Abstract
Current research in connectomics highlights that self-organized functional networks or “communities” of cortical areas can be detected in the adult brain. This perspective may provide clues to mechanisms of treatment response in psychiatric conditions. Here we examine functional brain community topology based on resting-state fMRI in adult Attention-Deficit/Hyperactivity Disorder (ADHD; n = 22) and controls (n = 31). We sought to evaluate ADHD patterns in adulthood and their modification by short term stimulants administration. Participants with ADHD were scanned one or two weeks apart, once with medication and once without; comparison participants were scanned at one time-point. Functional connectivity was estimated from these scans and community detection applied to determine cortical network topology. Measures of change in connectivity profile were calculated via a graph measure, termed the Node Dissociation Index (NDI). Compared to controls, several cortical networks had atypical connectivity in adults with ADHD when withholding stimulants, as measured by NDI. In most networks stimulants significantly reduced, but did not eliminate, differences in the distribution of connections between key brain systems relative to the control sample. These findings provide an enriched model of connectivity in ADHD and demonstrate how stimulants may exert functional effects by altering connectivity profiles in the brain.
Keywords: resting state, functional connectivity, stimulants, MRI, ADHD
Introduction
Brain connectomics, at the intersection of neuroscience and information theory, provides a framework by which we can begin to understand the complex architecture underlying human affect and cognition (Hagmann et al. 2012). One hypothesis, based on recent discoveries regarding large-scale brain organization, is that many mental health disorders will show a measurable atypical organization in structural and functional brain network topology (Bassett and Bullmore 2009; Bressler and Menon 2010; Power et al. 2011; van den Heuvel and Sporns 2011; Yeo et al. 2011). Network properties derived from the discipline of graph theory are likely to provide informative markers for conditions like attention-deficit/hyperactivity disorder (ADHD) (e.g. see Uddin et al. 2008; Fair et al. 2010) where the body of research up to this point indicates that no single locus in the brain may account for the heterogeneous and atypical behaviors that characterizes the disorder (Rubia et al. 2009; Wang et al. 2009; Nagel et al. 2011; Hart et al. 2013).
A key question concerning the nature of large-scale brain organization, for those presenting with neuropsychiatric disorders, is the detection and characterization of functional relations in terms of their community structure, which can also be called functional topology. Community structure is conceptually concerned with the segmenting or clustering of a set of regions (referred to as nodes) into unique, highly-connected subsystems which likely perform related functions (Fortunato 2010). The conceptual framework of community structure allows for a determination of how closely related any two brain systems are, how information may be passed between them, which regions are functionally segregated, and which are highly integrated. The ability to detect this type of system organization has been of significant practical importance for understanding the nature of complex brain systems in typical populations (Fair et al. 2009; Bressler and Menon 2010; Power et al. 2011; Stevens et al. 2012).
While prior studies have examined regional and networked brain activation patterns in adults with ADHD (e.g., Cortese et al. 2012; Hoekzema et al. 2014), few studies have examined brain wide community structure, network integration, and differentiation across multiple networks in adults (or children) with the disorder. Thus, the first aim of the current report is to examine the nature of community structure in a sample of adults with and without ADHD.
The second aim examines whether and in what way stimulant medication alters the organization of this large-scale functional topology in the short term. This can shed light on how stimulants exert their effects. In ADHD, symptoms can be modified by short acting psychostimulant medication (Tomasi et al. 2011; Volkow et al. 2012; Cubillo et al. 2014). In addition, several reports have documented either task related changes after stimulant medications in individuals with ADHD (Cubillo et al. 2014; Rubia et al. 2014), or circuit level connectivity changes after stimulant administration in individuals with ADHD (Li et al. 2013; Sripada et al. 2013; Mueller et al. 2014; Querne et al. 2014). Results in these studies indicate regions in the anterior cingulate, insula, inferior frontal cortex and parietal cortices. In general, findings have been distributed in various parts of the cortex depending on the study, suggesting that these medication effects are numerous and complex. These facts highlight the need to compliment fMRI and circuit based connectivity studies by experimentally examining systematic and specific alterations in large-scale functional topology associated with ADHD and stimulant administration (Hart et al. 2013). Such brain-wide, multi-system, state characteristics afforded by resting-state functional connectivity (rs-fcMRI) and graph theory will provide information on stimulant effects on brain organization that is complementary with the moment to moment reactivity induced by any given task fMRI experiment (Raichle 2010). Thus, in this report we test the hypothesis that changes in pharmacological intervention with stimulant medications does indeed alter brain network topology and the integration of multiple brain-wide networks as one of its mechanisms of short term action.
Materials and Methods
Participants
A group of 22 right-handed adult subjects (aged 18–35, mean(SD) 25.1(5.1), 14 female) who were previously diagnosed with ADHD (by self-report) and currently prescribed a stimulant medication and whose diagnosis was validated by us (below) formed the ADHD group. A group of 31 healthy control subjects, aged 19–35, mean 26.5(4.2), 18 females were also examined as an unaffected comparison group. All participants provided informed written consent consistent with the Oregon Health & Science University institutional review board.
All potential participants in the ADHD group underwent a diagnostic review to confirm diagnosis (described below), though diagnostic procedures were conducted on all participants to ensure comparability. The procedures included a structured clinical interview with the Structured Clinical Interview for DSM-IV (SCID-I/P) and Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Edition (K-SADS-PL) to enable confirmation of diagnostic criteria and co-occurring disorders, as well as severity of current ADHD symptoms. The K-SADS ADHD module was administered in modified form twice, to evaluate current symptoms and recalled childhood symptoms. The K-SADS provides a measure of inattentive and hyperactive symptom counts that were used for severity subgrouping.
A Wechsler Abbreviated Scale of Intelligence (WASI) was administered to estimate full-scale IQ, and the Wide Range Achievement Test (WRAT-4) word reading test was administered to enable approximation of possible learning disability.
Exclusion criteria for both groups included current (within past 6 months) major depressive episode or substance abuse disorder, significant head or neurological trauma, lifetime mania or psychotic disorder, as well as evidence of a learning disability or history of autism spectrum disorder. Additionally, an FSIQ < 85, reduced English proficiency (though not English as a second language), or a significant sensory motor handicap were also exclusion criteria.
Consensus diagnosis between two clinicians was then obtained via independent case review and case consultation considering reported childhood and current symptoms as well as comorbid disorders. ADHD was identified using DSM-IV criteria (i.e., requiring six symptoms and probable onset by age 7), which were current at the time of sample ascertainment. K-SADS combined inattentive and hyperactive scores of ADHD group; mean(SD) 11(3.6). Again using DSM-IV criteria, based on current symptom profile (ignoring symptom history) of the participants with ADHD, 12 met criteria for current combined subtype, nine for current predominantly inattentive subtype, and one for predominantly hyperactive-impulsive subtype (excluded from severity/subtype analysis due to excessive movement without medication). All control participants in our sample had 1.5 or fewer ADHD symptoms for either symptom domain (K-SADS-Control: mean(SD), 0.4(0.7)).
Study Design
A resting-state scan was collected for Control subjects at one time-point. ADHD subjects were scanned on two separate days, either one or two weeks apart according to availability, in order to match the day of the week and the time of the day on both scans. For one scan, the ADHD subjects (n = 22) were asked to administer their medication at the effective prescribed dosage at least 60 and no more than 180 min prior to the scan, depending on medication type. For the other scan, they abstained from their medication for 48 or 72 h prior, depending on medication type (n = 19), to achieve a 7 half-life washout. The loss of three participants at the off-medication scan was due to excessive motion during the scans. Subjects supplied their own doses of stimulant medication, comprised of dextroamphetamine/amphetamine (n = 15 at one time-point, n = 12 at both), or methylphenidate (n = 7). Prior history of stimulant medication use was not captured. The medication (on) and non-medication (off) visits were counterbalanced as first or second scanning visit across subjects, to control for effects of time or “test/retest.” Time between scans was intended to be either one or two weeks in duration, ideally on the same day of the week, at the same time of day. The final breakdown was 12 scans 7 days apart, 1 scan at 8 days, 5 at 14 days and 1 at 15 days for the 19 participants with two valid scans. A urine toxicology screening was performed for all subjects prior to each scanning session to confirm the absence of opioid, cocaine, and amphetamine/methamphetamine metabolites before the off-medication scan session, and clear of all but amphetamine/methamphetamine before the on-medication session. Importantly, while the stimulant medication washout here of 7 half-lives is standard practice in ADHD studies and is considered sufficient to obviate medication effects on behavioral measures, we recognize there is more controversy over whether ongoing stimulant treatment creates a “tolerance” that could affect results in neuroimaging studies via an extended withdrawal effect (Coghill et al. 2007).
For the 20-min resting-state scan, subjects were instructed to remain still, awake, and to keep their eyes open and fixed on a fixation cross (while blinking normally). In a supplementary analysis we also separated the ADHD group into “mild” and “severe” cases based on a K-SADS current total symptom count of 10.5–18 (n = 13 individuals) for the “severe,” versus a “mild” group of 4.5–9.5 symptoms (n = 9 individuals) – see Supplementary Material.
Imaging Parameters
A Siemens Tim Trio 3T system with a 12-channel head coil was used for data acquisition. High-resolution anatomical images were acquired using both T1 (TR = 2300 ms, TE = 4 ms, FOV = 240 × 256 mm, 1 mm isotropic, sagittal acquisition) and T2 weighted (TR = 2300 ms, TE = 497 ms, FOV = 256 × 256 mm, 1 mm isotropic, sagittal acquisition) contrast for registering a 20-min resting-state EPI sequence sensitive to BOLD contrast (TR = 2000 ms, TE = 30 ms, FOV = 240 × 240 mm, 3.75 × 3.75 × 3.8 × mm, 600 volumes, axial acquisition, and 90° flip angle).
Regions of Interest
For this report we rely on voxelwise maps of the cortex that are assigned to communities via community detection with the Infomap algorithm (Rosvall and Bergstrom 2008). In addition, we selected a set of 264 regions as reported in Power et al. (2011), comprising putative functional regions defined for task-based fMRI and by resting-state functional connectivity-mapping techniques (Nelson et al. 2010) as a means of validation and comparison for the voxelwise results. The 264 region set is defined as spheres, centered at their given Talairach atlas (Talairach and Tournoux 1988) coordinates. This particular region set provides an advantage in that it also includes subcortical regions where there are known effects of stimulant medications (note these regions are also part of specified networks, see Power et al. 2011).
Generating Time Series
The resting-state fMRI data is preprocessed via traditional preprocessing methods (Fair et al. 2012b), including removal of a central spike, slice time and motion correction, within-run intensity normalization, and registration to Talairach atlas space. The data is then subjected to connectivity-specific preprocessing for the region-wise analysis, consisting of the application of a low-pass temporal filter (0.08 Hz), spatial smoothing (6 × mm FWHM), and regression of the whole-brain, ventricle and white-matter time-series as well as their first derivatives. Additionally, a framewise displacement (FD) threshold of 0.2 × mm was enforced for both the region-wise and voxelwise analyses for every frame, to reduce the effects of motion on producing spurious correlation (see Power et al. 2012). This procedure ensures that the data utilized for the sample is not differentiated by condition (e.g. control, on medication, or off medication). Remaining motion did not differ between groups after this procedure (p = 0.48). Software used to conduct the study included “in-house” software in MATLAB (2012b, The MathWorks, Inc., Natick, Massachusetts, United States), as well as the 4dfp suite developed at Washington University (Fox et al. 2005; Fair et al. 2007; Dosenbach et al. 2010). Three off-medication scans from ADHD participants with fewer than 150 volumes (5.0 min) of “scrubbed” data were removed from the analyses at this point (see Fair et al. 2012b for rationale) reducing the off-medication group to 19 individuals.
Generating Connectivity Matrices
The Pearson correlation coefficient is calculated for all pairs of ROIs based on the aforementioned time series. In the voxelwise case, only voxels corresponding to cortical gray matter are included by masking all other voxels with a 3 mm cubic voxel volume rendering of the PALS-B12 (Population-Average, Landmark- and Surface-based) atlas surface (Van Essen 2005), resulting in a total of 15 989 cortical nodes. The voxelwise results are only calculated for voxel pairs with centers farther than 20 voxels or 6 cm to remove the influence of short-range connections when assigning communities (see Power et al. 2011 for rationale). Next, for both the voxelwise and region-wise data, correlation values for each subject are averaged in order to generate a mean connectivity matrix. The group connectivity matrix is then subjected to a tie-density threshold, where only the strongest connections (3% for the analysis presented here) are kept. The thresholded matrix is then used for community detection, and then binarized for the node dissociation index measures (NDI – see below).
Community Detection and Node Dissociation Measures
Community detection is performed on the resulting mean matrix, by way of the Infomap algorithm (Rosvall and Bergstrom 2008), and was conducted in the control sample, and the ADHD sample on and off medications (see Fig. 1). This particular approach was used in the original work by Power et al. (Power et al. 2011) and hence, is used again here. Other approaches, such as Newman's Spectral Approach, could also be used for this purpose; however, to maintain consistency and to show the robustness of our data (see Fig. 2) relative to the original report, we chose to use the same community detection method. Graph theoretical measures are calculated for the voxelwise and region-wise graphs. Importantly, throughout this report “system” generally refers to the whole brain or “multiple networks.” Community or “communities” refer to one system or network (e.g. default network) as defined by this community detection algorithm.
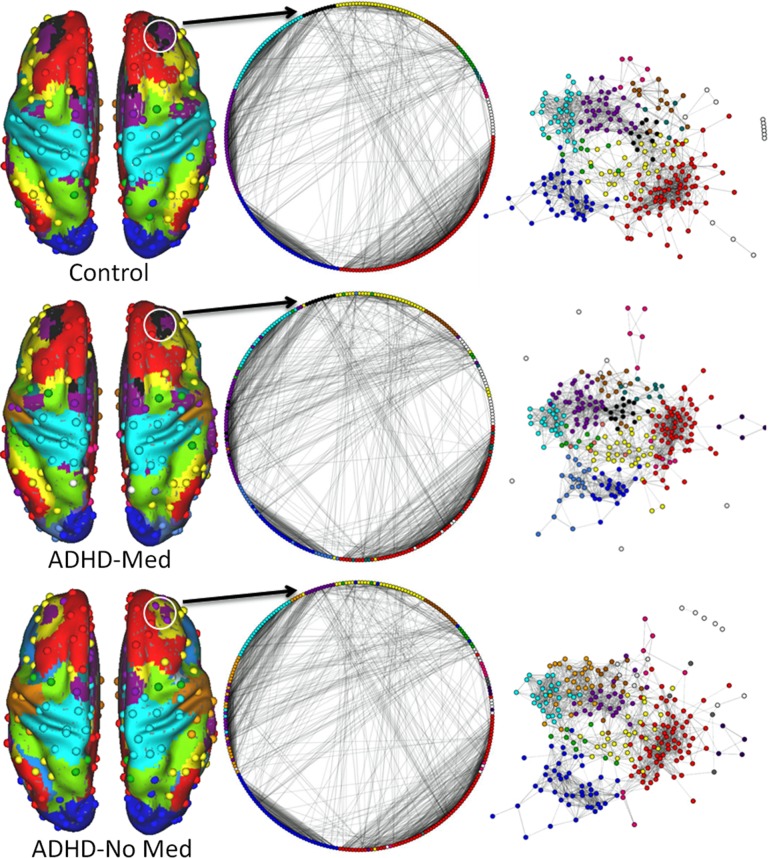
Figure 1.
Region-wise, voxel-wise, and spring embedded graphs. The correspondence and contrast between voxel-wise and region-wise networks are demonstrated. At left, the 264 spherical ROIs are shown on the brain surface, with an underlay of the corresponding voxel-wise communities. The white circles overlaying the anterior middle frontal gyrus (aMFG) highlight example regional nodes in the salience system that appear to switch communities across group and condition. The middle column is a circular representation of the 264 region-wise ROIs, where lines connecting each node signify only the strongest three percent of connections. All nodes belonging to communities with three members or fewer are left white. The pattern of highly modular connections, largely within instead of between communities (marked by color), indicates the connectivity profile for each community. At right we see the same representation, except that now the strength of connection between each node determines its distance from every other node, in what is known as a spring embedded graph. The colors in the figure represent the DMN – red, CON – purple, FPN – yellow, DAN – green, VAN – teal, SSM – cyan, VIS – blue, and SAL – black.
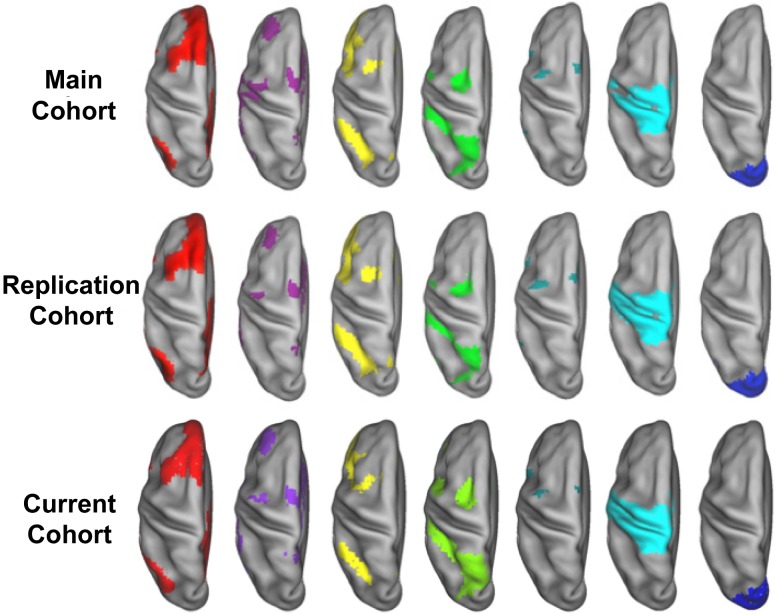
Figure 2.
Voxelwise community detection. Several voxel-based communities are shown projected on an idealized (PALS-B12; (Van Essen 2005)) left hemisphere, below the results of Power et al. (2011) for comparison. The voxelwise community assignments are shown for the previously reported cohorts in the top (n = 53) and middle (n = 52) rows, with the bottom row showing the control cohort from this study (n = 31). From left to right the represented networks are the DMN – red, , CON – purple, FPN – yellow, DAN – green, VAN – teal, SSM – cyan, and VIS – blue. The community detection process is applied to each voxel in a functional MRI scan, and can be seen to result in robust and reproducible communities.
Here we modify P, the participation coefficient (Guimerà and Amaral 2005), to indicate the movement of a given node between communities (see Fig. 3 and Supplementary Material). Instead of computing P, we examined the change in out-of-community connections relative to a reference community topology, what we term the NDI. Formally, NDI is defined in Eq. (1),
| (1) |
where M denotes the set of modules (s) over which the connections for node i are summed. The sum of connections between node i and each reference community is calculated for all modules except s(i), the module to which node i belongs. The term kis represents the number of connections between node i and module s, and ki is the total number of connections of node i (the degree).
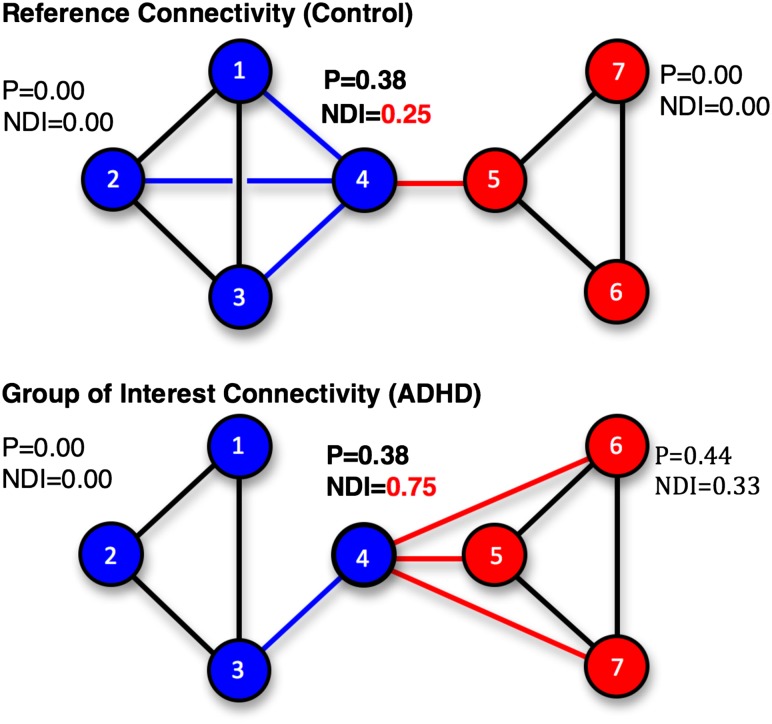
Figure 3.
Relative NDI for comparing community structure via connectivity. An idealized network of seven nodes is used to illustrate the NDI versus participation when comparing network properties across groups. Again, we note that this is only an illustration to give the reader an idea of how the measurement of the NDI relates and compares to the more common participation coefficient (P). In the reference case (top), nodes are divided into two communities, blue (nodes 1–4) and red (nodes 5–7), with a single inter-community connection between nodes 4 and 5. In the group of interest case (bottom), the community labels are the same for each node as in the reference case. Note that the links connecting node 4 to nodes 1 and 2 have shifted to connect node 4 to nodes 6 and 7, reflecting an altered pattern of connectivity. The increase in NDI for node 4 in the group of interest relative to the reference indicates that node 4 now has more connections outside of its reference community than within, and that it would likely have joined the red community in that case. The participation coefficient (P) for node 4, however, remains 0.38 in both cases, as it measures link distribution regardless of community assignment of the measured node.
In order to quantify the dissociation property for a community, the community dissociation index (CDI) is calculated as the mean NDI for all nodes in community s. CDI is calculated in each graph, and demonstrates how small changes in node association can change the profile of interconnectivity between communities. Critically, a measure of participation alone can hide shifts in connectivity (see Fig. 3). Significant differences in NDI are determined by paired t-tests between conditions.
Results
Community Topology and Connectivity Models
First, community (i.e. network) assignments were generated in our control population as in Power et al. (2011). Community detection was applied via a predefined region set of 264 ROIs (Power et al. 2011), and based on individual voxels (see Methods). For both approaches, community detection was performed by way of the Infomap algorithm (Rosvall and Bergstrom 2008). Figure 2 shows the surface-mapped voxelwise and pre-defined regional community makeup of the control group for the current study (n = 31) compared to previous work (n = 53 original sample: n = 52 replication sample) (Power et al. 2011).
As predicted, the findings show remarkable similarity to the prior report and confirmed the robustness of our sample and procedures. Importantly, several communities were identified, including the default mode network (DMN), cinguloopercular network (CON), frontoparietal network (FPN), dorsal attention network (DAN), ventral attention network (VAN), somatosensory motor system (SSM), visual (VIS) system, and salience (SAL) system (see Fig. 2). Each of these systems is reliably detected across a number of tie-density thresholds by removing all but the strongest 3–15% of connections (3% is used for the present analysis; however, similar findings were demonstrated across multiple thresholds). It should also be noted that the 264-region set (Power et al. 2011), displays a similar pattern to the voxelwise results, also reliably detecting the major systems of interest (see Fig. 1). Additionally, we point out that while the main report focuses on community structure or topology and network makeup, a supplementary analysis was performed on “hub” architecture (a hub is a node that connects many nodes) and the integration of information across these systems in the case of adult ADHD (see supplementary Figure S3).
Atypical Community Topology in ADHD is Modified with Stimulant Medication
This section of the report begins with a qualitative description of the different conditions, and patterns of how they appear to differ within group (Figs. 1–3). We follow this description with a quantitative analysis showing statistical reliability of the given qualitative findings.
Qualitative Description
Community detection is applied to the control cohort data, and the ADHD cohort with and without stimulant medications, resulting in three separate parcellations. A qualitative examination of the cortical community topology reveals a pattern where the off-medication condition displays communities with split, combined, greatly attenuated or absent community segments (Figs. 1 and 4) relative to the control cohort. In contrast, community topology of the same participants using stimulant medications is generally similar to the control cohort (see Figs. 1 and 4).
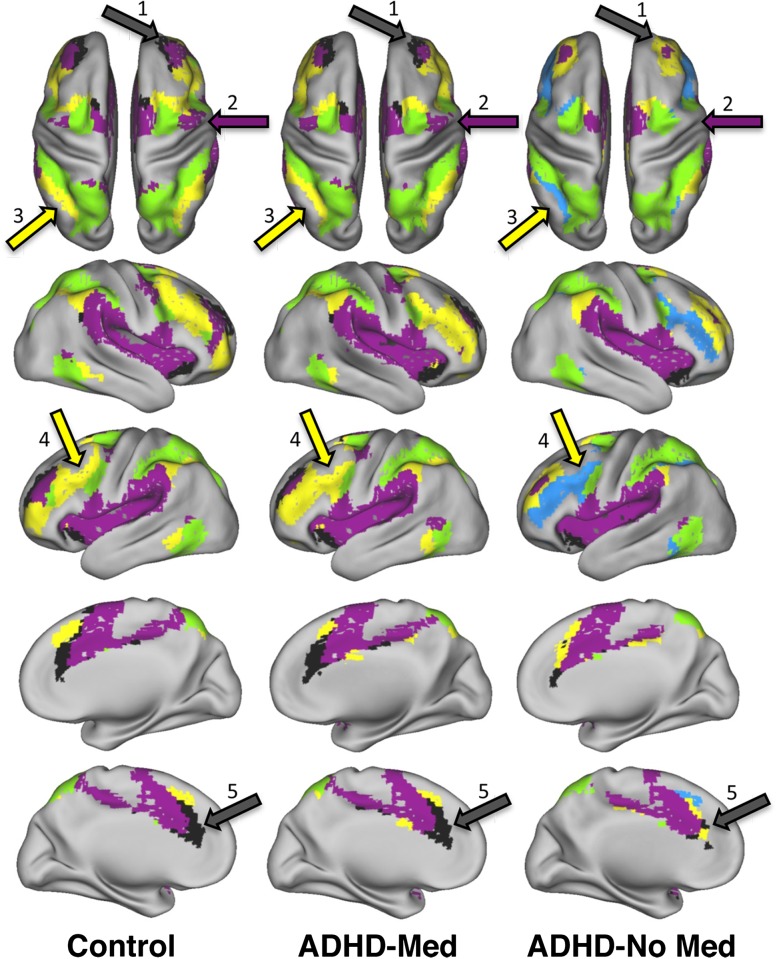
Figure 4.
Stimulant medication and examples of changes in cortical network extent. Voxelwise community detection was applied to control and ADHD cohort resting functional MRI scans. The ADHD cohort had two conditions, where one was measured after administration of stimulant medications (ADHD-Med) and the other was after observing a sufficient period for stimulant medication washout (ADHD-No Med). Standard views of the PALS-B12 atlas surface are shown with selected systems displayed for the control cohort, and ADHD cohort. The task-positive networks shown consist of the (DAN in green, CON in purple, FPN in yellow, and SAL in black. Regions that show gross differences between the control and ADHD-No Med state but not the control and ADHD-Med state are indicated by colored arrows. Region 1 denotes the anterior middle frontal gyrus (aMFG), where the SAL region (in black) is subsumed by a FPN system in the ADHD-No Med state. Region 2 indicates a similar pattern for the precentral gyrus, where the CON loses this region to the SSM system. Regions 3 and 4 highlight changes in the FPN for the dorsolateral prefrontal cortex and intraparietal sulcus. Region 5 indicates the attenuation of the salience system in the ADHD condition without medication that appears more control-like in the ADHD on medication condition.
More specifically, the anterior prefrontal cortex assigned to the SAL is “mis-assigned” to the CON for the ADHD group in the off-medication condition (see the purple regions of Fig. 4; top row, arrow “1”). This “misclassification” is corrected in the condition with stimulants. A similar pattern can be observed with the precentral gyrus where a change occurs in the ADHD cohort off-medication that is corrected when on stimulants (Fig. 4; top row, arrow “2”).
Other atypical patterns in the ADHD cohort in the off-medication condition that adapt with stimulant therapy are also present in the data. For example, the frontoparietal network begins as two separate subsystems in the off-medication condition. One of these frontoparietal subsystems (colored yellow in Fig. 4; rightmost column) contains a cortical area associated with the salience system in the anterior middle frontal gyrus (aMFG), but does not include the dorsolateral prefrontal cortex (dlPFC) and much of the posterior intraparietal sulcus (IPS) (see Fig. 4). Just as with the SAL and CON systems, stimulant medications appear to cause adaptive changes to the atypical topologic organization. Importantly, primary sensorimotor, visual and default systems showed little change across the systems (Fig. 1). In addition, the results based on 264-region set largely match the voxelwise results just summarized (Fig. 1). To determine the significance of the given findings, we next apply graph theory measures to the connectivity aspects of network topology. (Of note, these effects and the quantitative comparisons below were, in part, modulated by symptom severity – see Supplementary Material.)
Quantitative Comparisons
To quantify the community descriptions in the prior section we generated the node dissociation index (NDI – see Methods) for each condition, which allowed for statistical comparisons of community topology for a given network in the on and off medication conditions. NDI is able to provide a more meaningful between-group comparison measure than participation, a related measure, can provide (see Fig. 3). The NDI was calculated for each voxel in the control group and ADHD group (see Methods: Generating Connectivity Matrices), both on and off medication, based on the control group community assignment results, and restricted to the voxels assigned to eight communities of interest in the control cohort (see Figs. 1 and 2). The communities of interest are the DMN, CON, FPN, SAL, DAN, VAN, SSM, and VIS system. Increased NDI would represent a voxel less associated with its own community (as defined by the control population) and more associated with outside communities. The mean NDI per community (i.e. average across all voxels) is reported from here forward as the CDI.
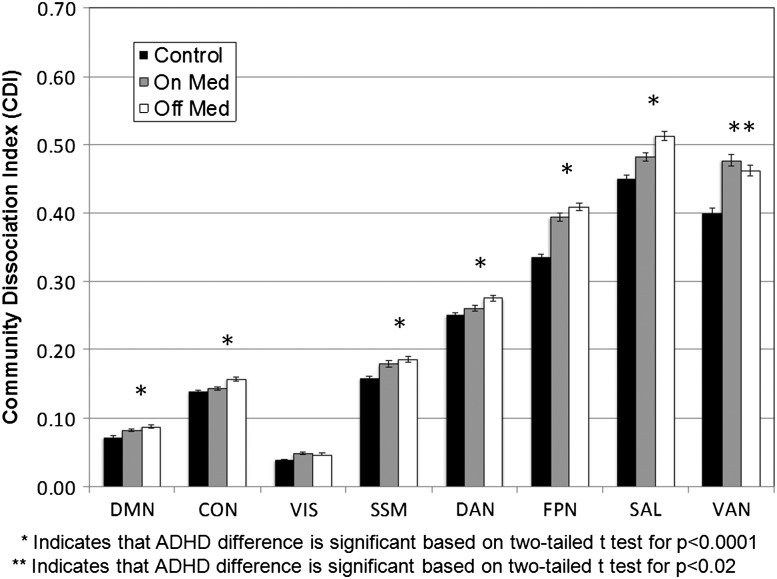
Figure 5 shows a trend amongst the examined systems, with a high CDI for the ADHD off medication condition, lower for the medication condition, and lowest for the control group as comparison. Medication is seen to significantly reduce CDI for six of the eight systems in the ADHD group (p < 0.0001, paired t-test), highlighting more similarities between the control and ADHD population when on stimulants. The visual system did not show significantly reduced CDI (p = 0.12), relative to the no medication condition. Interestingly, the ventral attention system (VAS) showed a weak, but significant trend (p < 0.02) in the opposite direction (of note: comparisons are only made between the on and off medication conditions, as the control group was used for the reference community structure. Asterisks are only highlighting significant differences between the on and off med condition).
Figure 5.
Community dissociation index (CDI) by medication. The graph shows the CDI (see Fig. 3), for each of 8 voxelwise communities of interest. The control community assignments are used as a reference partition when calculating the ADHD CDI results to provide a common set of regional community-wise comparisons. Communities are ranked left to right in terms of total community size (i.e., the number of voxels per community). The trend for the on versus off medication conditions is an ADHD group with stimulant medication showing a significantly lower distribution of connections between control-defined communities (i.e. CDI) than in the off medication state. Error bars represent standard errors. The communities examined are the DMN, CON, FPN, DAN, VAN, SSM, VIS, and SAL.
Discussion
A key question regarding the nature of large-scale brain organization, both for the typical population and those presenting neuropsychiatric disorders, is the detection and characterization of network topology. In this report we examine the nature of this structure in adults with ADHD. We also assess whether network topology is modified with stimulant medications. Two fundamental findings were observed. First, a robust network topology is reliably detected across multiple systems (i.e. DMN, CON, DAN, FPN, VAN, and SAL) in both Control and ADHD groups. Second, these systems are modified in ADHD with stimulant medications to a more “control-like” state (note our supplementary analysis, which shows that symptom severity is likely related to many of these alterations in connectivity). How these fundamental findings relate to highly connected hub regions, as measured by community density, is provided in Supplementary Material.
Atypical Network Topology Across Multiple Systems
Several large-scale networks were identified that appear atypical in adult ADHD when med-free (Fig. 4). The largest differences in topology were found in the VAN, SAL, and FPN networks. All of these systems are involved in higher order cognition and task level control – features that have often been found to be atypical in ADHD (Cortese et al. 2012; Fair et al. 2012b). Less reported in the literature is the influence on primary sensorimotor and visual systems and their involvement in ADHD. Here we see that while the network topology of the sensorimotor system more closely resembles that of the controls, they remain atypical. Such findings are consistent with work by Mostofsky and others (Mostofsky et al. 2006; Gilbert et al. 2011) and Castellanos and colleagues (Castellanos and Proal 2012) which highlight the potential importance of these primary systems in the pathophysiology of ADHD as well.
Interestingly, in regard to potential mechanisms of medication action, the default system tracked more closely with that of the primary motor and sensory systems than with higher order control systems (in that stimulants only slightly changed the NDI/CDI values). This finding, that the default system was minimally altered relative to other higher order systems, was somewhat surprising as well as interesting for two reasons. First, implications of the role of the default system in ADHD have been reported in several contexts (Uddin et al. 2008; Fair et al. 2010; Cortese et al. 2012). Second, the default system is often considered as an important network that interacts strongly, albeit negatively, with the control systems examined here. The current work, on the other hand, is more consistent with prior work suggesting its relationships, as measured via functional connectivity, tracks more closely to the primary motor and sensory systems (Power et al. 2011).
In all, these network-based findings highlight that it is unlikely that a single locus of dysfunction could account for the complex and atypical behaviors that characterize ADHD. We are quick to add, however, that ADHD is likely a heterogeneous disorder with multiple underlying brain profiles that can result in the DSM defined phenotype (Fair et al. 2012a, 2012b; Karalunas et al. 2014). Indeed, as highlighted in supplementary material (Figures S1, S2) it appears that symptom severity (here closely corresponding to ADHD subtype) plays a substantial role in the altered spatial cortical community organization observed in the whole-group off-medication ADHD state. Although the subject population was not large enough to identify potential additional (or alternative) sub-populations (Fair et al. 2012a; Gates et al. 2014; Karalunas et al. 2014), this finding potentially highlights two distinct populations as suggested by (Fair et al. 2012a) and (Solanto et al. 2009), a target for future, larger studies.
Effects of Medication on Functional Topology
Perhaps the most apparent effect of stimulant medications on network topology was that it appeared to improve relationships of the most affected subsystems, adapting both their spatial extent and connectivity profile to more closely reflect the reference organization (see Figs. 1, 4, 5). While not the only networks, the frontoparietal, salience and cinguloopercular systems all exhibit the greatest differences between the control and ADHD off medication condition (Fig. 5). While the work here is more akin to targeting “within” network connectivity, the systems most affected and the direction of effects are largely in line with similar work conducted in healthy adults and children with ADHD (Li et al. 2013; Mueller et al. 2014; Querne et al. 2014). In contrast, when examining the effect of medication, the ventral attention system was unique in that medication appeared to weakly increase, rather than strongly decrease intercommunity connectivity – moving it further away from the control condition. This is particularly interesting and may relate to recent findings in autism (Farrant and Uddin 2015).These types of effects (i.e. heterogeneous connectivity changes due to medication or disorder) suggest that the efficacy of stimulant therapy is not purely “normalization,” but rather a combination of effects that returns the network organization to typical topology for some systems, while re-organizing others – perhaps highlighting a compensatory mechanism.
In the context of task fMRI and stimulant therapy literature, these findings are both consistent and unique. While several studies have highlighted similar adaptive changes of task based responses with the administration of stimulant therapy, this finding is somewhat variable and dependent on age, brain location, and the specific task being conducted (Hart et al. 2013; Rubia et al. 2014). Our specified findings of the CON and SAL, however, which include the anterior insula and dorsal anterior cingulate are largely consistent with recent task based meta-analyses (Rubia et al. 2009; Hart et al. 2013). Interestingly, these systems have been shown to be important for higher order attentional control, including the maintanance of task sets (Dosenbach et al. 2008). Less consistent was the effect of the ventral attention system (Rubia et al. 2014). Again, this might be due to the age, specified tasks, measuring the ventral attention system in its entirety (via rsfMRI), and the specificity/demarcation of the inferior frontal cortex and anterior insula examined here with resting-state fcMRI, which is not always clear in prior fMRI work (Rubia et al. 2014). Nonetheless, determining how the task-based responses relate and interact with resting connectivity in the context of medications will be an important area of study in future research.
The resting-state fMRI literature has noted changes in brain connectivity of control subjects based on the administration of methylphenidate (Sripada et al. 2013), finding, in particular, an increased separation between default-mode regions and other network regions. The present work was consistent with this work in this regard by finding a decreased CDI in the DMN (albeit slightly). In other words, similar to Sripada et al. (2013), our findings indicate a decrease of between network connectivity of this network. In other work, the directions of medication effects were also in line with our general findings. For example, in youth subjects with ADHD (Li et al. 2013), a distributed effect of stimulant medications were found to eliminate activity differences between patients and controls on a measure of regional homogeneity (a measure of similarity in connectivity by spatial localization). Again, the present work found adaptive changes, bringing the connectivity profile closer to control subjects without the full “normalization” seen in the work of Li et al. (2013).
The question remains, however, how might stimulant medication lead to these types of system changes? The effect of stimulant medications on dopaminergic brain pathways (from the striatum to much of the prefrontal cortex) has been examined extensively, and may provide an avenue to consider (Mehta et al. 2000; Honey et al. 2003; Kobel et al. 2009; Swanson et al. 2011). Normalizing the dopamine levels in these subcortical structures may assist in stabilizing large-scale systems organization across the whole brain. For example, it has been shown that increased dopamine in the striatum is tied to improved clinical outcomes (Volkow et al. 2012). These medications, however, have effects not limited to subcortical structures and thus likely have some action related to network topology in the cortex as well (Di Martino et al. 2008). The inhibitory activity of dopamine on inter-neuron communication may be a key to understanding the normalizing effect of stimulants in the prefrontal circuits of the ADHD population. The observed differences, specifically of reduced CDI for ADHD subjects using stimulant medications toward that of the controls, may offer corroboration of these proposed models of stimulant therapy efficacy.
Limitations
It is important to note that the current work with voxelwise networks looks exclusively at cortico-cortical connections and does not account for the fronto-striatal connections, or cerebellar functions (in the voxelwise case). However, for this reason we used the previously defined 264-node set that includes subcortical and cerebellar regions (Power et al. 2011). These data showed largely similar structure in controls to that found in the original report, and followed the same patterns as was identified with the voxelwise analysis. Additionally, we are not able to properly assess the effects of stimulant medications on the hemodynamic responses themselves. With that said, we are able to identify effects in systems that are either unchanged (i.e. motor and visual), that decrease in the stimulant condition (e.g., dorsal attention system), or that increase in the stimulant condition (ventral attention system), which suggest that a “one-directional” effect on hemodynamics is unlikely. It is also possible that the modest sample size of participants with ADHD did not allow us to fully account for heterogeneity in the population. A larger sample could assist in refining the interpretation. As with all functional imaging studies where stimulants are withdrawn for a limited amount of time prior to scanning, one needs to consider the potential effects of cessation. The stimulant medication washout here of 7 half-lives is standard practice in many neuroimaging and other studies of ADHD (if the study accounts for stimulants, which is not always the case), and is generally thought sufficient to obviate medication effects on behavioral measures. However, we recognize there is more controversy over whether ongoing stimulant treatment creates a “tolerance” that could affect results in neuroimaging studies via an extended withdrawal effect (Coghill et al. 2007). More data and large studies on this question after longer withdrawal periods and potentially examining new patients who are “drug naïve” are sorely needed to be sure of the observed atypical connectivity. Last, the heterogeneous treatment history of the ADHD sample in this study does not allow us to properly examine or control for the effect of long term (years) versus short-term (months, weeks, days, or hours) treatment with stimulant medications, nor does it allow for us to examine the range of responses to stimulants (e.g. responders vs. non-responders). Differences in treatment duration, medication type (e.g., methylphenidate vs. amphetamine) and medication efficacy were represented at all of these time scales in the current sample. The action of these two stimulant classes in the brain is not identical and further examination of their distinct effects will be of interest. This consideration may be important, as long term treatment is known to correlate with cortical thickness measures during maturation (Shaw et al. 2009; Nakao et al. 2011) and increase dopamine transporter density in the striatum (Fusar-Poli et al. 2012; Wang et al. 2013), and should be considered in future studies.
Conclusions
In the current report, we show significant differences in community or network topology in an ADHD group with and without stimulant medication. Atypical network topology is not localized to one or two systems, but is present across multiple systems in the brain. Importantly, medication appears to modify the ADHD connectivity pattern to a more control-like state in most (but not all) networks. Symptom severity appears to reveal differential profiles in community structure, suggesting a possible difference in neurobiological connectivity between DSM-5 ADHD inattentive and combined presentations. Taken together, these results confirm that ADHD, in at least part of the adult population, may be characterized as a network-specific adaptation across the whole brain, for which stimulant medications partially reduce ADHD-related differences as compared to controls.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
James S. McDonnell Foundation Collaborative Activity Award (220020256), via Steven Petersen (PI), and Damien Fair (Site PI). Additional funding was supplied by National Institute of Health (R01 MH096773, Fair; R00 MH091238, Fair; and R01 MH086654, Nigg).
Supplementary Material
Notes
The authors would like to thank Steven Petersen, Ben Jarrett, Lisa Vingara, Marc Rudolph, Shannon Anderson, and Damion Demeter for contributing their useful comments and suggestions regarding this manuscript. Conflict of Interest: The authors declare no conflict of interest.
References
- Bassett DS, Bullmore ET. 2009. Human brain networks in health and disease. Curr Opin Neurol. 22:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. 2010. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 14:277–290. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. 2012. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 16:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Rhodes SM, Matthews K. 2007. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 62:954–962. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. 2012. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 169:1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer MJ, Simmons A, Rubia K. 2014. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb Cortex. 24:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. 2008. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. . 2010. Prediction of individual brain maturity using fMRI. Science. 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. 2012. a. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci. 109:6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, Schlaggar BL, Mennes M, Gutman D, Bangaru S, et al. . 2012. b. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE. 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2007. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 104:13507–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. 2010. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 68:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ. 2015. Atypical developmental of dorsal and ventral attention networks in autism. Dev Sci. 1–14. [DOI] [PubMed] [Google Scholar]
- Fortunato S. 2010. Community detection in graphs. Phys Rep. 486:75–174. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. 2012. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry. 169:264–272. [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PCM, Iyer SP, Nigg JT, Fair DA. 2014. Organizing heterogeneous samples using community detection of GIMME-derived resting state functional networks. PLoS One. 9:e91322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. 2011. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 76:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R, Amaral L a N. 2005. Cartography of complex networks: modules and universal roles. J Stat Mech Theory Exp. 2005:P02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Grant PE, Fair DA. 2012. MR connectomics: a conceptual framework for studying the developing brain. Front Syst Neurosci. 6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. 2013. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 70:185–198. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, Rovira M, Bulbena A, Tobeña A, Casas M, et al. . 2014. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp. 35:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SCR, Brown J, et al. . 2003. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 126:1767–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT. 2014. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions. JAMA Psychiatry. 71:1015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kobel M, Bechtel N, Weber P, Specht K, Klarhöfer M, Scheffler K, Opwis K, Penner IK. 2009. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 13:516–523. [DOI] [PubMed] [Google Scholar]
- Li A, Xiao-Hua C, Qing-Jiu C, Li S, Li Y, Qi-Hong Z, Rubia K, Yu-Feng Z, Yu-Feng W. 2013. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology. 38:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. 2000. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 20:RC65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JGB, Boyce A, Goldberg MC, Pekar JJ, Denckla MB. 2006. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 59:48–56. [DOI] [PubMed] [Google Scholar]
- Mueller S, Costa A, Keeser D, Pogarell O, Berman A, Coates U, Reiser MF, Riedel M, Möller H-J, Ettinger U, et al. . 2014. The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum Brain Mapp. 35:5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT. 2011. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 50:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. 2011. Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 168:1154–1163. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, et al. . 2010. A parcellation scheme for human left lateral parietal cortex. Neuron. 67:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. . 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querne L, Fall S, Le Moing A-G, Bourel-Ponchel E, Delignieres A, Simonnot A, de Broca A, Gondry-Jouet C, Boucart M, Berquin P. 2014. Effects of methylphenidate on default-mode network/task-positive network synchronization in children with ADHD. J Atten Disord. 1. First published January 13, 2014. DOI:10.1177/1087054713517542. [DOI] [PubMed] [Google Scholar]
- Raichle ME. 2010. Two views of brain function. Trends Cogn Sci. 14:180–190. [DOI] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. 2008. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci USA. 105:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. 2014. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 76:616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad A-M, Brammer M, Taylor E. 2009. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 57:640–652. [DOI] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. 2009. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 166:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH. 2009. Event-Related fMRI of inhibitory control in the predominantly inattentive and combined subtypes of adhd: Clinical investigative study. J Neuroimaging. 19:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Kessler D, Welsh R, Angstadt M, Liberzon I, Phan KL, Scott C. 2013. Distributed effects of methylphenidate on the network structure of the resting brain: a connectomic pattern classification analysis. Neuroimage. 81:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Tappon SC, Garg A, Fair DA. 2012. Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLoS One. 7:e30468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. 2011. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 36:207–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain, 1988. Theime, Stuttgart, Ger. 270:132. [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. 2011. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 54:3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, et al. . 2008. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 169:249–254. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2011. Rich-club organization of the human connectome. J Neurosci. 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. 2005. A Population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 28:635–662. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, Telang FW, Fowler JS, Logan J, Wong CT, et al. . 2012. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 32:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Wigal T, Kollins SH, Newcorn JH, Telang F, Logan J, Jayne M, Wong CT, Han H, et al. . 2013. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One. 8:e63023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y. 2009. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 30:638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.