Abstract
Important data on the social, epidemiological and clinical aspects of HIV-1, comorbidities and hepatitis as well as data on novel antiretroviral agents and the cure agenda were presented at AIDS 2018. This report covers some of the highlights.
Introduction
Important data on the social, epidemiological and clinical aspects of HIV-1, comorbidities and hepatitis as well as data on novel antiretroviral agents and the cure agenda were presented at the 22nd International AIDS Conference (AIDS 2018), 22–27 July 2018, Amsterdam, the Netherlands.
Epidemiological trends
Alison Rodger (University College London, UK) presented the results of the PARTNER2 study during the ‘Co-chair's Choice’ session [1]. This study investigated the risk of sexual HIV-1 transmission between ‘sero-different’ gay men when a condom is not used and the positive partner is virally suppressed on antiretroviral therapy (ART). The study recruited 972 couples from 14 European countries where one partner was HIV-positive and one HIV-negative and followed them between September 2010 and April 2018. The investigators were interested in follow-up time during which the couple were having condomless penetrative sex while the HIV-positive partner was on ART with a viral load<200 copies/mL. Of the couples, 783 met these criteria for a total of 1596 couple-years and an estimated 76,991 sex acts. During this time there were 15 transmissions, but none were phylogenetically linked to the initially positive partner's virus. Therefore, there were zero transmissions between the sero-different partners when the HIV-positive partner was on suppressive ART.
Thus, the estimated rate of transmission in this study was zero. The corresponding upper limits of the 95% confidence intervals (CI) were 0.23/100 couple-years for any sex; 0.27/100 couple-years for insertive anal sex and 0.57/100 couple-years for receptive anal sex with ejaculation. Note that the estimated transmission rate is zero for all types of sex: the wider confidence interval for receptive sex merely reflects larger uncertainty of the ‘true’ rate for the types of sex that were undertaken less frequently.
This study provides additional, strong, evidence that individuals living with HIV-1 who are virally suppressed on ART are sexually uninfectious in terms of HIV-1. As the authors concluded: ‘Our results give equivalence of evidence for gay men as for heterosexual couples and indicate that the risk of HIV transmission when HIV viral load is suppressed is effectively zero’.
Valerie Delpech (Public Health England, London, UK) analysed causes of death among participants living with HIV-1 in London in 2016 [2]. Among 206 deaths reported, non-AIDS malignancies were the most common cause followed by AIDS-defining illnesses. Seventy-seven percent of deaths were due to non-AIDS conditions with the majority of patients on ART and virally suppressed. Co-morbidities included: a history of depression (39%); hypertension (33%); dyslipidaemia (27%); HBV or HCV co-infections (18%); and diabetes (14%). Underlying risk factors, such as smoking, substance misuse and alcohol consumption were common.
David Magnuson (Gilead Sciences, Epidemiology, Foster City, CA, USA) described the use of emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) as PrEP for adolescents in the US from January 2012 through to September 2017 [3]. During this period 148,147 unique individuals began FTC/TDF, of whom 2388 (1.6%) were 12–17 years old. The total number of adolescents increased annually from 266 in 2012 to 805 in 2015, but decreased to 216 in 2016. Of adolescents prescribed FTC/TDF for PrEP, females accounted for 86.0% while adult women comprised only 18.4% of all adults given PrEP prescriptions (P<0.0001). Medicaid provided coverage for FTC/TDF in 59.1% of adolescents compared to 13.5% of adults. PrEP was most commonly prescribed by paediatricians (31.6%).
Acute HIV-1 infection (AHI) and the viral reservoir
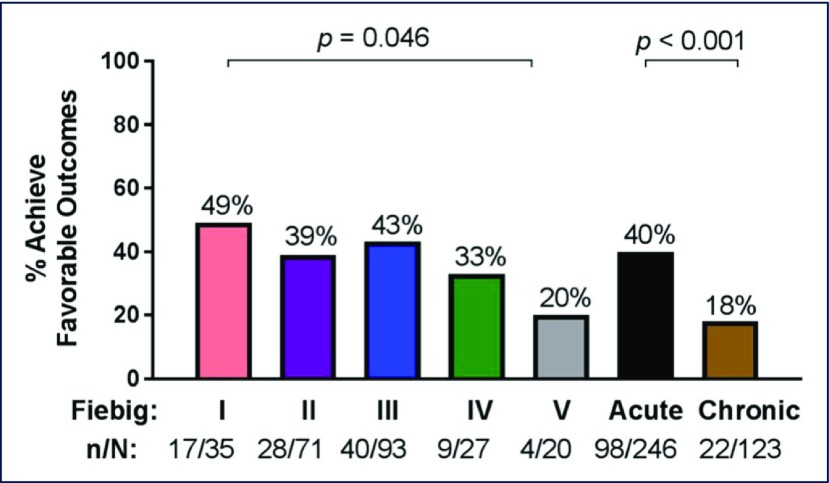
Jintanat Ananworanich (Henry M Jackson Foundation for the Advancement of Military Medicine, Bethesda, MA, USA) presented data from participants in the RV254/SEARCH 010 AHI cohort in Thailand regarding the proportion of antiretroviral-treated AHI individuals who displayed a favourable clinical phenotype (FCP] [4]. FCP was defined as fulfilling all three of the following criteria: (1) HIV-1 viral load (VL)<20 copies/mL at all visits from week 24 onwards; (2) the last CD4 T cell count was>500 cells/mm3; and (3) the last CD4/CD8 T cell ratio>1. There were 246 AHI participants in Fiebig stages I–V included in the study, the majority (99%) of whom initiated efavirenz (EFV)-based regimens. FCP was achieved in 40% with differences between Fiebig I and V (P=0.046) (Figure 1). FCP was associated with pre-ART CD4 T cell count (OR 1.27, 95% CI 1.11–1.46, P=0.001) and CD4/CD8 T cell ratio (OR 4.31, 95% CI 2.42–7.67, P<0.001) but not VL, age or sex. In comparison, FCP was less likely in a Thai cohort of 271 males treated during chronic HIV-1 infection (25%, P=0.002) who were older (31 years, range 25–37) but had a longer duration of ART (5.3 years, range 4.0–6.3).
Figure 1.
Proportions with FCP after treatment during AHI according to clinical stage
Véronique Avettand-Fenoel (Necker Hospital, APHP, Paris, France) described the dynamics over time of total and integrated HIV-1 DNA in blood in primary infection (PHI) and chronic infections in untreated individuals from the ANRS-PRIMO and the ANRS-SEROCO cohorts [5]. When modelling total and integrated HIV-1 DNA evolution, authors observed that PHI was characterised by high levels of total HIV-1 DNA (median 3.59 log10 copies/106 PBMCs, interquartile range [IQR] 3.29–4.03) and low levels of integrated forms among total HIV-1 DNA for most patients (2.15 log10 copies/106 PBMCs, IQR 0.95–3.16), suggesting a low contribution of integrated forms at PHI (stable reservoir). Among recent seroconverters, those who progressed towards AIDS during the study (rapid progressors, n=34) had higher total and integrated HIV-1 DNA levels at inclusion compared to others (progressors, n=63), and higher integrated/total HIV-1 DNA ratio (100% vs 44%, respectively). In multivariate analysis, integrated HIV-1 DNA was strongly associated with the risk of developing AIDS (adjusted relative risk [aRR] 2.6, P=0.002).
Laurent Hocqueloux (CHR d’Orléans - La Source, Infectious and Tropical Diseases, Orléans, France) reported the long-term outcome of 23 post-treatment controllers (PTC) from the time of their enrolment into the ANRS VISCONTI study [6]. The definition of PTC included individuals living with HIV-1 having started ART at the time of PHI, who achieved VL<400 copies/mL for at least 12 months after treatment interruption. Treatment was stopped after a median 3.7 years and PTC were followed-up for a median 11.9 years (until last visit or ART resumption). Intermittent viraemia (transient VL>400 HIV-1 copies/mL) was noted in seven PTC (30%). Five individuals (22%) resumed ART after a median 8.6 years off-ART: four owing to virological failure (two consecutive pVL>400 copies/mL); and one because of a non-AIDS defining cancer. Patients who had intermittent viraemia were more likely to resume ART than those without: 5/7 versus 0/16, respectively (P=0.0006). PTC without intermittent viraemia presented with low levels of inflammation when compared to HIV-1 controllers or individuals living with HIV-1 who were on ART (P<0.05 for IL-18, sCD163 and sCD14).
Ricardo Sobhie Diaz (Federal University of Sao Paulo, Sao Paulo, Brazil) investigated the effect of treatment intensification with dolutegravir (DTG) with and without maraviroc (MVC), nicotinamide (NA), and auranofin in decreasing total HIV-1 DNA [7]. Six arms in NCT02961829 with five patients each were followed up every 4 weeks for a total of 48 weeks. Groups were: (1) ART continuation; (2) ART intensification (ART+DTG and MVC); (3) ART, intensification and histone deacetylase inhibitors (HDACi; ART+DTG+MVC+NA); (4) ART intensification and auranofin (ART+DTG+MVC+auranofin); (5) partially intensified ART (ART+DTG); and (6) partially intensified ART (DTG+NA+auranofin). Treatment interventions were well tolerated. A decrease in viral DNA was observed in Group 6 as compared to all other groups (P= 0.022, odds ratio (OR) 9.75, 95% CI 1.1–72.39). Intensified ART with DTG+MVC showed a greater decrease in the total HIV-1 DNA as compared to intensified ART with DTG only (Group 2 vs Group 5, P=0014). Group 1 showed a significant linear trend towards an increase of the viral reservoir (P<0.05). An impact of these strategies on the proviral reservoir size is possible but not yet proven.
Antiretroviral strategies
Mariana Veloso Meireles (Ministry of Health of Brazil, Department of STI, AIDS and Viral Hepatitis, Brasilia, Brazil) presented results from a large real-world cohort in Brazil assessing the comparative effectiveness of first-line ART regimens using the 6-month VL results [8]. Of the 103,240 patients included in the analysis 76.9% achieved a VL<50 copies/mL. In a multivariable analysis, with lamivudine (3TC)+TDF+DTG as the reference, adjusted odds ratios (aOR) (95% CI) of failing to achieve virological suppression were: 1.42 (1.32–1.52) for 3TC+TDF+EFV; 1.51 (1.35–1.68) for 3TC+zidovudine (ZDV)+EFV; 2.11 (1.91–2.32) for 3TC+TDF+ atazanavir/ritonavir (ATV/r), 2.41 (2.18–2.66) for 3TC+AZT+LPV/r and 2.62 (2.32–2.95) for 3TC+TDF+lopinavir/ritonavir (LPV/r). These results support the decision made by the Ministry of Health of Brazil to switch its recommendations for preferred first-line ART from EFV- to DTG-containing regimens.
Dominique Braun (University Hospital Zurich, Switzerland) demonstrated efficacy, safety and non-inferiority of monotherapy with once daily DTG in patients with a documented PHI who had initiated ART<180 days after the estimated date of infection [9]. At week 48 in the per-protocol-population, 67/67 (100%) had virological response in the DTG monotherapy group versus 31/31 (100%) in the ART group (difference 0%, 95%-CI −1–0.047), showing non-inferiority. Overall, these results suggest that simplification strategies could be of benefit for stratification according to time of infection at start of first ART.
Laurent Hocqueloux presented results from the randomised non-inferiority MONCAY trial investigating whether DTG alone was able to maintain virological suppression in individuals living with HIV-1 on a successful DTG-based standardised triple therapy regimen [10]. From among 158 patients included (78 assigned to DTG and 80 to continue DTG/abacavir [ABC]/3TC), two patients in the DTG group experienced virological failure both at week 24 without the development of resistance to the integrase inhibitor (INSTI) class. Using intention-to-treat analysis, the success rate was 73/78 (93.6%) in the DTG arm and 77/80 (96.3%) in the DTG/ABC/3TC arm (difference 3.9%, 95% CI −5.0–10.8). During follow-up, three additional individuals in the DTG arm experienced virological failure (two at week 36 and one at week 48) with emerging resistance mutations to INSTI in two cases, whereas none occurred in the DTG/ABC/3TC group (difference 6.5%, 95% CI −1.8–15.6). Although non-inferior to DTG/ABC/3TC as per the primary endpoint at week 24, DTG monotherapy was not a valid option for maintaining virological suppression over time in individuals living with HIV-1 who were on a successful DTG/ABC/3TC triple therapy regimen and it favoured the emergence of INSTI resistance.
Pedro Cahn (Fundacion Huesped, Buenos Aires, Argentina) described the 48-week interim data from the GEMINI studies. GEMINI-1 and -2 included 714 and 719 individuals, respectively, with a screening HIV-1 RNA≤500,000 copies/mL ( ClinicalTrials.gov: NCT02831673/NCT02831764) [11]. Participants were randomly allocated 1:1 to treatment with DTG+3TC or DTG+TDF/FTC. The primary endpoint was the proportion of participants with plasma HIV-1 RNA<50 copies/mL at week 48. In the SNAPSHOT analysis, dual therapy was shown to be non-inferior to triple therapy, with a very high virological success rate in both arms (around 93%). This success rate was independent of baseline VL (less than or greater than 100,000 copies/mL) and no resistance mutations were detected in individuals who experienced virological failure in whom genotyping could be performed (six in the 3TC + DTG arm, four in the TDF/FTC + DTG arm). Overall rates of adverse events were similar between arms. Changes in markers of bone and renal function through week 24 favoured DTG+3TC. Even pending the long-term follow-up of these trials and the experience in real clinical practice, globally, the results of the GEMINI studies present dual therapy as an alternative to conventional triple therapies, even for those initiating ART.
Antiretroviral drug resistance
Ming Tain Lai (Merck & Co, Inc, Kenilworth, NJ, USA) opened the oral poster discussion session about antiretroviral drug resistance with a characterisation of doravirine (DOR)-selected resistance patterns from participants in treatment-naïve Phase 3 clinical trials [12]. From 747 participants through week 48 of treatment from the DRIVE-FORWARD and DRIVE-AHEAD trials, seven (0.9%) developed NNRTI resistance-associated mutations, which was lower than in the EFV arm (12/364, 3.3%) in the DRIVE-AHEAD trial. Laboratory mutant isolates were generated via a site-directed mutagenesis (SDM) method for the Y188L, V106I/F227C, A98G/F227C, V106I/H221Y/F227C, A98G/V106I/H221Y/F227C, V106A/P225H/Y318F, and V106M/F227C substitutions. Most of the mutants conferred a high level of resistance to DOR with a fold change (FC)>100 (where FC is the mutant EC50 versus wild-type EC50). Of the seven clinical DOR mutants, five displayed susceptibility to etravirine (FC<10) and hypersensitivity to some NRTIs with a low replicative capacity. Among the 12 EFV-resistant clinical mutants, nine were susceptible to DOR.
Emily Hyle (Massachusetts General Hospital, Boston, MA, USA) discussed the need to continue using baseline genotypes to detect primary resistance to ART in the era of integrase inhibitors [13]. In people newly diagnosed with HIV-1 in the USA, using the Cost-effectiveness of Preventing AIDS Complications (CEPAC) model to examine the value of standard genotype at HIV-1 diagnosis for people starting DTG, they concluded that the standard genotype test at diagnosis offers minimal clinical benefit, is more expensive, and not cost-effective. This communication is important because it provides the data to support the fact that clinical guidelines should consider removing genotyping from the recommended baseline evaluation.
All fired up: tackling inflammation
During long-term ART, persistent gut mucosal damage and microbial translocation appear to be the main source of immune activation. Markers of microbial translocation consisting of bacterial products including lipopolysaccharide (LPS) are released into the circulation [14]. Consequently, pro-inflammatory cytokines produced by myeloid cells exacerbate systemic immune activation that fuels the potential for memory CD4 T cells to be the targets of infection and remain exhausted and for an increased risk of non-AIDS events [15]. New information on mechanisms and interventions to prevent or limit inflammation is eagerly expected as well as on the benefits of early ART.
Maria Paola Caruso et al. (Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina) assessed the effect of ART on the TH17/ Treg balance in female genital mucosa [16]. After obtaining mononuclear cells and exocervical swabs in treated, untreated and control participants, cytokines secreted by cervical mononuclear cells were stimulated and quantified by cytometric bead array. The Th17 protective mucosal function was severely affected and only partially improved on ART contrasting with the absence of difference for the Treg function in the three groups. These findings in female genital mucosa seem to mirror previous reports on Th17/Treg balance in gut mucosa.
Jean-Pierre Routy et al. (Chronic Viral Illness Service, McGill University Health Centre, Montréal, Canada) studied a potential new plasma marker of fungal translocation [17]. LPS, LPS binding protein (LBP), and sCD14 are validated markers of bacterial translocation. (1-3)-β-D-glucan (βDG) is a major component of most fungal cell walls and binds to myeloid cells via Dectin-1 receptor. Elevated βDG plasma levels have been associated with inflammation markers and the development of non-AIDS events and are now considered a marker of gut fungal translocation. Using samples from PHI participants and HIV-1 ageing cohorts, investigators showed that the elevation of plasma βDG levels observed during acute and chronic infection did not decrease with either early ART initiation or long-term ART use. Elevated βDG levels, which correlated with markers of leaky gut and with CD4 and CD8 T cell activation, were likely to contribute to inflammation.
Shalena Naidoo et al. (Stellenbosch University, Medical Virology, Cape Town, South Africa) studied the effect of 8 years of ART on myeloid inflammation in children participating in the Children with HIV-Early ART (CHER) trial and with age-matched controls [18]. Again this group identified sCD14 and LBP, two markers of bacterial gut translocation, as remaining elevated and correlated with plasma level of IL-1b, IL-6, IL-8. TNF-a. This group of children is unique as early therapy and long-term viral suppression allowed low levels of cell-associated HIV-1 DNA, full CD4 recovery contrasting with the persistent myeloid immune activation and elevation of microbial translocation markers.
Hendrik Streeck et al. (Institute for HIV Research, University Hospital, University Duisburg-Essen, Essen, Germany) conducted a very large study in Tanzania and Kenya on 2240 HIV-1 positive participants and controls groups [19,20]. Using Random Forest analyses, an innovative technique to assess numerous markers of inflammation, investigators showed that inflammation can differ by geographical region, gender and age. Such findings are important for the future validation of new inflammatory markers in ART-treated individuals as geographical environment, economic milieu, age and sex can be significant confounding factors.
In conclusion the study findings presented at this session clearly showed the importance of persistent genital and gut mucosal damage, microbiota translocation, and myeloid cell activation even when ART is initiated very early in the infection. Globally, data presented highlight the importance of genital and gut immunity studies when combined with the related microbiota in order to attempt to implement strategies to reverse such damage [21].
Eliminating HIV-1 latency and reservoirs
Eliminating or substantially reducing the latent viral reservoir is absolutely necessary for an HIV-1 cure. Latency has traditionally been understood as transcriptional latency with persistent replication-competent proviruses that are transcriptionally silent but can be reactivated to produce infectious virus particles and reignite viral spread in the absence of ART. Hence most current latency-reversing agents (LRAs) function by stimulating viral transcription. Sarah Fidler (Imperial College London, UK) presented the results of the Research In Viral Eradication of HIV Reservoirs (RIVER) trial, the first randomised controlled trial of a ‘kick-and-kill’ strategy [22]. Patients who initiated ART within 4 weeks of HIV-1 diagnosis in PHI with suppressed plasma HIV-1 RNA were randomised to either ART plus therapeutic vaccination followed by 10 doses of vorinostat taken every 3 days, or ART alone. Participants in the intervention arm showed significantly higher HIV-1 specific CD4 and CD8 T cell responses, and histone acetylation was increased. Despite this, no difference between arms was observed in terms of the primary outcome (total HIV-1 DNA level in CD4 T cells at weeks 16 and 18 post-randomisation), or in the proportion of participants with undetectable viral outgrowth. The reasons for these negative results should be investigated further to inform future trials, but might include the insufficient potency of the LRA used (vorinostat) to fully reverse HIV-1 latency, as well as the limitations of the current assays to measure the viral reservoir and latency reversal.
Steven Yukl (University of California, San Francisco, CA, USA) presented a study that challenges the dogma that HIV-1 latency is transcriptional step, demonstrating that latency is maintained not at the transcription initiation level, but mainly at the subsequent levels of elongation, completion and splicing [23]. Yukl and colleagues developed a panel of assays specific for different HIV-1 transcripts, corresponding to distinct blocks to transcription, and applied these assays to ART-treated patient-derived peripheral blood and rectal tissue samples, with and without ex vivo activation. In peripheral blood, short viral transcripts were highly abundant, with almost all HIV-1 infected cells containing these transcripts, but HIV-1 RNA copy numbers progressively diminished, demonstrating a perfect gradient of abundance, as viral RNA became longer. This might be an HIV-1 specific latency mechanism, or simply reflect the dissociation of RNA polymerase II complexes from the DNA template as the nascent RNA strand elongates. Remarkably, latency blocks were partially reversible by ex vivo cellular stimulation. Different LRAs exerted differential effects on different blocks, with some LRAs only increasing the levels of unspliced HIV-1 RNA but having no effect on the multiply spliced RNA, and some increasing the latter as well. These results provide one possible explanation for the failure of a number of LRA clinical trials to reduce the HIV-1 reservoir. It remains to be seen if a combination of existing LRAs (or novel LRAs, designed to target the specific blocks) could more effectively reverse a number of latency blocks at the same time, which is necessary for efficient virus reactivation. In the rectum, median copy numbers of short transcripts were>10-fold lower that in the peripheral blood, with those of other transcripts roughly on the same level, indicating a much stronger block to the transcription initiation and less inhibition of elongation in the tissue compared to blood. These latter results should be interpreted with caution due to low patient numbers (n=7) and be confirmed in a larger study.
One of the most important current goals of HIV research is the search for biomarkers that could predict the time to viral rebound after treatment interruption. They biomarkers could be used to identify patients for whom it might be safer to interrupt therapy and who therefore might be better candidates for participation in clinical trials of novel curative interventions. A number of candidate predictive biomarkers have been identified, but it is unclear whether any of them is sufficiently robust. Marie-Angélique De Scheerder (University of Ghent, Belgium) presented the results obtained in the HIV-STAR cohort, where patients underwent transient analytical treatment interruption with extensive sampling before, during, and after the interruption [24]. De Scheerder and colleagues hypothesised that levels of host antiviral restriction factors might be predictive for the time to viral rebound after the ART interruption. The expression of known HIV-1 restriction factors (APOBEC3G, SAMHD1, MX2, PAF1, SLFN11, TRIM5α and BST2/tetherin), cofactors (NLRX1 and PSIP1) and interferon-stimulated genes (ISGs) IFIT1 and MX1 were evaluated. No associations were found between the expression of any restriction or cofactor and time to viral rebound, but a significant difference in restriction factor expression between at least two time points was identified for SLFN11, APOBEC3G and MX2. Further research is needed to establish whether these differences could be attributed to the function of these restriction factors.
Along the same lines, José Benito (Universidad Autónoma de Madrid, Spain) investigated potential genetic factors leading to loss of viral control (VC) in HIV-1 elite controllers (EC) [25]. A retrospective longitudinal study was performed to compare ECs who experienced VC loss (transient controllers, TC) with those with VC (persistent controllers, PC). The expression of 43 genes related to HIV-1 pathogenesis was measured by qPCR and principal component analysis (PCA) was conducted. PCA could differentiate TC from PC at one of the time points and significant downregulation of several genes in TC compared to PC was recorded. These genes could be considered potential biomarkers of loss of natural VC.
Kobus Bosman (Department of Medical Microbiology, Virology, University Medical Center Utrecht, Utrecht, Netherlands) studied the relative contribution of T cell subsets to the viral reservoir over decades of ART in seven patients whose HIV-1 was effectively suppressed on treatment since 1996 [26, 27]. Samples were taken at ART initiation and 1, 10 and 20 years post-treatment initiation. In the first year of ART, a significant reduction in the average HIV-1 DNA load in PBMCs was observed (from 3260 to 794 LTR copies/106, P=0.018), but HIV-1 DNA remained stable afterwards. Considering the differences in T cell subset abundance, contributions of Tcm and Ttm to the total reservoir were significantly higher than 17A-producing lymphocytes (Tn) and Tem (P<0.001) and did not change over time. T cell subset infection frequencies were also stable over 20 years of ART. These observations indicate that treatment alone does not shift the viral reservoir towards the most differentiated T cell subsets as was anticipated in the so-called ‘push and vanish’ eradication strategy.
The challenges of non-communicable diseases
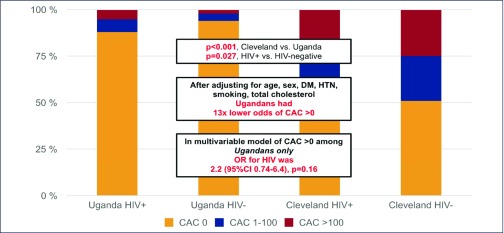
The Oral Abstract Session about Continued Challenges of Non-communicable Diseases opened with subclinical coronary disease among people living with HIV (PLWH) in sub-Saharan Africa by Christopher Longenecker (Case Western Reserve University School of Medicine, Cleveland, OH, USA). Coronary artery calcium (CAC) scores obtained from gated non-contrast computed tomography scans of the heart in 100 Ugandan individuals living with HIV who were on ART and over 40 years old, were compared to 100 age- and sex-matched HIV-1 uninfected controls (n=167) and uninfected controls (n=63) over 40 years old from a research database in Cleveland, OH, USA [28]. Despite a higher burden of some risk factors (age, cholesterol, diabetes, all P<0.01), Ugandans had over 13 times lower odds of CAC>0 (P<0.001) after adjustment for HIV serostatus, age, sex, and traditional risk factors. Variables associated with CAC>0 were sCD163 and oxidised LDL, even after adjustment for protease inhibitor use and current CD4 T cell count in Ugandan individuals living with HIV, and nadir CD4 T cell count was the only HIV-1 specific variable associated with CAC>0 in all persons living with HIV (Figure 2).
Figure 2.
Coronary artery calcium scores among people living with HIV-1 in Uganda and the US
Lisanne Demmer et al. (Rigshospitalet, Viro-Immunology Research Unit, Copenhagen, Denmark) assessed cardiac structural abnormalities and associated factors in people living with HIV and uninfected controls using Multidetector computed tomography (MDCT) [29]. Among 592 people living with HIV from the Copenhagen comorbidity in HIV infection (COCOMO) study and 1184 age and sex-matched uninfected controls from the Copenhagen General Population Study included in the study, no major structural cardiac abnormalities were found in the well-treated population of people living with HIV. Although HIV-1 was independently associated with a smaller left ventricular diastolic volume, larger right ventricular diastolic volume and greater left ventricular mass, their clinical impact remain uncertain when considering the increased CVD risk previously observed in people living with HIV.
Alan S Go et al. (Kaiser Permanente Northern California, Division of Research, Oakland, CA, USA) presented results from the HIV HEART study evaluating the independent association of HIV-1 infection with incident heart failure (HF) [30]. Among 38,868 HIV-positive and 386,586 matched HIV-1 negative adults studied, the rate (per 100 person-years) of incident HF was higher in HIV-1 positive (0.24, 95% CI 0.22–0.26) versus matched HIV-1 negative (0.16, 95% CI 0.15–0.16) individuals (P<0.0001). In multivariable analyses, HIV-1 infection was associated with a 75% increased rate of developing HF in the fully adjusted model. This excess risk did not appear to be mediated by atherosclerotic disease pathways or a differential use of cardiopreventive therapies.
Srishti Chhabra (Imperial College London, UK) presented a retrospective cohort analysis of all-cause mortality and malignancies in teenage young adults (TYA) living with perinatally acquired HIV (PaHIV) [31]. Among 290 TYAPaHIV who contributed 2644 person-years of follow-up, overall mortality rate was 2.3/1000 person-years, 9.4 times the age-matched general population (incidence rate ratio [IRR] 9.4, 95% CI 3.4–20.4, P<0.0001). The incidence of a malignancy was 3.0/1000 person-years in TYAPaHIV, IRR to the age-matched general population 12.9 (95% CI 5.6–25.5, P<0.0001), driven by lymphomas (IRR 44.2, 95% CI 16.1–96.7, P<0.0001). Since the median length of HIV-1 viraemia pre-cancer diagnosis was 15 years (IQR 12–17), earlier ART access may allow decreased mortality and risk for malignancy in the future.
Evelyn Verheij (Academic Medical Center, Department of Global Health and Division of Infectious Diseases, Amsterdam, Netherlands) presented longitudinal data on the impact of frailty on mortality/incident comorbidity risk and factors associated with frailty progression on behalf of the AGEhIV Cohort Study [32]. Among 598 people living with HIV and 550 demographically comparable HIV-1 uninfected individuals, 8.5% and 3.4% were frail, respectively and during 4423 person-years of follow-up (PYFU) 12 and five persons died, for an all-cause mortality rate of 5.2/1000 and 3.8/1000 PYFU, respectively. Time to death was shorter among frail persons. After adjustment for HIV-1 status, age and number of pre-existing comorbidities, frailty was independently associated with mortality (hazard ratio [HR] 12.3, 95% CI 4.5–33). After adjustment for confounding factors, frail participants had higher odds of developing ≥1 comorbidity (OR 1.93, 95% CI 1.15–3.322) independently of HIV status. Treated HIV disease increased the risk of frailty progression (OR 2.2, 95% CI 1.3–3.8), mediated by higher waist-to-hip ratio, higher comorbidity burden and presence of depressive symptoms.
Elizabeth Laidlaw (NIAID/NIH, Bethesda, MD, USA) analysed factors associated with weight gain in ART-naïve participants with a CD4 T cell count below 100 cells/mm3 who were followed for 160 weeks after ART initiation [33]. Among the 143 participants, median percent weight change was +14% (IQR 7.7–24%) at week 48 and +18% (IQR 7–28%) at week 160. Overweight or obese participants increased from 46 (32%) at week 0 to 102 (71%) at week160. Among five biomarkers studied, only CRP (P=0.009) and sCD14 (P=0.007) correlated with weight change at week 160. In a multivariate linear regression, adjusting for baseline opportunistic infections, CD4 T cell count, age, and gender, the ART regimen was significantly associated with weight change at week 48: negatively with NNRTI (P=0.012), positively with PI (P=0.001), but not with INSTI (P=0.38). Still, the difference between ART regimens may be related to a selection bias.
In terms of morbidity among HIV-1 positive people Patricia McGettigan (William Harvey Research Institute, London, UK) presented a population-based study in 50,000 UK patients [34]. The objective was to examine prevalent non-communicable disease (NCD) morbidities and multimorbidity among people with and without HIV-1 in England, comparing the findings with those of similar observational studies in Canada [35], the USA [30, 36], Denmark [37] and the Netherlands [38]. In a nested case control study, the authors included 10,071 patients with a diagnosis of HIV-1 and 40,284 controls from the CALIBER programme using Clinical Practice Research Datalink anonymised primary care electronic health record data. HIV-1 positive cases were matched to HIV-1 negative controls by sex and 5-year age groups in a 1:4 ratio. Amongst nine NCD morbidities that were examined (acute myocardial infarction [MI]), asthma, chronic pulmonary obstructive disease [COPD], diabetes, heart failure, hypertension, peripheral arterial disease [PAD], and renal disease) HIV-1 positivity was associated with an increased risk of renal disease and a reduced risk of heart failure. There was no association with the other morbidities or with multimorbidity. The studies from Canada, the USA, Denmark and the Netherlands individually reported various morbidity associations (Table 1). In all studies, patients with HIV-1 had a higher risk for renal disease than HIV-1 negative individuals. Non-disclosure has previously been estimated at 15–25% in HIV-1-positive cohorts in England [30] and may have impacted these findings, healthcare needs associated with renal disease have considerable resource implications for providers given the relative youth of the populations.
Table 1.
HIV-1-associated comorbidity risks in England, Canada, USA, Denmark and the Netherlands
| Study | CPRD, 2018 | Kendall et al. 2014 | Gallant et al. 2017 | Rasmussen et al. 2015 | Schouten et al. 2014 | ||
|---|---|---|---|---|---|---|---|
| Reported | Odds ratio | Prevalence ratio | P values for comparisons | Incidence rate ratio | P values for comparisons | ||
| Country | England | Ontario, Canada | Commercial population US | Medicaid population US | Medicare population US | Denmark | Netherlands |
| Average age, HIV vs no-HIV | Mean at matching 39.2y (SD 13.5) | 45.36y vs 46.65y | 46.2y vs 46.2y | 44.8y vs 44.7y | 66.8y vs 66.9y | 36.8y vs 36.8y | 52.1y vs 52.9y |
| Asthma | 1.06 (0.99, 1.14) | 1.31 (1.20, 1.43) | nr | nr | nr | nr | nr |
| COPD | 0.62 (0.26, 1.45) | 1.56 (1.39, 1.76) | nr | nr | nr | nr | nr |
| Diabetes | 1.08 (0.97, 1.19 | 1.19 (1.06, 1.33) | 0.704 | <0.001 | 0.089 | NR | ns |
| Heart Failure | 0.81 (0.72, 0.92) | 2.26 (1.74, 2.92) | nr | nr | nr | nr | nr |
| Hypertension | 0.96 (0.90, 1.03) | 0.95 (0.88, 1.04) | 0.005 | <0.001 | 0.106 | NR | <0.001 |
| MI | 1.04 (0.90, 1.21) | 1.12 (0.78, 1.60) | <0.001 | <0.001 | 0.932 | 2.02 (1.71, 2.38) | NR |
| PAD | 0.91 (0.72, 1.14) | 2.15 (1.35, 3.40) | <0.001 | 0.039 | 0.526 | nr | 0.008 |
| Renal disease | 4.92 (2.37, 10.23) | 2.57 (1.92, 3.44) | <0.001 | <0.001 | <0.001 | 4.01 (3.23, 4.98) | 0.044 |
| Stroke | 1.14 (0.98, 1.33) | 1.53 (1.15, 2.03) | nr | nr | nr | 1.84 (1.60, 2.13) | ns |
| Multi-morbidity | 0.94 (0.86, 1.02) | 1.30 (1.18, 1.44) | nr | nr | nr | nr | P = 0.009 |
| Significant risk association | Significantly reduced risk | nr=not reported; ns=not significant | |||||
HIV and pregnancy
In July 2017, the World Health Organisation (WHO) presented a series of guidelines and tools for transitioning from EFV-based to DTG-based first-line regimens [39]. This decision has been driven by efficacy, resistance and cost. However, recent findings from a Botswanan surveillance study regarding the risk of neural tube defects in early pregnancy has led to safety warnings issued in May 2018 by the European Medicines Agency and the US Food and Drug Administration [40,41]. Rebecca Zash presented this emerging evidence during a special session at the Conference [42].
The Tsepamo study started in August 2014 and takes place at 8 of the largest maternity wards in Botswana, covering around 45% of all births in the country. The primary aim of the study was to evaluate adverse birth outcomes by HIV-1 status and ART regimen. The study group have previously presented findings demonstrating that there is no increased risk of adverse birth outcomes when DTG is started during pregnancy. However, as the neural tube is closed by 28 days after conception, it is necessary to study DTG use at the time of, and in the first few days after, conception. In May 2018, the WHO guideline committee asked the study group to provide any preliminary data regarding adverse pregnancy outcomes among women who started DTG before pregnancy (pre-conception). At the time of the conference (data up to 15 July 2018), there were four neural tube defects in 596 births, corresponding to 0.67% risk (95% CI 0.26–1.7%). Clearly, this analysis is based on a small number of events, and these defects included various types (encephalocele, anencephaly, myelomeningocele, iniencephaly). None the less, this risk is higher than that among women who started DTG during pregnancy (1/3104, risk=0.03%, 95% CI 0.01–0.18%) or who were on EFV at conception (3/5787, risk=0.05%, 95% CI 0.02–0.15%). This difference is statistically significant. Dr Zash stressed the preliminary nature of these findings and stated that the group plans to present updated findings after April 2019, and will expand the number of study sites from eight to 18.
HIV vaccine session: from conception to delivery
The HIV vaccine session contained five talks: self-assembling nanoparticles mimicking HIV-1 envelope trimers [43], a small molecule CD4 mimetic approach [44], a novel approach to vaccine delivery [45], the APPROACH study [46] as well as a shortened immunisation schedule using the APPROACH vaccine.
The opening presentation demonstrated that soluble native-like HIV-1 envelope trimers (SOSIP trimers) were able to achieve the configuration of the native gp120 trimer found on the virus particle, something that previous attempts had failed to do [43]. These SOSIP particles, when presented on nanoparticles, were able to induce B cell activation in vaccinated rabbits and production of high levels of neutralising antibodies. This technology also allowed the successful incorporation of envelope proteins from different HIV-1 clades. It is believed that un-shielding of one of the major immunodominant epitopes is responsible for the improved immunogenicity.
Model data suggested that binding of a small molecule CD4 mimetic, BNM-III-170, to the closed form of gp120, increased the susceptibility of the protein to antibody neutralisation and antibody-dependent cell-mediated cytotoxicity, and inhibited HIV-1 infection [44]. This has led to the hypothesis that using this CD4 mimetic as an additive to gp120 vaccine could raise env-specific antibodies capable of preventing infection. The presence of the CD4 mimetic in combination with gp120 was able to generate the production of antibodies and prevent infection following up to three challenges and for up to 24 weeks following the last immunisation. The group are working on the development of a sustained release mechanism that would deliver drug to sites such as the vagina and could increase the efficacy of env-based vaccines.
The needle-free vaccine delivery system under development is based on a technology for delivering anaesthetics in dentistry [45]. It could be used to deliver vaccines orally to the sublingual and buccal regions. Studies performed in rhesus macaques using modified vaccinia Ankara produced higher levels of anti-gp 120 antibodies with the needle-free oral injection compared to intradermal/subcutaneous injections, with higher levels of mucosal IgG and IgA detected in plasma and a range of tissues. Subsequent challenge studies were able to demonstrate heterologous protection against SHIV challenges with up to six exposures. The exact mechanism involved in protection against the SHIV challenge is unclear as no neutralising antibodies directed against the challenge virus were detected. However, there was data to suggest that antibody-dependent cell mediated viral inhibition might be involved.
Details of the outcome from the first 48 weeks of the APPROACH vaccine study and its design had been published just prior to the conference [46]. The vaccine is based on an adenovirus type 26 vector delivering a mosaic of HIV-1 proteins from different HIV-1 clades (Ad26.Mos.HIV, MVA-mosaic and gp140 envelope protein) intramuscularly.
The eight study arms were based on a prime/boost strategy at four time points, the last one being at 48 weeks after the initial dose. Follow-up data was presented from the week 48 boost, at 96 weeks after the initial immunisation [47]. In terms of safety, no serious vaccine-related adverse events were reported. At week 96, antibody levels in the vaccine recipients were higher than those observed in the animal model, where protection from six challenges was achieved in 67% of vaccinated animals. At the same time point, the breadth of response was also found to be maintained, with about 80% of recipients of the lead regimen still having a detectable immune response against a range of nine different HIV-1 gp140 proteins from five different clades. A long-term follow-up of recipients is ongoing and a new trial to determine the efficacy of the vaccine was announced with the aim of enrolling 2600 young women in Southern Africa using the mosaic Ad26/Ad26 plus gp140.
The APPROACH study mentioned above, used a four-dose prime/boost schedule over the course of a year. But is it necessary to have such a prolonged immunisation schedule? Could similar results be achieved with a shorter duration and fewer immunisations? Kathryn Stephenson (BIDMC, Harvard, Boston, MA, USA) presented data comparing two shortened schedules of three immunisations over the course of 24 weeks with the schedule in the APPROACH study of four immunisations over the course of 48 weeks [48]. Results showed that the shorter schedule provided similar immune responses in terms of the persistence of the antibody response and the breadth of T cell response, leading the group to conclude that there may be flexibility in the dosing of the Ad26 mosaic/gp140 vaccine used in these studies.
Highlights from the HIV Cure Research with the Community workshop
One of the important meetings held in Amsterdam just before the 22nd International AIDS Conference (AIDS 2018) was the annual IAS one-day workshop to discuss the current state of research into an HIV cure. This year, the programme was developed by community activists, including people living with HIV (PLWH) together with leading researchers. The presentations included a review of the science, detailed analysis of HIV cure technologies, as well as results from ongoing research. Community speakers led a discussion about ethical and practical issues when developing cure studies. This included the direct experiences of people who had participated in cure research study protocols that involved an antiretroviral treatment (ART) interruption.
US activist Michael Louella, from the research collaboration defeatHIV [49] began the workshop with a lively discussion about the key considerations for designing future cure intervention trials. There was consensus amongst the workshop attendees that the first priority for any intervention must be safety. Therefore, the interest in developing novel interventions has to be balanced against the safety and effectiveness of current ART, which has already normalised life expectancy for many people. The discussion also included considerations of feasibility, acceptability and scalability of any innovation, at a cost that will be affordable in all settings. While global access to ART has enabled 22 million people to receive ART [50], nearly half of all people living with HIV are still not on therapy, and access to treatment is often dependent on international funding, which is itself vulnerable to political change [51]. Currently lifelong, ART can be easily threatened by resource limitations, availability of funding and economic and political change. Reporting from a recent cure meeting in Uganda [52], Moses Supercharger said people fear that they will lose their PEPFAR [53] medications and wanted a cure to overcome the lifelong vulnerability of HIV positive people who are dependent on donor-funded ART.
Very early ART and an unexpected role in research
Whilst there is a large array of complex laboratory tools that represent quantification and characterisation of the HIV-1 reservoir [54,55], none has been robust enough to accurately predict post-ART viral control. Hence to date, the only true determinate of remission is for individuals to interrupt ART. Experiences and challenges of what it feels like to interrupt ART were shared by Clark Hawley [56] who had begun very early ART after an indeterminate HIV-1 test result several days after starting PrEP. During an inspiring and charismatic interview, he detailed his unexpected role in research. Over several years, HIV was consistently undetectable on ART using multiple laboratory tests, from blood and tissue to evaluate viral RNA, DNA and antibody detection. After several years, his need to know whether or not he may have developed spontaneous post-treatment viral control, as well as the absence of detectable virus from a variety of different laboratory assays, drove his choice to stop ART as part of a research study. This protocol included weekly viral load testing [57]. Although his viral load rebounded after 9 months, he was stoical to the outcome and has since returned to having an undetectable viral load after restarting ART.
The unpredictability of viral rebound showed the need for the development and availability of an easy and inexpensive viral load home test – perhaps with a threshold of 1000 copies/mL. There is, in addition, a strong clinical need for such a point-of-care HIV viral load test for ART monitoring in many settings where routine viral load testing is not available, linking a joint research goal for both cure research and global health.
The safety of treatment interruptions
This was followed by a debate between John Frater (Oxford University, UK) and Steve Deeks (University of California, San Francisco, CA, USA) about the place for treatment interruption (TI) studies as a necessary evil [58]. Professor Frater (the lead investigator on studies that include a TI) argued that in many situations we are not there ‘yet’ in terms of scientific knowledge. We do not know who are the right participants to study based on surrogate biomarkers, nor when it is right to stop ART, how frequently to monitor the off-ART period, how high can viraemia be safely allowed to rebound and for how long, and when ART should be restarted. The vast majority of intervention trials to date have reported very rapid viral rebound, even amongst individuals having started ART very early after HIV acquisition [59]. To counter these points, Professor Deeks argued that as we have no meaningful laboratory assay that can accurately determine HIV remission, treatment interruption trials are essential. He presented data showing that if treatment interruption is undertaken with frequent viral load monitoring, with clear re-start criteria there is no evidence of short-term harm [60]. TIs are needed to advance the science [61] to evaluate with a clinically valuable endpoint the impact of a novel intervention. He argued that TI can be performed safely and with consent by HIV-1 positive people who share this goal.
Timothy Ray Brown [62], a participant at the meeting (and the only person to have so far been cured of HIV infection) emphasised that without having interrupted ART, it would not have been possible to definitely determine that he had been cured.
An advocacy perspective, featured in the discussions, was not so much focused on the inherent safety or risks in cure research, including when stopping treatment, but the difficulty of this taking place with informed consent. No matter how clearly the lack of personal benefit from joining a study is emphasised, most participants have a hope that they have a chance of being cured. This disconnect will remain an ethical challenge [58].
The diversity of research approaches – perhaps reflecting the lack of research consensus – is highlighted in an online database of more than 200 cure studies compiled by Richard Jefferys (TAG) [63].
ART in early acute infection, stem cell research and cure studies in children
Whilst many studies have included surrogate markers of the HIV reservoir, treatment interruption has been the only clear approach to determine true viral remission. The SPARTAC trial identified total HIV DNA in peripheral blood CD4+ T cells as an accurate predictor of post-treatment viral control, or time to viral rebound after treatment interruption [64, 65] although amongst individual cases these measures have not predicted HIV viral rebound after treatment interruption [66].
Jintanat Ananworanich (MHRP, Bethesda, MD, USA) presented data from an observational cohort study amongst individuals attending a Thai study centre that uses HIV-1 RNA screening to identify individuals with very early acute (Fiebig I/2) HIV-1 infection. Participants started immediate ART at the time of diagnosis, consenting to longitudinal follow-up with laboratory evaluation of reservoirs, clinical outcomes and immunological measures. Outcomes from very early ART included greater immunological recovery, a limited HIV-1 reservoir size and encouraging outcomes in terms of neurocognitive function [67,68]. However, even with such early treatment, viral load rebounded in all cases after ART discontinuation [59]. An additional negative outcome following ART interruption was that many of these volunteers, who were previously HIV negative on antibody testing have now seroconverted and test HIV-1 antibody positive. In Thailand this has important implications for their career, insurance and other aspects of life.
Research using stem-cell transplants, to mimic the case of Timothy Brown, was presented by Monique Nijhuis (UMC Utrecht, Netherlands). She described the detailed scientific evaluation of HIV-1 reservoirs amongst individuals enrolled into a cross-European collaboration, ICISTEM [69], where people living with HIV on ART undergo clinically indicated stem cell transplantation to treat malignancies. Within this group overall transplantation has cured the malignancy in over half the study participants. To date none have interrupted ART, although many have had marked reductions in measures of their reservoir and consideration of treatment interruption is ongoing for those with good levels of chimerism achieved post transplantation. Although the numbers of patients as yet are small, it appears that graft versus host disease might be associated with lower levels of HIV-1 reservoirs.
Caroline Tiemessen (NCID, South Africa) reviewed reported cases of post-treatment HIV-1 control. amongst very early treated adults [70], individual case-reports of ART-treated children [71] and adolescents [72]. In all cases these individuals controlled viraemia for several months to years after treatment interruption, but these remain rare cases and there has yet to be a prospective study exploring the prevalence of this phenotype and the best laboratory surrogate markers to predict remission.
The inclusion of children in cure research showed the diversity of views, even among paediatricians. While a cautious approach to any experimental intervention generates the belief that data should at least be available in adults first, the downside is that this might postpone paediatric studies for decades. To counter this view, the current limited treatments available for infants and children, including difficult formulations, and continued higher risk of early mortality suggest that paediatric cure research carries an even greater urgency.
A second debate on strategies to overcome HIV-1 latency featured Sharon Lewin (Doherty Institute, Melbourne, Australia) supporting the more established (but as yet unsuccessful [73] kick-and-kill approach against Susana Valente (Scripps Research Institute, FL, USA) who provided optimistic hopes for the newcomer block-and-lock approach, which rather than coaxing HIV out of hiding, aims to use transcription inhibitors to ensure HIV-1 remains permanently inactivated.
Conclusion
Taken together, the meeting provided an important opportunity for researchers and community activists from many countries to learn about the latest advances, engage in the issues of study design, raise the profile of ethical concerns and have an early preview of research to be presented at the main AIDS 2018 conference. There is no doubt that there is a strong support from the community of PLWH [74], advocates, funders and researchers to move the field into safe, feasible scalable and effective interventions that will cure HIV-1 infection, or induce remission which removes the need for lifelong ART.
The programme of the meeting and slides from the scientific presentations are available from the online conference programme [75].
References
All abstracts are from the 22nd International AIDS Conference. 23–27 July, 2018. Amsterdam, the Netherlands unless otherwise stated.
- 1. Rodger A, Cambiano V, Bruun T et al. . Risk of HIV transmission through condomless sex in gay couples with suppressive ART: the PARTNER2 study expanded results in gay men. Abstract WEAX0104LB.
- 2. Crowford S, Miller R, Post F et al. . Mortality and cause of death among HIV patients in London in 2016. Abstract TUPDC0106. [DOI] [PubMed]
- 3. Magnuson D, Hawkins T, Mera R. Adolescent use of Truvada (FTC/TDF) for HIV pre-exposure prophylaxis (PrEP) in the United States (2012–2017). Abstract TUAC0305.
- 4. Ananworanich J, Pinyakorn S, Avihingsanon A et al. . Favorable clinical phenotype reached in less than half of people treated in acute HIV infection. Abstract WEPDB0102.
- 5. Tremeaux P, Lenfant T, Boufassa F et al. . Increasing contribution of integrated forms to total HIV1-DNA in blood, in primary infection during natural history - ANRS PRIMO and SEROCO cohorts. Abstract WEPDB0103.
- 6. Hocqueloux L, Monceaux V, Avettand-Fenoel V et al. . Intermittent viremia after treatment interruption increased risk of ART resumption in post-treatment HIV-1 controllers. ANRS VISCONTI study. Abstract WEPDB0104.
- 7. Giron LB, Hunter J, Galinskas J et al. . Auranofin plus nicotinamide impact HIV reservoir among ART suppressed HIV individuals. Abstract WEPDB0105.
- 8. Meireles MV, Pascom AR, Perini F et al. . Comparative effectiveness of first-line antiretroviral therapy regimens: Results from a large real-world cohort in Brazil after the implementation of dolutegravir. Abstract TUAB0101. [DOI] [PubMed]
- 9. Braun DL, Turn T, Hampel B et al. . Simplification to dolutegravir monotherapy is non-inferior compared to continuation of combination antiretroviral therapy in patients who initiated combination antiretroviral therapy during primary HIV infection: A randomized, controlled, non-inferiority trial. Abstract TUAB0102.
- 10. Hocqueloux L, Allavena C, Prazuck T et al. . Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for HIV-1-infected virologically suppressed patients: results from the randomized non-inferiority MONCAY trial. Abstract TUAB0103.
- 11. Cahn P, Sierra Madero J, Arribas J et al. . Non-inferior efficacy of dolutegravir (DTG) plus lamivudine (3TC) versus DTG plus tenofovir/emtricitabine (TDF/FTC) fixed-dose combination in antiretroviral treatment-naïve adults with HIV-1 infection - 48-week results from the GEMINI studies. Abstract TUAB0106LB.
- 12. Lai M-T, Xu M, Ngo W et al. . Characterization of doravirine-selected resistance patterns from participants in treatment-naïve Phase 3 clinical trials. Abstract THPDB0101.
- 13. Hyle E, Scott J, Sax P et al. . Baseline resistance testing in the current treatment era – no longer cost-effective? Abstract THPDB0105.
- 14. Aounallah M, Dagenais-Lussier X, El-Far M et al. . Current topics in HIV pathogenesis, part 2: Inflammation drives a Warburg-like effect on the metabolism of HIV-infected subjects. Cytokine Growth Factor Rev 2016; 28: 1– 10. [DOI] [PubMed] [Google Scholar]
- 15. Wacleche VS, Landay A, Routy JP et al. . The Th17 lineage: from barrier surfaces homeostasis to autoimmunity, cancer, and HIV-1 pathogenesis. Viruses 2017; 9: E303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caruso MP, Holgado MP, Falivene J et al. . Antiretroviral treatment did not restore functionality of cervical mucosal cells for Th17-related cytokines altered after HIV infection. Abstract WEPDA0101.
- 17. Routy JP, Mehraj V, Ramendra R et al. . Effect of ART on reducing fungal translocation in HIV-infected patients. Abstract WEPDA0102.
- 18. Naidoo S, Veldsman K, Cotton MF et al. . Persistence of myeloid cell-associated inflammation in HIV-infected children after 8 years on early initiated therapy - the key role players in HIV persistence? Abstract WEPDA0103.
- 19. Streeck H, Son G, Habermann D et al. . Immune activation parameters are differentially expressed across four countries in sub-Saharan Africa and are associated with comorbidities in HIV+ and HIV- individuals. Abstract WEPDA0104.
- 20. Lu W, Feng Y, Jing F et al. . Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front Microbiol 2018; 9: 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen J, Xun J, Yang J et al. . Plasma indoleamine 2,3-dioxygenase activity is associated with the size of HIV reservoir in patients receiving antiretroviral therapy. Clin Infect Dis 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fidler S, Stohr W, Pace M et al. . A randomised controlled trial comparing the impact of antiretroviral therapy (ART) with a ‘Kick-and-Kill’ approach to ART alone on HIV reservoirs in individuals with primary HIV infection (PHI); RIVER trial. Abstract TUAA0202LB.
- 23. Yukl S. Low level transcription on ART – implications for latency elimination. Abstract WESY0904.
- 24. De Scheerder M-A, Van Hecke C, De Langhe N et al. . Increase in restriction factor expression in response to viral rebound after analytical treatment interruption in HIV-infected patients. Abstract WEAA0201.
- 25. Benito JM, Garcia M, Santisteban V et al. . Genetic factors leading to loss of viral control in HIV elite controller patients. Abstract WEAA0203.
- 26. Bosman K, Tesselaar K, Arends J et al. . Remarkable stability in size and composition of the latent reservoir over two decades of ART. Abstract WEPEA030.
- 27. Chahroudi A, Silvestri G, Lichterfeld M. T memory stem cells and HIV: a long-term relationship. Curr HIV/AIDS Rep 2015; 12: 33– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Longenecker CT, Alencherry B, Erem G et al. . Low prevalence of calcified coronary plaque among Ugandans with and without HIV infection: Comparison with a United States cohort and associations with biomarkers of inflammation. Abstract THAB0101.
- 29. Demmer L, Ronit A, Sigvardsen PE et al. . Cardiac chamber abnormalities and left ventricular mass in people living with HIV and matched uninfected controls assessed by multidetector computed tomography. Abstract THAB0102.
- 30. Go AS, Horberg M, Reynolds K et al. . HIV infection independently increases the risk of developing heart failure: The HIV HEART study. Abstract THAB0103.
- 31. Chhabra S, Fidler S, Bower M et al. . Malignancy and all-cause mortality: Incidence in teenage young adults living with perinatally acquired HIV. Abstract THAB0104. [PMC free article] [PubMed]
- 32. Verheij E, Kirk GD, Wit FW et al. . Increased risk of both mortality and incident comorbidity among frail HIV-positive and HIV-negative participants in the AGEhIV Cohort Study, and increased risk of frailty progression in those with HIV. Abstract THAB0105.
- 33. Laidlaw E, Gouel-Cheron A, Kuriakose S et al. . Weight gain after ARV initiation correlates with increased inflammatory biomarkers and protease inhibitor usage. Abstract TUPEB121.
- 34. McGettigan P, Daskalopoulou M, Denaxas S et al. . Morbidity among HIV-positive people: A population-based study in 43,545 UK patients. Abstract TUPEB085.
- 35. Kendall CE, Wong J, Taljaard M et al. . A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 2014; 14: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection–a trend analysis. J Infect Dis 2017; 216: 1525– 1533. [DOI] [PubMed] [Google Scholar]
- 37. Rasmussen LD, May MT, Kronborg G et al. . Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015; 2: e288– e298. [DOI] [PubMed] [Google Scholar]
- 38. Schouten J, Wit FW, Stolte IG et al. . Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59: 1787– 1797. [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization Transition to the use of dolutegravir. 2017. Available at: www.who.int/hiv/mediacentre/news/transition-to-new-arv-QA/en/index8.html ( accessed September 2018).
- 40. European Medicines Agency New study suggests risk of birth defects in babies born to women on HIV medicine dolutegravir. 2018. Available at: www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/05/news_detail_002956.jsp&mid=WC0b01ac058004d5c1 ( accessed September 2018).
- 41. Us Food and Drug Administration Medical product safety information. Available at: www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm608168.htm ( accessed September 2018).
- 42. Zash R, Holmes L, Mayhem J et al. . Surveillance for neural tube defects following antiretroviral exposure from conception. Abstract TUSY15.
- 43. Brouwer P, Ellis D, Antanasijevic A et al. . Two-component self-assembling nanoparticle vaccines that present multiple HIV-1 envelope trimers. Abstract TUAA0101.
- 44. Madani N, Princiotto A, Mach L et al. . A CD4 mimetic compound enhances vaccine efficacy against stringent immunodeficiency virus challenge. Abstract TUAA0102. [DOI] [PMC free article] [PubMed]
- 45. Jones A, Das R, Wyatt L et al. . Oral MVA/protein HIV vaccination with a needle-free injector induces robust systemic and mucosal antibody responses in rhesus macaques. Abstract TUAA0103.
- 46. Barouch DH, Tomaka FL, Wegmann F et al. . Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus macaques (NHP 13-19). Lancet 2018; 392, 232– 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomaka F, Stieh D, Barouch D et al. . Long-term data from APPROACH: Phase 1/2a randomized, double-blind, placebo-controlled study evaluating safety/tolerability and immunogenicity of vaccine regimens using combinations of Ad26.Mos.HIV, MVA-mosaic and gp140 envelope protein. Abstract TUAA0104.
- 48. Stephenson K, Ansel J, Walsh S et al. . HPX1002/IPCAVD010: A randomized controlled trial evaluating the safety and immunogenicity of shorter and simpler vaccine schedules using Ad26.Mos.HIV with gp140 Env protein. Abstract TUAA0105.
- 49. DefeatHIV Available at: defeathiv.org ( accessed September 2018).
- 50. UNAIDS Global HIV and AIDS statistics – 2018: fact sheet. Available at: www.unaids.org/en/resources/fact-sheet ( accessed September 2018).
- 51. Avert Available at: www.avert.org/professionals/hiv-around-world/global-response/funding ( accessed September 2018).
- 52. IAS Advocacy-for-Cure Academy. Available at: www.iasociety.org/HIV-Programmes/Programmes/Towards-an-HIV-Cure/Events/Advocacy-for-Cure-Academy ( accessed September 2018).
- 53. United States President's Emergency Plan for AIDS Relief Available at: www.pepfar.gov/about/270968.htm ( accessed September 2018).
- 54. Martin GE, Frater J. Post treatment and spontaneous viral control. Curr Opin HIV AIDS 2018; 13: 402– 407. [DOI] [PubMed] [Google Scholar]
- 55. Fidler S, Olson AD, Bucher HC et al. . Virological blips and predictors of post treatment viral control after stopping ART started in primary HIV infection. J Acquir Immune Defic Syndr 2017; 74: 126– 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. D’Adesky C. The man who was nearly cured of HIV. KQED, 2017. Available at: www.kqed.org/futureofyou/437567/frontiers-of-hiv-research-the-man-who-was-nearly-cured ( accessed September 2018).
- 57. Henrich TJ, Hatano H, Bacon O et al. . HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: an observational study. PLoS Med 2017; 14: e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eyal N, Holtzman LG, Deeks SG. Ethical issues in HIV remission trials. Curr Opin HIV AIDS 2018; 13: 422– 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colby D, Trautmann L, Pinyakorn S et al. . Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fieibig I acute HIV infection. Nat Med 2018; 24: 923– 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Papasavvas E, Lada SM, Joseph J et al. . Analytic antiretroviral treatment therapy interruption does not irreversibly change pre-interruption levels of cellular HIV. AIDS 2018; 32: 1763– 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Namazi G, Fajnzylber JM, Aga E et al. . The control of HIV after antiretroviral treatment pause (CHAMP) study: post treatment controllers identified from 14 clinical studies. J Infect Dis 2018: Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hutter G, Nowak D, Mossner M et al. . Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360: 692– 698. [DOI] [PubMed] [Google Scholar]
- 63. Treatment Action Group Research towards a cure trials. Available at: www.treatmentactiongroup.org/cure/trials ( accessed September 2018).
- 64. Williams JP, Hurst J, Stohr W et al. . HIV-1 DNA predicts disease progression and post-treatment virological control. ELife 2014; 3: e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hurst J, Hoffman M, Pace M et al. . Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun 2015; 6: 8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Persaud D, Luzuriaga K. Absence of HIV-1 after treatment cessation in an infant. N Engl J Med 2014; 370: 678. [DOI] [PubMed] [Google Scholar]
- 67. Samboju V, Philippi CL, Chan P et al. . Structural and functional brain imaging in acute infection. Neuroimage Clin 2018; 20: 327– 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. D’Antoni ML, Byron MM, Chan P et al. . Normalisation of soluble CD163 after institution of antiretroviral therapy during acute HIV infection tracks with fewer neurological abnormalities. J Infect Dis 2018; 218: 1453– 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. ICISTEM International Collaboration to guide and investigate the potential for HIV cure by stem cell transplant. Available at: www.icistem.org ( accessed September 2018).
- 70. Saez-Cirion A, Bacchus C, Hocqueloux L et al. . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early antiretroviral therapy ANRS Visconti Study. Plos Pathog 2013; 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shiau S, Abrams EJ, Arpadi SM, Kuhn L. Early antiretroviral therapy in HIV-infected infants: can it lead to HIV remission? Lancet HIV 2018; 5: e250– e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Frange P, Faye A, Avettand-Fenoel V et al. . HIV-1 remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 1: e49– e54. [DOI] [PubMed] [Google Scholar]
- 73. i-base First randomised kick-and-kill cure study fails to reduce HIV reservoir: RIVER study reports vorinostat and vaccines show activity, but not enough. Available at: i-base.info/htb/34618 ( accessed September 2018).
- 74. Simmons R, Kall M, Collins S et al. ; Survey collaboration A Global survey of HIV-positive people's attitudes towards cure research. HIV Med 2017; 18: 73– 79. [DOI] [PubMed] [Google Scholar]
- 75. Lewin S, Deeks S. HIV Cure Research with the Community Workshop; re-conference workshop. Available at: programme.aids2018.org/Programme/Session/178 ( accessed September 2018).