Abstract
Nearly all countries in sub-Saharan Africa (SSA) have adopted national policies to treat all persons with HIV, regardless of CD4 cell count or clinical stage (‘treat all’). With 10.3 million people untreated and a projected 1.2 million new infections per year in SSA, the current and anticipated unmet need for HIV treatment in SSA is substantial. Evidence to date from SSA suggests that, once linked to care, timely ART initiation with retention and viral suppression is the norm. However, ART initiation in SSA usually occurs late in the course of infection, driving high mortality and incidence rates. The ‘treat all’ era presents strategic opportunities for health systems to substantially reduce AIDS-related mortality and HIV incidence. This special issue of the Journal of Virus Eradication contains eight articles focused on issues critical to ensuring the success and impact of ‘treat all’ implementation in SSA.
Keywords: HIV, ‘treat all’, sub-Saharan Africa, HIV treatment guidelines
Introduction
The HIV pandemic has been among the major public health threats of our time. Since its beginning, an estimated 78 million people around the world have become infected with HIV, and 35 million have died of AIDS-related illnesses, driving major losses of life expectancy. Highly effective treatment for HIV, available in resource-rich settings since 1995, took another 10 years to reach those areas of the world hardest hit by HIV, such as sub-Saharan Africa (SSA), home to 25.7 million people or 70% of those living with HIV today [1]. The scale of the global public health response to the HIV pandemic is unprecedented. Through the combined efforts of people living with HIV, national public health programmes, global donors and a broad community of stakeholders, the number of people on antiretroviral therapy (ART) rose rapidly across SSA, going from about 100,000 in 2004 to 15.4 million by the end of 2017 [1,2]. This incredible accomplishment is saving millions of lives each year.
Scale-up and treatment guideline expansion have increased ART coverage in SSA, but substantial unmet needs persist
The early public health response to the HIV epidemic, including in SSA, began as an emergency response, with treatment initially recommended only for those with very advanced immunodeficiency and HIV disease, who were deemed at highest risk for death. Beginning in 2010 and then again in 2013, expansions in HIV treatment guidelines by WHO and national programmes in the region extended HIV treatment eligibility to individuals with less-advanced disease.
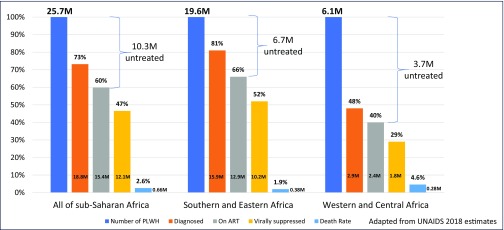
Expansion of HIV treatment guidelines led to substantial gains in ART coverage for people in SSA, and concomitant improvements in life expectancy and reduced mortality. Nonetheless, we are confronted with sobering statistics regarding the current state of SSA's regional HIV epidemics (Figure 1) – there are still an estimated 660,000 AIDS-related deaths per annum among SSA's 25.7 million people living with HIV [1]. The latest UNAIDS data suggest that there are 10.3 million people (6.7 million in Eastern and Southern Africa and 3.7 million in West and Central Africa) with untreated and often undiagnosed HIV infection; most of the AIDS-related deaths are in this group. Adding to this, at the current rate of 1.2 million new infections per year, there will be millions of people with new HIV infections anticipated in the coming 5 years in SSA alone who also will require diagnosis and treatment.
Figure 1.
HIV care continuum and number of people with untreated HIV in sub-Saharan Africa, 2017. Adapted from UNAIDS 2018 estimates [1,17].
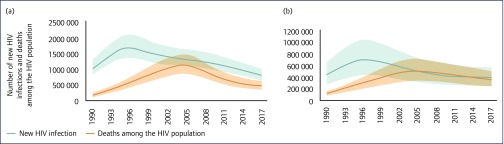
While trends in HIV incidence and deaths have improved substantially in SSA since the scale-up of HIV treatment began, they have slowed considerably (Figure 2), creating new implementation challenges to limit the epidemic's toll and trajectory (for country-specific trajectories and metrics, see: [1]).
Figure 2.
Trends in HIV incidence and deaths in sub-Saharan Africa, 1990–2017. (a) Eastern and southern Africa; (b) western and central Africa. Source: UNAIDS 2018 estimates [1,17].
Current evidence on the impact of ART guideline expansion and ‘treat all’ implementation
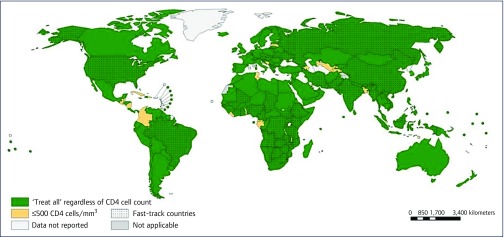
In late 2015, the WHO further expanded their HIV treatment guidelines to include all persons with HIV, regardless of CD4 cell count or clinical stage [3]. The ‘treat all’ guideline expansion heralds a new era in the response to the HIV epidemic in some of the hardest-hit areas of the world. Recent data from WHO suggest that, in less than 3 years, there has been nearly universal adoption of the WHO 2015 HIV treatment guidelines as national policy in SSA countries (Figure 3 [4]). ‘Treat all’ in SSA means that 10.3 million people living with HIV, and all people who subsequently acquire HIV infection, will be treated alongside the 15.4 million people in the region who are already receiving lifelong HIV treatment. The scale is enormous, and there will be numerous opportunities to learn what implementation models work best. ‘Treat all’ as national policy opens up new opportunities to reduce both AIDS-related mortality and new HIV infections.
Figure 3.
Uptake of WHO policy for ‘treat all’ ART initiation among adults and adolescents living with HIV (situation as of mid-2018). Map provided by courtesy of WHO (Global AIDS Monitoring [UNAIDS/WHO/UNICEF] and WHO HIV country intelligence tool, 2018).
First, ‘treat all’ has the potential to reduce AIDS-related mortality by improving both timely ART initiation and retention in HIV care, which have been found to occur with prior guideline expansions [5,6]. One recent randomised controlled trial (RCT) used a stepped wedge design to assess the impact of ‘treat all’ on timely ART initiation and retention in 14 real-world service delivery sites, among 3405 people enrolling in HIV care in eSwatini, from 2014 to 2017 [7]. Six months after enrolling in HIV care, patients enrolling under the intervention condition were seven times more likely to be retained in care with viral suppression than those enrolling under the standard-of-care condition (i.e. the national guidelines in place at the time). At 12 months after enrolment, retention remained 60% higher in the ‘treat all’ compared to the control group.
Second, ‘treat all’ has the potential to help drive down new HIV infections through treatment as prevention (TasP). To date, evidence of the impact of ‘treat all’ on HIV incidence in SSA has been mixed, and the reasons for this are not yet clear. There have been two large-scale randomised studies conducted to examine the impact of ‘treat all’. The first, known as the Ya Tse study (the Botswana Community Prevention Project), was conducted in Botswana from 2013 to 2018, and randomised 30 communities to a ‘treat all’ condition or the standard of care under the national guidelines (which included ‘treat all’ from June 2016) [8]. The viral suppression rate was higher in the intervention communities than in the control communities. HIV incidence in the intervention communities was 30% lower than that of the control communities (incidence ratio 0.70, 95% CI: 0.50–0.99). A second Africa-based cluster RCT, the SEARCH study, was conducted among 186,354 adults (with and without HIV) in 32 communities of approximately 10,000 people each in Uganda and Kenya from 2013 to 2016. SEARCH compared the standard of care to a community-based strategy to increase HIV testing alongside other disease screenings (e.g. for diabetes and hypertension), followed by immediate linkage and treatment for persons with an HIV diagnosis [9]. Among those with newly diagnosed HIV, 60% in the intervention communities and 17% in the control communities started ART within 6 months following their diagnosis, which reached 80% versus 40% at 24 months following diagnosis. Moreover, by 36 months, 79% of those living with HIV in intervention communities, compared to 68% in control communities, had achieved viral suppression. However, the rate of new HIV infections did not differ between study arms (0.8% per year). Early findings of both trials around retention and viral suppression under ‘treat all’ conditions are also encouraging.
The way forward: ‘treat all’ implementation in SSA, with a focus on earlier diagnosis and linkage, can bend the curves of AIDS-related deaths and new HIV infections
To help ensure the success of ‘treat all’ on reducing both HIV mortality and incidence, it is essential to identify and scale up strategies capable of getting people with HIV in SSA diagnosed and initiated on treatment at much higher than current CD4 cell counts. The large numbers of both deaths and new infections in SSA are driven by distressingly low median CD4 cell counts at care enrolment and ART initiation [10–14], which were still below 300 cells/mm3 in 2015 [10]. To put these CD4 cell counts into perspective, the median CD4 cell count at ART initiation in the treatment arm of the HPTN 052 trial, which drove a >90% reduction in onward transmission among serodiscordant partners, was 445 cells/mm3 [15]. The median CD4 cell count in the treatment arm of the INSIGHT START trial, which demonstrated a reduction in mortality at higher CD4 counts, was 650 cells/mm3 [16].
Another reason why HIV treatment has not had a sustained substantial public health impact on HIV incidence could be suboptimal HIV care outcomes among adolescents and young adults, who tend to be more sexually active than older persons. Historically, in these groups, there has been delayed treatment uptake and poor retention under CD4 cell count-based treatment guidelines. Treatment guideline expansions have resulted in increases in timely ART initiation among younger persons [6]. Targeted efforts to improve outcomes among adolescents and young adults as part of ‘treat all’ implementation are critical and could result in substantial impact on HIV incidence [17]. Strategies to more effectively reach adolescents must be highly scalable, since demographic trends anticipate a massive rise in the number of adolescents in SSA in the coming decade, and by extension, the potential number of adolescents with HIV [18].
A special issue on ‘treat all’ in SSA
To support the goal of identifying knowledge gaps that need to be addressed in order to best inform, guide and increase the impact of ‘treat all’ implementation in SSA, the African regions of IeDEA convened a ‘Treat All’ Consensus Statement Working Group (TACSWG) and the All-Africa IeDEA Meeting in Kigali, Rwanda (November 2017) to identify research priorities with the potential to inform and guide ‘treat all’ implementation in SSA [19]. The Working Group summarised relevant literature from SSA in key areas, and the dissemination of this information is the motivation for this special issue of the Journal of Viral Eradication, which is themed around identifying research priorities to inform ‘treat all’ implementation in SSA. The articles are led by investigators from the African regions of IeDEA, as well as other stakeholders and collaborators.
The first article in this issue by Ford and colleagues [20] from the WHO, summarises the ways in which observational data in general, and data from IeDEA specifically, have supported the work of WHO in developing treatment guidelines and monitoring their implementation. After more than a decade of ad hoc collaboration between IeDEA and WHO, the partnership was formalised in 2014. Zaniewski et al. [21] describe the genesis and structure of the collaboration and its outcomes.
It is difficult to treat all persons with HIV in SSA without examining how poor mental health and substance use may serve as barriers to timely HIV diagnosis, and initiation and adherence to lifelong treatment. These important issues have long been neglected in the region, and represent new areas for implementation research. Parcesepe et al. [22] along with Lancaster and colleagues [23] provide overviews of mental health and substance use research, respectively, among those living with HIV in SSA, and interventions or strategies to address comorbid mental illness and substance use among individuals living with HIV. They also highlight research gaps relevant to ‘treat all’ implementation, including the need for implementation science research to evaluate promising models of integrated mental health and substance use screening and treatment within HIV care delivery systems.
SSA is home to 85% of children living with HIV. Lifelong ART has been the policy for infants since 2010, expanding to children under 5 years, and pregnant and breastfeeding women (Option B+) in 2013. Although ART coverage among pregnant and breastfeeding women has surpassed 90% in some Southern and Eastern African countries, the rate of mother-to-child transmission (MTCT) in the region remains higher than expected, suggesting serious gaps along the prevention of MTCT cascade. For children living with HIV, ART coverage still lags substantially behind that of adults. Abuogi and colleagues [24] provide a review of the progress, gaps, and research needs to achieve the 90-90-90 targets and optimise ART outcomes for pregnant and postpartum women. Enane et al. [25] highlight the complex challenges in optimising the ART cascade for children and adolescents, ranging from underdiagnosis to lower rates of ART initiation and viral suppression compared to adults.
Policy development and resource allocation greatly benefit from the ability to forecast the potential impact of different implementation scenarios using mathematical models. Kimmel et al. [26] examine how mathematical models have informed scale-up and implementation of ‘treat all’ in Southern Africa and highlight the need for local, pragmatic policy assessments with realistic assumptions. Related to this, a scoping review by de Waal and colleagues [27] describes gaps in current knowledge related to HIV drug resistance in SSA, and how to address current and future resistance challenges by combining real-world data from IeDEA and mathematical modelling.
‘Treat all’ sets the stage for moving beyond the status quo approaches to ART scale-up
Evidence and experience from SSA to date suggests that if people can be linked to HIV care, most will get onto treatment rapidly and be retained in care [5–7]. However, without better strategies for earlier testing and linkage, those living with HIV may remain undiagnosed and out of care until they develop symptoms, driving up mortality and ongoing transmission. With national policies expanded to ‘treat all’ across SSA, the stage is set for a new era in the response to the region's epidemic that must identify, implement and scale test-and-treat strategies, and accelerate diagnosis and treatment initiation, at higher CD4 cell counts. Importantly, with diminishing resources for HIV service provision [28], this cannot be achieved simply by using the same approaches that national programmes have used in the past. Populations and sub-populations that have not been reached are likely to have greater individual, health system, and cultural barriers to HIV testing and treatment. Achieving success and impact sooner rather than later in SSA's ‘treat all’ era will require identification and deployment of contextually appropriate, locally informed strategies that can be more effective and efficient at achieving earlier diagnosis and linkage than the status quo.
Acknowledgements
The authors would like to acknowledge the IeDEA Treat All Consensus Statement Working Group (TACSWG): Diane Addison, Keri Althoff, Ellen Brazier, Barbara Castelnuovo, Craig R Cohen, Morna Cornell, Mary-Ann Davies, Geraldina Dominguez, Stephany N Duda, Aimee Freeman, Antoine Jaquet, Olivia Keiser, April D Kimmel, Kathryn E Lancaster, Valeriane Leroy, Janne Markus, Rosemary McKaig, Pamela M Murnane, Denis Nash (co-Chair), Dominique Nsonde, Amobi Onovo, Angela M Parcesepe, Jean d'Amour Sinayobye, Annette H Sohn, Per M von Groote, Rachel C Vreeman, Gilles Wandeler, Radhika Wikramanayake, Carolyn F Williams, Kara Wools-Kaloustian, Constantin Yiannoutsos, Marcel Yotebieng (co-Chair).
The authors would also like to acknowledge the contributions of Lynn Murchison, Jill Raufman, and the IeDEA Rwanda team for their organisational and logistical support for the All-Africa meeting of IeDEA in Kigali, Rwanda in November 2017.
Funding
DN was supported by funding from the US National Institutes of Health, including Central Africa IeDEA (U01AI096299), the Einstein, Rockefeller, CUNY Center for AIDS Research (ERC CFAR, P30 AI124414), and the HIV Center for Clinical and Behavioral Studies (P30 MH043520).
MY was supported by funding from the US National Institutes of Health, Central Africa IeDEA (U01AI096299) and R01HD087993.
The All-Africa meeting of IeDEA was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R13AI134393.
References
- 1. UNAIDS UNAIDS data 2018. Geneva, Switzerland: UNAIDS; Available at: www.unaids.org/en/resources/documents/2018/unaids-data-2018 ( accessed November 2018).
- 2. AVERT.org Global HIV and AIDS statistics. AVERT.org; 2017. Available at: www.avert.org/global-hiv-and-aids-statistics ( accessed November 2018).
- 3. WHO Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva ISBN: 978 92 4 150956 5 2015. Available at: www.who.int/hiv/pub/guidelines/earlyrelease-arv/en ( accessed November 2018). [PubMed]
- 4. WHO Uptake of WHO policy for Treat All ART initiation among adults and adolescents living with HIV (situation as of mid-2018). Personal communication to D Nash, 2018. [Google Scholar]
- 5. Bor J, Fox MP, Rosen S et al. Treatment eligibility and retention in clinical HIV care: a regression discontinuity study in South Africa. PLoS Med 2017; 14: e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tymejczyk O, Brazier E, Yiannoutsos C et al. HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med 2018; 15: e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan S, Spiegelman D, Walsh F et al. Universal test and treat (UTT) versus standard of care for access to antiretroviral therapy in HIV clients: the MaxART stepped-wedge randomized controlled health systems trial in Swaziland AIDS 2018. July 2018. Amsterdam, the Netherlands. Abstract WEAX0102LB.
- 8. Makhema MJ, Wirth K, Pretorius Holme M et al. Impact of prevention and treatment interventions on population HIV incidence: primary results of the community-randomized Ya Tsie Botswana prevention project. AIDS 2018. July 2018. Amsterdam, the Netherlands. Abstract WEAX0105LB.
- 9. Havlir D, Charlebois E, Balzer L et al. SEARCH community cluster randomized study of HIV ‘test and treat’ using multi- disease approach and streamlined care in rural Uganda and Kenya. AIDS 2018. July 2018. Amsterdam, the Netherlands. Abstract WEAX0106LB.
- 10. IeDEA, Cohere Cohort Collaborations Global trends in CD4 cell count at the start of antiretroviral therapy: Collaborative Study of Treatment Programs. Clin Infect Dis 2018; 66: 893– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lahuerta M, Ue F, S. H et al. The problem of late ART initiation in sub-Saharan Africa: a transient aspect of HIV care and treatment scale-up or a long-term phenomenon? J Health Care Poor Underserved 2013; 24: 359– 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffman S, Wu Y, Lahuerta M et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS 2014; 28: 2429– 2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lahuerta M, Wu Y, Hoffman S et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-saharan African countries. Clin Infect Dis 2014; 58: 432– 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lahuerta M, Nash D. Reply to Pattern et al. Advanced HIV disease at ART initiati’on despite implementation of expanded ART eligibility guidelines during 2007–2012 in Khayelitsha, South Africa’. Clin Infect Dis 2014; 59: 457– 458. [DOI] [PubMed] [Google Scholar]
- 15. Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Curr Opin HIV AIDS 2012; 7: 99– 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Insight Start Study Group, Lundgren JD, Babiker AG et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373: 795– 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UNAIDS Miles to go—closing gaps, breaking barriers, righting injustices Geneva: 2018. Available at: www.unaids.org/en/resources/documents/2018/global-aids-update ( accessed November 2018).
- 18. Slogrove AL, Sohn AH. The global epidemiology of adolescents living with HIV: time for more granular data to improve adolescent health outcomes. Curr Opin HIV AIDS 2018; 13: 170– 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yotebieng M, Brazier E, Addison D et al. Research priorities to inform ‘Treat All’ policy implementation for people living with HIV in sub-Saharan Africa: a consensus statement from the International Epidemiology Databases to Evaluate AIDS (IeDEA). J Int AIDS Soc 2018. ( In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford N, Penazzato M, Vitoria M et al. The contribution of observational studies in supporting the WHO ‘treat all’ recommendation for HIV/AIDS. J Virus Erad 2018; 4 Suppl 2: 5– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaniewski E, Tymejczyk O, Kariminia A et al. IeDEA-WHO Research-Policy Collaboration: contributing real-world evidence to HIV progress reporting. J Virus Erad 2018; 4 Suppl 2: 9– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parcesepe AM, Bernard C, Agler R et al. Mental health and HIV: research priorities related to the implementation and scale up of ‘treat all’ in sub-Saharan Africa. J Virus Erad 2018; 4 Suppl 2: 16– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lancaster E, Hetrick A, Jaquet A. Substance use and universal access to HIV testing and treatment in sub-Saharan Africa: implications and research priorities. J Virus Erad 2018; 4 Suppl 2: 26– 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abuogi LL, Humphrey JM, Mpody C et al. Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: progress, gaps and research needs. J Virus Erad; 4 Suppl 2: 33– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enane LA, Davies M-A, Leroy V et al. Traversing the cascade: urgent research priorities for implementing the ‘treat all’ strategy for children and adolescents living with HIV in sub-Saharan Africa. J Virus Erad 2018; 4 Suppl 2: 40– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimmel AD, Bono RS, Keiser O et al. Mathematical modelling to inform ‘treat all’ implementation in sub-Saharan Africa: a scoping review. J Virus Erad; 4 Suppl 2: 47– 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Waal R, Lessells R, Hauser A et al. HIV drug resistance in sub-Saharan Africa: public health questions and the potential role of real-world data and mathematical modelling. J Virus Erad; 4 Suppl 2: 55– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bekker LG, Alleyne G, Baral S et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society-Lancet Commission. Lancet 2018; 392: 312– 358. [DOI] [PMC free article] [PubMed] [Google Scholar]