Abstract
Reward and motivation have powerful effects on cognition and brain activity, yet it remains unclear how they affect sustained cognitive performance. We have recently shown that a variety of motivators improve accuracy and reduce variability during sustained attention. In the current study, we investigate how neural activity in task-positive networks supports these sustained attention improvements. Participants performed the gradual-onset continuous performance task with alternating motivated (rewarded) and unmotivated (unrewarded) blocks. During motivated blocks, we observed increased sustained neural recruitment of task-positive regions, which interacted with fluctuations in task performance. Specifically, during motivated blocks, participants recruited these regions in preparation for upcoming targets, and this activation predicted accuracy. In contrast, during unmotivated blocks, no such advanced preparation was observed. Furthermore, during motivated blocks, participants had similar activation levels during both optimal (in-the-zone) and suboptimal (out-of-the-zone) epochs of performance. In contrast, during unmotivated blocks, task-positive regions were only engaged to a similar degree as motivated blocks during suboptimal (out-of-the-zone) periods. These data support a framework in which motivated individuals act as “cognitive investors,” engaging task-positive resources proactively and consistently during sustaining attention. When unmotivated, however, the same individuals act as “cognitive misers,” engaging maximal task-positive resources only during periods of struggle.
Keywords: reward, motivation, sustained attention, task-positive networks, dorsal attention network
Introduction
The ability to sustain attention over time is critical to most everyday tasks and underlies many other cognitive functions (Barkley 1997; Sarter et al. 2001; Silver and Feldman 2005). Sustaining attention does not represent a unitary, fixed process but rather involves dynamic fluctuations between periods of relative focus and periods of distraction or inattention. Interestingly, though motivation and performance-based rewards have been shown to enhance numerous transient aspects of attention (for reviews, see Botvinick and Braver 2015; Pessoa 2015), less work has examined the effects of reward on sustained attention (Sipowicz et al. 1962; Esterman et al. 2014a). This leaves unanswered how motivation/reward interacts with these natural fluctuations in sustained attention and how behavioral enhancements may be accomplished via differential recruitment of task-positive, attentional control regions of the brain.
Numerous studies have demonstrated the effects of performance-based reward on goal-directed attention, both in terms of behavioral enhancements and co-occurring neural recruitment (e.g., Engelmann et al. 2009; Etzel et al. 2015). These tasks typically have discrete trial-based designs, sometimes involving both cues indicating upcoming payoffs, as well as targets for which performance-based rewards are accrued. In these tasks, reward has been shown to facilitate transient acts of attention, such as improving orienting and reorienting attention to features, objects, and spatial locations (e.g., Buschschulte et al. 2014; Hopf et al. 2015). Performance-based motivation has also been associated with increased recruitment of brain regions known to be involved in the engagement of attentional resources (e.g., visual/stimulus specific areas, frontal–parietal control regions, and subcortical structures in the basal ganglia; for review, see Pessoa 2015). These quantitative differences in activation have been associated with two strategies aimed to maximize reward: (1) cue-related processing, thought to represent proactive and preparatory engagement of attentional resources, and (2) target-related processing, thought to represent greater attentional selection and visual processing.
While most of the quantitative effects of reward on brain activity are transient or involve relatively short trials on the order of seconds, some sustained, proactive effects have been observed, which help inform predictions about reward's effects on sustained attention. For example, neural recruitment has been shown to persist at higher levels across blocks of rewarded trials within a Posner-type spatial task (Engelmann et al. 2009). Within the domain of working memory, there is also evidence that motivation induces qualitative differences in cue-related neural activation, such that reward encourages a more sustained mode of activation thought to reflect greater preparation (Jimura et al. 2010; Etzel et al. 2015). Moreover, Taylor and colleagues (2004) found that reward induced greater activity in specific regions of the dorsal attention network, which were also generally engaged in working memory maintenance. Boksem and colleagues (2006), however, demonstrated more transient target-related, reactive brain activation effects of reward. Reward-based motivation was found to enhance accuracy on a response-cueing paradigm, particularly after the effects of fatigue had set in. These effects were seen in error-related event-related potentials and post-error adjustments, suggesting increased error monitoring due to motivation. Finally, some studies have demonstrated both sustained and transient incentive-related activation in the distinct regions of the parietal and prefrontal cortex, reflecting both the processing of immediate rewards and sustained reward contexts (Engelmann et al. 2009; Kouneiher et al. 2009). Together, the literature points to both transient and sustained motivation-based modulations of brain activity that may differ across task-positive networks as attentional demands change.
Several important studies have attempted to theoretically integrate these proactive and reactive reward-based modulations. Specifically, Braver and colleagues (e.g., Locke and Braver 2008; Braver et al. 2009) have proposed the dual mechanisms of control (DMC) theory, in which they postulate that reward induces a proactive mode of processing that both increases sustained and anticipatory activation in task-related regions (particularly the prefrontal cortex), and in turn decreases reactive, probe-related processing. In contrast to this proactive mode, un-incentivized performance invokes a reactive mode, reflecting decreased cue-related, anticipatory activation, and increased probe-related, reactive activation. While these studies use a working memory paradigm, with discrete trials and cues separated by a number of seconds, they help make predictions regarding the potential effects of motivation on prolonged sustained attention tasks.
With regard to more continuous and less trial-based measures of sustained attention, we have recently demonstrated that performance-based motivators (e.g., money, completing the experiment sooner) enhance sustained attention ability, as measured by increased accuracy and reduced response variability (Esterman et al. 2014a). Still, it remains unclear how these behavioral effects of motivation/reward on sustained attention are supported by differential neural recruitment. Proactive/sustained and quantitative increases in neural recruitment are likely to be associated with motivation/reward (e.g., Locke and Braver 2008; Engelmann et al. 2009). Furthermore, it is also possible that target-evoked, reactive activation is greater in unrewarded epochs, consistent with DMC. However, one important unknown, both with regard to sustained attention and motivated cognition in general, is how these changes in neural recruitment interact with performance fluctuations on a moment-to-moment basis. For example, because of the resource-demanding nature of sustaining attention (Warm et al. 2008), unmotivated individuals may take an approach where they only selectively engage attentional resources and maximal effort when performance falls off or in response to errors (Esterman et al. 2013). Conversely, when motivated, participants may be more willing to continuously exert attentional resources regardless of fluctuations in performance.
In the current study, we address these questions by investigating how reward affects engagement of task-positive brain areas (i.e., visual/stimulus specific areas, and frontal–parietal control regions) with respect to fluctuations in accuracy and response time variability on a well-validated sustained attention task (Fortenbaugh et al. 2015). We demonstrate both quantitative and qualitative changes in neural recruitment induced by motivation and provide a new framework for understanding the relationship between motivation, resource recruitment, and sustained performance.
Materials and Methods
Participants
Sixteen participants (10 males; mean age = 22 years, range: 19–29) performed the gradual-onset continuous performance task (gradCPT) during fMRI. Fourteen participants were right handed and all were considered healthy, had normal or corrected-to-normal vision, and no reported history of major illness, head trauma, or neurological/psychiatric disorders. All were screened to confirm no metallic implants or history of claustrophobia. Drug/medication use was not explicitly assessed. The study protocol was approved by the VA Boston Healthcare System Institutional Review Board, and all participants gave written informed consent.
Paradigm and Stimuli
The gradCPT uses 20 gray-scale photographs of cities and mountain scenes that are presented randomly and gradually transition every ~800 ms. Participants are instructed to respond via button press to frequently occurring city scenes (90% of stimuli) and withhold response to rare mountain scenes (10%). More detailed information on this task may be found elsewhere (Esterman et al. 2013; 2014a; Fortenbaugh et al. 2015). Note that each participant received a random sequence of cities and mountains, such that on each trial, there was a 10% chance of a mountain (no-go trials).
In the current study, each 8-min task run was divided into alternating 1-min rewarded and unrewarded blocks, which were differentiated by a continuous color border (green for rewarded; blue for unrewarded). To have the background colors be more intuitive and avoid confusion, we chose “green” for money-rewarded blocks in all participants rather than counterbalancing green and blue colors. This yielded 4 min of each block-type per run. Similar to our previous study (Esterman et al. 2014a), participants earned $0.01 for correctly pressing to city scenes and $0.10 for correctly withholding a response to mountain scenes during rewarded blocks. However, if a participant failed to press to a city scene, they would lose $0.01, and if a participant incorrectly pressed to a mountain scene they would lose $0.10. During the unrewarded blocks, no money could be gained or lost. These identical reward contingencies were shown to produce reliable improvements in accuracy and reaction time (RT) variability in our recent study with 54 participants (Esterman et al. 2014a), thus a priori, we expected the payoff matrix to successfully modulate sustained attention performance.
Procedure
A MacBook Pro with MATLAB (Mathworks, Natick, MA) delivered stimuli to a rear-facing projector. Subjects viewed the stimuli on a rear-projector screen via a mirror inside of the MRI bore. Responses were collected using a fiber optic button box. Before scanning, participants were given a 1-min practice of the task. Inside of the scanner, participants completed 3–5 8-min runs of the task (13 participants completed five runs, 2 completed four runs, and 1 completed three runs). Participants were informed of their accrued reward after each run (mean = $4.84, range: $2.93–6.63) and were told in advance that two would randomly be selected for bonus payment at the end of the experiment; however, the two highest runs were actually selected as the additional bonus payment. An anatomical magnetization prepared rapid gradient echo (MP-RAGE) sequence and a resting-state scan (not used in this study) were also acquired and interspersed to provide breaks between task runs, such that no more than two runs were done consecutively.
Imaging Parameters
Scanning was performed on a 3T Siemens MAGNETOM Trio system equipped with a 32-channel head coil at the VA Boston Neuroimaging Research Center for Veterans (NeRVe). Each gradCPT functional run included 248 whole-brain volumes acquired using an echo-planar imaging sequence with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, acquisition matrix = 64 × 64, in-plane resolution = 3.0 × 3.0 mm2, 33 oblique slices aligned to the anterior and posterior commissures, slice thickness = 3 mm with a 0.75 mm gap. MP-RAGE sequence parameters were as follows: TE = 3.32 ms, TR = 2530 ms, flip angle = 7°, acquisition matrix = 256 × 256, in-plane resolution = 1.0 mm2, 176 sagittal slices, slice thickness = 1.0 mm.
Behavioral Analyses
Reaction Time
RTs were calculated relative to the beginning of each image transition, such that an RT of 800 ms indicated a button press at the moment image n was 100% coherent and not mixed with other images. A shorter RT indicated that the current scene was still in the process of transitioning from the previous, and a longer RT indicated that the current scene was in the process of transitioning to the subsequent scene. For example, an RT of 720 ms would be at a time point of 90% image n and 10% image n−1. An iterative algorithm was used to assign ambiguous responses that maximized correct response assignment (e.g., Esterman et al. 2013). Reaction times were used to compute mean RT, RT variability (standard deviation of RT/mean RT), and the variance time course analysis (see below).
Accuracy
Trials in which participants correctly inhibited a button press to mountain scenes were considered correct omissions (COs). Trials in which participants erroneously pressed to mountains were considered “lapses” or commission errors (CEs). Trials in which participants pressed correctly to city scenes were considered correct commissions and constituted the vast majority of trials. Conversely, errors of omission (OEs), or failing to press to city scenes, occurred rarely, 1.0% of the time, and were not present in all runs. Thus, OEs were not considered in fMRI analyses, although we report these behaviorally for completeness.
Variance Time Course Analysis
To assess trial-to-trial changes in RT, we conducted a within-subject analysis called the variance time course analysis (VTC; Esterman et al. 2013; Rosenberg et al. 2013; Esterman et al. 2014a; Kucyi et al. 2016). VTCs were computed from the correct responses in each run (following z-transformation of RTs within-subject to normalize the scale of the VTC), where the value assigned to each trial represented the absolute deviation of the trial RT from the mean RT of the run. Like in previous studies, to define attentional states and reduce high-frequency noise, the VTC was smoothed using a Gaussian kernel of 20 trials (∼16 s) full-width at half-maximum (FWHM), and divided into low- or high-variability periods (in-the-zone and out-of-the-zone epochs) with a median split for each run. This yielded 4 min each (per run) of being in the zone and out of the zone. This initial analysis was agnostic to reward, and thus could be used to evaluate percentage differences in being in versus out of the zone across the reward states (i.e., if participants were more frequently in the zone when rewarded). For region-of-interest (ROI) fMRI analyses examining the effects of reward on RT fluctuations, we performed VTC analyses separately for rewarded and unrewarded blocks. This allowed us to equate relative periods of in versus out of the zone during rewarded versus unrewarded states, preserve an equal number of time points in each (relative) state, and control for the overall effect of variability. In whole-brain fMRI analyses, we likewise used the mean/standard deviation of RT for rewarded and unrewarded blocks separately for VTC normalization, although no such dichotomous states were defined, as VTC is treated continuously (see below). These procedures ensured normalization both between subject and conditions.
fMRI Analyses
General Preprocessing Methods
Preprocessing of fMRI data included slice-timing correction; motion correction; spatial smoothing to an 8-mm FWHM Gaussian kernel; automated co-registration and normalization of anatomical and functional volumes to Talairach space; the scaling of functional data set values to percent signal change; and motion, white matter, and ventricle nuisance regression. These data were analyzed using Analysis of Functional NeuroImages (AFNI; Cox 1996) and custom scripts in MATLAB.
Whole-Brain Analyses
Multiple whole-brain mass-univariate general linear model (GLM) analyses were conducted with several regressors, including two for rewarded and unrewarded blocks, and four for the different target events (CORewarded, COUnrewarded, CERewarded, CEUnrewarded). Additionally, the RT variability analysis was implemented via amplitude modulation regression using the non-smoothed/raw VTC, as convolution with a hemodynamic response/gamma function smoothed the VTC prior to regression (separately computed for both rewarded and unrewarded blocks). In all cases, regressors were computed by convolution with a 1-parameter gamma variate hemodynamic response function. Regression coefficients for effects of interest were compiled across participants and evaluated via voxel-wise group-level t-tests. Whole-brain statistical maps were corrected for multiple comparisons using a new and appreciably more conservative voxel-cluster Monte-Carlo-type α simulation rather than the previous standard Gaussian model in AFNI (Forman et al. 1995). First, the AFNI 3dFWHMx function was run using the spatial autocorrelation function (ACF) option to estimate the smoothness of the data with a mixed Gaussian plus mono-exponential model to generate random noise fields. The estimated parameters were then used with the 3dClustSim function, again using the ACF option, to estimate the minimum cluster sizes needed to reach statistical significance. Based on results from these analyses cluster-corrected thresholds for P < 0.05 were given by nominal P = 0.01 and cluster size ≥79 voxels.
Region-of-Interest Analyses
Event-related averages were extracted from several a priori task-positive networks/regions of interest to examine how fluctuations in accuracy and variability interact with reward condition. Linear time interpolation was conducted to estimate the blood oxygen level-dependent (BOLD) response at each image transition (rate of 0.8 s), assuring that any interpolated response only considered the nearest TRs for estimation. In addition, β values were extracted from whole-brain GLMs within these ROIs for examination of similar interactions.
The dorsal attention (DAN), ventral attention (VAN), and frontoparietal (FPN) networks were examined as the three main task-positive cognitive control networks. These networks were based on previously published cortical 7-network surface parcellations (Yeo et al. 2011) that were converted into volumetric Talairach space. The bilateral parahippocampal place areas (PPAs), task-positive visual regions involved in scene processing, were also examined and were based on a previous data set with the same task and stimuli (Esterman et al. 2013).
fMRI: Event-Related Fluctuations in Accuracy
Target accuracy fluctuated such that participants both correctly withheld responses (CO) and failed to withhold responses (CE) to mountains in both reward conditions. To examine these trial-based events, both rewarded and non-rewarded success and lapse event-related averages were extracted from the networks/regions of interest. To determine the degree to which preparatory activity was modulated by reward and related to subsequent accuracy, we extracted signal from a pre-trial window. BOLD signal values were averaged across the pre-trial window spanning 0.8–3.2 s prior to target appearance, excluding the interpolated TR time-locked to target appearance to avoid any contamination of the BOLD from the event itself (4 trials, ~1 TR).
In addition, to examine the evoked responses to these target events (both COs and CEs), we extracted the average β values in each ROI/network, importantly controlling for the block/sustained effects in the model. Thus, the β values represent the transient responses to COs and CEs for each reward condition.
fMRI: Fluctuations in Reaction Time
Rather than discrete events, the VTC defines attentional states, based on local RT variability, in which participants were in the zone (consistent responses) versus out of the zone (erratic responses). These estimates were made separately for each reward condition. Thus, event-related averages focused on the response 3.2–6.4 s following a city trial that was estimated to be in versus out of the zone based on the VTC, in order to represent the neural response associated with each state of response variability (Esterman et al. 2013; 2014b). In the case of in-the-zone versus out-of-the-zone comparisons, pre-trial analyses were less interpretable, given the variable duration of these states and the fact that transitions from in the zone to out of the zone (and vice versa) are not distinct transitions in time, but rather gradual (e.g., behavioral differences emerge after ~12 trials in each direction, see Rosenberg et al. 2013). Furthermore, the exact transition from in/out-of-zone periods is somewhat arbitrary, given the median split assignment.
Importantly, the VTC itself is a continuous metric, and thus whole-brain regression examines coupling with variability that occurs continuously, from trial to trial, without discrete definition of states (see above; Esterman et al. 2013; 2014b; Kucyi et al. 2016). To corroborate our in/out-of-zone ROI analyses, we also extracted β values from the whole-brain VTC analyses in our networks of interest as a direct comparison (Fig. 3A–D).
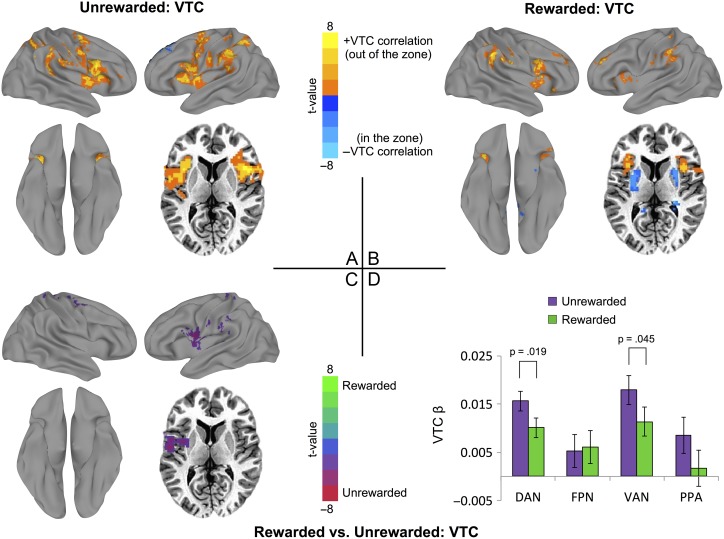
Figure 3.
Whole-brain fMRI analysis of reaction time variability/VTC-BOLD signal correlation for (A) unrewarded blocks; (B) rewarded blocks and (C) the contrast of reward and unrewarded VTC maps. For panels A and B, regions in orange were positively correlated with the VTC and thus were associated with relative instability of RTs (higher variability, out of the zone). Blue regions were associated with a negative VTC correlation and being in the zone. All maps are displayed after correction for multiple comparisons (corrected P < 0.05; nominal P < 0.01, cluster size >78 voxels; see Table 1 for clusters). (D) VTC β-values extracted for each task-positive network/ROI, reflecting coupling with variability with and without reward. Error bars represent standard error of the mean.
It is important to note that in order to provide direct comparisons between mountain and city events using the same time series (Fig. 2A,B), we used the residuals based on a GLM without mountain trial regressors. Results and statistics of these analyses, however, were identical when using the residual time series of a first-stage analysis that regressed the mountain trials (also see Esterman et al. 2013; 2014b). Note that the whole-brain analyses do separately model the mountain trials and VTC (Fig. 3) and corroborate the results of these event-related average analyses.
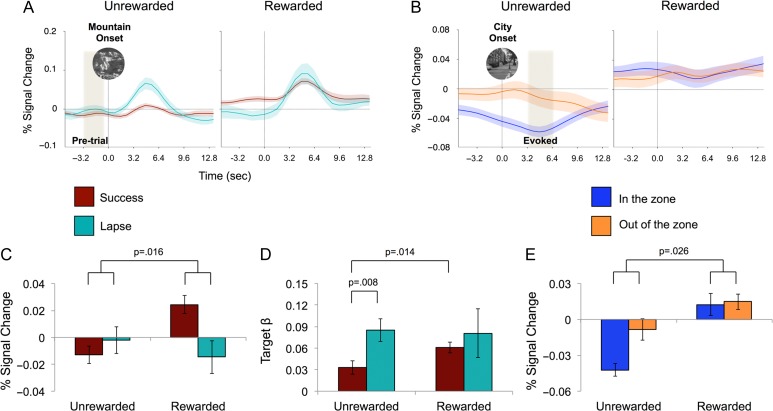
Figure 2.
Interactions between reward and behavioral performance. (A) Event-related averages associated with successes (maroon) and lapses (blue) for target mountain trials during unrewarded and rewarded blocks. Time courses are expressed as percent signal change from the mean and were averaged across individuals and task-positive ROIs (see Supplementary Figure 1 for individual ROIs). Time points across each of the pre-trial windows (shaded) were averaged and extracted in panel “C”. (B) Event-related time courses associated with high (orange; out of the zone) and low (blue; in the zone) RT variability for non-target city trials during unrewarded and rewarded blocks (see Supplementary Figure 2 for individual ROIs). Time points across the city trial-evoked window (shaded) were averaged and extracted in panel “E”. (C) Pre-trial activation following success and lapse trials during rewarded and unrewarded blocks exhibiting a significant interaction between reward and accuracy on target mountain trials. (D) Mountain-evoked activations (β values), when controlling for sustained reward effects. (E) City trial-evoked activation when in and out of the zone during the rewarded and unrewarded blocks exhibiting a significant interaction between reward and state of variability (in vs. out of zone). Error bars represent standard error of the mean.
Network/ROI Analysis Strategy
To look for general patterns across our a priori task-positive networks/ROIs and to protect against multiple comparisons, we conducted ANOVAs for our dependent measures of interest (see above), in which we have network/ROI, rewarded/unrewarded, and performance (e.g., in/out of zone, or correct/incorrect) included in a 3-factor model. Hypothesized interactions between reward and performance were tested at α = 0.05. While no interactions with network were significant, we performed planned analyses of the (two-way) interactions in each network, as one of our goals was to determine the differential contributions of task-positive regions in reward-based modulations of sustained attention. For these analyses, we report both the nominal P-value and false discovery rate (FDR) corrected P-value.
Results
Behavioral Performance: Effects of Reward
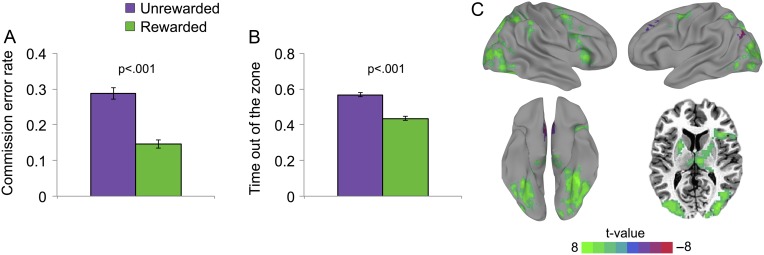
As can be seen in Fig. 1A,B, when comparing rewarded to unrewarded blocks, participants made fewer lapses/errors to mountain scenes [CEs: t(15) = 6.35, P = 0.000013], spent less time out of the zone/more time in the zone [t(15) = 5.53, P = 0.000058], and exhibited lower RT variability in responses to city scenes [t(15) = 6.28, P = 0.000015]. Specifically, participants, on average, made 14.7% CEs (range: 5.3–26.4%), as compared with 28.5% during unrewarded blocks (range: 11.3–52.8%). They were also out of the zone 43% of the time when rewarded (range: 34–51%), and 57% of the time when not rewarded (range: 49–66%). No significant differences were observed in mean RT or OE rate between reward and unrewarded conditions (678 vs. 686 ms, respectively, P = 0.06; 0.7% vs. 1.4%, respectively, P = 0.22). Note that although there was a trend for differences in mean RT, it is in the opposite direction of a possible speed/accuracy trade-off, as rewarded epochs have marginally faster RTs and significantly fewer CEs. No systematic differences in performance were observed between runs.
Figure 1.
Overall effects of reward. (A) Commission error rate during rewarded versus unrewarded blocks. (B) Time spent out of the zone during rewarded versus unrewarded blocks. When rewarded, participants had a significantly lower lapse/CE rate and spent less time out of the zone (P < 0.001). Error bars represent standard error of the mean. (C) Overall sustained activation differences between rewarded and unrewarded bocks, when controlling for transient target-evoked activations (map displayed after correction for multiple comparisons: corrected P < 0.05; nominal P < 0.01, cluster size >78 voxels; see Table 1 for clusters).
fMRI: Sustained Effects of Reward
We first sought to examine the whole-brain effects of reward and confirm that rewarded blocks were associated with greater engagement of task-positive regions. To determine whether this increased engagement of task-positive regions was independent of transient, target event-related differences in activation between rewarded and unrewarded trials, we performed a GLM with regressors for each target event-type (CORewarded, COUnrewarded, CERewarded, and CEUnrewarded) included in the model. Similar to previous studies of cognitive control (Engelmann et al. 2009), this revealed substantially greater sustained widespread activation in task-positive regions during reward versus without reward (Fig. 1C; Table 1). This demonstrates that even when accounting for transient responses, reward during sustained attention was associated with increased sustained recruitment of regions primarily overlapping with our a priori networks/regions of interest: DAN, VAN, FPN, and PPA (networks/ROIs shown in Supplementary Figure 1). Subcortically, bilateral putamen and thalamus also showed significantly greater recruitment during rewarded blocks (Fig. 1C; Table 1). Because our hypotheses were directed toward task-positive regions, our subsequent analyses primarily focused on our a priori regions (DAN, VAN, FPN, and PPA).
Table 1.
Significant clusters for contrast of rewarded – unrewarded blocks, and correlation with RT variability time course (VTC) in both block types, as well as the contrast of the two block types.
| Cluster/subcluster* | Cluster size | Peak coordinates (RAI) | T-value | Anatomical location | |
|---|---|---|---|---|---|
| Rewarded – unrewarded Fig. 1C | |||||
| 1 | 4336 | — | — | (Multiple regions) | |
| A | 746 | −35 80 18 | 9.32 | Right middle occipital gyrus; right middle temporal gyrus | |
| B | 188 | 29 47 −16 | 6.66 | Left cerebellar culmen | |
| C | 138 | 32 83 18 | 7.10 | Left middle occipital gyrus | |
| D | 103 | 8 68 −34 | 6.71 | Left cerebellar uvula | |
| E | 48 | −29 53 42 | 6.42 | Right superior parietal lobule | |
| F | 36 | −50 −14 30 | 5.70 | Right middle frontal gyrus | |
| G | 35 | −38 5 48 | 6.84 | Right middle frontal gyrus | |
| H | 27 | −32 −26 3 | 5.41 | Right inferior frontal gyrus | |
| I | 26 | 35 8 42 | 6.27 | Left middle frontal gyrus | |
| J | 21 | −8 20 12 | 5.01 | Right thalamus | |
| 2 | 100 | 20 −2 15 | 4.86 | Left lentiform nucleus; left putamen | |
| 3 | 93 | 5 −17 −1 | −5.93 | Left caudate; left anterior cingulate | |
| 4 | 88 | 44 71 30 | −7.22 | Left angular gyrus; left middle temporal gyrus | |
| 5 | 87 | 35 8 42 | 6.27 | Left precentral gyrus; left middle frontal gyrus | |
| 6 | 83 | −47 38 36 | 4.86 | Right inferior parietal lobule; right supramarginal gyrus | |
| VTC: unrewarded Fig. 3A | |||||
| 1 | 3597 | — | — | (Multiple regions) | |
| A | 231 | −47 −11 9 | 7.47 | Right precentral gyrus; right inferior frontal gyrus | |
| B | 214 | 53 −5 27 | 6.99 | Left inferior frontal gyrus | |
| C | 205 | 26 5 51 | 8.32 | Left middle frontal gyrus | |
| D | 92 | 56 26 27 | 5.74 | Left inferior parietal gyrus | |
| E | 62 | −32 5 51 | 7.15 | Right middle frontal gyrus | |
| F | 47 | 29 47 42 | 5.42 | Left precuneus; left inferior parietal lobule | |
| G | 30 | −62 38 27 | 6.12 | Right inferior parietal lobule | |
| H | 29 | −23 53 42 | 5.59 | Right precuneus | |
| I | 22 | −53 −2 45 | 5.44 | Right precentral gyrus | |
| 2 | 164 | 17 −41 39 | −5.35 | Left superior frontal gyrus | |
| VTC: rewarded Fig. 3B | |||||
| 1 | 310 | −62 41 30 | 9.97 | Right inferior parietal lobule | |
| 2 | 223 | −2 −23 33 | 6.56 | Right cingulate gyrus | |
| 3 | 197 | −38 −11 12 | 5.81 | Right insula | |
| 4 | 170 | −53 −8 12 | 6.54 | Right precentral gyrus | |
| 5 | 163 | 53 32 36 | −5.87 | Left lentiform nucleus; left putamen | |
| 6 | 163 | 26 2 9 | 5.76 | Left inferior parietal lobule | |
| 7 | 132 | −35 35 −4 | −6.98 | Right cingulate gyrus; right caudate | |
| 8 | 120 | 44 −38 27 | 4.66 | Left middle frontal gyrus | |
| 9 | 118 | 35 −17 15 | 4.71 | Left insula | |
| 10 | 106 | −35 −47 27 | 4.88 | Right middle frontal gyrus | |
| 11 | 85 | 5 47 27 | −4.62 | Left posterior cingulate | |
| 12 | 80 | −29 2 15 | −5.46 | Right lentiform nucleus; right putamen | |
| VTC: rewarded – unrewarded Fig. 3C | |||||
| 1 | 442 | 5 44 63 | −6.13 | Left paracentral lobule | |
| 2 | 257 | 50 2 15 | −5.73 | Left precentral gyrus | |
| 3 | 115 | 38 41 27 | −4.98 | Left inferior parietal lobule | |
| 4 | 93 | −26 11 42 | −5.96 | Right middle frontal gyrus | |
All clusters were significant at a corrected P < 0.05 (nominal P < 0.01, minimum cluster size of 79 voxels).
*For clusters larger than 1000 voxels, we used a more stringent threshold (nominal P < 0.001, minimum cluster size 21 voxels), to define and report subclusters. All subclusters were thus significant at corrected P < 0.05.
fMRI: Event-Related Fluctuations in Accuracy, Preparatory Activity
To explore interactions between reward and trial-to-trial accuracy, we first extracted the event-related average time courses of target mountain trials (COs and CEs) in the a priori networks/ROIs for both rewarded and unrewarded blocks (Fig. 2A, Supplementary Figure 1). We then examined pre-trial activity in each network as a function of future outcome in both reward conditions (Fig. 2C).
A three-way ANOVA (ROI × reward × accuracy) revealed a significant two-way interaction between reward and accuracy (F(1,15) = 7.42, P = .016, Fig. 2C). As can be observed in the event-related averages across all task-positive regions (Fig. 2A, Supplementary Figure 1), recruitment was significantly higher preceding successful (CO) trials compared with lapse (CE) trials, but only during the rewarded blocks. This effect was consistent across all ROIs [DAN: F(1,15) = 5.5, P = 0.033, P(FDR) < 0.05; FPN: F(1,15) = 6.375, P = 0.023, P(FDR) < 0.05; PPA: F(1,15) = 8.33, P = 0.011, P(FDR) < 0.05; VAN: F(1,15) = 4.35, P = 0.054]. Thus, during motivated blocks, participants exerted relatively higher preparatory activity in regions responsible for goal-directed attention that predisposed them for a greater probability of success. Relatively less proactive activity can be seen, however, prior to errors on rewarded blocks. In fact, pre-trial activity for lapses during rewarded blocks did not differ from unrewarded block trials, as if during those rare errors (14% of targets) recruitment is at unrewarded-like levels. The relative failure to proactively engage these regions in the unrewarded blocks was associated with a roughly 2-fold increase in error rate.
fMRI: Event-Related Fluctuations in Accuracy, Evoked Activity
To further explore interactions between reward and trial-to-trial accuracy, we also extracted the β values for target mountain trials (COs and CEs) in a priori networks/ROIs for both rewarded and unrewarded blocks (Fig. 2D). This assessed the reactive or evoked response to targets across accuracy and reward, while importantly controlling for sustained block differences.
A three-way ANOVA (ROI × reward × accuracy) revealed no significant interaction between reward and accuracy (F(1,15) = 1.45, P = 0.24; Fig. 2D). This indicates that the interaction in the preparatory/pre-trial signal was distinct from the evoked responses. However, as can be observed in the event-related averages (Fig. 2A, Supplementary Figure 1) and β values (Fig. 2D), there was a markedly greater response to correct trials between the rewarded and unrewarded conditions (t(15) = 2.78, P = 0.014), such that correctly withholding to mountains evoked a greater response when the trial was rewarded. However, when unrewarded, errors evoked a significantly greater response than correct trials (t(15) = 3.03, P = 0.008), and only errors evoked a similar response across rewarded conditions. Thus, there is some evidence that reactive activation is also greater for rewarded targets, but only in the case of trials in which successful inhibitory control is exerted.
fMRI: Fluctuations in Reaction Time, In Versus Out of the Zone
Previously, our work has demonstrated that task-positive recruitment, particularly in regions associated with the VAN and DAN, is greater during out-of-the-zone epochs, potentially reflecting periods of struggle and/or increased perceptual load (Esterman et al. 2013, 2014b; Kucyi et al. 2016). Importantly, these studies were conducted without reward. In order to explore interactions between reward and fluctuations in RT variability, we extracted the event-related average time courses for in- versus out-of-the-zone “city trials” during the rewarded and unrewarded blocks (Fig. 2B, Supplementary Figure 2).
Replicating our previous studies, during unrewarded blocks, we observed greater recruitment in task-positive regions, including DAN and VAN, when out versus in the zone (Fig. 2B, Supplementary Figure 2; also see Fig. 3). In contrast, during rewarded blocks, we observed little or no differences in recruitment. This was confirmed with a three-way ANOVA (ROI × reward × zone) which revealed a significant two-way interaction between reward and zone across all regions (F(1,15) = 6.13, P = 0.026; Fig. 2E). Examining each network, there were significant interactions between variability state (in versus out of zone) and reward in the VAN and DAN (VAN: F(1,15) = 6.55, P = 0.022, P(FDR) < 0.05; DAN: F(1,15) = 8.14, P = 0.012, P(FDR) < 0.05; Supplementary Fig. 2) and less robustly in PPA and FPN (PPA: F(1,15) = 2.54, P = 0.13; FPN: F(1,15) = 1.49, P = 0.24; Supplementary Fig. 2). This suggests that during motivated performance, participants exert more similar levels of engagement/effort regardless of whether they are performing optimally or suboptimally. When unmotivated, these resources are only fully deployed during periods of suboptimal performance or struggle (when out of the zone). Thus, when unmotivated, optimal performance (in the zone) is accomplished with fewer task-positive resources, at the cost of lower accuracy and less ability to stay in the zone (Fig. 1). However, periods of struggle (out of the zone) evoked similar engagement as measured by task-positive recruitment.
fMRI: Fluctuations in Reaction Time, Variance Time Course Regression
To provide corroborating evidence for this variability versus reward interaction, we conducted whole-brain analyses with the continuous VTCs as regressors during unrewarded blocks (Fig. 3A; Table 1), rewarded blocks (Fig. 3B; Table 1), and contrasted these two conditions (Fig. 3C; Table 1). These whole-brain results were consistent with the ROI analyses above, demonstrating greater task-positive activation associated with high states of variability, mainly in regions overlapping with the DAN and VAN, especially in the unrewarded blocks. Furthermore, we extracted the β values from these maps to examine the individual networks, and as predicted, VTC coupling with variability was greater in DAN and VAN (t(15) = 2.19, P = 0.045; t(15) = 2.64, P = 0.019, respectively). Greater variability coupling with these task-positive regions without reward reflects greater differences between in/out of zone when unrewarded and more consistent recruitment when rewarded.
fMRI: Fluctuations in Reaction Time, Putamen, and Default Mode Network
As in previous studies, low variability periods, or in-the-zone performance, were further associated with activity in the putamen as well as parts of the default mode network (Esterman et al. 2013, 2014b). These regions demonstrated main effects of reward and attentional state/zone, but not an interaction between the two (Supplementary Figure 3). Interestingly, the putamen exhibited greater activity during rewarded blocks as well as in the zone periods, suggesting it may uniquely track optimal periods of performance.
Discussion
Using fMRI, we revealed both quantitative and qualitative changes in neural recruitment accompanying the behavioral effects of motivation/reward on sustained attention. After replicating our previous behavioral findings that performance-based rewards improve accuracy and reduce response variability in sustained attention, we found reward-related quantitative changes in neural recruitment of task-positive and visual regions, and further qualitative changes that interacted with performance fluctuations. In particular, without the external motivation of reward, participants acted as “cognitive misers”, in that they only engaged attentional resources maximally when performance was relatively poor, namely during out-of-the-zone periods. When motivated, however, the same participants acted as “cognitive investors”, engaging attentional resources more proactively and consistently, regardless of performance.
Behavioral improvements from reward were specific to decreased CEs and reaction time variability, similar to our previous work (Esterman et al. 2014a). Commission errors, a common measure of attentional and/or inhibitory lapses (Robertson et al. 1997), were reduced by nearly 50% during rewarded blocks (Cohen's d = 1.6, large effect size). RT variability and time in the zone, measures of attentional fluctuations (MacDonald et al. 2006; Esterman et al. 2013), showed similarly large effect sizes (Cohen's d = 1.6 and 1.4, respectively). The size of these behavioral effects is notable given the relatively modest rewards. That said, without reward, performance indicates that participants were still putting forth reasonable effort (in the zone 43% of the time and 71% accurate), similar to previous unrewarded versions of this task.
Turning to the neural signatures present in the motivated (rewarded) condition, we propose that participants proactively behave as “cognitive investors”, in order to minimize attention failures. Three pieces of evidence support this framework. First, pre-trial recruitment was broadly higher across task-positive and visual regions on correct versus incorrect trials, indicative of a proactive strategy that maximizes success (Fig. 2A,C). Second, evoked activation for correct acts of cognitive control (COs) was greater when rewarded than unrewarded (Fig. 2D), indicative of greater reactive cognitive control that further maximized success. Third, task-positive networks during stable, in-the-zone periods showed greater recruitment when rewarded (Fig. 2B,E), more modest difference between in-the-zone and out-of-the-zone states, and similarly less coupling with variability (Fig. 3C,D). Together, these results indicate more consistent sustained recruitment of visual and task-positive regions when motivated. These findings suggest that when acting as cognitive investors, participants proactively and reactively engage task-positive regions to minimize errors, and engage task-positive regions more consistently across fluctuations in performance to reduce variability.
Turning next to the unmotivated (unrewarded) condition, we propose that when less motivated, participants act as “cognitive misers” and only engage maximal attentional resources during periods of struggle. Again, we uncover three pieces of evidence to support this framework. First, when unmotivated, whether correct or incorrect, no proactive activation in task-positive networks was evident pre-trial, indicating that participants did not invest comparable resources in advance of a target trial without motivation, likely increasing error rates (Fig. 2A,C). Second, when participants were unmotivated, they only reactively engaged comparable task-positive regions when making an error (Fig. 2A,D), as if successful performance was accomplished with fewer resources, thus likely increasing error-proneness. Third, when participants were in the zone and more consistent, there was less activation relative to the motivated condition, again, as if better performance was accomplished with less exertion of resources. When out of the zone, or struggling, task-positive networks were engaged similar to when motivated, suggesting a mode in which task-positive networks are engaged only when needed, during periods of struggle (Fig. 2B,E; Fig. 3). Thus, the “cognitive miser” shifts between a more automated (“autopilot”) task set while in the zone and a reactive mode while out of the zone or making errors.
These results replicate many of the main findings of motivation/reward found in more trial-based transient attention tasks (e.g., Engelmann et al. 2009), and are partly consistent with a prominent theory of motivation in cognition, the “dual mechanisms of cognitive control”, or DMC theory (Braver 2012; Botvinick and Braver 2015). The DMC theory suggests two modes of operation for cognitive control. The first mode, induced by the prospect of reward, is a more proactive mode of processing, accompanied by sustained preparatory control. Similar to this, during rewarded blocks, we observed increased activation preceding correct trials in several task-positive networks. Furthermore, this proactive strategy is also evident during periods of relative success and stability, when in the zone, such that task-positive activity is sustained to likely maximize and prolong such optimal periods of performance when participants are motivated. Our work extends DMC findings by linking the former proactive approach to better sustained performance from trial to trial.
The second mode in DMC theory, typically observed when less motivated, is a more transient reactive mode engaged only in response to the necessary demands of target stimuli (Locke and Braver 2008). Consistent with this, the current findings show less proactive activity during unrewarded compared with rewarded blocks. However, in contrast we observed, if anything, greater evoked responses to correct targets in the rewarded versus unrewarded (Fig. 2D), inconsistent with DMC. One possible interpretation of the current results is that the greater activation during out-of-the-zone periods (versus in the zone) and to errors (versus correct trials) when unrewarded reflects a related type of reactivity where task-positive resources are recruited only in response to more challenging epochs of the task. However, the overall similar activation patterns for out-of-the-zone periods across motivation states may indicate that reactive activation is consistent during periods of relatively poor sustained attention, regardless of motivational state. Together, the current results uniquely demonstrate that even without a cue, or predictability as to when high-reward trials will appear (mountains), individuals can generally maintain a proactive strategy, but only when motivated by reward.
A unique aspect of unmotivated blocks is the observation (also see Esterman et al. 2013, 2014b; Kucyi et al. 2016) that relatively successful periods of performance (being in the zone) were associated with lower task-positive network recruitment, particularly DAN and VAN (or salience network). This may be a unique property of performing a continuous sustained attention task that does not offer breaks. In such a task, it may be most efficient to only engage these resources when needed, such as when struggling or only to avoid catastrophic attention failures. Along these lines, it may be that unrewarded blocks act as relative breaks for participants, where effort is reduced due to greater opportunity cost (Kurzban et al. 2013). Thus, this may reflect a “demotivated” rather than an unmotivated approach, although arguing against this notion is the fact that unrewarded block accuracy was comparable to a previous study without reward (current CE rate 29% versus 26% in Esterman et al. 2013). However, in that study, proactive (pre-trial) DAN activation did predict successful performance, indicating that participants without the promise of reward may have engaged some proactive strategies. Taken together, it may be that the current design and task encourage, and are particularly sensitive to detecting these different cognitive strategies.
Another important aspect of our results is that task-positive network recruitment, particularly in the DAN, was associated with both better and worse behavioral performance. Specifically, while this network exhibited greater activation during rewarded blocks, and before rewarded correct trials, the network also exhibited greater recruitment when participants were out of the zone (Esterman et al. 2014b). These disparate results may be explained by the difference between proactive and reactive activation of these regions, such that proactive engagement of the goal-oriented DAN may support better performance, but reactive activation of this network may represent a response to errors or periods of struggle and thus less efficient engagement. Supporting this notion, we previously observed that out-of-the-zone periods were accompanied by signatures of high perceptual load, as if visual processing was performed less efficiently (Esterman et al. 2014b). Furthermore, using transcranial magnetic stimulation, we recently found that the inhibition of the right frontal eye field of the DAN (Esterman et al. 2015) disrupted performance specifically during in-the-zone periods. This indicates that even when DAN activation is lower in magnitude (in the zone), there is greater reliance on DAN, potentially reflecting a more efficient processing strategy.
Other regions/networks displayed slightly different patterns with regard to reward and performance fluctuations. Interestingly, the FPN exhibited strong proactive effects with regard to accuracy, but less coupling with moment-to-moment fluctuations in variability. It could be that FPN, and lateral prefrontal cortex specifically, represents more abstract maintenance of task set and goals (Badre 2013). On the other hand, the VAN, DAN, and to a lesser extent PPA may represent less abstract aspects of task set and more subtle visual processes and response control more tied to RT fluctuations. This is consistent with other studies linking fluctuations in RT to DAN and VAN specifically, even in the absence of visual stimuli or complex task set (Kucyi et al. 2016). While the task-positive regions show mainly consistent patterns, these differences deserve future study via potentially different sustained attention tasks, stimulus sets, or complementary analyses (e.g., functional connectivity; Poole et al. 2016; Rosenberg et al. 2016).
As highlighted by Fig. 2C and E, rewarded sustained attention had two neural signatures in task-positive networks surrounding fluctuations in accuracy and variability. The accuracy effect is such that additional preparatory activity is observed before correct mountain trials when comparing reward and no reward (Fig. 2C, maroon bars). The variability effect is such that greater activity is observed in response to being in the zone (time-locked to city trials of low variability) when comparing reward and no reward (Fig. 2E, blue bars). These effects were both present in the DAN. To examine how they were related, we correlated the correct mountain trial and in-the-zone differences between rewarded conditions. These reward-induced differences were related (r = 0.58, P < 0.05) in the DAN, suggesting a common underlying strategic process. To ensure that these were not numerically related based on derivation from the same time series, we examined the correlation of the in-the-zone difference with the accuracy difference for both in-the-zone periods (same portion time course) and out-of-the-zone periods (different portion of time course). Importantly, there was no appreciable difference (in the zone: r = 0.49; out of the zone: r = 0.45), indicating while these effects are likely psychologically related, they are not based on dependencies within the data set.
There are several limitations of the current study. First, the experimental design combines losses and gains, which may have unique effects on cognition (Yechiam and Hochman 2013). Previous work suggests that avoiding losses has distinct effects on attention relative to receiving reward. In addition, individual variation in sensitivity to monetary rewards may mask more nuanced effects or interactions (Dreher et al. 2009). Another limitation is that the alternation of reward/unrewarded conditions and only 4-mins of each per run is not conducive for examination of vigilance (time-on-task) effects (Warm et al. 2008; Esterman et al. 2014a) and how they interact with motivation and neural recruitment. Finally, a general concern about the interpretation of the in/out of zone analyses rests on the temporal relationship between the behavior states and (strategic) neural recruitment. Specifically, if one realizes that they are out of the zone and need to focus more, any resulting adjustment could bleed into future periods of in-the-zone performance and vice versa. By averaging across all in/out of zone trials, regardless of temporal position, we partly alleviated this concern. However, the slow nature of BOLD response and the gradual nature of the behavioral fluctuations point to challenges to be considered in future investigations. Despite these concerns, there has been promise that our in/out-of-the-zone framework may be useful for exploring more “intrinsic fluctuations” outside of gradCPT (Rosenberg et al. 2015; Kucyi et al. 2016) and that its interaction with motivation may also extend to other paradigms and domains of cognition (e.g., working memory). Overall, we believe the current study provides new evidence and a novel framework for understanding the relationship between neural recruitment, motivation, and sustained performance that can drive future research.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the US Department of Veteran Affairs through a Clinical Science Research and Development Career Development Award (grant number 1IK2CX000706-01A2) to M.E.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Badre D. 2013. Hierarchical cognitive control and the functional organization of the frontal cortex. In: Ochsner KN, Kosslyn S, editors, The Oxford Handbook of Cognitive Neuroscience. Vol. 2: The Cutting Edges, (Oxford: Oxford University Press), pp. 300–317. [Google Scholar]
- Barkley RA. 1997. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 121(1):65–94. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM.. 2006. Mental fatigue, motivation and action monitoring. Biol Psychol. 72(2):123–132. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T.. 2015. Motivation and cognitive control: from behavior to neural mechanism. Ann Rev Psychol. 66:83–113. [DOI] [PubMed] [Google Scholar]
- Braver TS. 2012. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 16(2):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM.. 2009. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci USA. 106(18):7351–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschschulte A, Boehler CN, Strumpf H, Stoppel C, Heinze HJ, Schoenfeld MA, Hopf JM.. 2014. Reward- and attention-related biasing of sensory selection in visual cortex. J Cogn Neurosci. 26(5):1049–1065. [DOI] [PubMed] [Google Scholar]
- Cox RW. 1996. Afni: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29(3):162–173. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF.. 2009. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci USA. 106(2):617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L.. 2009. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Liu G, Okabe H, Reagan A, Thai M, DeGutis J.. 2015. Frontal eye field involvement in sustaining visual attention: evidence from transcranial magnetic stimulation. Neuroimage. 111:542–548. [DOI] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, Degutis J.. 2013. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex. 23(11):2712–2723. [DOI] [PubMed] [Google Scholar]
- Esterman M, Reagan A, Liu G, Turner C, DeGutis J.. 2014. a. Reward reveals dissociable aspects of sustained attention. J Exp Psychol Gen. 143(6):2287–2295. [DOI] [PubMed] [Google Scholar]
- Esterman M, Rosenberg MD, Noonan SK.. 2014. b. Intrinsic fluctuations in sustained attention and distractor processing. J Neurosci. 34(5):1724–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel JA, Cole MW, Zacks JM, Kay KN, Braver TS.. 2015. Reward motivation enhances task coding in frontoparietal cortex. Cereb Cortex. 26(4):1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC.. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fmri): use of a cluster-size threshold. Magn Reson Med. 33(5):636–647. [DOI] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, Germine L, Wilmer JB, Grosso M, Russo K, Esterman M.. 2015. Sustained attention across the life span in a sample of 10,000 dissociating ability and strategy. Psychol Sci. 26(9):1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf J-M, Schoenfeld MA, Buschschulte A, Rautzenberg A, Krebs R, Boehler C.. 2015. The modulatory impact of reward and attention on global feature selection in human visual cortex. Vis Cogn. 23(1–2):229–248. [Google Scholar]
- Jimura K, Locke HS, Braver TS.. 2010. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci USA. 107(19):8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E.. 2009. Motivation and cognitive control in the human prefrontal cortex. Nature Neurosci. 12(7):939–945. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hove M, Esterman M, Hutchison R, Valera E.. 2016. Dynamic brain-network correlates of spontaneous fluctuations in attention. Cerebral Cortex. doi: 10.1093/cercor/bhw029 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzban R, Duckworth A, Kable JW, Myers J.. 2013. An opportunity cost model of subjective effort and task performance. Behav Brain Sci. 36(6):661–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS.. 2008. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 8(1):99–112. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Backman L.. 2006. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 29:474–480. [DOI] [PubMed] [Google Scholar]
- Pessoa L. 2015. Multiple influences of reward on perception and attention. Vis cogn. 23(1–2):272–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole VN, Robinson ME, Singleton O, Degutis J, Milberg WP, McGlinchey RE, Salat DH, Esterman M.. 2016. Intrinsic functional connectivity predicts individual differences in distractibility. Neuropsychologia. 86:176–182. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J.. 1997. Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 35(6):747–758. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Noonan S, DeGutis J, Esterman M.. 2013. Sustaining visual attention in the face of distraction: a novel gradual-onset continuous performance task. Atten Percept Psychophys. 75(3):426–439. [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Finn ES, Constable RT, Chun MM.. 2015. Predicting moment-to-moment attentional state. Neuroimage. 114:249–256. [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM.. 2016. A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neurosci. 19(1):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP.. 2001. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 35(2):146–160. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P.. 2005. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 17(3):391–398. [DOI] [PubMed] [Google Scholar]
- Sipowicz RR, Ware JR, Baker RA.. 1962. The effects of reward and knowledge of results on the performance of a simple vigilance task. J Exp Psychol. 64:58–61. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ.. 2004. A functional neuroimaging study of motivation and executive function. Neuroimage. 21(3):1045–1054. [DOI] [PubMed] [Google Scholar]
- Warm JS, Parasuraman R, Matthews G.. 2008. Vigilance requires hard mental work and is stressful. Hum Factors. 50(3):433–441. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Hochman G.. 2013. Losses as modulators of attention: review and analysis of the unique effects of losses over gains. Psychol Bull. 139(2):497–518. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.