Abstract
Creativity is widely recognized as an essential skill for entrepreneurial success and adaptation to daily-life demands. However, we know little about the neural changes associated with creative capacity enhancement. For the first time, using a prospective, randomized control design, we examined longitudinal changes in brain activity associated with participating in a five-week design-thinking-based Creative Capacity Building Program (CCBP), when compared with Language Capacity Building Program (LCBP). Creativity, an elusive and multifaceted construct, is loosely defined as an ability to produce useful/appropriate and novel outcomes. Here, we focus on one of the facets of creative thinking—spontaneous improvization. Participants were assessed pre- and post-intervention for spontaneous improvization skills using a game-like figural Pictionary-based fMRI task. Whole-brain group-by-time interaction revealed reduced task-related activity in CCBP participants (compared with LCBP participants) after training in the right dorsolateral prefrontal cortex, anterior/paracingulate gyrus, supplementary motor area, and parietal regions. Further, greater cerebellar–cerebral connectivity was observed in CCBP participants at post-intervention when compared with LCBP participants. In sum, our results suggest that improvization-based creative capacity enhancement is associated with reduced engagement of executive functioning regions and increased involvement of spontaneous implicit processing.
Keywords: creativity, fMRI, functional connectivity, intervention, randomized control trial
Introduction
Creativity is considered the driving force behind all human progress—in science, business, politics, arts and even interpersonal relationships. It is no surprise that creativity is now widely recognized as an essential skill for personal and entrepreneurial success (Stavridou and Furnham 1996; Amabile 1997; Kern 2010), successful adaptation to daily-life demands (Csikszentmihalyi 1997; Reiter-Palmon et al. 1998; Sternberg and O'Hara 1999; Carson et al. 2003), psychological well-being (Cropley 1990; Solso 2001; Runco 2004; Gobel et al. 2011) and resilience (Runco 1991; Metzl 2009). Given the importance of this cognitive faculty, it is vital to examine the neural correlates of creative capacity enhancement so that effective augmentation strategies, informed by cognitive neuroscience, can be developed.
Although the lay definition of creativity can be simply stated as the ability to create novel and useful outcomes, the construct of creativity is elusive and complex (Abraham 2014). Creativity is not a unitary construct and researchers agree that a multifaceted approach is required to fully understand creativity. Thus, creativity has been a subject of intense research across disciplines. To evaluate creative potential in individuals, researchers have studied novelty and appropriateness of products or outcomes generated by established artists and writers (Colangelo et al. 1992; Carson et al. 2005). Prior work has also focused on understanding how individual differences in personality and outlook affect creativity (Csikszentmihalyi 1997; Wolfradt and Pretz 2001). Similarly, different environmental factors (e.g., upbringing or work culture) can affect creative capacity in individuals (Runco 2004). From the perspective of brain mechanisms underlying creative thinking, neuroscientific research has largely focused on studying individual components of creative thinking (Dietrich and Kanso 2010; Kozbelt et al. 2010; Abraham 2014). For example, investigators have used innovative experimental designs to study insight (or Aha! Moments) (Jung-Beeman et al. 2004; Aziz-Zadeh et al. 2009), conceptual expansion (Abraham et al. 2012), mental imagery (LeBoutillier and Marks 2003), and spontaneous improvization (Limb and Braun 2008; Saggar et al. 2015).
Given the fundamental importance of creativity in everyday life, as well as the fact that creativity can decline during early childhood (Torrance 1968; Kim 2011), a wide array of experimental studies have quantitatively examined and shown that creativity can be enhanced at the individual and team level (see Scott et al. 2004 for review). However, it can be argued that describing the neural correlates of creativity and its enhancement are essential for developing more neuroscience-informed approaches for fostering and sustaining creativity across the lifespan. For example, in future, neuroscience-informed training approaches could help us understand how brain development affects creative thinking, so that efficient interventions can be developed to reduce the impact of the so-called creative “slump” during middle childhood (Torrance 1968; Claxton et al. 2005). Additionally, researchers have also argued that better understanding of the neuroscience of creativity and including those insights into training is crucial for enhancing creative confidence in individuals (Onarheim and Friis-Olivarius 2013). However, very little is known about the neural correlates of creative capacity enhancement. Recently, Fink et al. employed functional Magnetic Resonance Imaging (fMRI) to examine longitudinal task-related changes in brain activity associated with a 3-week (20 min a day) at-home computerized verbal divergent thinking training program (Fink et al. 2015). Whole-brain voxel-wise analysis revealed longitudinal changes in brain activity during a divergent thinking task (when compared with a control task) in regions within the left inferior parietal cortex and the left middle temporal gyrus. In another study, Cousijn et al. examined changes in resting-state functional connectivity (RSFC) associated with a short 2-week at-home computerized divergent-thinking-based training program (comprised of 8 20-min sessions) in adolescents (Cousijn et al. 2014). Although no training-related changes in RSFC were observed, Cousijn et al. reported that performance over time was predicted by connectivity between left supramarginal gyrus and right occipital cortex at baseline.
Taken together, these studies provide first steps towards understanding changes in brain activity/connectivity associated with creative capacity enhancement. However, several knowledge gaps need to be addressed to further advance our understanding of this area. For example, both the Fink et al. and Cousijn et al. studies used short, at-home computerized training programs to enhance creativity in individuals. As such, the results from these studies may not be germane to understanding the neural basis of creativity occurring in group-settings or involving “hands-on” activities. Thus, it can be argued that brain activity changes associated with training programs such as those employed in the study described here could be more relevant to naturally occurring educational and vocational settings. Further, the training programs as well as assessments of creative capacity enhancement in both these studies were specifically limited to the divergent thinking component of creativity. Thus, it is not clear whether the knowledge or expertise gained in such focused training programs would apply broadly to other domains of creativity such as improvization, imagination, etc. Lastly, to tease apart the longitudinal changes in brain activity associated with creative capacity enhancement from those associated with training regimen itself (i.e., cognitive engagement and motivation), a parallel active control-training program is required.
To address some of these gaps, we conducted a 5-week design-thinking-based Creative Capacity Building Program (CCBP) and a parallel, control training program (Language Capacity Building Program [LCBP]) with healthy adults. Participants were randomly assigned to either training program after initial behavioral and neuroimaging assessments (Fig. 1). The CCBP was designed as an interactive group studio where students learned to improve their improvizational skills by increasing their bias towards taking action through hands-on experiences, rapid prototyping, and fail-faster exercises (see Materials and Methods; Hawthorne et al. 2013). The LCBP was designed as active control training where participants learned basic Chinese (Mandarin) vocabulary, character writing, and commonly used phrases via hands-on exercises. The LCBP was also performed in a group setting to simulate the shared environment atmosphere of the CCBP studio. We endeavored to keep CCBP and LCBP trainings identical in terms of hands-on exercises, stimulating group environment and instructor's motivation, while minimizing creative idea generation and consolidation during the LCBP training (Hawthorne et al. 2013). The overall study design incorporated a crossover component such that participants enrolled in the LCBP later received the CCBP training and vice versa (see Materials and Methods). However, the focus of current paper is limited to comparison of the initial parallel-group phase (i.e., before crossover).
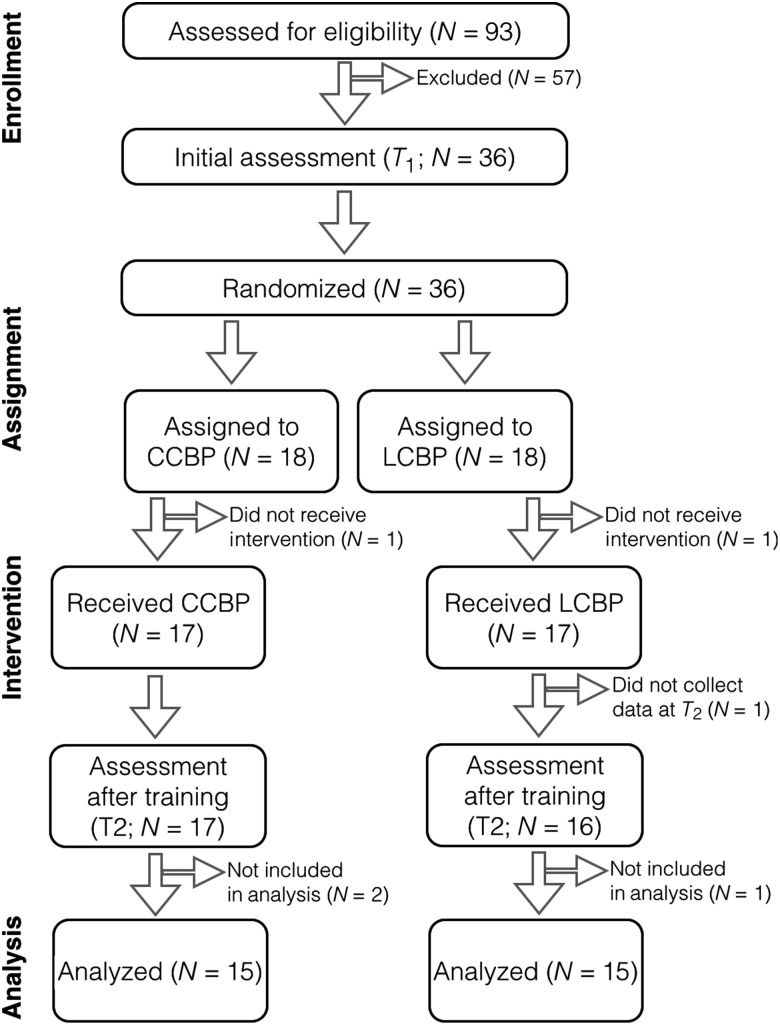
Figure 1.
Randomized control design to examine the neural correlates of creative capacity enhancement.
Using a standardized figural creative thinking assessment (i.e., the Torrance Test of Creative Thinking [TTCT-Figural; Torrance 1998]), we recently showed that participation in the CCBP, when compared with LCBP, led to increased creative capacity at post-intervention (Kienitz et al. 2014). For the TTCT-F, participants are given a set of incomplete figures and are asked to complete them in a set amount of time such that the final figure should portray a unique and interesting story (Torrance 1998). Out of the 5 subscales on which the final figures are (blindly) scored, 2 subscales (i.e., elaboration and resistance to closure) showed significant enhancement in the CCBP when compared with the LCBP participants after training. As the elaboration score on the TTCT-F captures details and imagination of responses to stimuli (Torrance 1998), enhanced elaboration scores suggest that CCPB participants generated detailed and more imaginative responses than participants in the LCBP. Furthermore, the resistance to premature closure on the TTCT-F is associated with the tendency to persist with a creative undertaking in the service of finding novel outcomes as opposed to stopping at more routine or predictable solutions (Torrance 1998). Thus, an increase in resistance to closure in CCBP participants after training suggests that they demonstrated greater persistence in arriving at novel outcomes.
As part of the overall study, we also examined changes in executive functioning associated with participation in the CCBP when compared with LCBP training (Bott et al. 2014). The Color-Word Interference Test (CWIT) was used as our primary outcome measure (Delis 2001). The CWIT is based on the Stroop measure (Stroop 1935) and it consists of four conditions. The first two conditions (color-naming and word-reading) assess processing speed, whereas the last two assess “higher-level” inhibition and cognitive flexibility. Interestingly, while no significant group by time interaction was observed for inhibition and cognitive flexibility scores, significant improvements in processing speed was observed in CCBP participants when compared with LCBP participants after training (Bott et al. 2014). As the color-naming and word-reading conditions of CWIT are highly automatic and prepotent processes in adults, we argued that participation in the CCBP led to increased productivity of automatic or implicit processes.
As creativity is not a unitary construct, examining the neural correlates of creative thinking or its enhancement is a daunting task. Thus, researchers are increasingly adopting a “process-driven” or component-based approach for examining neural correlates of creative thinking (Dietrich and Kanso 2010; Kozbelt et al. 2010; Abraham 2014). In this paper, to examine the longitudinal changes in brain activity associated with CCBP when compared with LCBP, we focused specifically on the spontaneous improvization component of creative thinking. Though just a component of creative thinking, improvization can also encapsulate prototypical creative behavior as a whole (Bengtsson et al. 2007). For example, during a musical improvization, a musician can freely generate behavioral choices, adapt these choices to the ongoing performance, monitor outcomes through auditory and somatosensory feedback, and preserve the overall esthetic goal (Pressing 1988). Consistent with this view, several studies have employed free-form spontaneous improvization in musicians to examine the neural correlates of creative thinking (Bengtsson et al. 2007; Limb and Braun 2008; Pinho et al. 2014).
In previous work, we investigated the neural correlates of drawing-based spontaneous improvization in the same nonartist/musician cohort described here before intervention. To engage participants in drawing-based improvization, we developed a novel fMRI task based on the social game of Pictionary™ (Saggar et al. 2015). In this task, using an MR-safe drawing tablet, participants were asked to improvize and draw representations of a given verb in 30 s with the caveat that others would later guess the word by their drawing alone. In line with musical improvization, during this Pictionary task, participants had to generate free choices to represent the given verb, adapt and monitor their sketching, while preserving the overall goal that the given verb should later be easily guessed by their drawing alone. To reduce anxiety and performance pressure, no explicit instructions were given to the participants to be “creative” during the task. However, the drawings were later scored for creative content and subjective ease of guessing by two creativity experts from Stanford's Design School (A.R. and G.H.). The neuroimaging results showed that higher expert-rated creative content in the drawings was associated with increased engagement of bilateral cerebellum, while task-related activation in the cingulate and dorsolateral prefrontal cortices negatively influenced task performance (i.e., ease of guessing the word) (Saggar et al. 2015). These results suggested a putative negative role of conscious monitoring and volitional control and a potentially positive role of implicit processing via cerebral-cerebellar interaction during spontaneous improvization (Saggar et al. 2015).
Extending this previous work, for the first time, here we report longitudinal changes in brain activity associated with a “live” group-based and domain-general creativity-training program (i.e., CCBP) when compared with an active control-training program (i.e., LCBP). The Pictionary-based fMRI task described above was used to assess the behavioral correlates of spontaneous improvization at both pre- and post-intervention. Based on the nature of the CCBP training (see Materials and Methods) as well as previous findings pertaining to the neural correlates of improvization (Limb and Braun 2008; Pinho et al. 2014; Saggar et al. 2015), we predicted that enhanced creativity in CCBP relative to LCBP participants would be associated with increased engagement of implicit cognitive processes and reduced engagement of conscious monitoring and volitional control processes. Specifically, at post-intervention, we predicted increased cerebellar–cerebral interaction and reduced prefrontal activation in CCBP participants relative to LCBP participants.
Materials and Methods
Participants and Study Design
Thirty-six healthy adults (18M, 18F) were enrolled in the study. Half of the participants were randomly assigned to either a 5-week CCBP or a parallel 5-week LCBP. Participants were assessed before and after intervention. See Figure 1 for a visual representation of the study design. Additional details regarding assessments are provided in Supplementary Methods.
Interventions
Details of the CCBP and LCBP interventions have been described elsewhere (Hawthorne et al. 2013; Bott et al. 2014; Kienitz et al. 2014). To summarize, both interventions included 5 2-h group classes conducted weekly. The CCBP was an abbreviated version of a highly popular class offered at the Stanford's Hasso Plattner Institute of Design called “Creative Gym” (http://dschool.stanford.edu/classes/#creative-gym-a-design-thinking-skills-studio), taught by the author G.H. Creative Gym course immerses students in a learning environment based in experimentation with an overall goal to enhance improvization and creative skills. To improvize novel solutions, students rapidly cycle through a series of phases: observe, brainstorm, synthesize, prototype, and implement; repeating as necessary. The class includes many hands-on exercises based on the following elements: 1) “see” (reducing perceptual bias to identify multiple perspectives to a give problem/issue); 2) “start/build” (inducing bias toward action by rapidly prototyping using limited everyday materials); 3) “feel” (enhancing perspective taking and empathy); 4) “communicate/inspire” (seeking active inspiration from everyday incidents and situations) and 5) “synthesize/navigate” (combining disparate constructs to transform independent sets of ideas into new directions). Taken together, the underlying goal of CCBP training was to make participants repeatedly exercise their improvizational skills in a group setting, thereby allowing them to improvize creative outcomes, even in situations where resources were limited or the results were uncertain.
We endeavored to keep CCBP and LCBP conditions comparable in terms of hands-on exercises, stimulating group environment and instructor's motivation, while minimizing improvization and creative thinking during LCBP training. As an active control, the LCBP was of same duration as CCBP and was conducted in parallel (at the same time) in a group setting and in a similar interactive studio as CCBP. The LCBP classes consisted of many hands-on exercises to learn basic Chinese vocabulary, character writing (Calligraphy), commonly used phrases (e.g., How are you?) and language learning group games. The author N.L., who is a bilingual native Mandarin speaker, was the LCBP instructor.
fMRI Task Design
Details of this task are presented elsewhere (Saggar et al. 2015). Briefly, the word-guessing fMRI task is based on a block-design paradigm, with 30 s block duration for each of the two conditions (word-drawing and zigzag-drawing). In the first condition, word-drawing, participants were asked to draw a given word (mainly actions/verbs) to the best of their ability using the MR-safe drawing tablet, with the caveat that others would later try to guess the word by their drawing alone (see Supplementary Fig. 2 for a set of sample drawings). To control for the basic motor and visuospatial aspect during the word-drawing condition, participants were also asked to make a drawing representing a control word (zigzag) in the second condition. Participants were asked to fully utilize the given 30 s in each block and continue to add elements to the illustration in case they wanted to finish early. Each block was separated by a fixation period with a random duration within the range of 10–15 s. There were a total of 10 blocks per condition and the total duration of task was approximately 14.5 min. For more details, see Supplementary Methods.
Behavioral Assessments
Two task-related behavioral assessments were employed in this study: 1) expert-rated representation and creativity ratings for each drawing (from the word-drawing condition) and 2) self-rated subjective rating of difficulty (as difficult, medium, or easy) in drawing each word by the participants during postscan questionnaire. Two raters from the Stanford Design School (authors A.R. and G.H.) were chosen to blindly rate each drawing (from the word-drawing condition) on the scales of representation and creativity. The instructions for the “representation” scale were as follows: “how easily do you think another person can guess the word represented by the drawing”. The ratings were obtained on a 5-point scale (1–5).
The creativity ratings of each drawing were assessed based on the 3 subscales of—fluency, elaboration, and originality. These subscales were chosen based on the assessment scheme used in the standardized test of creativity (TTCT-F; Torrance 1998). Scores from these three subscales were averaged to get the final score of creativity. Each subscale was defined as follows: 1) Fluency—total number of elements/ideas in the drawing; 2) Elaboration—imagination and exposition of detail; and 3) Originality—the statistical infrequency and unusualness/uniqueness of the response. The rating for each subscale was also done on a 5-point scale (1–5). The 2 raters were trained on a small sample of drawings (36 drawings) and their inter-rater reliability index for all the drawings (as measured by Intra-class Correlation Coefficient [ICC]) were 0.80 for representation and 0.884 for the creativity scale.
Although participants were highly encouraged to attend all 5 sessions (i.e., 10 h), a few participants still missed sessions partly or fully due to unanticipated family or work events. Thus, we recorded attendance based on the number of hours participants were present in these sessions. Further, for each 2-h session missed, participants were given 1-h worth of make-up homework to be completed before the next assessment. Overall, within the CCBP, 1 participant received 8 h of training, 6 received 9 h of training, and the rest received full 10 h of training.
MRI Image Acquisition and Data Analysis
Participants were imaged on a 3Tesla scanner (GE MR750, Milwaukee, WI, USA) at Stanford University's Center for Cognitive and Neurobiological Imaging (CNI) using a 32-channel radiofrequency receive head coil (Nova Medical, Inc., Wilmington, MA, USA). A total of 435 whole-brain volumes were collected on 42 axial-oblique slices (2.9 mm thick) prescribed parallel to the intercommissural (AC-PC) line, using a T2*-weighted gradient echo pulse sequence sensitive to blood oxygen level-dependence (BOLD) contrast with the following acquisition parameters: echo time (TE) = 30 ms, repetition time (TR) = 2000 ms, flip angle = 77°, FOV = 23.2 cm, acquisition matrix = 80 × 80, approximate voxel size = 2.9 × 2.9 × 2.9 mm. A high-resolution T1-weighted 3D BRAVO pulse sequence acquisition was acquired for co-registration with the following parameters: echo time (TE) = 2.8 ms, repetition time (TR) = 7.2 ms, flip angle = 12°, FOV = 23 cm, slice thickness = 0.9 mm, 190 slices in the sagittal plane; matrix = 256 × 256; acquired resolution = 0.9 × 0.9 × 0.9 mm. The images were reconstructed as a 256 × 256 × 190 matrix.
Functional MRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Details regarding the data preprocessing and functional analysis are provided in Supplementary Methods.
Results
Task-Related Behavioral Performance
To examine the intervention-related changes in behavioral performance, we ran repeated measures MANOVA on the factors of time (pre- and post-intervention) and rating scores (expert creativity score, expert representation score and subjective difficulty ratings) between the two groups (CCBP and LCBP). Only a trend towards significance was observed for the main interaction of time x rating scores x group (F2,27 = 2.945, P = 0.07, η2 = 0.18). As an exploratory post hoc analysis, we separately examined the effect size for between-group longitudinal differences in creativity ratings. We observed a medium effect size (Cohen's d = 0.44) for between-group longitudinal differences in creativity ratings, suggesting a tendency towards larger increase in creativity ratings in CCBP when compared with LCBP. Table 1 reports descriptive statistics for pre- and post-intervention scores for each rating.
Table 1.
Task-related behavioral performance
| CCBP |
LCBP |
|||
|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | |
| Mean expert creativity ratings (scale of 1–5) | 2.66 (SD = 0.30) | 2.78 (SD = 0.28) | 2.70 (SD = 0.21) | 2.74 (SD = 0.26) |
| Mean expert representation ratings (scale of 1–5) | 3.43 (SD = 0.72) | 3.25 (SD = 0.78) | 3.69 (SD = 0.77) | 3.24 (SD = 0.91) |
| Mean subjective difficulty in drawing each word (scale of 1−3) | 1.83 (SD = 0.23) | 1.78 (SD = 0.24) | 1.84 (SD = 0.26) | 1.95 (SD = 0.18) |
Task-Related Correlates of Changes in Brain Activity Associated with CCBP
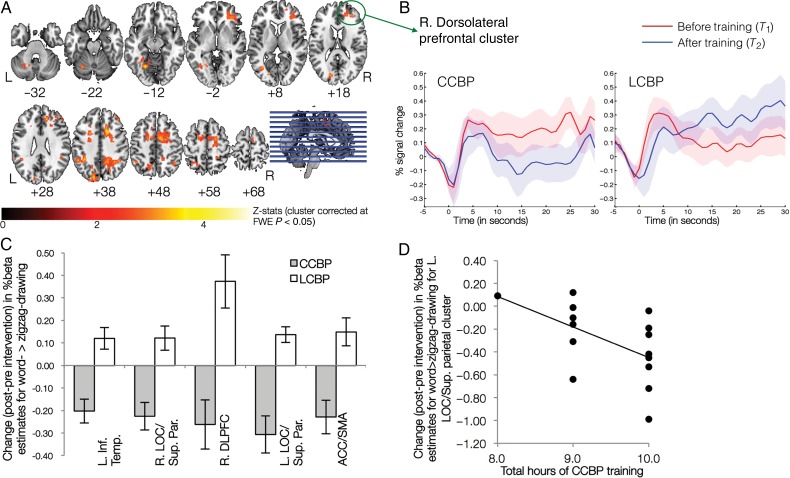
To reveal task-related changes in brain activity associated with CCBP when compared with LCBP, group (CCBP vs. LCBP) × time (pre- vs. post-intervention) interaction was performed for the primary task contrast (i.e., word- vs. zigzag-drawing). Five significant clusters were observed encompassing the supplementary motor area (SMA), anterior cingulate cortex (ACC), paracingulate gyrus, right dorsolateral prefrontal cortex (DLPFC), bilateral lateral occipital complexes (LOC) and parietal lobules, left temporal-occipital fusiform, lingual gyrus, and cerebellum (Fig. 2A and Supplementary Table 1). The same pattern of direction was evident across all clusters, i.e., the CCBP participants had reduced activity after training relative to baseline, whereas LCBP participants had increased activity after training (Fig. 2B,C).
Figure 2.
Neural correlates of creative capacity enhancement. (A) Shows the clusters found for the group by time interaction. (B) Average time course for a representative cluster (R. dorsolateral prefrontal) before and after training. Similar pattern of longitudinal change in activation, across groups, was observed in other 4 clusters. Band around the mean time course represents SEM across participants. (C) Mean change in activation for each of the 5 clusters, bars represent SEM across participants. (D) Observed relation between reduction in activity and hours of CCBP training (r(15) = −0.563, P = 0.029).
Although we asked participants to attend all sessions, due to unavoidable circumstances, a few participants received less than 10 h of training (see Materials and Methods). This discrepancy, however, provided an opportunity to assess whether longitudinal changes in neural activity were related to the amount of CCBP training received. Significant negative association was observed between training hours and reduced activity in the left occipital/parietal regions [r(15) = −0.563, P = 0.029], suggesting that the “dose” of CCBP training affected task-related reductions in brain activity at post-training (Fig. 2D). Negative associations were also observed between training hours and reduced activity in the other four clusters but did not reach significance.
Changes in Cerebellar-Cerebral Connectivity with Training
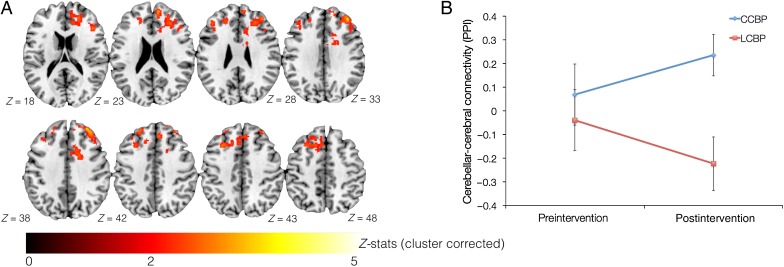
To examine longitudinal changes in cerebellar–cerebral connectivity associated with participation in the CCBP when compared with LCBP, longitudinal between-group PPI analyses were performed. Similar to examining neural correlates of creative capacity enhancement, we first calculated within-participant differences in cerebellar–cerebral connectivity across time (i.e., pre- vs. post-intervention), followed by comparing these longitudinal differences between groups (CCBP vs. LCBP). No significant group x time interaction was observed for the PPI analysis. However, at post-intervention, differences in the cerebellar–cerebral connectivity between groups were observed to be significant (Fig. 3 and Supplementary Table 2). Specifically, increased post-intervention cerebellar–cerebral connectivity was observed in the CCBP participants when compared with LCBP participants for the right ACC, bilateral paracingulate gyrus, right inferior frontal gyrus, bilateral DLPFC, bilateral frontal pole, and bilateral superior frontal gyrus. A similar between-group analysis at pre-intervention revealed no significant difference between the groups. Lastly, to visualize change in cerebellar–cerebral connectivity, beta estimates were extracted from pre- and post-intervention data (separately for each group) using the significant group-difference cluster observed at post-intervention. Qualitatively, an increase in cerebellar–cerebral connectivity was observed in CCBP participants, while a reduction was observed for LCBP participants (Fig. 3). Using the significant group-difference cluster observed at post-intervention as a mask, we also estimated the effect size for between-group longitudinal differences in cerebellar–cerebral connectivity. A medium-size effect was observed (Cohen's d = 0.52), suggesting a tendency toward larger increase in connectivity after participating in the CCBP when compared with LCBP.
Figure 3.
Differences in cerebellar–cerebral (PPI) connectivity between groups (CCBP > LCBP) at post-intervention. No significant group differences in PPI connectivity were observed at pre-intervention. (A) Shows the cluster with significant PPI differences between-group (CCBP > LCBP). (B) Visualization of the change in cerebellar–cerebral connectivity over time (i.e., before and after training). The cluster found at post-intervention (see A) was used to extract values at pre-intervention.
Discussion
Using a randomized control study design, we previously showed that participation in CCBP relative to LCBP was associated with: 1) increased creative capacity as measured by the standardized test of figural creativity (TTCT-F; Kienitz et al. 2014) and 2) increased automatic or implicit processing as measured by standardized neuropsychological testing (Bott et al. 2014). In this paper, using the same cohort from whom these behavioral data were obtained, we examined longitudinal changes in task-related brain activity/connectivity associated with improvization-based design-thinking training to enhance creativity when compared with an active control language-based training. Participants were assessed pre- and post-intervention for spontaneous improvization while performing the word-drawing social game of Pictionary during an fMRI scan. As predicted, at post-intervention, widespread task-related reduction in prefrontal and parietal brain activity was observed in the CCBP participants when compared with LCBP participants. Furthermore, the amount of decrease in parietal activation was associated with the quantity of training received (in hours), suggesting a “dose–response” relationship from CCBP training. Greater cerebellar–cerebral connectivity was also observed in the CCBP group post-intervention relative to LCBP. However, despite the fact that the two groups did not show significant differences at baseline, the group by time interaction for cerebellar–cerebral connectivity did not reach significance. The lack of a significant group by time interaction, despite a medium estimated effect size, may be related to the sample sizes used in this first of its kind study to examine the neural correlates of creative capacity enhancement. In sum, our data suggest that reduced engagement of executive functioning and volitional control, and increased involvement of implicit processing (via cerebellar–cerebral connectivity) might be associated with improvization-based creativity training.
A significant group by time interaction revealed widespread reduction of task-related activity in the CCBP participants when compared with LCBP participants at post-intervention. This training-related drop in activity was observed in several brain regions including the ACC/DLPFC, SMA, paracingulate gyrus, LOC and parietal lobules, left temporal-occipital fusiform, lingual gyrus, and cerebellum. The ACC and DLPFC regions are known to preferentially activate during effortful monitoring, evaluation and selection of relevant responses (MacDonald et al. 2000; Koechlin et al. 2003). Reduced activity in these regions after training could be explained by two plausible mechanisms. First, it is possible that CCBP training led to efficient utilization of cognitive control regions and thus, after design-thinking-based training, participants could exert such control effortlessly, that is, with less neural resources. Previous research using various cognitive tasks indicated that “efficient” utilization of neural resources occur with other kinds of training (a.k.a. expertise or practice effect) (Gobel et al. 2011). Alternatively, it is also possible that after CCBP training, participants focused less on monitoring, evaluating or selecting ideas and more on generating and synthesizing ideas. We argue that the second interpretation is more likely, because of the fact that CCBP was specifically designed to enhance improvization skills in participants by increasing their bias toward action. Thus, by regularly practicing improvization and rapid prototyping, participants in the CCBP training learned to increase engagement of implicit processing while reducing the involvement of volitional cognitive processing during improvization and creative thinking. Further, in our previous work in the same cohort, using only baseline (pre-intervention) data, we showed that activation in the ACC/DLPFC regions was negatively associated with representation ratings of the drawings (Fig. 2 in Saggar et al. 2015). Thus, a drop of task-related brain activity in these regions after CCBP training also suggests that participants may have focused less on selecting or inhibiting ideas and more on generating and synthesizing ideas during the word-drawing condition.
Other studies have also indicated that greater creativity is associated with reduced cognitive inhibition (Stavridou and Furnham 1996; Carson et al. 2003; Takeuchi et al. 2011). For example, using a latent-inhibition paradigm, Carson et al. (2003) showed that highly creative individuals have reduced early selective attention processing, thereby providing access to a larger inventory of unfiltered stimuli and increasing opportunities for synthesis of original (or unusual) ideas. Similarly, Stavridou and Furnham (1996) used a negative-priming paradigm to suggest that reduced cognitive inhibition during selective attention is responsible for “widening” of associative connections, thus allowing creative individuals to attend to many aspects of a given stimulus and produce more diverse associations. To deal with the large inventory of unfiltered stimuli, however, creative individuals are thought to engage additional intellectual qualities, for which overall intelligence might serve as a proxy (Sternberg and O'Hara 1999; Carson et al. 2003). Building upon these previous results and the fact that our participant sample had high intelligence scores (∼120; Table 2), reduced activity in the top-down cognitive control regions after CCBP training (when compared with LCBP training) might have enhanced improvization and creative capacity by facilitating diverse associations between ideas.
Table 2.
Participant demographics
| Number of participants | Total | CCBP | LCBP | t | P | |
|---|---|---|---|---|---|---|
| 30 (16F; 8 students) | 15 (8F; 4 students) | 15 (8F; 4 students) | ||||
| Age (years) | Mean | 28.77 | 27.67 | 29.87 | −1.09 | 0.28 |
| SD | 5.54 | 5.15 | 5.87 | |||
| I.Q. | Mean | 120.67 | 119 | 122.33 | −0.86 | 0.40 |
| SD | 10.52 | 11.81 | 9.16 | |||
| Gross income | Mean | 5.23 | 5.2 | 5.27 | −0.08 | 0.94 |
| SD | 2.22 | 1.61 | 2.76 | |||
| Creative achievement (CAQ) | N | 20.93 | 23.2 | 18.67 | 0.87 | 0.39 |
| SD | 14.14 | 13.27 | 15.08 | |||
| TTCT-F average | Mean | 110.31 | 111.267 | 109.286 | 0.49 | 0.63 |
| SD | 10.7 | 9.25 | 12.33 |
The two groups had equal number of participants, females, and students. Groups did not differ at baseline in terms of age, intelligence, gross household income, previous creative achievements and performance on the standardized test of creativity (TTCT-F).
It is important to question, however, whether creativity requires evaluation or selection of ideas at all. Some might argue that to choose an original or unusual response, one has to evaluate and reject other less novel responses (Runco 1991). Thus, evaluation seems to be a necessary component in creative thinking. In a recent study, Beaty et al. (2015) showed engagement of large-scale brain networks of executive functioning, in addition to spontaneous thought processes, during a divergent thinking task (i.e., to find alternate uses of a given object). Several other studies have also reported engagement of executive function and/or cognitive control regions during creative thinking for similar divergent thinking tasks (Kowatari et al. 2009; Aziz-Zadeh et al. 2013). Our finding of reduced engagement of executive functioning regions with improvization-based training and associated creative capacity enhancement may sound contradictory at first, but the observed differences could be mainly due to the task design. Instead of using divergent thinking tasks where participants are explicitly asked to produce an “alternate” or “unusual” answer to a given problem or question, we used spontaneous improvization as a means to examine the neural correlates of implicit creative thinking. We argued that implicit paradigms could play an essential role in reducing variation in creativity neuroimaging results by minimizing confounding influences of cognitive processes that might not be directly related to creative thinking, but are employed due to task design (Saggar et al. 2015). Thus, it is possible that different components of creativity (e.g., improvization vs. divergent thinking) may exploit different cognitive resources. Future research is required to systematically compare longitudinal changes in brain activity associated with creativity trainings that are focused on different components of creativity.
Our finding of reduced engagement of executive functioning areas with creative capacity enhancement in nonartists/musicians is in line with the previous literature in expert musicians where improvization tasks were used to assess creativity. For example, using a musical improvization task, Limb and Braun (2008) have found that enhanced creativity in expert Jazz musicians was associated with reduced recruitment of executive functioning regions. Similarly, more recent studies have shown deactivations in the executive functioning regions (especially the DLPFC) during musical improvization (Liu et al. 2012; Pinho et al. 2014) and poetry generation (Liu et al. 2015) as a sign of reduced monitoring and volitional control.
Interestingly, although we observed a reduction in task-related activity in some cerebellar areas associated with CCBP training, we also observed greater functional connectivity between the dorsolateral prefrontal cortex and cerebellum in CCBP (when compared with LCBP) participants at post-intervention. Cerebellar–cerebral functional connectivity has been previously hypothesized to facilitate implicit processing during creative thinking (Vandervert et al. 2007; Ito 2008). Especially when confronted with novel situations (as in the case of improvization), prefrontal-cerebellar connectivity has been postulated to provide “rapid” manipulation of conceptual ideas for making a quick decision (Leiner et al. 1986). These hypotheses are based on the premise that the cerebellum might facilitate efficient manipulation of mental representations as well as movements; the widespread anatomical connectivity between cerebellum and prefrontal regions in humans supports this premise (Schmahmann 1991; Ito 1993, 2006; Vandervert et al. 2007; Buckner 2013).
To better understand the role of cerebellar–cerebral connectivity and implicit processing during creative thinking, Ito proposed a theoretical analog of how the cerebellum facilitates motor learning from the viewpoint of control (Ito 2008). To achieve speed, accuracy, and automaticity in executing motor commands, researchers have proposed that the commands directed towards movement control regions in the cerebral cortex also get copied as “internal models” (forward or inverse) in the cerebellum for simulating natural body movements (Ito 2005). Through repeated and parallel simulations, the cerebellum facilitates acquisition of advanced motor skills via forward models and eventually provides automaticity via inverse models. In humans, based on the extensive cerebellar–cerebral anatomical connectivity (Ito 1997), it can be speculated that this theoretical model of motor learning can be extended to higher order cognitive functioning and thought processing (Ramnani 2006; Vandervert et al. 2007; Ito 2008; Koziol et al. 2014). Thus, analogous to voluntary movement, one could speculate the forward models in cerebellum could facilitate faster acquisition and processing of information (e.g., quick arithmetic calculation), whereas the inverse models in cerebellum could enable automaticity in thinking and idea generation (e.g., “aha” moments) (Ito 1997).
Although the postulated role of cerebellum in thought control mechanism by Ito et al. is appealing, admittedly we know very little about how our brain stores and manipulates a thought (Ito 2011, pp. 19–20). Empirical evidence, however, is beginning to confirm the role of cerebellar–cerebral connectivity in thinking in general and creative improvization in particular. In previous work, also using PPI analysis, de Manzano and Ullén (2012) demonstrated cerebellar–cerebral connectivity was higher during improvization when compared with other control conditions. Similarly, in a recent study, Pinho et al. (2014) also showed increased cerebral-cerebellar functional connectivity in expert musicians during improvization. In our previous work, using the same participant-set and Pictionary-based fMRI task as presented in this paper, we showed that activation in the cerebellum uniquely and linearly increased with increasing expert creativity ratings at pre-intervention (Saggar et al. 2015). When considered in the light of previous theoretical and empirical findings, our current result of higher cerebellar–cerebral connectivity associated with CCBP training (when compared with LCBP) provides preliminary evidence for a potentially direct role of cerebellar–cerebral connectivity in improvization-based creative capacity enhancement. Using Ito's model for the cerebellum's role in mental activities (Ito 2008), we can speculate that an increase in functional connectivity between dorsolateral prefrontal cortex and cerebellum would allow the cerebellum to emulate the “controller” function of prefrontal cortex using inverse internal models and, in turn, facilitate efficient improvization of word-drawings.
Previous neuroimaging work has shown activations in specific cerebellar regions while participants learned to acquire input–output properties of controlled objects, including tools (see Imamizu and Kawato 2012 for review). In the current work, participants used an MR-safe drawing tablet for sketching word representation. Thus, it is possible that some of the longitudinal changes in cerebellar activation/connectivity could be due to learning how to dexterously use the tablet. However, we argue that it is unlikely that the primary driver for observed longitudinal changes in cerebellar activity/connectivity is tablet-related dexterity, due to following reasons. First, we endeavored to reduce tablet-related effects on results by training the participants on how to use the drawing tablet before they entered the MR scanner, both at pre- and post-intervention assessments. Second, due to the study design, any longitudinal effects associated with tablet-use should get canceled as both groups (CCBP and LCBP) employed the tablet during the fMRI task at pre- and post-intervention. Third, and specific to cerebellar–cerebral connectivity results, if dexterity of tablet-use was driving longitudinal changes in cerebellum, we should have observed changes in connectivity between sensory/motor areas and cerebellum. Instead, we observed greater connectivity between cerebellum and prefrontal regions, thereby suggesting that cerebellum was facilitating idea manipulation rather than movement manipulation.
Cerebro-cerebellar connectivity has also been previously hypothesized to facilitate creative thinking and innovation by enhancing working memory functioning (Vandervert 2003, 2015; Vandervert et al. 2007). As per Baddeley's well-known working memory model, three components—central executive, phonological loop, and visuospatial sketchpad—work together for successful implementation of working memory (Baddeley 2003). Using the internal models of cerebellum (i.e., forward and inverse modeling), the cerebro-cerebellar connectivity has been hypothesized to enhance information processing in each of the three components of working memory; such increase in efficient processing of working memory operations is in turn thought to increase the likelihood of creative solutions (Vandervert 2003; Koziol et al. 2014). Recent neuroimaging findings support the premise that cerebro-cerebellar connectivity plays a role in efficient processing of working memory operations (Marvel and Desmond 2010, 2012; Sobczak-Edmans et al. 2016). Thus, from the perspective of working memory, it can be argued that our figural task could have enlisted the visuospatial sketchpad for temporary storage and manipulation of visuospatial information as well as for bottom-up planning of hand movements to draw the representation. Additionally, our task could have also employed the central executive component to facilitate coordination between the low-level components and in selectively attending and inhibiting ideas for drawing the word. The neuroimaging results for task-related activation, using just the pre-intervention data, supports the enlisting of visuospatial and central executive components of working memory during the word-drawing task (Saggar et al. 2015). The longitudinal analysis presented here shows greater cerebro-cerebellar connectivity between the dorsolateral prefrontal regions and cerebellum associated with creativity training. As the dorsolateral prefrontal region has been previously implicated to host the central executive component of working memory (Baddeley 2003), our results again suggest that creative capacity enhancement could be associated with emulation of the prefrontal “controller” function by internal cerebellar models.
Although we mainly focused on examining the neural correlates of creative capacity enhancement, a brief discussion is warranted regarding the longitudinal changes associated with LCBP. Opposite to the pattern observed in CCBP participants, the group × time interaction revealed an increase in task-related activity in LCBP participants after training. Furthermore, the PPI analysis revealed reduced cerebro-cerebellar task-related connectivity at post-intervention in LCBP (when compared with CCBP) participants. Given that the LCBP included exercises on learning Mandarin language, which presumably requires higher mental control (Tu et al. 2015), it can be argued that the task-related increase in activity in the cognitive control regions and SMA might be due to increased evaluation and motor planning. Furthermore, as the LCBP did not include improvization-based exercises, we did not expect cerebro-cerebellar connectivity to be stronger at post-intervention.
It is important to note that due to the nature of our fMRI task (i.e., improvization and drawing) and our design-thinking-based training paradigm, our results might not generalize to other components of the broadly defined construct of creativity. Future work is required to extend our creativity-conducive task and training paradigms to other components of creative thinking. In designing our fMRI task, we used zigzag-drawing as a control for basic motor and visuospatial processing that is also required during the word-drawing condition. However, it is possible that zigzag-drawing might not provide an optimal control for the degree of language processing required for the word-drawing condition. Furthermore, it might be argued that the overall cognitive load required during the word-drawing condition was not matched in the zigzag-drawing condition. The previous work has suggested that such imbalance between creative and control conditions can produce activation maps that are not necessarily specific to creative thinking (Abraham et al. 2012). However, this potential limitation of the control condition should not affect the between-group task-related results presented in this paper as both groups participated in the study across both time points. Furthermore, as a post hoc analysis, we also compared between-group longitudinal changes in brain activity associated with just the word-drawing blocks (i.e., without the zigzag-drawing condition). Comparing just the word-drawing blocks across time and groups resulted in similar reduction in task-related activity in the cingulate and prefrontal regions in CCBP (when compared with LCBP) participants as observed with including zigzag-drawing as a control condition (Supplementary Fig. 3).
We did not observe a significant group by time interaction for expert creativity ratings for the drawings made during the fMRI task. The lack of significance could be attributed to low statistical power or the fact that our task was not standardized. However, using the same set of individuals, we previously showed that participation in the CCBP led to increased standardized scores of creativity (TTCT-F) after training compared with LCBP participation (Kienitz et al. 2014). Additionally, we did not observe a significant group by time interaction for the whole-brain PPI analysis, which was conducted to examine between-group longitudinal changes in cerebellar–cerebral connectivity. PPI analyses tend to lack power and hence are prone to higher false negatives (O'Reilly et al. 2012), as the psychophysiological interaction time series is likely correlated with both the psychological and seed ROI time series. Post hoc power analysis revealed that given α = 0.05 and observed effect size of 0.52, future studies with a group size of 98 participants may be required to observe significant differences for the group by time interaction of interest. Nonetheless, significant group differences in cerebellar–cerebral connectivity at post-intervention provide an interesting avenue for future research.
Lastly, although we endeavored to keep the CCBP and LCBP trainings comparable in all aspects, except that of creativity, it is possible that some of the results presented in this study could be associated with differences in instructor motivation and teaching style.
Altogether, for the first time, we reveal the neural correlates of improvization-based creative capacity enhancement in adults using a randomized control design. Our data suggest reduced engagement of cognitive monitoring and volitional control and putatively higher cerebellar–cerebral connectivity associated with improvization-based creativity training. We anticipate that these results can guide future efforts to develop and measure the efficacy of interventions to enhance improvization-based creativity in adults and children across lifespan.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by a Hasso Plattner Design Thinking Research Program grant to A.L.R. Additional support was provided by the NIH K99-MH104605 grant to M.S.
Supplementary Material
Notes
We thank the staff at Stanford's Design School, Center for Interdisciplinary Brain Sciences Research, and Center for Cognitive and Neurobiological Imaging for their support. Conflict of Interest: None declared.
References
- Abraham A. 2014. Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Front Hum Neurosci. 8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Pieritz K, Thybusch K, Rutter B, Kröger S, Schweckendiek J, Stark R, Windmann S, Hermann C. 2012. Creativity and the brain: uncovering the neural signature of conceptual expansion. Neuropsychologia. 50:1906–1917. [DOI] [PubMed] [Google Scholar]
- Amabile TM. 1997. Entrepreneurial creativity through motivational synergy. J Creative Behavior. 31:18–26. [Google Scholar]
- Aziz-Zadeh L, Kaplan JT, Iacoboni M. 2009. “Aha!”: The neural correlates of verbal insight solutions. Hum Brain Mapp. 30:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Liew S-L, Dandekar F. 2013. Exploring the neural correlates of visual creativity. Soc Cogn Affect Neurosci. 8:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. 2003. Working memory: looking back and looking forward. Nat Rev Neurosci. 4:829–839. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Barry Kaufman S, Silvia PJ. 2015. Default and executive network coupling supports creative idea production. Sci Rep. 5:10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Csíkszentmihályi M, Ullén F. 2007. Cortical regions involved in the generation of musical structures during improvisation in pianists. J Cogn Neurosci. 19:830–842. [DOI] [PubMed] [Google Scholar]
- Bott N, Quintin E-M, Saggar M, Kienitz E, Royalty A, Hong DW-C, liu N, Chien Y-H, Hawthorne G, Reiss AL. 2014. Creativity training enhances goal-directed attention and information processing. Thinking Skills Creat. 13:120–128. [Google Scholar]
- Buckner RL. 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 80:807–815. [DOI] [PubMed] [Google Scholar]
- Carson SH, Peterson JB, Higgins DM. 2003. Decreased latent inhibition is associated with increased creative achievement in high-functioning individuals. J Pers Soc Psychol. 85:499–506. [DOI] [PubMed] [Google Scholar]
- Carson SH, Peterson JB, Higgins DM. 2005. Reliability, validity, and factor structure of the creative achievement questionnaire. Creat Res J. 17:37–50. [Google Scholar]
- Claxton AF, Pannells TC, Rhoads PA. 2005. Developmental trends in the creativity of school-age children. Creat Res J. 17:327–335. [Google Scholar]
- Colangelo N, Kerr B, Hallowell K. 1992. The Iowa inventiveness inventory: toward a measure of mechanical inventiveness. Creat Res. 5:157–163. [Google Scholar]
- Cousijn J, Zanolie K, Munsters RJM, Kleibeuker SW, Crone EA. 2014. The relation between resting state connectivity and creativity in adolescents before and after training. PLoS ONE. 9:e105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley AJ. 1990. Creativity and mental health in everyday life. Creat. Res. J. 3:167–178. [Google Scholar]
- Csikszentmihalyi M. 1997. Creativity: Flow and the Psychology of Discovery and invention. New York: HarperCollins Publications. [Google Scholar]
- Delis DC. 2001. Delis-Kaplan Executive Functioning System (D-KEFS). San Antonio: The Psychological Corporation. [Google Scholar]
- de Manzano Ö, Ullén F. 2012. Activation and connectivity patterns of the presupplementary and dorsal premotor areas during free improvisation of melodies and rhythms. NeuroImage. 63:272–280. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. 2010. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull. 136:822–848. [DOI] [PubMed] [Google Scholar]
- Fink A, Benedek M, Koschutnig K, Pirker E, Berger E, Meister S, Neubauer AC, Papousek I, Weiss EM. 2015. Training of verbal creativity modulates brain activity in regions associated with language- and memory-related demands. Hum Brain Mapp. 36:4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel EW, Parrish TB, Reber PJ. 2011. Neural correlates of skill acquisition: decreased cortical activity during a serial interception sequence learning task. NeuroImage. 58:1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne G, Quintin E-M, Saggar M, Bott N, Keinitz E, liu N, Chien Y-H, Hong D, Royalty A, Reiss AL. 2013. Impact and Sustainability of Creative Capacity Building: The Cognitive, Behavioral, and Neural Correlates of Increasing Creative Capacity. Cham: Springer International Publishing; p. 65–77. [Google Scholar]
- Imamizu H, Kawato M. 2012. Cerebellar internal models: implications for the dexterous use of tools. Cerebellum. 11:325–335. [DOI] [PubMed] [Google Scholar]
- Ito M. 2005. Bases and implications of learning in the cerebellum--adaptive control and internal model mechanism. Prog Brain Res. 148:95–109. [DOI] [PubMed] [Google Scholar]
- Ito M. 2006. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 78:272–303. [DOI] [PubMed] [Google Scholar]
- Ito M. 1997. Cerebellar microcomplexes. In: Schmahmann JD, editor. The cerebellum and cognition. New York: Academic; p. 475–487. [Google Scholar]
- Ito M. 2008. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 9:304–313. [DOI] [PubMed] [Google Scholar]
- Ito M. 1993. Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci. 16:448–450. [DOI] [PubMed] [Google Scholar]
- Ito M. 2011. The cerebellum: brain for an implicit self. Upper Saddle River: FT: Press. [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, Reber PJ, Kounios J. 2004. Neural activity when people solve verbal problems with insight. PLoS Biol. 2:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F. 2010. What Chief Executives Really Want. Bloomberg Businessweek. [Google Scholar]
- Kienitz E, Quintin E-M, Saggar M, Bott NT, Royalty A, Hong DW-C, liu N, Chien Y-H, Hawthorne G, Reiss AL. 2014. Targeted intervention to increase creative capacity and performance: a randomized controlled pilot study. Thinking Skills Creat. 13:57–66. [Google Scholar]
- Kim KH. 2011. The creativity crisis: the decrease in creative thinking scores on the torrance tests of creative thinking. Creat Res J. 23:285–295. [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. 2003. The architecture of cognitive control in the human prefrontal cortex. Science. 302:1181–1185. [DOI] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M. 2009. Neural networks involved in artistic creativity. Hum Brain Mapp. 30:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbelt A, Beghetto RA, Sternberg RJ. 2010. Theories of creativity. In: Kaufman JC, Sternberg RJ, editors. The Cambridge Handbook of Creativity. Cambridge: p. 20–47. [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K et al. 2014. Consensus paper: the cerebellum's role in movement and cognition. In: Presented at the Cerebellum London, England p. 151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoutillier N, Marks DF. 2003. Mental imagery and creativity: a meta-analytic review study. Br J Psychol. 94:29–44. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. 1986. Does the cerebellum contribute to mental skills? Behav Neurosci. 100:443. [DOI] [PubMed] [Google Scholar]
- Limb CJ, Braun AR. 2008. Neural substrates of spontaneous musical performance: an FMRI study of jazz improvisation. PLoS ONE. 3:e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chow HM, Xu Y, Erkkinen MG, Swett KE, Eagle MW, Rizik-Baer DA, Braun AR. 2012. Neural correlates of lyrical improvisation: an FMRI study of freestyle rap. Sci Rep. 2:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Erkkinen MG, Healey ML, Xu Y, Swett KE, Chow HM, Braun AR. 2015. Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Hum Brain Mapp. 36:3351–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 288:1835–1838. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. 2010. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex. 46:880–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. 2012. From storage to manipulation: How the neural correlates of verbal working memory reflect varying demands on inner speech. Brain and Language. 120:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzl ES. 2009. The role of creative thinking in resilience after hurricane Katrina. Psychol. Aesthet Creat. Arts. 3:112–123. [Google Scholar]
- Onarheim B, Friis-Olivarius M. 2013. Applying the neuroscience of creativity to creativity training. Front Hum Neurosci. 7:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. 2012. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho AL, de Manzano Ö, Fransson P, Eriksson H, Ullén F. 2014. Connecting to create: expertise in musical improvisation is associated with increased functional connectivity between premotor and prefrontal areas. J Neurosci. 34:6156–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. 2006. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 7:511–522. [DOI] [PubMed] [Google Scholar]
- Reiter-Palmon R, Mumford MD, Threlfall KV. 1998. Solving everyday problems creatively: the role of problem construction and personality type. Creat Res J. 11:187–197. [Google Scholar]
- Runco MA. 2004. Creativity. Annu Rev Psychol. 55:657–687. [DOI] [PubMed] [Google Scholar]
- Runco MA. 1991. The evaluative, valuative, and divergent thinking of children. J Creat Behav. 25:311–319. [Google Scholar]
- Saggar M, Quintin E-M, Kienitz E, Bott NT, Sun Z, Hong W-C, Chien Y-H, liu N, dougherty RF, Royalty A et al. 2015. Pictionary-based fMRI paradigm to study the neural correlates of spontaneous improvisation and figural creativity. Sci Rep. 5:10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. 1991. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 48:1178–1187. [DOI] [PubMed] [Google Scholar]
- Scott G, Leritz LE, Mumford MD. 2004. The effectiveness of creativity training: A quantitative review. Creat Res J. 16:361–388. [Google Scholar]
- Sobczak-Edmans M, Ng THB, Chan YC, Chew E, Chuang KH, Chen SHA. 2016. Temporal dynamics of visual working memory. NeuroImage. 124:1021–1030. [DOI] [PubMed] [Google Scholar]
- Solso RL. 2001. Brain activities in a skilled versus a novice artist: an fMRI study. Leonardo. 34:31–34. [Google Scholar]
- Stavridou A, Furnham A. 1996. The relationship between psychoticism, trait-creativity and the attentional mechanism of cognitive inhibition. Pers Individual Differ. 21:143–153. [Google Scholar]
- Sternberg R, Ohara PT. 1999. Handbook of Creativity. Cambridge University Press. [Google Scholar]
- Stroop JR. 1935. Studies of interference in serial verbal reactions. J Exp Psychol. 18:643–662. [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R. 2011. Failing to deactivate: the association between brain activity during a working memory task and creativity. NeuroImage. 55:681–687. [DOI] [PubMed] [Google Scholar]
- Torrance EP. 1968. A longitudinal examination of the fourth grade slump in creativity. Gifted Child Q. 12:195–199. [Google Scholar]
- Torrance EP. 1998. Torrance tests of creative thinking: Norms-technical manual: Figural (streamlined) forms A & B. Earth City: Scholastic Testing Services. [Google Scholar]
- Tu L, Wang J, Abutalebi J, Jiang B, Pan X, Li M, Gao W, Yang Y, Liang B, Lu Z et al. 2015. Language exposure induced neuroplasticity in the bilingual brain: a follow-up fMRI study. Cortex. 64:8–19. [DOI] [PubMed] [Google Scholar]
- Vandervert L. 2015. How music training enhances working memory: a cerebrocerebellar blending mechanism that can lead equally to scientific discovery and therapeutic efficacy in neurological disorders. Cerebellum Ataxias. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervert LR. 2003. How working memory and cognitive modeling functions of the cerebellum contribute to discoveries in mathematics. New Ideas Psychol. 21:159–175. [Google Scholar]
- Vandervert LR, Schimpf PH, Liu H. 2007. How working memory and the cerebellum collaborate to produce creativity and innovation. Creat Res J. 19:1–18. [Google Scholar]
- Wolfradt U, Pretz JE. 2001. Individual differences in creativity: personality, story writing, and hobbies. Eur J Pers. 15:297–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.