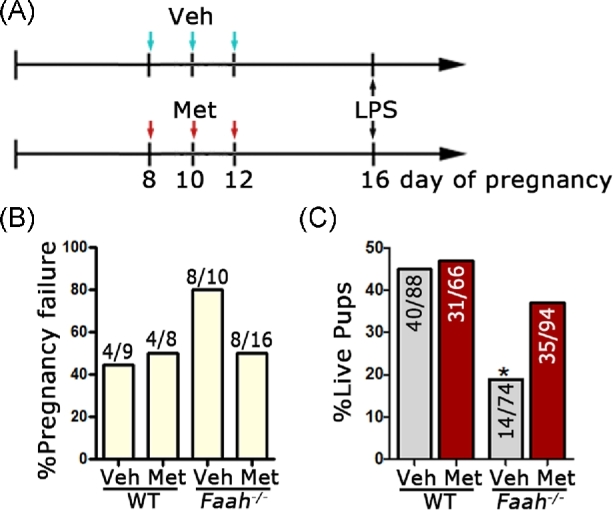
Metformin attenuates inflammation-induced preterm birth in Faah−/− mice. (A) Scheme of metformin and LPS administration. (B) Rates of pregnancy failure in WT and Faah−/− mice after LPS injection. (C) Rate of live pups over all pups generated by LPS-challenged WT and Faah−/− mice at full term. Numbers on bars are no. of live pups/no. of total pups. (*P < 0.05, Chi-square tests). Veh, vehicle; Met, metformin.
Materials and methods
Animals and treatments
Faah−/− mice were generated as described [19] and maintained on a C57BL6/CD-1 mixed background. Faah−/− mice and their wild type (WT) littermates were housed in the animal care facility at the Cincinnati Children's Hospital Medical Center according to National Institutes of Health and institutional guidelines for laboratory animals. All protocols of this study were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee. All mice were housed in wall-mount negative airflow polycarbonate cages with corn cob bedding. They were provided ad libitum with double distilled autoclaved water and rodent diet (LabDiet 5010).
Female mice were mated with WT fertile males to induce pregnancy (vaginal plug = day 1 of pregnancy). Mice were euthanized by cervical dislocation right before tissue collection under deep anesthesia. Pregnant WT and Faah−/–females were treated with metformin by oral gavages (1 mg kg−1 body weight) on days 8, 10, and 12 of pregnancy, and tissue was collected on day 16. Highly purified LPS (Escherichia coli 0111:B4, tlrl-3pelps, Invivogen) was dissolved in saline and given intraperitoneally (10 μg) in the morning of day 16. The dose and treatment regimen of metformin are based on our previous work [26]. We lowered the dose of LPS compared to our previous study [4], since the potency of LPS acquired from the same vendor varies from batch to batch. We tested different doses of LPS in WT and Faah−/− pregnant mice. We found that about 80% of Faah−/− pregnant females gave preterm birth at 10 μg of LPS, which is comparable to our previous study [4]. Parturition events were monitored from days 17 through 21 by observing mice daily in the morning and evening. Preterm birth was defined as birth occurring earlier than day 19.
In situ hybridization
In situ hybridization was performed as previously described [31]. Both radioactive (35S GTP) and Digoxigenin (DIG) labeling methods were used. In brief, frozen sections (12 μm) were mounted onto poly-L-lysine-coated slides and fixed in cold 4% paraformaldehyde in phosphate-buffered saline (PBS). The sections were prehybridized and hybridized at 45°C for 4 h in 50% (vol/vol) formamide hybridization buffer containing 35S-labeled antisense RNA probes (Perkin Elmer). RNase A-resistant hybrids were detected by autoradiography. For DIG labeling, sections were hybridized at 65°C overnight in 50% (vol/vol) formamide hybridization buffer containing DIG-labeled probes (Roche). All sections were poststained with hematoxylin and eosin. prostaglandin-endoperoxide synthase 2 (Ptgs2), prolactin receptor (Prlr), and trophoblast specific protein alpha (Tpbpa) probes were synthesized as previously described [32].
Measurement of estradiol-17β and progesterone levels
Serum levels of estradiol-17β (E2) and progesterone (P4) were measured by enzyme immunoassay kits (Cayman).
Senescence-associated-β-galactosidase staining
Staining of SA-β-gal activity was performed as described previously [33]. The staining was performed at pH 5.5. To compare the intensity of SA-β-gal staining, sections from different genotypes and on different days of pregnancy were processed on the same slide.
Measurement of N-acylethanolamines, 2-acyl-glycerols, and prostaglandins profiles
Maternal decidual tissues of implantation sites were collected on day 16 of pregnancy. These tissues were flash frozen and stored at –80°C until used for extractions. Methanolic extracts of tissues were partially purified using C18 solid-phase extraction columns (Agilent), and lipids including AEA, 2-AG, and prostaglandins (PGs) were quantified by high-performance liquid chromatography-tandem mass spectrometry as previously described [33].
Immunofluorescence
Immunofluorescence for platelet/endothelial cell adhesion molecule 1 (PECAM; BD Pharmingen) and phosphorylated histone-2AX (γH2AX; Millipore) was performed using secondary antibodies Cy3-conjugated donkey anti-Rat and Cy3-conjugated donkey anti-mouse (Jackson Immunoresearch), respectively. Nuclear staining was performed using Hoechst 33342 (H1399, Molecular Probes, 2 μg/ml). Immunofluorescence was performed on fresh-frozen sections. Sections were fixed in 10% neutral buffered formalin and incubated with primary antibody at 4°C overnight, followed by incubation in secondary antibody for 1 h in PBS. Immunofluorescence was visualized under a confocal microscope (Nikon Eclipse TE2000).
Western blotting
Protein extraction and western blotting were performed as previously described [33]. Antibodies to p38, phospho-p38 (p-p38), S6, p-S6, and Tubulin were obtained from cell signaling. Bands were visualized by using an ECL Prime Western blotting detection system (GE Healthcare), and band intensities were quantified by ImageJ. Experiments were repeated three times, and a set of representative gel images is presented. Band intensities of p-p38 and p-S6 were normalized against p38 and S6, respectively. Antibodies to Tubulin were also used as a loading control. Values are mean ± SEM.
Statistical analysis
Data were analyzed by the Student t-tests, Mann–Whitney tests, or Chi-square tests as indicated in figure legends. Data of N-acylethanolamines (NAEs), 2-acyl-glycerols, and PGs levels were analyzed by analysis of variance including three-way interaction to test the effect of genotype (WT VS Faah−/−), LPS treatment, and metformin treatment, and their interaction on the outcomes. Posthoc analysis was conducted to compare the pairwise difference in the eight group combinations. Tukey method was used to control for multiple comparisons. P < 0.05 was considered significant. Values are mean ± SEM.
Results
Metformin attenuates inflammation-induced preterm birth in Faah−/− mice
After LPS injection on day 16 of pregnancy, 44% of WT and 80% of Faah−/− pregnant females had pregnancy failure (Figure 1B), confirming our previous observation that Faah−/− mothers are more vulnerable to preterm birth and/or resorptions after an LPS challenge [4]. Metformin treatment reduced the rate of pregnancy failure from 80% to 50% in Faah−/− females. Among all pregnancy failures, preterm birth rates decreased from 40% (4/10) to 19% (3/16) in Faah−/− females and resorption rates decreased from 40% (4/10) to 31% (5/16) (Supplemental Table S1). The percentage of metformin-treated Faah−/− females that had term births (50%) is higher than that of vehicle-treated Faah−/− females (20%), whereas the percentage of WT females with term birth did not change significantly after metformin or vehicle treatment (Supplemental Table S1). The rate of live pups delivered from metformin-treated Faah−/− females (37%) significantly increased compared to those vehicle-treated Faah−/− females (19%) (Figure 1C); metformin at the same dose had limited effects on WT animals. These results suggest that metformin reduces inflammation-induced preterm birth in mice with higher endocannabinoid levels.
Metformin treatment does not compromise embryonic growth
Metformin has been used in treating gestational diabetes mellitus and PCOS during pregnancy [30, 34, 35]. In the current study, we tested the effects of metformin exposure during the midgestational stage on placentation and embryo development in mice. Following the same metformin treatment regime on days 8, 10, and 12 as shown in Figure 1A, we examined the placental development on day 16. Weights of implantation sites in both WT and Faah−/− females are comparable after Metformin treatment (Supplemental Figure S1). Since the placental labyrinth zone consists mainly of blood vessels, we also examined the vascular density and morphology in this zone by immunostaining of PECAM, an endothelial cell marker. The morphology of vasculature in the labyrinth layer and decidua is comparable in metformin- or vehicle-treated WT and Faah−/− females (Figure 2A and B). We also examined the spongy layer of the placenta by its marker Tpbpa expression. The morphology of the spongy layer in metformin-treated animals appeared similar to that of vehicle-treated WT and Faah−/− females (Figure 2A and B). The results suggest that exposure to metformin during midgestation has little or no adverse effects on placentation. The effect of metformin on embryo development was further monitored by examining litter size and neonatal birth weight after metformin treatment. Without LPS and metformin treatment, WT and Faah−/− females produced healthy offspring with normal litter sizes, birth weights, and neonatal survival rates, and metformin treatment apparently has no adverse effects on offspring health (Figure 2C–E). In sum, the results showed that females receiving metformin during pregnancy have normal placentation and embryo development.
Figure 2.
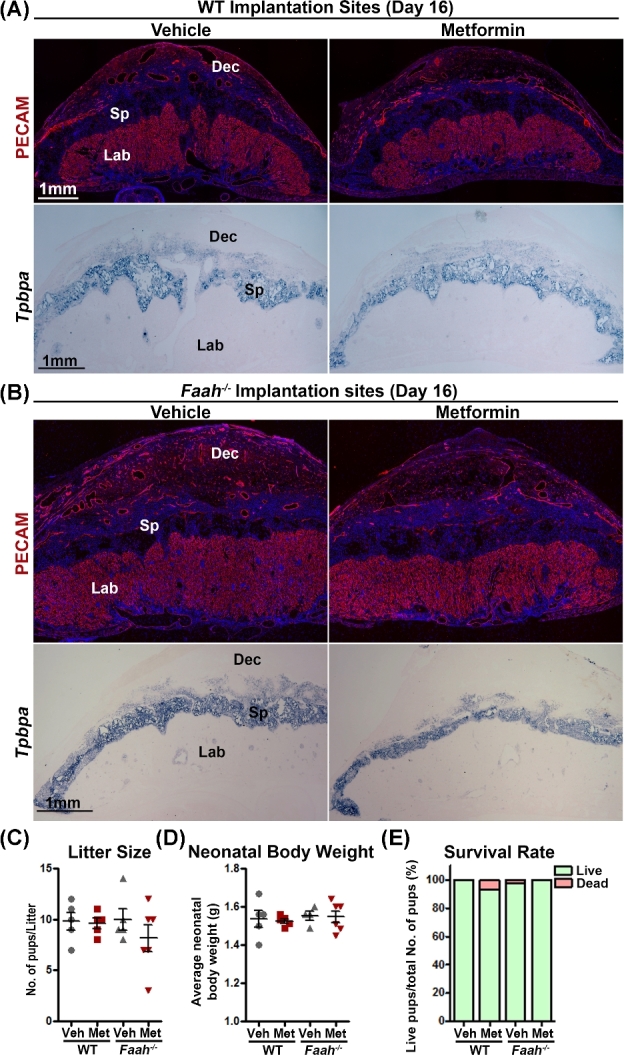
Metformin treatment does not compromise the development of placenta and embryos (A and B) Immunostaining of PECAM and in situ hybridization of Tpbpa on day 16 in WT and Faah−/− implantation sites. Positive PECAM signals highlighted all endothelial cells, whereas Tpbpa signals are localized on spongy trophoblast cells. Three implantation sites from different females were examined, and a representative image for each staining is shown in the figure. (C) Litter size of WT and Faah−/− mice with or without metformin treatment. (D and E) Body weights and survival rates of neonatal pups of WT and Faah−/− mice with or without metformin treatment. All neonatal pups were derived from litters shown in panel C. Dec, decidua; Sp, spongy layer; Lab, Labyrinth zone; Veh, vehicle; Met, metformin.
Metformin treatment elevates N-acylethanolamines in pregnant Faah−/–females exposed to lipopolysaccharide
To study the impact of metformin on maternal decidual endocannabinoids and structurally similar bioactive lipids in Faah−/–females, levels of 2-acyl-glycerols and NAEs, including 2-AG and AEA, in decidual tissues were measured 12 h after vehicle or LPS (10 μg) injections. We found that levels of the endocannabinoid 2-AG, as well as other 2-acyl-glycerol lipids, showed no significant changes in Faah−/− decidua as compared to those in WT mice with or without LPS injection, and no significant differences in tissues treated with metformin compared to corresponding vehicle-treated groups were noted in WT or Faah−/− decidua except for 2-palmitoylglycerol levels in LPS-challenged Faah−/− mice (Figure 3). As expected, levels of AEA and other NAEs were significantly elevated in Faah−/− decidua as compared to those in WT mice (Figure 4), and NAE levels were also elevated after LPS stimulation in both WT and Faah−/− deciduae (Figure 4). Interestingly, metformin increased the levels of NAEs only in LPS-challenged Faah−/− decidua, but not in WT decidua (Figure 4). The results suggest that metformin augments NAEs in Faah−/–mice after an injection of LPS. Collectively, the results show that the increased rate of LPS-induced preterm birth occurs in Faah−/–mice with higher levels of NAEs.
Figure 3.
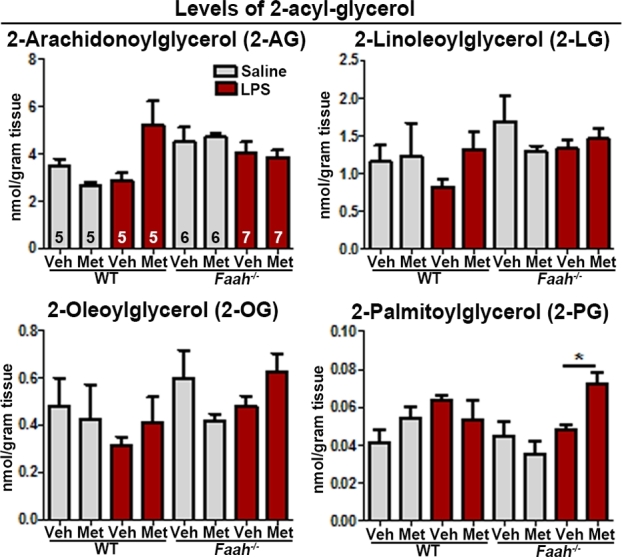
Levels of 2-acyl-glycerols with or without LPS challenge in maternal decidua with or without metformin treatment on day 16 of pregnancy. Levels of 2-acyl-glycerols in maternal decidua on day 16. Sample sizes (n) are indicated in the first bar diagrams, and the sample sizes are the same throughout all graphs. Veh, vehicle; Met, metformin. (*P < 0.05, Mann–Whitney tests).
Figure 4.
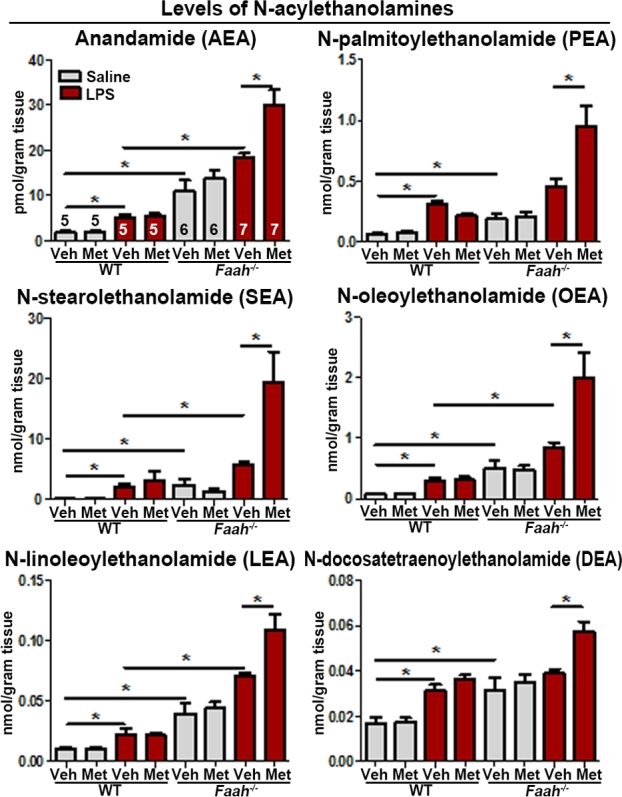
Levels of NAEs with or without LPS challenge in maternal decidua with or without metformin treatment on day 16 of pregnancy. Levels of NAEs in maternal decidua on day 16. Sample sizes (n) are indicated in the first bar diagrams, and the sample sizes are the same throughout all graphs. Veh, vehicle; Met, metformin (*P < 0.05, Mann–Whitney tests).
Metformin treatment tempers premature decidual senescence in Faah−/− females
Our previous studies have shown that increased endocannabinoid signaling causes premature decidual senescence [4]. To investigate the effect of metformin on decidual cells, we examined SA-β-gal staining in vehicle- and metformin-treated WT and Faah−/− females. We found that metformin treatment decreased SA-β-gal staining in both WT and Faah−/–decidual cells on day 16 of pregnancy (Figures 5A and 6A), suggesting that metformin effectively lessened decidual senescence. In further support of this finding, we examined γH2AX expression, another marker of senescence associated with DNA damage response [36]. The number of γH2AX positive decidual cells was substantially reduced in metformin-treated WT or Faah−/− females when compared to vehicle-treated groups (Figure 5A and B; Figure 6A and B). These results were associated with reduced decidual senescence in Faah−/− females after metformin treatment as evident by the increased height of the decidual zone, demarcated by Prlr expression compared with those in vehicle-treated Faah−/− implantation sites (Figure 6). Collectively, these results show that metformin attenuates premature decidual aging in Faah−/− females.
Figure 5.
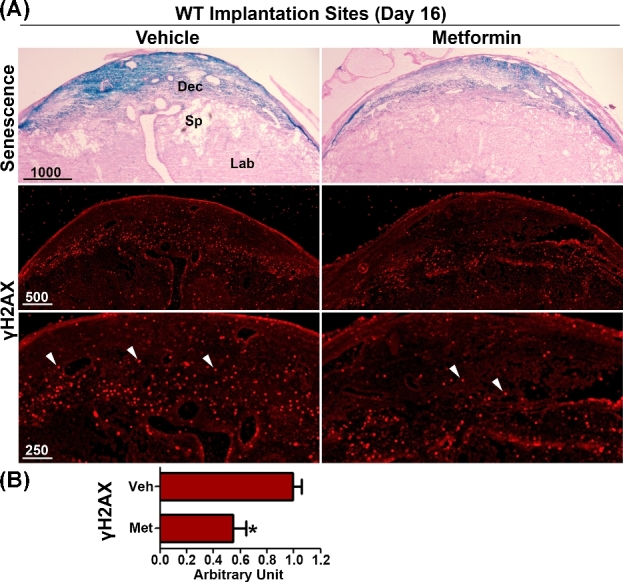
Metformin treatment reduces decidual senescence in WT females. (A) SA-β-gal staining (blue) and immunostaining of γH2AX in sections of WT implantation sites 12 h after LPS injection on day 16 of pregnancy. White arrowheads indicate signals of γH2AX. Three implantation sites from different pregnant females were examined, and a representative image for each staining is shown in the figure. (B) Quantification of γH2AX positive signals in WT implantation sites. γH2AX positive signals were counted in three randomly selected fields in each of three implantation sites examined. Veh, vehicle; Met, metformin. (*P < 0.05, Mann–Whitney tests).
Figure 6.
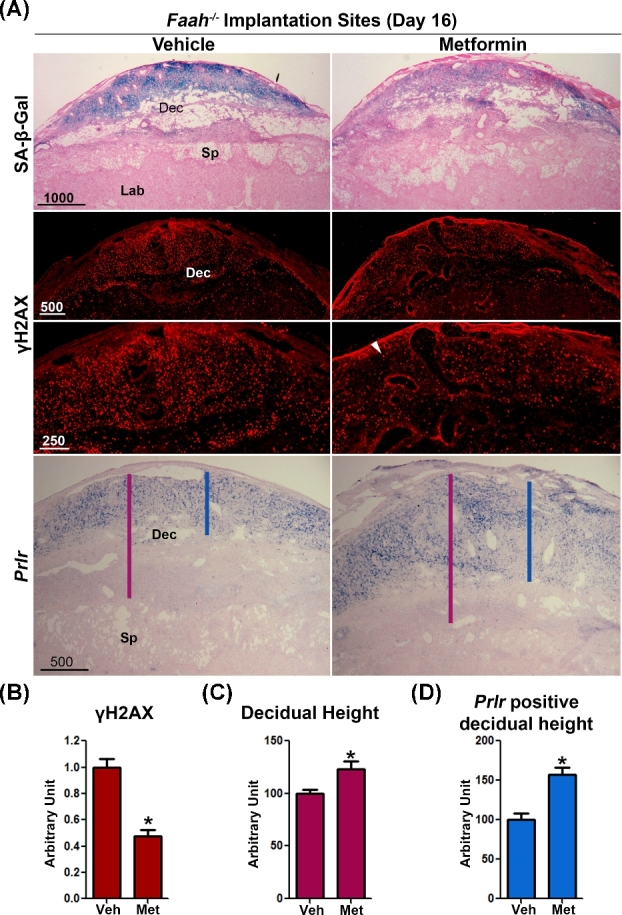
Metformin treatment tempers premature decidual senescence in Faah−/− female. (A) SA-β-gal staining, immunostaining of γH2AX, and in situ hybridization of Prlr in sections of Faah−/− implantation sites 12 h after LPS injection on day 16 of pregnancy. White arrowheads indicate signals of γH2AX. Presented pictures are representative pictures from three different implantation sites. Decreased signals for γH2AX, a senescence, and DNA damage response marker, was observed in metformin-treated samples, which is quantitated in panel (B). (*P < 0.05, Mann–Whitney tests). The height of the whole decidual zone and Prlr positive decidual zone are marked by red and blue lines respectively. (C and D) Quantitation of heights of the whole decidual zone and Prlr positive decidual zone. Decidual heights increased in metformin-treated Faah−/− implantation sites. Dec, decidua; Sp, spongy layer; Lab, Labyrinth zone; Veh, vehicle; Met, metformin. (n = 9; *P < 0.05, Student t tests).
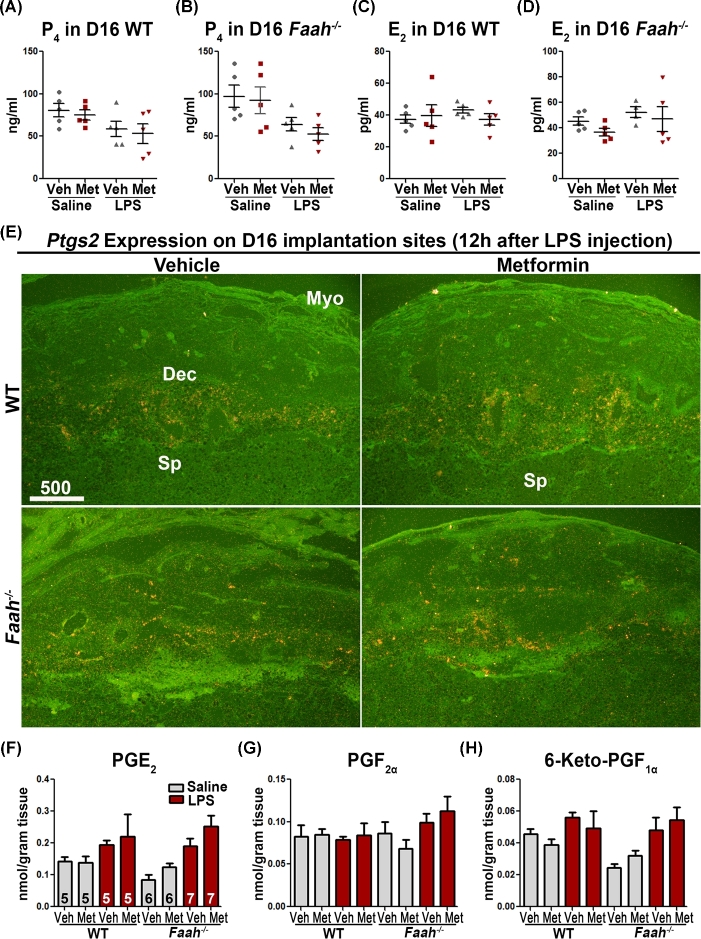
Higher serum P4 levels maintain myometrial quiescence during pregnancy. LPS-induced inflammation promptly diminishes P4 levels [37], predisposing pregnant females to preterm birth [38]. To examine whether the effect of metformin in Faah−/− mice correlated with ovarian hormone levels, we quantified serum levels of estradiol-17β (E2) and P4 12 h after LPS injection on day 16 by enzyme immunoassays. Although an LPS injection decreased P4 levels in both metformin- and vehicle-treated Faah−/− and WT mice, the levels were comparable between the two groups (Figure 7A and B). E2 levels were also comparable in all groups examined in WT and Faah−/− females (Figure 7C and D).
Figure 7.
Levels of ovarian hormones and PGs are not affected by metformin treatment. (A–D) Serum P4 and E2 levels 12 h after LPS or saline injection on day 16 of pregnancy. Faah−/− and WT mice had comparable P4 and E2 levels in metformin or vehicle-treated groups. (n = 5 in each group; mean ± SEM). (E) In situ hybridization of Ptgs2 on day 16 implantation sites 12 h after LPS injection. Metformin-treated WT and Faah−/− mice had comparable Ptgs2 signals as compared to those treated with vehicle. Dec, decidua; Sp, spongy layer; Lab, Labyrinth zone. (F–H) Profiles of PGs in maternal decidua 12 h after LPS injection on day 16. Sample sizes (n) are indicated in bar diagrams. Veh, vehicle; Met, metformin.
Prostaglandins play a major role in parturition. There is evidence that prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), and PGI2 levels are upregulated in the uterus and decidua at the onset of parturition [39], which enhance contractile response/activity in the myometrium [40, 41]. Cyclooxygenases Ptgs1 (Cox1) and Ptgs2 (Cox2) are two major enzymes that catalyze PG biosynthesis. Ptgs1 is constitutively expressed in many tissues, while Ptgs2 is induced by growth factors, cytokines, and various inflammatory stimuli [42]. Therefore, we examined the expression of Ptgs2 on day 16 by in situ hybridization in vehicle- and metformin-treated Faah−/− implantation sites 12 h after LPS challenge. We found that Ptgs2 expression appeared at the maternal–conceptus interface, primarily in the decidual cells, but the expression pattern and signal intensity were comparable in vehicle- or metformin-treated Faah−/− implantation sites (Figure 7E). This observation was reflected in levels of PGE2, PGF2α, and 6-keto prostaglandin F1α (the stable metabolite of PGI2) as measured by High-performance liquid chromatography-tandem mass spectrometry [43] (Figure 7F–H). These results implicate that PG levels were not affected by metformin treatment in Faah−/− mice.
Metformin regulates decidual senescence by targeting mechanistic target of rapamycin and p38 pathway
Our recent studies have shown that elevated endocannabinoid signaling advances decidual senescence by activating p38 MAPK signaling [4], and our present results show that metformin reverses premature decidual aging in Faah−/− females (Figure 4A). Thus, we further investigated the signaling pathways by which metformin regulates decidual senescence in tissues collected from vehicle or metformin-treated WT and Faah−/− females on day 16 of pregnancy. Western blotting results of p38 showed that levels of p-p38 were higher in Faah−/− decidua than those in WT decidua on day 16, and that metformin significantly decreased p38 activation in both WT and Faah−/− decidua (Figure 8). In addition, the levels of pS6, a downstream effector of mechanistic target of rapamycin complex 1 (mTORC1) signaling, were also decreased by metformin treatment in WT and Faah−/–decidua (Figure 8). The results suggest that metformin suppresses both p38 and mTORC1 signaling pathways in decidua.
Figure 8.
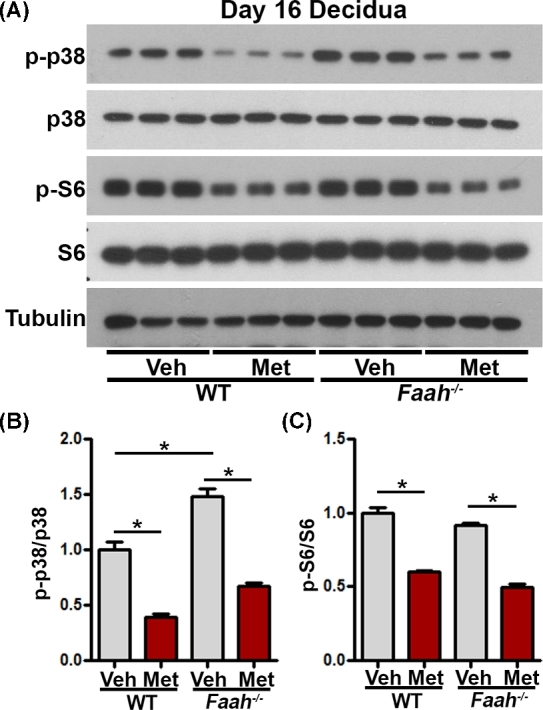
Metformin regulates decidual senescence by targeting mTORC1 and p38 pathways. (A) Western blotting of p-p38 and p-S6 in WT and Faah−/− decidual tissues on day 16. Band intensities quantification were shown in bar diagrams in panel (B). Western blotting results showed that p-p38 and p-S6 levels were decreased in metformin-treated WT and Faah−/− decidual tissues on day 16 of pregnancy. Veh, vehicle; Met, metformin. (*P < 0.05, Student t-tests).
Discussion
In this study, we show that decidua in Faah−/− mice have increased levels of AEA and related NAEs, which are associated with premature decidual senescence and increased susceptibility to LPS-induced preterm birth. Metformin rescues Faah−/− mice from inflammation-induced preterm birth by suppressing p38 and mTORC1 signaling in decidual tissues without altering levels of ovarian hormones and PGs, suggesting that targeting MAPK and mTORC1 signaling is an alternative approach to protect pregnancy under increased endocannabinoid tone and inflammation.
Placentation is initiated after day 8 of pregnancy, and decidual cells in the mesometrial domain connect the placenta with the maternal system throughout pregnancy until parturition occurs. Our previous research showed that a higher amount of senescent cells could be detected in the decidua as early as day 12 of pregnancy in Faah−/− females [4]. Therefore, we speculated that metformin administered during the midgestational stage and LPS given on day 16 of pregnancy would provide meaningful information. Metformin has been used in humans to treat PCOS and gestational diabetes [30, 34, 35]. Our observation of normal litter size and neonatal birth weight following metformin treatment in WT and Faah−/− suggests that metformin has little, if any, adverse side effects on fetal health. However, we cannot exclude the possibility that fetal exposure to metformin may have adverse effects in adulthood. A previous study showed compromised fetal testis development if males are exposed to metformin during pregnancy [44].
Endocannabinoid signaling is generally considered to offer protection against aging (reviewed in [45]). Mice with genomic Cnr1 deletion or conditional deletion in hippocampal GABAergic neurons show accelerated cognitive decline, an indicator of brain aging [46]. Cnr1−/− mice were also shown to have deficits in social memory starting from 3 months of age with early onset of aging-like histological changes in skin, but not in other organs [47]. Cnr2−/− mice show accelerated aging in bone [48]. These results suggest that lack of either CB1 or CB2 induces accelerated aging in specific tissues. Interestingly, studies on Caenorhabditis elegans show that N-eicosapentaenoylethanolamine (EPEA), the most abundant NAE in worms, inhibits lifespan extension induced by dietary restriction [49], implicating NAE signaling in the accelerated aging process in worms. Our current study together with our previous report [4] demonstrates that CB1-mediated cannabinoid signaling advances the decidual aging process, which again reinforces the concept that the role of endocannabinoid in aging is tissue specific.
Decidual tissues produced more NAEs in response to LPS challenge in WT and Faah−/− females, including AEA, N-oleoylethanolamine (OEA), N-palmitoylethanolamine (PEA), N-linoleoylethanolamine (LEA), and N-stearoylethanolamine (SEA). A recent study showed increases in plasma levels of NAEs after LPS injection in pregnant mice [43]. The significance of these increases is not clearly understood at this time. However, endocannabinoids are generally produced in response to injury, aiming to decrease proinflammatory mediators. This role may extend to nonendocannabinoid NAEs. For example, PEA is well known for its anti-inflammatory effects mediated by PPARα [50]. PEA was shown to reduce microglia activation and proinflammatory cytokine production in the spinal cord [51] and suppress rat mast cell activation in vitro [52]. OEA also plays an inflammatory role, though studies mostly focus on its anorexigenic effects [53]. OEA suppresses the expression of interleukin-6, interleukin-8, vascular adhesion molecule-1, and intercellular adhesion molecule-1 in TNFα-treated human umbilical vein endothelial cells [54]. LEA and SEA are another set of NAEs with anti-inflammatory roles. In mouse RAW264.7 macrophages, LEA suppressed LPS-induced expression of proinflammatory cytokines, and in a contact dermatitis animal model, applying LEA to affected ear skin ameliorated dermatitis and proinflammatory cytokine expression at inflamed sites [55]. SEA downregulates allergic inflammation in mouse skin [56]. Our observations together with these reports suggest that increased levels of NAEs in response to LPS challenge may suppress inflammatory decidual responses when exposed to an environmental insult. However, we cannot rule out the possibility that NAEs also have proinflammatory roles. Recent reports showed that plasma AEA levels increase with active labor in humans [57], and endocannabinoids promote production of PGs in gestational tissues in humans and mice [58, 59].
In the absence of LPS, metformin treatment had minimal impact on levels of NAEs and 2-monoacylglycerols. Metformin influenced NAE levels in LPS-injected Faah−/− decidual tissues. Normally, NAE levels are higher due to inefficient degradation in the absence of FAAH [18, 19]. The result that LPS injection further increased the levels of NAEs in Faah−/− tissues suggests that LPS may suppress alternative pathway(s) for NAE degradation or increase NAE synthesis. Under this condition, metformin can further increase NAEs levels, suggesting that metformin augments LPS’s effects on NAE degradation or synthesis. However, metformin has limited impact on NAEs metabolism in the presence of FAAH.
Our results suggest that metformin prevents preterm birth without affecting PGs. There are two critical factors in preterm labor: (1) the strength and frequency of muscle contractions and (2) the strength of the placenta/fetus attachment with the maternal decidua. Prostaglandins are major players in determining the strength and frequency of myometrial contractions [60, 61]. Metformin treatment makes the anchoring of placenta/fetus attachment with maternal decidua much stronger by slowing the premature decidual senescence and thus ameliorates preterm birth in response to LPS.
On day 16 of pregnancy, Prlr is expressed in maternal decidual cells close to the myometrium (Figure 6); decidual cells in the same domain showed the highest intensity of SA-β-Gal staining, suggesting that decidual cells close to the myometrium undergo premature decidual senescence. Interestingly, our previous study also showed that p-P38 positive signals are present in the same domain [4]. All these results suggest that cells in the decidual basalis did not behave uniformly and could be classified into two layers: the decidual zone close to the placenta, which is penetrated by fetal trophoblasts, has little or no Prlr expression and reduced SA-β-Gal staining; the decidual zone close to the myometrium, which expresses Prlr, has a higher likelihood of undergoing cellular senescence. Prlr−/− females are infertile due to compromised ovulation, impaired fertilization, and implantation failure [62]. Since the formation of corpus luteum in Prlr−/− females is defective, we and others previously showed that implantation failure in Prlr−/− females is rescued by P4 supplement, suggesting its critical role for ovarian function [63]. Our current study and our previous work [32] show that Prlr is also expressed in the decidua. Prlr−/–mice with continuous P4 supplement have an increased number of resorption sites, emphasizing the significance of decidual Prlr in pregnancy. Although the function of decidual PRLR is not clear yet, the expression pattern of Prlr is a useful marker in outlining decidual senescent cells.
Decidual health is critical to parturition timing. The current study suggests a possible approach to strengthen pregnant females’ resistance to environmental insults during pregnancy by restraining the decidual aging process. Metformin treatment suppressing both mTORC1 and p38 signaling is an effective way to rescue premature decidual aging in Faah−/− decidua. These findings provide new insights to improve maternal and fetal health during pregnancy and encourage further investigation in the field.
Supplementary Material
Acknowledgments
We thank Katie A. Gerhardt for efficient editing of the manuscript and Nanhua Zhang for statistical analysis.
Notes
Edited by Dr. Peter J. Hansen, PhD, University of Florida
Footnotes
Grant Support: This work was supported in parts by grants from National Institutes of Health (DA006668 and HD068524) and March of Dimes (21-FY12-127 and 22-FY13-543) to SKD.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplemental Figure S1. Weights of implantation sites of WT and Faah–/– mice with or without metformin treatment on day 16 of pregnancy (mean ± SEM). Veh, vehicle; Met, metformin.
Supplemental Table S1. LPS-induced pregnancy failure is alleviated by metformin treatment.
Authors’ contribution
XS, AT, WD, EL, and HBB have contributed to the experimental design and acquisition, and analysis of data. XS, HBB, and SKD have contributed to the interpretation of data and manuscript writing.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1. Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and Prevention. National Academies Press, Washington, DC; 2007: 1–5. [PubMed] [Google Scholar]
- 2. Dey SK, Gupta JS, Deb C. Histochemical studies on the Leydig-cell-leucine aminopeptidase activity in the guinea-pig testis. Reproduction 1973; 34(3):475–479. [DOI] [PubMed] [Google Scholar]
- 3. Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci USA 2011; 108(44):18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun X, Deng W, Li Y, Tang S, Leishman E, Bradshaw HB, Dey SK. Sustained endocannabinoid signaling compromises decidual function and promotes inflammation-induced preterm birth. J Biol Chem 2016; 291(15):8231–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258(5090):1946–1949. [DOI] [PubMed] [Google Scholar]
- 6. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G et al. . Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995; 50(1):83–90. [DOI] [PubMed] [Google Scholar]
- 7. Sugiura T, Kudo N, Ojima T, Mabuchi-Itoh K, Yamashita A, Waku K. Coenzyme A-dependent cleavage of membrane phospholipids in several rat tissues: ATP-independent acyl-CoA synthesis and the generation of lysophospholipids. Biochim Biophys Acta 1995; 1255(2):167–176. [DOI] [PubMed] [Google Scholar]
- 8. Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988; 34:605–613. [PubMed] [Google Scholar]
- 9. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346(6284):561–564. [DOI] [PubMed] [Google Scholar]
- 10. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365(6441):61–65. [DOI] [PubMed] [Google Scholar]
- 11. Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 2004; 279(7):5298–5305. [DOI] [PubMed] [Google Scholar]
- 12. Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N -Acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 2006; 45(15):4720–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA 2006; 103(36):13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho- N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem 2006; 281(36):26465–26472. [DOI] [PubMed] [Google Scholar]
- 15. Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996; 384(6604):83–87. [DOI] [PubMed] [Google Scholar]
- 16. Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci 1997; 94(6):2238–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim Biophys Acta 1998; 1392(2–3):153–175. [DOI] [PubMed] [Google Scholar]
- 18. Leishman E, Cornett B, Spork K, Straiker A, Mackie K, Bradshaw HB. Broad impact of deleting endogenous cannabinoid hydrolyzing enzymes and the CB1 cannabinoid receptor on the endogenous cannabinoid-related lipidome in eight regions of the mouse brain. Pharmacol Res 2016; 110:159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 2001; 98(16):9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol 1992; 106(2):231–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci USA 2003; 100(25):14914–14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci USA 1995; 92(21):9460–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J 1995; 312(2):637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rueda D, Galve-Roperh I, Haro A, Guzman M. The CB(1) cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol Pharmacol 2000; 58:814–820. [DOI] [PubMed] [Google Scholar]
- 25. Pucci M, Pasquariello N, Battista N, Di Tommaso M, Rapino C, Fezza F, Zuccolo M, Jourdain R, Finazzi Agro A, Breton L, Maccarrone M. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J Biol Chem 2012; 287(19):15466–15478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, Viollet B, Bradshaw HB, Hirota Y, Dey SK. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest 2016; 126(8):2941–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, McPherson C, Warnick RE, Kendler A, Giri S et al. . Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci USA 2014; 111(4):E435–E444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdelsaid M, Prakash R, Li W, Coucha M, Hafez S, Johnson MH, Fagan SC, Ergul A. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes 2015; 64(5):1804–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril 2002; 77(3):520–525. [DOI] [PubMed] [Google Scholar]
- 30. Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, Walker BR, Quenby S, Wray S, Weeks A, Lashen H, Rodriguez A, Murray G, Whyte S et al. . Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2015; 3(10):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999; 140(11):5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cha J, Bartos A, Egashira M, Haraguchi H, Saito-Fujita T, Leishman E, Bradshaw H, Dey SK, Hirota Y. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest 2013; 123(9):4063–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 2010; 120(3):803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, Pastides A, Shehata H. Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med 2016; 374(5):434–443. [DOI] [PubMed] [Google Scholar]
- 35. Nawaz FH, Khalid R, Naru T, Rizvi J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res 2008; 34(5):832–837. [DOI] [PubMed] [Google Scholar]
- 36. Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 2004; 6(2):168–170. [DOI] [PubMed] [Google Scholar]
- 37. Fidel PI Jr., Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med 1998; 7:222–226. [DOI] [PubMed] [Google Scholar]
- 38. Casey ML, MacDonald PC. The endocrinology of human parturition. Ann NY Acad Sci 1997; 828:273–284. [DOI] [PubMed] [Google Scholar]
- 39. Winchester SK, Imamura T, Gross GA, Muglia LM, Vogt SK, Wright J, Watanabe K, Tai HH, Muglia LJ. Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology 2002; 143(7):2593–2598. [DOI] [PubMed] [Google Scholar]
- 40. Houlihan DD, Dennedy MC, Morrison JJ. Effects of abnormal cannabidiol on oxytocin-induced myometrial contractility. Reproduction 2010; 139(4):783–788. [DOI] [PubMed] [Google Scholar]
- 41. Dennedy MC, Friel AM, Houlihan DD, Broderick VM, Smith T, Morrison JJ. Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol 2004; 190(1):2–9; discussion 3A. [DOI] [PubMed] [Google Scholar]
- 42. Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 1996; 62:167–215. [DOI] [PubMed] [Google Scholar]
- 43. Wolfson ML, Correa F, Leishman E, Vercelli C, Cymeryng C, Blanco J, Bradshaw HB, Franchi AM. Lipopolysaccharide-induced murine embryonic resorption involves changes in endocannabinoid profiling and alters progesterone secretion and inflammatory response by a CB1-mediated fashion. Mol Cell Endocrinol 2015; 411:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tartarin P, Moison D, Guibert E, Dupont J, Habert R, Rouiller-Fabre V, Frydman N, Pozzi S, Frydman R, Lecureuil C, Froment P. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod 2012; 27(11):3304–3314. [DOI] [PubMed] [Google Scholar]
- 45. Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 2015; 16(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Albayram O, Alferink J, Pitsch J, Piyanova A, Neitzert K, Poppensieker K, Mauer D, Michel K, Legler A, Becker A, Monory K, Lutz B, Zimmer A, Bilkei-Gorzo A.. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc Natl Acad Sci USA 2011; 108(27):11256–11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bilkei-Gorzo A, Drews E, Albayram O, Piyanova A, Gaffal E, Tueting T, Michel K, Mauer D, Maier W, Zimmer A. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1(-)/(-) mice. Neurobiol Aging 2012; 33(1):200.e211–200.e222. [DOI] [PubMed] [Google Scholar]
- 48. Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I.. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 2006; 103(3):696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, Gill MS. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 2011; 473(7346):226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 2005; 67(1):15–19. [DOI] [PubMed] [Google Scholar]
- 51. Loria F, Petrosino S, Mestre L, Spagnolo A, Correa F, Hernangomez M, Guaza C, Di Marzo V, Docagne F. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur J Neurosci 2008; 28(4):633–641. [DOI] [PubMed] [Google Scholar]
- 52. Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA 1995; 92(8):3376–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 2003; 425(6953):90–93. [DOI] [PubMed] [Google Scholar]
- 54. Xu X, Guo H, Jing Z, Yang L, Chen C, Peng L, Wang X, Yan L, Ye R, Jin X, Wang Y. N-oleoylethanolamine reduces inflammatory cytokines and adhesion molecules in TNF-alpha-induced human umbilical vein endothelial cells by activating CB2 and PPAR-alpha. J Cardiovasc Pharmacol 2016; 68(4):280–291. [DOI] [PubMed] [Google Scholar]
- 55. Ishida T, Nishiumi S, Tanahashi T, Yamasaki A, Yamazaki A, Akashi T, Miki I, Kondo Y, Inoue J, Kawauchi S, Azuma T, Yoshida M, Mizuno S.. Linoleoyl ethanolamide reduces lipopolysaccharide-induced inflammation in macrophages and ameliorates 2,4-dinitrofluorobenzene-induced contact dermatitis in mice. Eur J Pharmacol 2013; 699(1–3):6–13. [DOI] [PubMed] [Google Scholar]
- 56. Dalle Carbonare M, Del Giudice E, Stecca A, Colavito D, Fabris M, D’Arrigo A, Bernardini D, Dam M, Leon A. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: a neglected story. J Neuroendocrinol 2008; 20(s1):26–34. [DOI] [PubMed] [Google Scholar]
- 57. Nallendran V, Lam PM, Marczylo TH, Bankart MJ, Taylor AH, Taylor DJ, Konje JC. The plasma levels of the endocannabinoid, anandamide, increase with the induction of labour. BJOG 2010; 117:863–869. [DOI] [PubMed] [Google Scholar]
- 58. Mitchell MD, Sato TA, Wang A, Keelan JA, Ponnampalam AP, Glass M. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab 2008; 294:E352–E356. [DOI] [PubMed] [Google Scholar]
- 59. Bariani MV, Dominguez Rubio AP, Cella M, Burdet J, Franchi AM, Aisemberg J. Role of the endocannabinoid system in the mechanisms involved in the LPS-induced preterm labor. Reproduction 2015; 150:463–472. [DOI] [PubMed] [Google Scholar]
- 60. Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F. Failure of parturition in mice lacking the prostaglandin F receptor. Science 1997; 277:681–683. [DOI] [PubMed] [Google Scholar]
- 61. Riemer RK, Heymann MA. Regulation of uterine smooth muscle function during gestation. Pediatr Res 1998; 44:615–627. [DOI] [PubMed] [Google Scholar]
- 62. Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 1997; 11:167–178. [DOI] [PubMed] [Google Scholar]
- 63. Binart N, Helloco C, Ormandy CJ, Barra J, Clement-Lacroix P, Baran N, Kelly PA. Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinology 2000; 141:2691–2697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.