Summary
Reduced functional activity of CYP2A6 leads to less intense smoking and possible reduced CYP2A6-catalyzed activation of tobacco-specific lung carcinogen NNK, which results in approximately 3-fold reduction in risk of lung cancer development among smokers.
Abstract
Cytochrome P450 2A6 (CYP2A6) catalyzes the metabolism of nicotine and the tobacco-specific lung carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Genetic variation in CYP2A6 may affect smoking behavior and contribute to lung cancer risk. A nested case-control study of 197 lung cancer cases and 197 matched controls was conducted within a prospective cohort of 63 257 Chinese men and women in Singapore. Quantified were five genetic variants of CYP2A6 (*1A, *4, *7, *9 and *12) and urinary metabolites of nicotine [total nicotine, total cotinine, total trans-3′-hydroxycotinine (3HC)] and NNK (total NNAL, free NNAL, NNAL-glucuronide, NNAL-N-glucuronide, and NNAL-O-glucuronide). Higher urinary metabolites of nicotine and NNK were significantly associated with a 2- to 3-fold increased risk of lung cancer after adjustment for smoking intensity and duration. Lower CYP2A6-determined nicotine metabolizer status was significantly associated with a lower ratio of total 3HC over total cotinine, lower total nicotine equivalent and reduced risk of developing lung cancer (all Ptrend < 0.001). Compared with normal metabolizers, odds ratios (95% confidence intervals) of developing lung cancer for intermediate, slow and poor metabolizers determined by CYP2A6 genotypes were 0.85 (0.41–1.77), 0.55 (0.28–1.08) and 0.32 (0.15–0.70), respectively, after adjustment for smoking intensity and duration and urinary total nicotine equivalents. Thus the reduced risk of lung cancer in smokers with lower CYP2A6 activity may be explained by lower consumption of cigarettes, less intense smoking and reduced CYP2A6-catalyzed activation of the tobacco-specific lung carcinogen NNK.
Introduction
The primary cause of lung cancer is tobacco smoking, and in the United States it is estimated that as many as 90% of lung cancer deaths are attributable to smoking (1). However, the susceptibility of smokers to this disease varies significantly. Among all smokers it is estimated that 11–24% will develop lung cancer (2). The large inter-individual variation in smoking-related lung cancer risk may be determined in part by variability in the uptake and metabolism of tobacco carcinogens. The uptake of tobacco carcinogens parallels the uptake of nicotine, the primary addictive component of tobacco. Smokers who metabolize nicotine poorly will have to smoke less, either use fewer cigarettes or smoke each cigarette less efficiently to attain the same levels of nicotine. Therefore, a smoker’s nicotine metabolism capacity can directly influence their level of carcinogen exposure.
In the majority of smokers, cytochrome P450 2A6 (CYP2A6) is by far the primary enzyme responsible for nicotine metabolism (3). CYP2A6 catalyzes the formation of the 5’-iminium ion of nicotine, which is then oxidized to cotinine, either by CYP2A6 or aldehyde oxidase (3,4). In most smokers, 70–80% of the inhaled nicotine is converted to cotinine (3). Cotinine is converted, primarily by CYP2A6 to trans-3′-hydroxycotinine (3HC), which is the most abundant urinary metabolite of nicotine (3). Nicotine, cotinine and 3HC all are glucuronidated (3). The sum of urinary total nicotine (nicotine plus nicotine-glucuronide), total cotinine (cotinine plus cotinine-glucuronide), and total 3HC (3HC plus its glucuronide) accounts for >80% of the excreted nicotine dose and is referred to as total nicotine equivalents (TNE) (3,5). TNE are an excellent biomarker for daily nicotine intake and total tobacco smoking exposure (5).
There are 72 established carcinogens in cigarette smoke (6). Among these, polycyclic aromatic hydrocarbons (PAH) and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) are widely considered to be among the most important causative agents for lung cancer (7,8). We previously reported that the urinary level of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a metabolite of NNK (9,10), and the urinary level of phenanthrene tetraol (10), a biomarker of PAH, were associated with increased risk of lung cancer in two prospective cohorts of Chinese smokers.
The carcinogenicity of NNK and its prime metabolite NNAL is dependent on their metabolic activation. Detoxification is mediated by glucuronidation (11). NNAL is glucuronidated in humans to produce NNAL-N-Gluc and NNAL-O-Gluc (12). Cytochrome P450 enzymes catalyze the metabolic activation of NNK and NNAL by α–hydroxylation (13), which generates reactive metabolites that covalently modify DNA and lead to the initiation of lung carcinogenesis. These enzymes include CYP2A6 and CYP2B6 in the liver and CYP2A6, CYP2A13 and CYP2B6 in the lung (14,15). CYP2B6 is similar in catalytic efficiency to CYP2A6, but is typically less abundant in the liver (14,16). CYP2A13 is a more efficient catalyst of NNK α-hydroxylation than CYP2A6. However, little CYP2A13 is present in the liver (16) and in human liver microsomes, up to 70% of NNK α-hydroxylation is inhibited by CYP2A6 antibodies (14). These data suggest that CYP2A6 plays an important role in the metabolic activation of NNK. Therefore variability in CYP2A6 activity will influence the carcinogenicity of NNK.
There are two potential mechanism by which genetic polymorphisms in the CYP2A6 gene may contribute to inter-individual variation in risk of lung cancer among smokers by altering cigarette consumption and smoking intensity due to altered nicotine metabolism and excretion, and affecting NNK activation pathways. Several epidemiological studies have examined the relationship between individual polymorphisms of CYP2A6 and lung cancer risk. The results are inconsistent. Earlier studies reported a null association or an increased risk of lung cancer with the low-activity CYP2A6 alleles (17–19) while more recent studies, including our recent report, found a reduced risk of lung cancer with the low-activity CYP2A6 alleles (20–23).
The CYP2A6 gene is highly polymorphic (http://www. cypalleles.ki.se/cyp2a6.htm). There are large inter-individual and interethnic variations in both protein expression and activity of CYP2A6. Genetic polymorphisms of the CYP2A6 gene that result in no or reduced CYP2A6 activity alter nicotine metabolism and tobacco consumption (21,24,25). The ratio of 3HC to cotinine, also called the nicotine metabolite ratio (NMR), in plasma or urine has been used to assess the relative activity of CYP2A6 (3,24). A somewhat better measure of CYP2A6 activity in urine is the ratio of total 3HC to cotinine, since a portion of the 3HC is excreted as its glucuronide conjugate (3,24). Alternatively, the urinary ratio of total 3HC to total cotinine has been used for phenotyping CYP2A6 activity (26).
Using the resources of the Singapore Chinese Health Study, urinary metabolites of nicotine (TNE, total nicotine, total cotinine, total 3HC) and NNK (total NNAL, free NNAL, NNAL-Glucs, NNAL-N-Gluc and NNAL-O-Gluc) were examined with respect to risk of developing lung cancer. The impact of CYP2A6 genotypes on NMR, cigarette smoking and biomarkers of tobacco smoke constituents in urine were also assessed in the present study. We also evaluated the association between genetically and phenotypically determined CYP2A6 activity levels and risk of lung cancer. The results of this study improve our understanding of the inter-individual variation in smoking-related lung cancer susceptibility.
Materials and methods
Subjects
Study subjects were drawn from the Singapore Chinese Health Study (27). Briefly, from April 1993 through December 1998, the Singapore Chinese Health Study enrolled 63 257 Chinese men and women aged 45–74 years who resided in government-built housing estates. At the time of recruitment, each subject was interviewed in person by a trained interviewer to obtain information on tobacco and alcohol use. Sixty-one percent of eligible subjects donated blood, buccal and urine samples at baseline. The present study has been approved by the Institutional Review Boards of the University of Minnesota and the University of Pittsburgh.
Identification of incident lung cancer cases was accomplished by annual record linkage of all cohort participants with the database of the population-based Singapore Cancer Registry (28). To date, only 47 (<1%) cohort participants were known to be lost to follow-up due to migration out of Singapore. The present study included 244 incident lung cancer cases of current smokers who donated both blood and urine specimens at baseline. For each case, one control subject was randomly selected from all eligible cohort participants who were alive and free of cancer on the date of cancer diagnosis of the index case. The control subject was individually matched to the index case by smoking status at baseline (i.e., current smoker), gender, dialect group (Hokkien, Cantonese), age at enrollment (±3 years), date of baseline interview (±2 years) and date of biospecimen collection (±6 months).
Laboratory measurements
Urinary metabolites of cigarette smoke constituents
Urine samples of all study subjects were retrieved from the biospecimen bank. Specimens from matched control subjects and their index cases were always assayed in the same batch. All urine aliquots were identified only by unique codes, and laboratory personnel had no knowledge of the case/control status of the test samples. The assays for quantifying total NNAL, NNAL-N-Gluc and NNAL-O-Gluc in urine were previously described (29). Quantification of total nicotine, total cotinine and total 3HC in urine that had been treated with β-glucuronidase was carried out by gas chromatography-mass spectrometry or liquid chromatography tandem mass spectrometry as previously described (10,30). Urinary creatinine (Cr) was assayed by Fairview-University Medical Center Diagnostic Laboratories (Minneapolis) with a Kodak Ektachem 500 chemistry analyzer. The urinary concentration of creatinine was used to quantify levels of urinary analytes per mg creatinine that adjusted for varying water content of the individual spot urine samples.
CYP2A6 genotyping
Genomic DNA was extracted from buffy coat or buccal samples using QIAmp DNA mini kit (Qiagen Inc, Valencia, CA). Quality and quantity of purified DNA were evaluated using a Nanodrop UV-spectrometer (Thermo Fisher Scientific Inc., Wilmington, DE). DNA samples were stored at −20°C until analysis.
The methods for CYP2A6 genotyping were described in detail in our recent report (23). Briefly, the structural variants of CYP2A6*4 and *12 (a hybrid allele with CYP2A7) were determined using quantitative PCR assays designed by ABI (Applied Biosystems). The CYP2A6*7 (rs5031016) and *9 (rs28399433) alleles were determined using nested PCR followed by pyrosequencing (Pyromark Q96MD instrument, Qiagen). The *1A variant was captured with an allelic discrimination (TaqMan) assay for rs1137115. For quality control (QC) purpose, DNA samples of Han Chinese ethnicity from the Coriell Institute (Coriell.org) were inserted in all test samples in one-tenth by frequency.
CYP2A6*1A,*9 and *12 were considered a ‘decrease of function (D)’ allele whereas *4 and *7 were considered ‘loss of function (L)’ (24,31,32). The CYP2A6 genotype grouping according to the predicted pharmacokinetic effects was as follows: (1) ‘normal metabolizers’ were those carrying both alleles of normal function (i.e. *1/*1), (2) ‘intermediate metabolizers’ were those carrying only one D allele (i.e. *1A, *9 or *12), (3) ‘slow metabolizers’ were those carrying either one L allele or two D alleles (e.g. *1/*4 or *9/*9) and (4) ‘poor metabolizers’ were those carrying one L plus one D allele or two L alleles (e.g. *9/*7 or *7/*4).
Of the 244 case–control pairs, 20 cases and 23 control subjects were excluded due to missing information on CYP2A6 genotypes. In addition, 26 cases and 24 controls were excluded due to urinary total cotinine levels below 35 ng/ml, indicating that they were from nonsmokers (or very light/infrequent smokers) at the time of urine collection. An additional case with unknown total cotinine was also excluded. Thus, the present study included 197 cases and 197 control subjects.
Statistical analysis
All urine biomarker measurements were expressed in their molecular weight per mg creatinine as described above. NMR, the total 3HC:total cotinine ratio in urine, was used to evaluate the CYP2A6 activity. TNE, the sum of total nicotine, total cotinine and total 3HC, was used for total tobacco smoke exposure. The NNAL-Gluc:TNE ratio was used as a detoxification biomarker for NNK after controlling for nicotine uptake while the NNAL-Glucs:free NNAL ratio as detoxification biomarker for NNAL. Given the markedly skewed distributions of these urinary biomarkers, formal statistical testing was performed on logarithmically transformed values, and geometric means are presented.
The χ2 test and the t-test were used to compare the distributions of selected variables between lung cancer cases and controls. The analysis of covariance (ANCOVA) method was used to examine the difference in the concentrations of urinary biomarkers or their ratios across different CYP2A6 genotype predicted metabolizers among control subjects.
We used the unconditional logistic regression, for maximizing the number of subjects included in the analysis, to calculate odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) and P values to assess the relationship between urinary metabolites of cigarette smoke constituents and lung cancer risk with adjustment for matching factors including age, sex, dialect and year of sample collection. For each urinary biomarker, study subjects were grouped into quartiles according to its distribution among control subjects. The linear trend test for the association between urinary biomarker and lung cancer risk was based on the ordinal value of quartiles. Similarly, we examined the association between the CYP2A6 genotype-predicted metabolizer status or NMR and lung cancer risk. Covariates included in the logistic regression models were matching factors, smoking intensity and duration and urinary metabolites of nicotine.
Statistical analyses were carried out using SAS software version 9.3 (SAS Institute, Cary, NC). All P values reported are two-sided, and those that were less than 0.05 were considered to be statistically significant.
Results
Of the 197 cases, 173 (87.8%) were histopathologically confirmed while the remaining 24 (12.2%) were diagnosed based on radiography or computer-assisted tomography evidence. Among the histopathologically confirmed cases, 51 (29.5%) were adenocarcinomas, 48 (27.8%) were squamous cell cancers, 25 (14.5%) were small cell cancers and 49 (28.2%) were other cell types. The mean age (standard deviation) at cancer diagnosis of all case patients was 72.2 (6.3) years. The average time interval between baseline biospecimen collection and cancer diagnosis was 4.2 (2.5) years, ranging from 0.5 month to 10.2 years. Current smokers with incident lung cancer reported more cigarettes per day, years of smoking and pack-years of smoking than did controls at baseline. The percentage of regular drinkers of alcohol was slightly higher in cases than in controls (Table 1).
Table 1.
Baseline demographic and lifestyle characteristics and urinary biomarkers of current smokers who developed lung cancer (Cases) and those who remained cancer-free (Controls), The Singapore Chinese Health Study 1993–2014
| Characteristics or biomarkers | Cases | Controls | P† |
|---|---|---|---|
| Number of subjects | 197 | 197 | |
| Mean age (SD), years | 60.8 (6.2) | 60.8 (6.2) | 1.000 |
| Sex, % | |||
| Men | 83.8 | 83.3 | 0.892 |
| Women | 16.2% | 16.7% | |
| Mean body mass index (SD), kg/m2 | 21.8 (2.8) | 22.3 (2.7) | 0.069 |
| Level of education, % | |||
| No formal education | 28.9 | 25.4 | 0.431 |
| Primary (1–6 years) | 56.4 | 55.3 | |
| Secondary and above | 14.7 | 19.3 | |
| Mean no. of cigarettes/day (SD) | 18.3 (10.6) | 15.2 (10.1) | 0.003 |
| Mean no. of years of smoking (SD) | 39.3 (9.3) | 37.2 (11.3) | 0.0497 |
| Mean no. of pack-years of cigarettes (SD) | 37.2 (22.5) | 30.1 (21.5) | 0.002 |
| Alcohol drinking, % | |||
| Nondrinkers | 62.4 | 72.1 | 0.041 |
| Regular drinkers | 37.6 | 27.9 | |
| Mean no. of drinks/day (SD)a | 1.4 (1.9) | 1.1 (1.6) | 0.458 |
| Urinary biomarkersb | Geometric mean (95% CI) | ||
| Total nicotine (nmol/mg Cr) | 9.70 (8.34–11.28) | 7.70 (6.62–8.94) | 0.033 |
| Total cotinine (nmol/mg Cr) | 14.00 (12.50–15.68) | 10.64 (9.50–11.90) | <0.001 |
| Total 3HC (nmol/mg Cr) | 14.28 (12.20–16.72) | 9.00 (7.68–10.52) | <0.001 |
| TNE (nmol/mg Cr)c | 42.68 (38.26–47.60) | 31.24 (28.00–34.84) | <0.001 |
| Total NNAL (pmol/mg Cr)d | 1.08 (0.96–1.22) | 0.90 (0.80–1.02) | 0.0498 |
| Free NNAL (pmol/mg Cr)d | 0.40 (0.34–0.46) | 0.32 (0.28–0.36) | 0.007 |
| NNAL-Glucs (pmol/mg Cr)d | 0.66 (0.58–0.76) | 0.56 (0.48–0.62 | 0.052 |
| NNAL-N-Gluc (pmol/mg Cr)d | 0.14 (0.12–0.18) | 0.12 (0.10–0.14) | 0.061 |
| NNAL-O-Gluc (pmol/mg Cr)d | 0.50 (0.42–0.56) | 0.42 (0.38–0.48) | 0.151 |
| NNAL-Glucs:TNE ratio (pmol/µmol)d | 15.50 (13.92–17.28) | 17.42 (15.64–19.42) | 0.133 |
| NNAL-Glucs:free NNAL ratiod | 1.68 (1.54–1.84) | 1.74 (1.60–1.90) | 0.558 |
| NMR | 1.02 (0.92–1.14) | 0.84 (0.76–0.94) | 0.016 |
Cr, creatinine; 3HC, trans-3′-hydroxycotinine; NMR, nicotine metabolite ratio; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNAL-Glucs, NNAL glucuronides; NNAL-N-Gluc, NNAL N-glucuronide; NNAL-O-Gluc; NNAL O-glucuronide; SD, standard deviation; TNE, total nicotine equivalents.
aAmong regular alcohol drinkers only.
bAll urinary biomarkers and their ratios were presented as geometric means (95% confidence intervals).
cThe sum of total nicotine, total cotinine and total 3HC.
dVarious number of subjects excluded due to missing data on following analysis: 9 (3 cases and 6 controls) for total NNAL; 4 (all controls) for free NNAL; 13 (7 cases and 6 controls) for NNAL-Glucs; 16 (10 cases and 6 controls) for NNAL-N-Gluc; and 23 (11 cases and 12 controls) for NNAL-O-Gluc.
†Two-sided Ps were based on t test for continuous variables or chi-square test for categorical variables.
The geomeric means of urinary total nicotine, total cotinine, total 3HC, TNE, total NNAL and free NNAL in cases were statistically significantly higher than those in controls (Table 1). Higher quartiles of all nicotine metabolites, total and free NNAL and NNAL-N-Gluc (see quartile cutoffs in Supplementary Table 1, available at Carcinogenesis Online) were significantly associated with increased risk of lung cancer (all Ptrend < 0.030) whereas the NNAL-Gluc:TNE ratio and the NNAL-Glucs:free NNAL ratio were associated with reduced risk of lung cancer, although they were not statistically significant, after adjustment for smoking intensity and duration (Table 2).
Table 2.
Urinary metabolites of cigarette smoke constituents in relation to risk of lung cancer The Singapore Chinese Health Study 1993–2014
| Urinary biomarkers | Odds ratio (95% CI) by levels of biomarkersa | ||||
|---|---|---|---|---|---|
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P for trend | |
| Total nicotine | 1.00 | 0.90 (0.49–1.67) | 0.87 (0.47–1.61) | 2.00 (1.12–3.55) | 0.015 |
| Total cotinine | 1.00 | 1.22 (0.65–2.31) | 1.54 (0.82–2.88) | 2.64 (1.44–4.81) | <0.001 |
| Total 3-hydroxy cotinine | 1.00 | 1.74 (0.90–3.36) | 2.18 (1.14–4.16) | 3.16 (1.67–5.96) | <0.001 |
| Total nicotine equivalent | 1.00 | 1.26 (0.65–2.44) | 2.01 (1.07–3.77) | 2.93 (1.59–5.41) | <0.001 |
| Total NNAL | 1.00 | 0.90 (0.47–1.70) | 1.38 (0.75–2.54) | 1.73 (0.95–3.15) | 0.029 |
| Free NNAL | 1.00 | 1.14 (0.61–2.12) | 0.97 (0.51–1.84) | 2.26 (1.25–4.09) | 0.007 |
| NNAL-Glucs | 1.00 | 0.90 (0.48–1.69) | 1.23 (0.67–2.25) | 1.58 (0.87–2.86) | 0.076 |
| NNAL-N-Gluc | 1.00 | 0.97 (0.51–1.82) | 1.16 (0.62–2.14) | 1.92 (1.04–3.54) | 0.025 |
| NNAL-O-Gluc | 1.00 | 0.92 (0.48–1.74) | 1.25 (0.67–2.33) | 1.51 (0.83–2.76) | 0.104 |
| NNAL-Glucs:TNE ratio | 1.00 | 0.93 (0.51–1.66) | 0.92 (0.51–1.63) | 0.65 (0.35–1.19) | 0.200 |
| NNAL-Glucs:Free NNAL ratio | 1.00 | 0.98 (0.55–1.74) | 0.85 (0.48–1.53) | 0.67 (0.37–1.24) | 0.182 |
NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNAL-Glucs, NNAL glucuronides; NNAL-N-Gluc, NNAL N-glucuronide; NNAL-O-Gluc; NNAL O-glucuronide; TNE, total nicotine equivalents.
aAll odds ratios were adjusted for number of cigarettes per day, number of years of smoking and matching factors including age, gender, year of enrollment and dialect group.
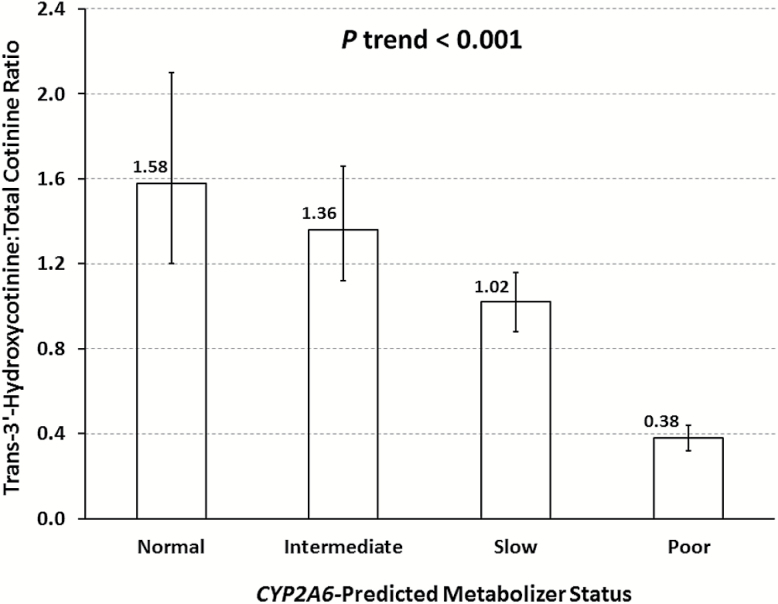
The allele frequencies of CYP2A6*1, *1A,*4, *7, *9 and *12 among controls were 0.267, 0.218, 0.081, 0.162, 0.267 and 0.005, respectively. The corresponding figures among cases were 0.411, 0.221, 0.061, 0.096, 0.208 and 0.003. The joint distribution of CYP2A6 genotypes slightly deviated from Hardy–Weinberg equilibrium among controls (P = 0.036) whereas it was in agreement with Hardy–Weinberg equilibrium among cases (P = 0.276) (Supplementary Table 2, available at Carcinogenesis Online). Among control subjects, CYP2A6 metabolizer status was highly correlated with NMR in a dose-dependent manner (Ptrend <0.001) (Figure 1).
Figure 1.
Geometric means and 95% confidence intervals of nicotine metabolite ratio in urine by CYP2A6-predicted metabolizer status among control subjects, the Singapore Chinese Health Study. See details in the Material and Methods for the classification of CYP2A6-predicted metabolizer status.
The distribution of smoking intensity and duration and urinary metabolites of tobacco smoke constituents across four different levels of CYP2A6-predicted metabolizer status is shown in Table 3. The CYP2A6 poor metabolizers smoked fewer cigarettes per day and pack-years of smoking compared with normal metabolizers whereas no difference was seen in age at starting to smoke and years of smoking between metabolizer status groups. Compared with normal metabolizers, poor metabolizers had a statistically significant 41% lower total cotinine (Ptrend = 0.008), 86% lower total 3HC (Ptrend < 0.001), 53% lower TNE (Ptrend < 0.001) and 36% lower total NNAL (Ptrend = 0.042) (Table 3).
Table 3.
Average levels of smoking intensity and duration and urinary metabolites of cigarette smoke constituents by CYP2A6 genotypes-predicted metabolizers status among control subjects, The Singapore Chinese Health Study 1993–2014
| Smoking related variables | CYP2A6 genotype predicted metabolizer statusa | P for trend | |||
|---|---|---|---|---|---|
| Normal | Intermediate | Slow | Poor | ||
| Number of subjects (%) | 19 (9.6) | 41 (20.8) | 80 (40.6) | 57 (28.9) | |
| Number of cigarettes/day, mean (SD) | 15.4 (11.2) | 19.7 (11.4) | 13.6 (8.5) | 14.1 (10.1) | 0.052 |
| Age start smoking (years), mean (SD) | 18.9 (6.3) | 20.1 (5.9) | 20.4 (6.5) | 20.1 (6.6) | 0.600 |
| Number of years of smoking, mean (SD) | 38.9 (10.6) | 39.0 (9.4) | 36.5 (12.0) | 36.4 (11.8) | 0.210 |
| Pack-years of smoking, mean (SD) | 31.3 (23.1) | 39.7 (23.6) | 27.1 (18.8) | 27.1 (21.6) | 0.031 |
| Urinary biomarkersb | |||||
| Total nicotine (nmol/mg creatinine) | 9.4 (5.8–15.1) | 7.5 (5.4–10.3) | 7.1 (5.6–8.9) | 8.3 (6.3–10.9) | 0.883 |
| Total cotinine (nmol/mg creatinine) | 14.2 (9.8–20.8) | 11.9 (9.2–15.4) | 11.2 (9.3–13.4) | 8.3 (6.7–10.3) | 0.008 |
| Total 3HC (nmol/mg creatinine) | 22.5 (14.6–34.8) | 16.2 (12.0–21.8) | 11.3 (9.2–14.0) | 3.1 (2.4–4.0) | <0.001 |
| TNE (nmol/mg creatinine)c | 49.5 (34.7–70.5) | 37.6 (29.5–47.8) | 31.6 (26.6–37.6) | 23.1 (18.8–28.4) | <0.001 |
| Total NNAL (pmol/mg creatinine)d | 1.16 (0.80–1.66) | 0.94 (0.72–1.22) | 0.96 (0.80–1.16) | 0.74 (0.60–0.92) | 0.042 |
| Free NNAL (pmol/mg creatinine)d | 0.40 (0.26–0.60) | 0.30 (0.24–0.40) | 0.34 (0.28–0.40) | 0.26 (0.20–0.32) | 0.090 |
| NNAL-Glucs (pmol/mg creatinine)d | 0.72 (0.48–1.08) | 0.60 (0.46–0.80) | 0.60 (0.48–0.72) | 0.44 (0.34–0.54) | 0.022 |
| NNAL-N-Gluc (pmol/mg creatinine)d | 0.12 (0.08–0.20) | 0.14 (0.12–0.20) | 0.12 (0.10–0.16) | 0.10 (0.08–0.14) | 0.151 |
| NNAL-O-Gluc (pmol/mg creatinine)d | 0.58 (0.40–0.88) | 0.46 (0.34–0.60) | 0.44 (0.36–0.54) | 0.34 (0.26–0.42) | 0.017 |
| NNAL-Glucs:TNE ratio (pmol/µmol)d | 14.5 (10.2–20.6) | 15.5 (12.1–19.7) | 18.5 (15.6–22.0) | 18.5 (15.0–22.8) | 0.138 |
| NNAL-Glucs:free NNAL ratiod | 1.80 (1.38–2.36) | 1.88 (1.56–2.28) | 1.76 (1.54–2.00) | 1.62 (1.38–1.92) | 0.314 |
3HC, trans-3′-hydroxycotinine; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNAL-Glucs, NNAL glucuronides; NNAL-N-Gluc, NNAL N-glucuronide; NNAL-O-Gluc; NNAL O-glucuronide; SD, standard deviation; TNE, total nicotine equivalents.
aSee the Materials and Methods and Supplementary Table 1, available at Carcinogenesis Online, for the CYP2A6 grouping.
bGeometric mean (95% confidence interval).
cThe sum of total nicotine, total cotinine and total 3HC.
dNumber of subjects excluded from analysis due to missing data: 6 for NNAL, NNAL-Glucs, and NNAL-N-Gluc; 12 for NNAL-O-Gluc and 4 for free NNAL.
The association between CYP2A6 and lung cancer risk is shown in Table 4. There was a dose-dependent relation between CYP2A6 activity determined by genotype or phenotype (NMR), and lung cancer risk (both Ptrend ≤ 0.010). Adjustment for smoking intensity and duration and urinary TNE significantly attenuated the association for lung cancer with CYP2A6 phenotype (Ptrend = 0.132). However the association between CYP2A6-genotype determined metabolizer status and lung cancer risk remained statistically significant (Ptrend < 0.001). Compared with normal metabolizers, adjusted ORs (95% CI) of developing lung cancer for intermediate, slow and poor metabolizers was 0.85 (0.41–1.77), 0.55 (0.28–1.08) and 0.32 (0.15–0.70), respectively.
Table 4.
CYP2A6 genotypes-predicted metabolizer status in relation to the risk of developing lung cancer, The Singapore Chinese Health Study 1993–2014
| CYP2A6 activity | Median NMR among controls | Cases/controls | OR (95% CI)a | OR (95% CI)b |
|---|---|---|---|---|
| CYP2A6 genotype-predicted | ||||
| Normal | 1.60 | 35/19 | 1.00 | 1.00 |
| Intermediate | 1.40 | 60/40 | 0.81 (0.40–1.64) | 0.85 (0.41–1.77) |
| Slow | 0.99 | 74/79 | 0.47 (0.25–0.91) | 0.55 (0.28–1.08) |
| Poor | 0.41 | 28/59 | 0.23 (0.11–0.49) | 0.32 (0.15–0.70) |
| P for trend | <0.001 | <0.001 | ||
| NMR | ||||
| 4th quartile | 2.00 | 59/49 | 1.00 | 1.00 |
| 3rd quartile | 1.17 | 61/49 | 1.03 (0.60–1.76) | 1.09 (0.62–1.91) |
| 2nd quartile | 0.73 | 48/49 | 0.77 (0.44–1.36) | 0.86 (0.48–1.54) |
| 1st quartile | 0.37 | 29/50 | 0.45 (0.24–0.83) | 0.62 (0.32–1.18) |
| P for trend | 0.010 | 0.132 | ||
CI, confidence interval; NMR, nicotine metabolite ratio; OR, odds ratio.
aOdds ratios were calculated using unconditional logistic regression models with adjustment for matching factors including age, gender, year of enrollment, and dialect group.
bIn addition to matching factors, odds ratios were adjusted for cigarettes per day, years of smoking and urinary total nicotine equivalents.
We conducted stratified analysis to examine if exposure to different levels of tobacco constituents would modify the association between the CYP2A6 predicted metabolizer status and risk of lung cancer. The number of cigarettes per day, number of years of smoking, pack-years of smoking, urinary total TNE and urinary total NNAL did not have a significant impact on the CYP2A6-lung cancer risk association (Supplementary Table 3, available at Carcinogenesis Online). None of the interaction terms between CYP2A6 genotype-determined metabolizer status and levels of exposure to cigarette smoking were statistically significant (all Pinteraction > 0.150).
When analyses were conducted for lung cancer cases separated by histological types, the low-activity CYP2A6 genotypes were associated with 60% reduced risk of lung adenocarcinoma, 42% of squamous cell cancer and 74% of other/unknown histological types (Supplementary Table 4, available at Carcinogenesis Online). The association for CYP2A6-determined metabolizer status with risk of adenocarcinoma was comparable with risk of squamous cell cancer (P = 0.408). We also examined and found no difference in the association between urinary nicotine and NNK metabolites and risk of lung cancer by histological type (data not shown).
Discussion
The present study demonstrates an approximately three-fold risk reduction in developing lung cancer for current smokers with the CYP2A6-determined poor metabolizer status compared with normal metabolizers even after adjustment for smoking intensity and duration and urinary TNE. The significant association between CYP2A6 phenotype measure (NMR) and lung cancer risk was considerably weakened and became statistically non-significant after adjustment for TNE and smoking intensity and duration because NMR decreased with decreasing number of cigarettes per day and urinary TNE (Table 3). These findings are consistent with those in our recent study from the Shanghai Cohort Study (23), further lending support to our hypothesis that CYP2A6 plays a significant role in contributing to the inter-individual variation in smoking-related lung cancer susceptibility
Nicotine is the main addictive compound in tobacco (33). In smokers with functional CYP2A6, approximately 80% of nicotine is inactivated to cotinine (3), and an association between genetic variation in CYP2A6 activity and smoking behavior has been reported in several, but not all study populations (20–22,34). A recent study in a Chinese population found that the CYP2A6 genotype-predicted poor metabolizers consumed fewer cigarettes per day, initiated smoking at a later age, had a shorter duration of smoking, and were more likely to quit smoking than normal metabolizers (35). Consistent with these findings, the present study showed statistically significant differences in the number of cigarettes per day and pack-years of smoking among different CYP2A6 genotypes in control subjects. More importantly, the present study demonstrated that TNE, an objective measure of daily nicotine intake, was more than doubled in smokers with normal compared with poor functional alleles of CYP2A6 (Table 3). These results showed that genotypic polymorphisms of CYP2A6 have a significant impact on smoking behavior–individuals with low CYP2A6 activity consume fewer cigarettes per day and over their lifetime, are exposed to lower amounts of tobacco smoke carcinogens, and have a lower risk of lung cancer. The dose-dependent relationship between CYP2A6 activity and lung cancer risk remained after taking into account difference in the consumption of cigarettes and intake of nicotine, suggesting that another mechanism for CYP2A6 activity and lung cancer risk may exist, such as the metabolic activation of the procarcinogen NNK by CYP2A6.
CYP2A6 catalyzes the α-hydroxylation-mediated bioactivation of the tobacco-specific lung carcinogen NNK and NNAL (13). It’s not possible at present to directly measure either NNK or NNAL α-hydroxylation pathway in smokers. If CYP2A6 is an important catalyst of NNK α-hydroxylation, one would predict that poor CYP2A6 metabolizers would have a reduced amount of NNK metabolized by the α-hydroxylation pathway and increased conversion to NNAL (36). In addition, further metabolism of NNAL by α-hydroxylation would also be decreased in these individuals. Therefore, we investigated and found that the NNAL-Gluc:TNE ratio (TNE is serving as a proxy for NNK dose) was higher in poor metabolizers than normal metabolizers, although this difference was not statistically significant. Future studies with large sample size are warranted to produce definitive results.
Pharmacokinetic studies have established that plasma NMR is a standard measure of CYP2A6 activity (3,24). More recent studies (37–39), including ours, demonstrated that urinary NMR is an excellent measure for CYP2A6 enzyme activity. Similar to our recent report (23), there was a more than 10-fold difference in urinary NMR between the CYP2A6 *1/*1 and *7/*4 genotypes in the present study (data not shown), and a strong dose-dependent relation between combined CYP2A6 genotypes and NMR (Figure 1). CYP2A6 genotypes explained approximately 35% of variation in NMR in the present study, which is consistent with findings from a similar study in the Multiethnic Cohort using similar CYP2A6 genotypes (39). In addition, genome-wide association (GWA) studies identified a number of single-nucleotide polymorphisms (SNPs) in the CYP2A6 gene region that have a significant impact on plasma NMR (40,41). This was confirmed in a recent GWA study using urinary NMR (42). These data demonstrate that the urinary NMR, as well as plasma NMR, is a robust functional measure for CYP2A6 activity for ad libitum smoking.
The frequencies of CYP2A6 alleles vary widely among different racial/ethnic populations. Consistent with previous studies, the CYP2A6 *4, *7, and *9 alleles are common and the *12 allele is rare in Asian populations (24,31,35,43). A recently identified SNP rs1137115 captures the CYP2A6*1A allele and results in a significant reduction in nicotine metabolism in European American smokers (32,44). Similar to our previous study in Shanghai (23), the present study showed a 10–20% reduced NMR in individuals carrying homozygous variants of CYP2A6*1A relative to normal functional genotype (*1/*1) (data not shown).
A number of studies have assessed the association between the deletion polymorphism, CYP2A6*4 and risk of lung cancer in different populations. However, the results are inconsistent. One study in Japan reported a statistically significant 50% reduced risk of lung cancer associated CYP2A6*4 (45), whereas a similar study in China found a two-fold increased risk of lung cancer in individuals carrying CYP2A6*4 (18). Several meta-analyses reported a statistically significant approximately 50% lower crude OR of lung cancer for poor metabolizers in Asians, especially in smokers (46,47). Consistent with results of previous studies including ours (23), the present study also found that CYP2A6*4 alone was associated with approximately 30% reduced risk of lung cancer (data not shown).
The structural alteration in the CYP2A6 gene can obscure the detection of genetic variants using a genome-wide genotyping approach. The Lung Cancer Oncoarray Project genotyped more than 500 000 SNPs in 57 775 individuals using the Oncoarray platform. As part of the consortium, we submitted our DNA samples from 268 of the 394 subjects included in the present analysis. The Oncoarray platform did not cover the CYP2A6*4 allele. In addition, the Oncoarray could not distinguish the homozygous variant from heterozygous genotypes for both rs5031016 (CYP2A6*7) and rs28399433 (CYP2A6*9). For rs5031016 (CYP2A6*7), the GG genotype was identified in 6 of the 268 subjects by our genotyping method, but none in the Oncoarray platform. Similarly for rs28399433 (CYP2A6*9), the CC genotype was called for on 25 of the 268 subjects but again none by the Oncoarray. Alternatively, the Oncoarray identified 11 SNPs in the CYP2A6 gene region that had a significant impact on the CYP2A6 activity in the Multiethnic Cohort study participants, but only one (rs56113850) was confirmed to be significantly associated with the risk of lung cancer. Nonetheless, the association between the rs56113850 and lung cancer risk became statistically non-significant (P = 0.279) after adjustment for smoking status and pack-years (42). GWA studies that incorporate both copy number polymorphism (CNP) and SNP at the CYP2A6 locus may increase the capability to capture the information on gene structural alteration. Kumasaka and colleagues (48) conducted an integrated GWA study involving more than 17 000 Japanese smokers and identified a common CNP with a strong effect on cigarettes per day (rs8102683; P = 3.8 × 10−42) in the 19q13 region, encompassing the CYP2A6 locus. After adjustment for the associated CNP, an additional associated SNP (rs11878604; P = 9.7 × 10−30) in the CYP2A6 gene was also identified. The haplotypes of CNP (linked to CYP2A6*4) and the rs11878694 SNP (linking to deleterious alleles CYP2A6*7, *9 and other SNPs) were significantly associated with 3–4 fewer cigarettes per day and approximately 50% reduced risk of lung cancer, although there was no adjustment for smoking intensity and duration in the report. Therefore, the inconsistent results for an independent association between CYP2A6 genetic polymorphisms and lung cancer risk in GWA studies could be due to the challenges in genotyping this complex gene using a high-throughput platform.
Other explanations for the inconsistent results of CYP2A6 and lung cancer risk from previous studies using targeted genotyping approaches could be as follows: (1) possible measurement error in genotyping of CYP2A6 due to the altered gene structure resulting from the deletion polymorphism; (2) different distributions of CYP2A6 variants and allele frequencies across different racial/ethnic groups. For example, the CYP2A6*7 allele is absent in both Caucasian and African populations but is common in Asian populations (5.7–12.5%), whereas CYP2A6*2 and*12 are present in Caucasians (3–5%) but absent or extremely rare in Asians (25,35); (3) inclusion of both smokers and nonsmokers in prior studies; (4) the incomplete adjustment for total tobacco smoking exposure; (5) potential selection bias due to hospital-based retrospective study design and (6) small sample size and low statistical power. Similar to our recent study within the Shanghai Cohort Study, the present study circumvented most of these limitations by using a prospective study design, including only current smokers whose smoking status was biochemically verified, enrolling only Han Chinese, and genotyped for CYP2A6 variants that are relatively common in Asian populations. The CYP2A6 genotype-determined metabolizer status was verified by NMR, a robust functional measure of CYP2A6 activity. The consistent results from these two Chinese cohorts support an independent role of CYP2A6 in the development of lung cancer among smokers.
The present study was the first to examine the possible association between different metabolites of NNAL and lung cancer risk. Among the three NNAL metabolites measured, the association between free NNAL and risk of lung cancer was the strongest (Table 2). This finding is consistent with the notion that NNAL glucuronidation is a detoxification pathway. The present study also confirmed our previous findings on urinary total NNAL and TNE in relation to lung cancer risk that are independent from smoking intensity as measured by cigarettes per day, and duration (10,23).
In summary, the present study demonstrated a strong dose-dependent association between reduced CYP2A6 activity determined by genotypes and reduced risk of lung cancer. This CYP2A6-lung cancer risk association is independent from measures of cigarette smoking and nicotine uptake, suggesting that CYP2A6 may play a role in NNK metabolic activation, independent of its influence on smoking behavior in contributing to the inter-individual variation in smoking-related lung cancer susceptibility.
Supplementary material
Supplementary data are available at Carcinogenesis Online.
Funding
USPHS grants R01 CA129534, R01 CA144034, and UM1 CA182876. W.-P. Koh is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013).
Supplementary Material
Acknowledgements
We thank Katherine Wickham for carrying out all nicotine and metabolite analyses, Elizabeth Vielguth for performing the NNAL and NNAL-glucuronide analyses, Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study, and the Singapore Cancer Registry for assistance with the identification of cancer outcomes.
Conflict of Interest Statement: None declared.
Abbreviations
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NMR
nicotine metabolite ratio
- PAH
polycyclic aromatic hydrocarbons
- TNE
total nicotine equivalent
References
- 1. International Agency for Research on Cancer (2004) Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. IARC Scientific Publications, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- 2. Brennan P., et al. (2011) Genetics of lung-cancer susceptibility. Lancet. Oncol., 12, 399–408. [DOI] [PubMed] [Google Scholar]
- 3. Hukkanen J., et al. (2005) Metabolism and disposition kinetics of nicotine. Pharmacol. Rev., 57, 79–115. [DOI] [PubMed] [Google Scholar]
- 4. von Weymarn L.B., et al. (2006) Inactivation of CYP2A6 and CYP2A13 during nicotine metabolism. J. Pharmacol. Exp. Ther., 316, 295–303. [DOI] [PubMed] [Google Scholar]
- 5. Scherer G., et al. (2007) Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol., 47, 171–183. [DOI] [PubMed] [Google Scholar]
- 6. Hecht S.S. (2012) Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob. Res., 14, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hecht S.S. (2003) Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer, 3, 733–744. [DOI] [PubMed] [Google Scholar]
- 8. International Agency for Research on Cancer (2007) Smokeless tobacco and tobacco-specific nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Scientific Publications, Lyon, France. [Google Scholar]
- 9. Yuan J.M., et al. (2009) Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res., 69, 2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan J.M., et al. (2011) Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res., 71, 6749–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Upadhyaya P., et al. (1999) Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers and metabolites in the A/J mouse. Carcinogenesis, 20, 1577–1582. [DOI] [PubMed] [Google Scholar]
- 12. Carmella S.G., et al. (2002) Analysis of N- and O-glucuronides of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Chem. Res. Toxicol., 15, 545–550. [DOI] [PubMed] [Google Scholar]
- 13. Jalas J.R., et al. (2005) Cytochrome P450 enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Chem. Res. Toxicol., 18, 95–110. [DOI] [PubMed] [Google Scholar]
- 14. Dicke K.E., et al. (2005) Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-butanone metabolism by cytochrome P450 2B6. Drug Metab. Dispos., 33, 1760–1764. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X., et al. (2007) CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J. Pharmacol. Exp. Ther., 323, 570–578. [DOI] [PubMed] [Google Scholar]
- 16. Gervot L., et al. (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics, 9, 295–306. [PubMed] [Google Scholar]
- 17. Loriot M.A., et al. (2001) Genetic polymorphisms of cytochrome P450 2A6 in a case-control study on lung cancer in a French population. Pharmacogenetics, 11, 39–44. [DOI] [PubMed] [Google Scholar]
- 18. Tan W., et al. (2001) Frequency of CYP2A6 gene deletion and its relation to risk of lung and esophageal cancer in the Chinese population. Int. J. Cancer, 95, 96–101. [DOI] [PubMed] [Google Scholar]
- 19. Wang H., et al. (2003) Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco-specific carcinogen NNK. Cancer Res., 63, 8057–8061. [PubMed] [Google Scholar]
- 20. Fujieda M., et al. (2004) Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis, 25, 2451–2458. [DOI] [PubMed] [Google Scholar]
- 21. Wassenaar C.A., et al. (2011) Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J. Natl. Cancer Inst., 103, 1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wassenaar C.A., et al. (2015) CYP2A6 reduced activity gene variants confer reduction in lung cancer risk in African American smokers–findings from two independent populations. Carcinogenesis, 36, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuan J.M., et al. (2016) Genetic determinants of cytochrome P450 2A6 activity and biomarkers of tobacco smoke exposure in relation to risk of lung cancer development in the Shanghai cohort study. Int. J. Cancer, 138, 2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benowitz N.L., et al. (2006) CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther., 80, 457–467. [DOI] [PubMed] [Google Scholar]
- 25. Mwenifumbo J.C., et al. (2007) Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics, 8, 1385–1402. [DOI] [PubMed] [Google Scholar]
- 26. Derby K.S., et al. (2008) Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol. Biomarkers Prev., 17, 3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan J.M., et al. (2003) Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol. Biomarkers Prev., 12, 890–898. [PubMed] [Google Scholar]
- 28. Parkin D.M., et al. (2005) Cancer Incidence in Five Continents. International Agency for Research on Cancer, Lyon. [Google Scholar]
- 29. Carmella S.G., et al. (2013) High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem. Res. Toxicol., 26, 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy S.E., et al. (2014) Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis, 35, 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakajima M., et al. (2006) Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin. Pharmacol. Ther., 80, 282–297. [DOI] [PubMed] [Google Scholar]
- 32. Bloom A.J., et al. (2013) A compensatory effect upon splicing results in normal function of the CYP2A6*14 allele. Pharmacogenet. Genomics, 23, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benowitz N.L. (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol., 49, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malaiyandi V., et al. (2006) Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol. Psychiatry, 11, 400–409. [DOI] [PubMed] [Google Scholar]
- 35. Liu T., et al. (2011) Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction, 106, 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hecht S.S., et al. (1996) Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol. Biomarkers Prev., 5, 645–652. [PubMed] [Google Scholar]
- 37. Swan G.E., et al. (2009) Genetic and environmental influences on the ratio of 3’hydroxycotinine to cotinine in plasma and urine. Pharmacogenet. Genomics, 19, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Binnington M.J., et al. (2012) CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogenet. Genomics, 22, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park S.L., et al. (2016) Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risks of lung cancer and effect on their smoking intensity. Carcinogenesis, 37, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loukola A., et al. (2015) A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism. PLoS Genet., 11, e1005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baurley J.W., et al. (2016) Genome-wide association of the laboratory-based nicotine metabolite ratio in three ancestries. Nicotine Tob. Res., 18, 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel Y.M., et al. (2016) Novel association of genetic markers affecting CYP2A6 activity and lung cancer risk. Cancer Res, 76:5768–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bloom A.J., et al. (2012) Use of a predictive model derived from in vivo endophenotype measurements to demonstrate associations with a complex locus, CYP2A6. Hum. Mol. Genet., 21, 3050–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bloom J., et al. (2011) The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet. Genomics, 21, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyamoto M., et al. (1999) CYP2A6 gene deletion reduces susceptibility to lung cancer. Biochem. Biophys. Res. Commun., 261, 658–660. [DOI] [PubMed] [Google Scholar]
- 46. Liu Y.L., et al. (2013) CYP2A6 deletion polymorphism is associated with decreased susceptibility of lung cancer in Asian smokers: a meta-analysis. Tumour Biol., 34, 2651–2657. [DOI] [PubMed] [Google Scholar]
- 47. Liu T., et al. (2013) Association between CYP2A6 genetic polymorphisms and lung cancer: a meta-analysis of case-control studies. Environ. Mol. Mutagen., 54, 133–140. [DOI] [PubMed] [Google Scholar]
- 48. Kumasaka N., et al. (2012) Haplotypes with copy number and single nucleotide polymorphisms in CYP2A6 locus are associated with smoking quantity in a Japanese population. PLoS One, 7, e44507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.