Abstract
Partnerships between researchers and policymakers can improve uptake and integration of scientific evidence. This article describes the research-policy partnership between the International epidemiology Databases to Evaluate AIDS (IeDEA) ( www.iedea.org) and the World Health Organization (WHO), which was established in 2014. IeDEA is an international research consortium, which analyses data on almost 2 million people living with HIV under care in routine settings in 46 countries in Asia-Pacific, the Caribbean, Central and South America, North America and sub-Saharan Africa. Five multiregional analyses were identified to inform the WHO on progress towards the second and third 90s of the 90-90-90 targets in adults and children: (i) trends in CD4 cell counts at the start of antiretroviral therapy (ART); (ii) delays from enrolment in HIV care to ART initiation; (iii) the impact of ART guideline changes; (iv) retention in care, mortality and loss to follow-up; and (v) viral suppression within the first 3 years after initiating ART. Results from these analyses were contributed to the 2015 and 2016 WHO global HIV progress reports, will contribute to the 2018 report, and were published in academic journals. The partnership has been mutually beneficial: discussion of WHO policy agendas led to more policy-framed, relevant and timely IeDEA research, and the collaboration provided the WHO with timely access to the latest data from IeDEA, as it was shared prior to peer-review publication.
Keywords: research-policy partnerships, HIV, cohort data, observational data, World Health Organization
Background
Over the past decade, the World Health Organization (WHO) has published a series of global HIV progress reports, documenting the successes and challenges of the massive antiretroviral therapy (ART) scale-up in resource-limited settings that resulted in nearly 21 million people receiving ART by mid-2017 [1–5]. National governments, international donor agencies, and implementing agencies use these reports to assess progress and reorient priorities. The WHO also supports ART scale-up through a series of evidence-based recommendations, ranging from standardising ART regimens to promoting service delivery models to assist people living with HIV, healthcare workers and country-level policymakers, in making informed decisions about healthcare interventions [6–9].
WHO progress reporting and evidence-based guidelines aim to translate and incorporate the best available research evidence into well-informed guidelines and recommendations [6,7,10]. However, production and dissemination of scientific evidence does not necessarily lead to its integration into policy [11,12]. Partnerships between researchers and policymakers can facilitate and improve uptake and integration of scientific evidence [12–16]. Communication between stakeholders allows researchers to be aware of policy-related evidence gaps and upcoming policy priorities, leading to more pertinent and opportune policy-relevant research [10,14,17]. Such research-policy partnerships can also provide the opportunity for critical information to be communicated rapidly and prior to completion of the often lengthy peer-review and publication process.
This article summarises the process, output and challenges of a research-policy partnership between the International epidemiology Databases to Evaluate AIDS (IeDEA) and the WHO that aims to formalise and facilitate uptake and integration of IeDEA scientific evidence into WHO ART progress reporting and HIV health policy development.
The International epidemiology Databases to Evaluate AIDS (IeDEA)
IeDEA ( www.iedea.org) is an international research consortium of HIV cohorts funded by the National Institutes of Health (NIH) since 2006 [18–20]. IeDEA pools existing clinical and epidemiological data on people living with HIV under care in routine settings as a cost-effective way of generating large data sets to address high priority and evolving research questions in HIV/AIDS treatment and care. The seven regions included in IeDEA are: Asia-Pacific; the Caribbean, Central and South America (CCASA); North America; and four regions in Africa (Figure 1). Across these regions, IeDEA has individual-level data on over 1.7 million patients from over 480 clinic and research centres in 46 countries, in both high- and low-HIV burden settings.
Figure 1.
Map of the regions of IeDEA. Adapted from www.iedea.org. CCASAnet: Caribbean, Central and South America; NA-ACCORD: North America.
IeDEA collects routine data from both urban and rural settings, in primary through tertiary care facilities, and from small private clinics to large programmes run by national health systems. Patient information, clinic visit history, laboratory measurements, medications and clinical outcomes are some of the data collected by IeDEA regional cohorts. Regional cohorts do not collect data through a single standardised protocol, so IeDEA implements a prospective data exchange standard ( www.IeDEADES.org) for a selection of existing data – based on the HIV Cohorts Data Exchange Protocol ( www.hicdep.org) – to facilitate sharing and merging of data across IeDEA regions. A series of site assessment surveys obtain up-to-date information on facility policies and procedures and the clinical and support services provided to HIV patients enrolled at IeDEA clinics [21–26]. IeDEA is also a network of epidemiologists, clinicians, statisticians and data management specialists, who participate in topic-specific working groups to foster collaboration between regional cohorts, facilitate data harmonisation and dissemination, and advance the international HIV scientific research agenda.
The IeDEA-WHO collaboration
The IeDEA-WHO collaboration was launched at the end of 2014, after more than a decade of ad hoc work between IeDEA and the WHO. The WHO was seeking to formally collaborate with IeDEA so that up-to-date IeDEA data and analyses could contribute in a predictable and sustainable way to WHO annual global HIV progress reporting and guideline development. The WHO wanted to rapidly assess implementation of new HIV policy recommendations, and identify and characterise gaps in the response, which randomised control trials and observational studies based on outdated data cannot address. The IeDEA Southern Africa region was awarded an NIH grant supplement to fund a part-time project manager to initiate, plan and develop a formal collaboration between the IeDEA consortium and the WHO.
At that time, UNAIDS had just released ambitious 90-90-90 fast-track targets: 90% of all people living with HIV know their status, 90% of people diagnosed with HIV are on sustained ART, and 90% of all people receiving ART are virally suppressed by 2020 [27]. These fast-track targets aimed to improve the HIV cascade of care with the goal of ending the AIDS epidemic as a public health threat by 2030 [27]. In the following year, WHO released the ‘treat all’ guideline update, recommending immediate ART for all people living with HIV, eliminating prerequisites for initiating treatment [28].
The collaboration commenced with a face-to-face meeting at the WHO headquarters in Geneva, Switzerland in November 2014, where four members of the WHO Department of HIV/AIDS and four investigators of the IeDEA Southern Africa team based in Bern, Switzerland, discussed potential collaborative work. Four video conference calls were subsequently held in early February 2015 to discuss analyses that the IeDEA consortium could undertake to support the WHO 2015 global HIV progress report. Each call had between 10 and 12 participants from the seven IeDEA regions, the NIH-funding institutions and the WHO. Later that month, during the IeDEA Scientific Symposium at the Conference on Retroviruses and Opportunistic Infections (CROI), attended by more than 20 IeDEA investigators representing every IeDEA region, the IeDEA consortium agreed to undertake this collaboration with the WHO, and identified five core multiregional cascade analyses and the teams that would undertake them. These cascade analyses aimed to inform the WHO on progress towards the 90-90-90 targets, guideline implementation and trends in the epidemic, using routinely collected IeDEA programme data (Table 1).
Table 1.
Analysis of data from IeDEA to inform progress on 90-90-90 targets
| 90-90-90 target | Analyses performed | Data sources |
| First 90: 90% of all people living with HIV know their status | No data available in IeDEA | None |
| Second 90: 90% of people diagnosed with HIV are on sustained ART | Analyses of CD4 cell counts at the start of ART [29–31] | All seven IeDEA regions, ART-CC, COHERE, NISDI, PHACS and IMPACCT |
| Analysis of delays from enrolment in HIV care to ART initiation and the influence and impact of ART guideline changes among adults and children [32,33] | All seven IeDEA regions | |
| Analysis of retention in care, mortality and loss to follow-up among HIV-infected children and adults on ART [34] | All seven IeDEA regions | |
| Third 90: 90% of all people receiving ART are virally suppressed | Analysis of routine viral load data to assess viral suppression among adults and children within the first 3 years after initiating ART [35] | All seven IeDEA regions |
ART: antiretroviral therapy; ART-CC: ART Cohort Collaboration; COHERE: Collaboration of Observational HIV Epidemiological Research Europe in EuroCoord; IMPAACT: International Maternal Pediatric Adolescent AIDS Clinical Trials Group; NISDI: NICHD Site Development Initiative; PHACS: Pediatric HIV/AIDS Cohort Study.
IeDEA-WHO analyses
Cohorts cannot provide information on the number of people living with HIV or the number who know their status in their settings, so the collaboration is unable to assess the first 90 (percentage knowing their status). The IeDEA collaboration can, however, examine how quickly patients are started on ART after linkage to care and enrolment at their clinics, and how many of those who start ART have stopped treatment (the second 90 target), while those cohorts that collect routine viral load data can also assess the third 90 target. With more than 15 years of longitudinal data and accompanying facility information, IeDEA can monitor and examine temporal trends in patient care uptake and outcomes, and the impact of HIV guideline changes.
Progress towards the second 90 target was evaluated by two multiregional IeDEA-WHO analyses that focused on the pre-ART care cascade, and assessed delays from linkage to and enrolment in HIV care to ART initiation and the influence and impact of ART guideline changes among adults and children, separately [32,33]. The IeDEA West Africa data centre undertook the multiregional analysis that focused on children, and the IeDEA Central Africa data centre performed the multiregional analysis on adults. These analyses were undertaken in 2015, 2016 and 2017. The IeDEA Central Africa team additionally collaborated with the WHO to collect historical data on country-level ART guideline expansions by searching the internet for national ART policies and contacting IeDEA and WHO in-country experts.
A third IeDEA-WHO analysis described retention in care, mortality and loss to follow-up among children and adults living with HIV on ART, and compared outcomes under different ART eligibility guidelines to examine whether people who start ART remain on ART [34]. This multiregional analysis was performed at the IeDEA Southern Africa data centre in 2015, 2016 and 2017. A fourth IeDEA-WHO analysis assessed outcomes in younger (10–14 years of age) and older (15–19 years of age) adolescents to provide insights into long-term retention for the mixed population of perinatally and behaviourally infected youth. The IeDEA Asia-Pacific data centre undertook this analysis in 2016. The last core multiregional analysis of the collaboration used IeDEA routine viral load data to assess viral suppression (<1000 copies/mL) among adults and children within the first 3 years after initiating ART [35]. This multiregional analysis was undertaken in 2015 and 2017 by the IeDEA Asia-Pacific data centre. Additionally, one analysis took advantage of South Africa's National Population Register, with nearly complete enumeration of deaths and extensive long-term follow-up data of patients on ART, to assess advances in life expectancy over time [36]. The IeDEA Southern Africa data centre performed this analysis in 2015 using data from South African cohorts.
Since the IeDEA-WHO collaboration began, IeDEA has produced 12 multiregional analyses and one region-specific analysis – an average of four analyses per year using newly reported IeDEA data. The WHO received a summary report for each analysis within 6 months from data collection. Over the years, these analyses have supported three WHO global progress reports. Several analyses have also supported the development of WHO guidelines; these are summarised in a separate article in this supplement [37].
Project organisation and management
More than 65 IeDEA regional investigators across the seven IeDEA regions, 10 representatives of the NIH funding institutions, and nine WHO staff members have been involved with this collaboration since its inception. The collaboration project manager liaised between the IeDEA network and the WHO, and provided updates on work and progress in monthly IeDEA working group conference calls and by email. IeDEA held project debriefs to discuss and evaluate the process and work of the collaboration following publication of the annual WHO progress reports.
IeDEA multiregional data are only available after review and approval of a concept proposal by the IeDEA Executive Committee. This committee is composed of principal investigators from the seven IeDEA regions and representatives of the NIH funding institutions, including the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver Institute on Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health and the National Institute on Drug Abuse. For each iteration of the above analyses, IeDEA, in consultation with the WHO, drafted new concept proposals that were reviewed by the IeDEA Strategic Data Working Group (WG) prior to submission to the Executive Committee. The Strategic Data WG holds monthly conference calls to promote and guide IeDEA multiregional research conducted in collaboration with external partners, and consists of representatives from each IeDEA region and programme staff from the NIH and the WHO. Following Executive Committee approval, the concept proposal teams worked with the IeDEA Data Harmonization WG, comprised of IeDEA regional data managers and analysts, to clarify data elements and organise data transfer.
To help navigate, streamline and facilitate IeDEA procedures, a timeline was drafted, outlining deliverables and deadlines to ensure research occurred in a timely manner and aligned with the timeline for the development of annual WHO Progress Reports. IeDEA also undertook efforts to synchronise and increase the frequency of data collection to ensure that the most up-to-date data were available for these purposes.
Reports and publications
The results from the IeDEA-WHO collaboration analyses were presented in two WHO reports and several academic publications.
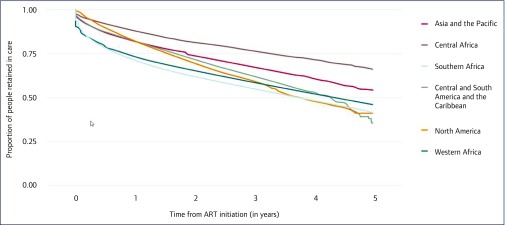
The Global health sector response to HIV, 2000–2015: focus on innovations in Africa was the 2015 WHO global HIV progress report released in November of that year [3]. The report relied on evidence produced by the IeDEA-WHO collaboration to highlight gaps between HIV diagnosis and treatment initiation. Evidence on over 800,000 adults from 10 African countries enrolled in IeDEA clinics from 2004 to 2014 showed that about 22% of adults were lost to follow-up before initiating ART. Despite this shortfall, IeDEA data indicated a steady shift towards earlier enrolment in HIV care as illustrated in a graph showing increasing median CD4 cell count at enrolment in HIV care across IeDEA programmes from 2004 to 2014. The report stressed the importance of retention in care and the need to retain more people on ART. It highlighted another collaboration analysis that found only 45% of adults on ART in IeDEA achieved viral suppression after 3 years, increasing to over 92% when excluding losses to follow-up and deaths. A graph showing the declining proportion of people on ART retained in care during the first 5 years, and across IeDEA regions, provided further evidence of high attrition (Figure 2).
Figure 2.
IeDEA evidence included in WHO Global Health Sector Response to HIV, 2000–2015: Focus on Innovations in Africa, published November 2015 [3]. Retention rates of people on ART in the first 5 years after initiating ART between 2009 and 2014 in IeDEA.
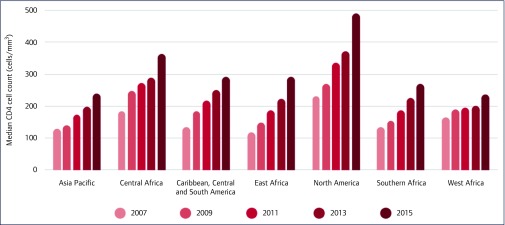
The 2016 WHO global HIV progress report Prevent HIV, test and treat all – WHO support for country impact, published in November 2016, focused on the latest WHO recommendation to initiate all people living with HIV on ART regardless of CD4 cell count or clinical stage [4]. This report highlighted key gaps in achieving the ‘treat all’ recommendation, including linkage from testing to treatment, citing the IeDEA-WHO collaboration's latest findings on delays from enrolment in HIV care to ART initiation in adults. Despite these challenges, the report found people have been starting ART earlier and at higher CD4 cell counts, as illustrated in a figure showing increasing median CD4 cell count at ART initiation at IeDEA clinics up to 2015 (Figure 3). This report also highlighted another collaboration analysis as evidence that a high level of viral suppression can be achieved, even in resource-limited settings.
Figure 3.
IeDEA evidence included in the WHO Progress Report 2016 [4]: Prevent HIV, Test and Treat all – WHO support for country impact. Median CD4 cell count at ART initiation among adults by IeDEA regions over time.
At present, the collaboration is undertaking four multiregional cascade analysis updates to assess the impact of recent guideline changes and progress towards the 90-90-90 targets across all age groups, to support the 2018 WHO global progress report that is currently under development for release at the end of 2018.
Beyond supporting WHO, these analyses have also generated 14 abstracts presented at international research conferences, including the International Workshop on HIV and Hepatitis Observational Databases (IWHOD), CROI, the International AIDS Society conferences, and Australian HIV and AIDS conferences. Several collaborative analyses have also been published in peer-reviewed journals including AIDS, Journal of Acquired Immune Deficiency Syndromes, Journal of the International AIDS Society and PLoS Medicine [32–36].
Discussion
Over the past 4 years, IeDEA has produced 13 analyses based on IeDEA multiregional cohort data to support WHO HIV guideline development and ART progress reporting. Evidence from IeDEA contributed to the 2015 and 2016 WHO global HIV progress reports and two WHO HIV guideline updates. Research evidence from these analyses also informed audiences outside of the WHO. Fourteen oral abstract presentations and posters were presented at international HIV conferences and workshops. The five core analyses and the one regional analysis have also resulted in five peer-reviewed publications [32–36].
The IeDEA-WHO collaboration is an example of a research-policy partnership overcoming barriers that often hinder the rapid uptake and integration of research evidence into policy development [12,14,16,38]. Increased interaction and regular communication strengthened links between IeDEA and WHO, as each became more aware of how IeDEA could be a resource to WHO and vice versa. Opportunities for discussion of upcoming WHO policy agendas led to more policy-framed, relevant and timely IeDEA research. The science has also been strengthened through this opportunity for the WHO and IeDEA research teams to communicate on the strengths and weaknesses of reporting indicators. The collaboration also provided the WHO with more timely access to the latest IeDEA scientific evidence, as it was shared with them prior to peer-review publication. Efforts to synchronise and accelerate data extraction in IeDEA resulted in more timely and up-to-date data that benefited the collaboration work as well as other multiregional research. The collaboration timelines ensured IeDEA procedures were navigated efficiently, allowing analyses to be completed annually. This research-policy partnership provided a unique opportunity for the WHO to monitor current implementation progress of their action-oriented guidelines and for IeDEA to produce more innovative policy-relevant observational research evidence.
Although IeDEA successfully produced and shared multiregional analyses with WHO, combining large volumes of data from such a wide range of settings on tight timelines can present a number of challenges. Issues of missing data on key variables, and varying definitions and data collection protocols, led to a duplication of time-consuming data cleaning efforts undertaken simultaneously by research teams. Inconsistent data distribution and a large variability in the volume of data across regions, due to the uneven burden of disease and temporal trends, limited the ability to provide region-specific and age-specific stratified estimates, complicating the interpretation of findings. Service delivery models, clinical protocols, standards of care, monitoring schedules and efforts in place to trace patients lost to follow-up also vary widely. The tight timeline dictated by the WHO publication schedule increased and concentrated the workload of IeDEA regional data managers responsible for preparing and transferring data for up to five separate research proposals requesting varying data elements and eligibility criteria within the same timeframe, and alongside other regional and multiregional data requests, each year. However, engagement with WHO also highlighted areas where IeDEA and its data workflows could be improved or made more efficient.
Data and structural challenges experienced in this collaboration with WHO propelled IeDEA to develop and implement improvements to expand and increase data collection and analytic capacity. The Strategic Data WG was established in the second year of the collaboration to facilitate regular dialogue between the IeDEA network and the WHO through monthly teleconference calls. Data harmonisation improvements were undertaken to ensure IeDEA data standards were up-to-date and met current multiregional research needs. The IeDEA data exchange standard ( www.IeDEADES.org) was translated from paper format to an online research electronic data capture (REDcap) database to simplify, coordinate and accelerate updates across multiple platforms, and enable faster and better data exchange and collaborative research [39]. In response to the growing number and frequency of multiregional concept proposals, IeDEA developed an online review hub to simplify, streamline and expedite the concept proposal review and approval process for multiregional research.
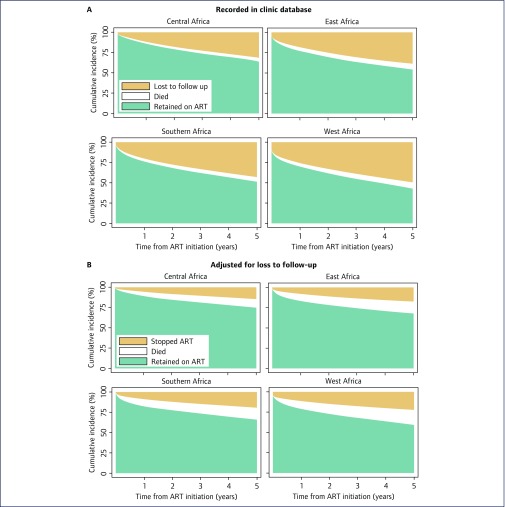
Limitations of the data have led IeDEA researchers to develop methods and approaches to improve the reliability of findings. For example, loss to follow-up can be substantial in ART programmes with unknown outcomes for patients lost. Analyses of programme-level outcomes that are based on patients retained in care may be biased in this situation [40]. IeDEA researchers developed novel approaches and tools to correct estimates of programme-level mortality for loss to follow-up, taking into account the results of tracing of patients who were lost to follow-up [29,41–44]. For instance, a study in an IeDEA site in Malawi found that among patients lost to follow-up, and found to be alive on tracing, a majority (56%) were still taking ART, sourced from another clinic [45]. Figure 4 shows the results from a recent attempt to adjust ART programme-level outcomes for unrecorded deaths and transfers among patients lost to follow-up [34]. In South Africa, IeDEA investigators took advantage of the nearly complete recording of deaths and linked birth, laboratory and death registries to improve mortality outcome information among patients lost to follow-up [46]. Some IeDEA regions have now implemented standardised tracing efforts to bring patients back to care and to ascertain outcomes of patients lost to follow-up. In the analysis of global trends in CD4 cell count at the start of ART, multiple imputation was used to deal with missing CD4 cell counts at the start of ART [47]. In the same analysis [47], estimates for World Bank country income groups were weighted by the number of patients starting ART in a given country and year (as reported by UNAIDS [48]), so that countries with many patients were appropriately represented.
Figure 4.
IeDEA evidence from the IeDEA-WHO collaboration: Cumulative incidence of antiretroviral therapy outcomes among adults. Panel A: outcomes recorded in clinic databases. Panel B: outcomes adjusted for unrecorded deaths and transfers among patients lost to follow-up. Reproduced from Haas et al. [34].
Now 12 years old, the IeDEA consortium is uniquely positioned to provide operational and clinical research that is highly relevant to WHO policy development and progress reporting. With close to two million patients from nearly 500 sites in 46 countries, including both high- and low-HIV burden settings and across a range of contexts, IeDEA can assess outcomes at both the individual and programme level. IeDEA site assessment surveys can be used to inform implementers and policymakers about site-level needs and capacity, guideline uptake and implementation, patient-level impact of practices on HIV testing and counselling, and CD4 and viral load monitoring, and how they evolve over time. The diversity of data and the heterogeneity of the HIV epidemic, as well as the ability to perform state-of-the-art statistical analyses that improve the reliability of findings, give IeDEA substantial ability to generalise findings across a variety of care delivery settings and geographic contexts.
Conclusion
The research-policy collaborative partnership between IeDEA and WHO allows for a better understanding of current policy priorities and data and research limitations, leading to more well-timed and policy-relevant research. Regular communication provides a pathway to facilitate and expedite exchange of crucial knowledge and scientific evidence prior to peer-reviewed publication. Such partnerships that promote dialogue between stakeholders should be encouraged, to facilitate and improve uptake and integration of research evidence, to provide timely and reliable insights into progress and challenges in the global response to HIV, and to support the development of health policy.
Acknowledgements
We thank all patients, care providers and data managers in the seven IeDEA regions for contributing data for this project. We would also like to thank all the investigators participating in Data Harmonization WG (Beverly Musick, Chair), the IeDEA Pediatrics WG (Rachel Vreeman, Chair), the Strategic Data WG (Constantin Yiannoutsos, Chair) and the IeDEA Executive Committee (Annette Sohn, Chair), as well as the WHO (Meg Doherty, Jesus M Garcia Calleja, Daniel Low-Beer, Marina Penazzato and Marco Vitoria).
Funding sources
The International Epidemiology Databases to Evaluate AIDS (IeDEA) collaboration is supported by the National Institute of Allergy And Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute of Mental Health (NIMH) and the National Institute on Drug Abuse (NIDA) under core grants: U01AI069907 (Asia-Pacific), U01AI069923 (CCASAnet), U01AI096299 (Central Africa), U01AI069911 (East Africa), U01AI069918 (NA-ACCORD), U01AI069924 (Southern Africa), U01AI069919 (West Africa). ME was supported by special project funding (Grant No. 174281) from the Swiss National Science Foundation.
References
- 1. UNAIDS Fact sheet – Latest statistics on the status of the AIDS epidemic. 2018. Available at: www.unaids.org/en/resources/fact-sheet ( accessed September 2018).
- 2. World Health Organization Global update on the health sector response to HIV. 2014. Available at: www.who.int/hiv/pub/progressreports/update2014/en ( accessed September 2018).
- 3. World Health Organization Progress report. Global health sector response to HIV, 2000–2015. Focus on innovations in Africa. 2015. Available at: www.who.int/hiv/pub/progressreports/2015-progress-report/en ( accessed September 2018).
- 4. World Health Organization Progress report 2016. Prevent HIV, test and treat all. WHO support for country impact. 2016. Available at: www.who.int/hiv/pub/progressreports/2016-progress-report/en ( accessed September 2018).
- 5. World Health Organization, UNAIDS, UNICEF Global update on HIV treatment 2013: results, impact and opportunities. 2013. Available at: www.who.int/hiv/pub/progressreports/update2013/en ( accessed September 2018).
- 6. Ford N, Ball A, Baggaley R et al. . The WHO public health approach to HIV treatment and care: looking back and looking ahead. Lancet Infect Dis 2017; 18: e76– e86. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization Guidelines for WHO guidelines. 2003. Available at: http://apps.who.int/iris/bitstream/handle/10665/68925/EIP_GPE_EQC_2003_1. pdf;jsessionid=578717350D35BE04C3BE22116E10EBEA?sequence=1 ( accessed September 2018).
- 8. World Health Organization Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. Available at: www.who.int/hiv/pub/guidelines/earlyrelease-arv/en ( accessed September 2018). [PubMed]
- 9. World Health Organization Guideline for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017. Available at: www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en ( accessed September 2018). [PubMed]
- 10. Oxman AD, Lavis JN, Lewin S et al. . SUPPORT Tools for evidence-informed health policymaking (STP)1: What is evidence-informed policymaking? Health Res Policy Syst 2009; 7: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hutchinson E, Droti B, Gibb D et al. . Translating evidence into policy in low-income countries: lessons from co-trimoxazole prevention therapy. Bull World Helath Organ 2011; 89: 312– 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oliver K, Innvar S, Lorenc T et al. . A systematic review of barriers to and facilitators of the use of evidence by policymakers. BMC Health Serv Res 2014; 14: 1– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cairney P, Oliver K, Wellstead A. To bridge the divide between evidence and policy: reduce ambiguity as much as uncertainty. Public Administration Review 2016; 76: 399– 402. [Google Scholar]
- 14. Goor IVD, Hämäläinen R-M, Syed A et al. . Determinants of evidence use in public health policy making: Results from a study across six EU countries. Health Policy 2017; 121: 273– 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richards GW. How research-policy partnerships can benefit government: A win-win for evidence-based policy-making. Canadian Public Policy 2017; 43: 165– 170. [Google Scholar]
- 16. Walter I, Davies H, Nutley S. Increasing research impact through partnerships: evidence from outside health care. J Health Serv Res Policy 2003; 8: 58– 61. [DOI] [PubMed] [Google Scholar]
- 17. Moynihan R, Oxman AD, Lavis JN et al. . Evidence-informed health policy: using research to make health systems healthier. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 01-2008. 2008. Available at: www.fhi.no/en/publ/2009-and-older/evidence-informed-health-policy-using-research-to-make-health-systems-healt ( accessed September 2018). [PubMed]
- 18. Egger M, Ekouevi DK, Williams C et al. . Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41: 1256– 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gange SJ, Kitahata MM, Saag MS et al. . Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mcgowan CC, Cahn P, Gotuzzo E et al. . Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol 2007; 36: 969– 976. [DOI] [PubMed] [Google Scholar]
- 21. Ballif M, Nhandu V, Wood R et al. . Detection and management of drug-resistant tuberculosis in HIV-infected patients from lower income countries. Int J Tuberc Lung Dis 2014; 18: 1327– 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coffie PA, Egger M, Vinikoor MJ et al. . Trends in hepatitis B virus testing practices and management in HIV clinics across sub-Saharan Africa. BMC Infect Dis 2017; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duda SN, Farr AM, Lindegren ML et al. . Characteristics and comprehensiveness of adult HIV care and treatment programmes in Asia-Pacific, sub-Saharan Africa and the Americas: results of a site assessment conducted by the International epidemiologic Databases to Evaluate AIDS (IeDEA) Collaboration. J Int AIDS Soc 2014; 17: 1– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. IeDEA Pediatric Working Group A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa - The International epidemiologic Databases to Evaluate AIDS (IeDEA). J Int AIDS Soc 2013; 16: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parcesepe AM, Mugglin C, Nalugoda F et al. . Screening and management of mental health and substance use disorders in HIV treatment settings in low- and middle-income countries within the global IeDEA consortium. J Int AIDS Soc 2018; 21: e25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spaar A, Graber C, Dabis F et al. . Prioritising prevention strategies for patients in antiretroviral treatment programs in resource-limited settings. AIDS Care 2010; 22: 775– 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. UNAIDS 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014. Available at: www.unaids.org/en/resources/documents/2017/90-90-90 ( accessed September 2018).
- 28. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/ ( accessed September 2018). [PubMed]
- 29. Anderegg N, Johnson LF, Zaniewski E et al. . All-cause mortality in HIV-positive adults starting combination antiretroviral therapy. AIDS 2017; 31: S31– S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Avila D, Althoff KN, Mugglin C et al. . Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2014; 65: e8– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koller M, Patel K, Chi BH et al. . Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2015; 68: 62– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Desmonde S, Tanser F, Vreeman R et al. . Access to antiretroviral therapy in HIV-infected children aged 0–19 years in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Global Cohort Consortium, 2004–2015: a prospective cohort study. PLoS Med 2018; 15: e1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tymejczyk O, Brazier E, Yiannoutsos C et al. . HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries. PLoS Med 2018; 96299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haas AD, Zaniewski E, Anderegg N et al. . Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc 2018; 21: e25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiamsakul A, Kariminia A, Althoff KN et al. . HIV viral load suppression in adults and children receiving antiretroviral therapy – results from the IeDEA collaboration. J Acquir Immune Defic Syndr 2017; 76: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson LF, Keiser O, Fox MP et al. . Life expectancy trends in adults on antiretroviral treatment in South Africa. AIDS 2016; 30: 2545– 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ford N, Penazzato M, Vitoria M et al. . The role of observational studies in supporting the implementation and uptake of Treat All guidelines for HIV/AIDS. J Virus Erad 2018; 4 ( Suppl 2): 5– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin G, Currie G, Lockett A. Prospects for knowledge exchange in health policy and management: institutional and epistemic boundaries. J Health Serv Res Policy 2011; 16: 211– 217. [DOI] [PubMed] [Google Scholar]
- 39. Harris PA, Taylor R, Thielke R et al. . Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377– 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yiannoutsos CT, Johnson LF, Boulle A et al. . Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect 2012; 88: I33– I43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Egger M, Spycher BD, Sidle J et al. . Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med 2011; 8: e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geng EH, Emenyonu N, Bwana MB et al. . Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA 2008; 300( 5): 506– 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schomaker M, Gsponer T, Estill J et al. . Non-ignorable loss to follow-up: correcting mortality estimates based on additional outcome ascertainment. Stat Med 2014; 33: 129– 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zürcher K, Mooser A, Anderegg N et al. . Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health 2017; 22: 375– 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tweya H, Feldacker C, Estill J et al. . Are they really lost? ‘True’ status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in urban Malawi. PLoS One 2013; 8: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson LF, Dorrington RE, Laubscher R et al. . A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. J Int AIDS Soc 2015; 18: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. IeDEA and COHERE Cohort Collaborations Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66: 893– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. UNAIDS AIDSinfo Online Database. 2018. Available at: http://aidsinfo.unaids.org/ ( accessed September 2018).