Abstract
As universal testing and treatment for HIV, or ‘treat all’, expands across sub-Saharan Africa (SSA), substance use will likely have a negative impact on the success of scale-up efforts for antiretroviral treatment (ART). Overwhelming evidence highlights the negative impact of substance use on HIV care and treatment outcomes. Yet, as many countries in SSA expand ART, evidence of the extent of substance use, and its impact in the region, is more limited. Stigma, and the psychoactive effects of substance use, are barriers to seeking HIV treatment and adhering to ART regimens for persons with heavy alcohol use or substance use. As a result, we identified several implementation and operations research priorities and metrics for monitoring the impact of substance use and Treat All. Identifying barriers and facilitators to the integration of the prevention and treatment of substance use with HIV care, and assessing effects on HIV outcomes, through longitudinal studies are priorities that will determine the impacts of substance use on ‘treat all’ in SSA. Future research must use existing infrastructure, including large networks of HIV clinics, to enhance our understanding of the implementation and service delivery of substance use screening, referral and treatment. These networks will also inform robust and standardised substance use estimates and interventions within the ‘treat all’ era in SSA.
Keywords: injection drug use, non-injection drug use, alcohol, antiretroviral treatment, Africa
Introduction
Substance use among people living with HIV (PLWH) is a major public health challenge globally and within sub-Saharan Africa (SSA) [1-4]. While alcohol is the most commonly used substance, injection drug use and non-injection drug use are growing [5]. An estimated 500,000 to 3 million persons reported injecting drugs in SSA and nearly one-fifth are estimated to be living with HIV [3,4]. Non-injection drug use in Africa ranges widely across substances; in 2014 an estimated 1.6 million persons used opiates, 2.8 million persons used cocaine, and 5.5 million persons used amphetamines and prescription stimulants [5]. In 2010, approximately 30%, or 314 million persons, consumed alcohol in Africa, and among those, 92 million were heavy episodic drinkers [6]. As Africa becomes more integrated within drug trafficking routes, injection and non-injection drug use are expected to expand [1]. The intertwining relationships between substance use and HIV are complex in many ways, with each simultaneously hindering optimal physical and mental health, well-being and quality of life. PLWH who use substances, and are not engaged in substance use treatment, may be less likely to achieve optimal engagement into HIV care and treatment when compared to non-substance-using PLWH [7-9]. In parallel, those who are engaged in substance use treatment are likely not offered HIV testing and do not receive HIV risk-reduction counselling [10-12].
In 2015, the World Health Organization expanded their global recommendations on the use of antiretroviral drugs for treating and preventing HIV infection by implementing a universal test and treat strategy also known as ‘treat all’ [13]. The ‘treat all’ policy encompasses initiating antiretroviral treatment (ART) as soon as possible after a diagnosis of HIV infection, to both maximise the benefits for individual health and minimise the potential for forward transmission [14,15]. The elimination of ART eligibility criteria for PLWH is anticipated to have tremendous effects on achieving universal access to HIV treatment [16]. Globally, adoption of the ‘treat all’ policy has been high, and by 2017, nearly all countries within SSA had implemented some form of immediate ART initiation [17]. The International Epidemiology Databases to Evaluate AIDS (IeDEA) consortium, funded by the US National Institutes of Health, is unique, and to our knowledge, the largest network of HIV clinics in SSA to monitor the implementation of ‘treat all’.
‘Treat all’ implementation is the critical first step toward universal testing and treatment, improved clinical outcomes for patients, and the prevention of new infections. However, to achieve the full benefits, all PLWH must be fully engaged in HIV care and treatment, including those engaging in substance use [18,19]. If left unaddressed, substance use could hinder potential advances of the ‘treat all’ policy in SSA. In this viewpoint, we characterise the current literature on substance use, including injection drug use, non-injection drug use, alcohol use and their potential impact on ‘treat all’ implementation in SSA. We conclude by identifying several knowledge gaps and providing examples of research priorities for the rollout of ‘treat all’ in the region.
Alcohol use
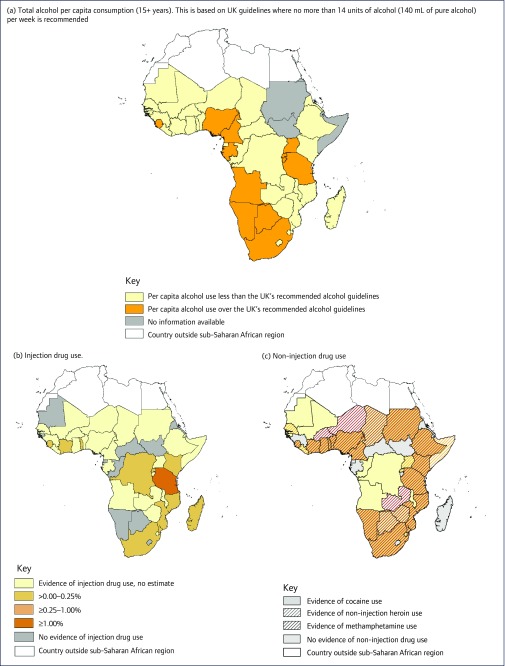
Alcohol continues to be the most predominantly used substance within SSA (Figure 1a) [6,20]. SSA has the highest prevalence of both HIV and heavy episodic drinking in the world [6,21]. In many SSA countries, over half of key populations, such as female sex workers (FSW) and men who have sex with men (MSM), supplement sexual encounters with frequent heavy alcohol use, which may ultimately lead to alcohol use disorders [22-24]. The association between heavy alcohol use and HIV acquisition and transmission has been well documented [25]. Alcohol use can directly affect cognitive ability and judgement [26,27], which can lead to high-risk sexual behaviours, including unprotected, multiple sexual partners, and coercive sex [28-35]. Heavy alcohol use is also biologically linked with increased genital viral shedding, increasing the potential for HIV transmission [36,37]. In addition to HIV risk, heavy alcohol use among PLWH directly leads to sub-optimal ART adherence, decreasing the likelihood for plasma viral suppression, the ultimate goal for ART [23,27,36,38,39]. Often, due to concerns of potential interactions, ART medication is missed when drinking [40], which can lead to ART resistance [41]. These outcomes lead to higher mortality and morbidity rates, particularly among key populations, and are barriers to achieving the second and third 90-90-90 targets proposed by UNAIDS (by 2020, 90% of all people living with HIV will know their HIV status, 90% of those will be in care, and 90% of those will be virally suppressed).
Figure 1.
All panels were created using ArcGIS Desktop (Release 10, Environmental Systems Research Institute, Redlands, CA, USA). Evidence of substance use, including: (a) Total annual alcohol per capita consumption of those 15 years or older in litres of pure alcohol as stated in each country's profile in the WHO's 2014 Global Status Report on Alcohol and Health was used [6,20]. In an effort to discern low and high rates of alcohol use in each country, the United Kingdom's alcohol guidelines were used to differentiate levels of alcohol use. The UK guidelines recommend no more than 14 units of alcohol (140 mL of pure alcohol) per week or 7.28 L of pure alcohol per year; (b) Injection drug use-prevalence data were pulled from a systematic review by Degenhardt et al [42]; and (c) non-injection drug use within sub-Saharan Africa. A combination of search techniques was implemented to find evidence of non-injection drug use of cocaine [43-55], heroin [4,43,52,56-69] and methamphetamine [5,53,55,70-72] within each country in SSA. The United Nations Office on Drugs and Crime Statistics Online Tool was used to determine prevalence of amphetamine and cocaine use by country. If no data were available, then PubMed and Google Scholar search functions were used with each country's name and ‘cocaine’, ‘heroin’, ‘methamphetamine’, or ‘amphetamine’ to find studies that documented non-injection use from the year 2000 to the present. All non-injection drug use sources were combined to create Figure 1c.
Injection drug use
Injection drug use is now documented in many countries in SSA, with prevalence estimates ranging from 0% to 2.3% in the general adult population (Figure 1b) [42]. The number of persons who inject drugs (PWID) is growing, particularly within Kenya, Tanzania, Nigeria, Mauritius and South Africa [4,8,73]. The emergence of injection drug use provides an additional route for HIV transmission. Serial use and sharing of drug injection equipment, preparing drugs for injection, and collectively using shared drug preparations, create risks for acquiring and transmitting HIV [74]. Additionally, blood sharing practices, known as ‘flashing’, can directly increase transmission potential as a person injects a syringe full of blood drawn back immediately after initial injection to another person as a way of sharing drugs [75,76]. Injection drug use, particularly of heroin, is commonly associated with poor ART adherence [7]. However, involvement in substance use treatment, such as methadone substitution therapy, significantly improves HIV clinical outcomes, including ART adherence and viral suppression [9,77,78]. Aside from the negative impact of injection drug use on HIV treatment outcomes, this ‘hard-to-reach’ population is less likely to be aware of their HIV infection and entered/retained into care. PWID face persistent social barriers to HIV testing and linkage to care, including stigma and punitive legal systems [79,80]. It then will represent a major challenge in achieving the first 90 of the 90-90-90 targets proposed by UNAIDS [81]. Therefore, the integration or linkage of harm-reduction services with HIV care programmes might constitute a central element to achieve universal access to treatment. Evidence-based interventions that have been documented to prevent HIV transmission and improve HIV treatment outcomes are largely unavailable within SSA [8,82]. In 2009, only three African countries included harm reduction services, including syringe exchange and/or methadone substitution programmes, as part of their national plans [82]. However, since 2013, availability of these harm reduction services has expanded, particularly within East Africa [83-88].
Non-injection drug use
Non-injection substance use is emerging and is a major concern for several SSA countries (Figure 1c) [4,5,43-72]. Specifically, smoking or ingesting heroin, methamphetamine and amphetamine-type stimulants has been increasing [4,8,89]. Although injection drug use has received much attention for its direct link to HIV transmission, heroin is also commonly smoked, particularly in developing markets. Several countries within Africa have become major international transport corridors for heroin and cocaine trafficking [1,2,4]. As a result, cocaine use throughout West Africa and heroin consumption throughout East Africa have been documented [4,76,90]. South Africa is also experiencing a severe methamphetamine epidemic, the majority of which is with non-injection routes, particularly among key populations at risk for HIV, including sex workers and men who have sex with men [89,91]. Similar to other substances, non-injection substance use can lead to delays in HIV diagnosis, poor linkage to care and treatment, as well as sub-optimal ART adherence for viral suppression [8,70,92]. Without adequate surveillance, the impact of these emerging non-injection drug use epidemics on HIV acquisition, transmission and treatment within the context of high HIV prevalence is unknown [8]. Furthermore, harm-reduction strategies are often tailored for injection drug use to improve HIV treatment outcomes and need to include strategies for sexual and non-injection drug-related risk reduction [70].
Research priorities
The growing substance use epidemic has the strong potential to aggravate the HIV epidemic in SSA. For a successful rollout of ‘treat all’ in SSA, several knowledge gaps in understanding and addressing substance use require attention and resources. To address these gaps, we have identified a set of research priorities, including four implementation and operations priorities that address screening and treatment and three needed metrics for monitoring the impact of ‘treat all’ (Table 1).
Table 1.
Research priorities for ‘treat all’ policy in sub-Saharan Africa
| Implementation and operations | Metrics for monitoring impact |
|---|---|
|
|
Implementation and operations
Screening
Substance use screening tools that are culturally appropriate and reliable must be prioritised. Within several areas of SSA, screening for substance use is either non-existent or severely limited [93]. Substance use screening can include biomarkers and self-report behavioural surveys, particularly within treatment settings. Biological tests for biomarkers of substance use, such as urinalysis, hair testing and saliva tests, can often be rapid and accurate for detecting recent substance use. However, these tests require proper training, specialised laboratories and adequate resources, which may be challenging in overburdened HIV clinical settings. Self-report screening tools are likely more feasible to implement within the current HIV care settings [94]. Several tools have been developed and validated within the United States, yet few have been evaluated with regard to their sensitivity and specificity for measuring substance use, misuse and disorders in other cultures [95]. For example, studies within Kenya have used generic questions to identify types of substances used as well as a pattern of use, while others have adapted the WHO Model Core Questionnaire among students and prison populations for the same purpose [96-99]. Cultural adaptations and validation of standardised screening tools must be undertaken to integrate local nomenclature for the various substances used in the region [100]. Common modifications have included the addition of contextual items such as factors associated with introduction to substance use and its continued use, as well as potential complications of use. Additionally, contextualisation will often require the inclusion of local names for various substances, information that would otherwise be missed without cultural context. Epidemiological studies require this ongoing ethnographic work to monitor the on-the-ground, and often rapidly changing, drug markets [101].
As alcohol use remains highly prevalent in SSA, strategies are needed to integrate low-cost and reliable point-of-care testing for alcohol use among patients receiving HIV care. As an example, the assessment of excessive alcohol use presents various challenges in the SSA context. An accurate measure of alcohol intake in a given setting requires a minimum knowledge of types of locally brewed alcoholic beverages regularly consumed as well as conversions into standard alcohol drinks. Reproducibility of alcohol intake measures across various settings and populations is also an issue to enable reliable comparisons. To this end, the diffusion and promotion of a common tool to assess alcohol use, such as the Alcohol Use Disorder Identification Test (AUDIT), or its short version, AUDIT-C, constitutes a key step in the documentation of alcohol use in SSA [102]. Besides these technical considerations, relying on self-report to diagnose excessive alcohol use is subject to desirability bias. This is particularly sensitive in a context of access to care for chronic and lifelong conditions, such as HIV infection. Indeed, recent research using alcohol biomarkers, such as phosphatidylethanol, has found significant rates of underreported alcohol intake among HIV-infected persons [103,104]. Other factors, such as religion, ethnicity and employment, might also significantly affect self-reported alcohol use. Previous prevalence estimates on alcohol use from SSA, based on self-report, showed a particularly low level of alcohol use in predominantly Muslim countries [105,106]. Whether or not these assessments reflect the true consumption of alcohol in these countries remains to be confirmed. The use of inexpensive and reliable point-of-care testing to complement self-report measurements of alcohol intake might provide more reliability in alcohol-use estimates [107].
Treatment
Written guidelines and protocols for substance use disorder treatment and referrals must be developed and implemented by non-specialists within HIV clinics. For example, the World Health Organization's Mental Health Gap Action Programme (mhGAP) provides guidance for the integration of substance use care [108]. Until integration can be achieved, timely referral strategies can be employed to address treatment gaps in settings where referral services exist [109]. Referral strategies for those in need of substance use disorder treatment can be successful through collaboration between the patient, service providers and related treatment organisations. However, it is critical to note that throughout much of SSA, referral services are nonexistent or inaccessible, highlighting the need for development of new and innovated models for substance use care and support [110].
To facilitate an understanding of the barriers and facilitators to integrating substance use disorder and HIV treatments, we advocate for an implementation science framework approach for generating critical and actionable evidence. In HIV care settings in SSA, a complex set of patient, community, provider, organisational and systemic factors must come together to create integrated substance-use treatment among PLWH. At the patient and community levels, the challenges include the inability to engage and retain HIV-positive substance users in care due to varying levels of motivation, loss to follow-up, stigma associated with substance abuse (including from service providers), lack of social support, and co-morbid conditions including psychiatric and mental health disorders, as well as complex socioeconomic and contextual factors that inhibit access to and retention in care [109,111-114]. Provider and organisational level barriers include lack of knowledgeable and skilled providers, personnel shortages, inadequate diagnostic tools and poor treatment infrastructure, all within an environment that continues to prioritise HIV care over conditions that increases the risk of transmission. Systemic challenges are related to the absence of coherent and comprehensive substance abuse policies and programmes that support an integrated care model [3,115,116]. To further address the barriers to treatment among vulnerable drug-using populations, including MSM and FSW, non-HIV sector approaches that use dedicated substance abuse services and more community-based outreach models should be considered [109].
Metrics for monitoring impact
The focus of implementation science research must enhance our understanding of what resources are needed, how to most efficiently address multiple barriers to integrating substance use treatment and HIV prevention programmes, and how to develop and implement optimal interventions to maximise the benefits of ART in the ‘treat all’ era. This should include highlighting optimal models of care delivery, efficiency, and effects of interventions and policy innovations on HIV treatment [117]. More specifically, implementation science research should be designed to assess context-specific barriers at individual, community, health provider and structural levels to facilitate increased access to HIV prevention and treatment for substance use among PLWH. It is also necessary to identify the cost-effective approaches to facilitate the integration of substance use and HIV treatment because of the potential to dramatically improve the success of ‘treat all’ in SSA [117,118].
Multiple metrics will be necessary for monitoring the impact of substance use within the ‘treat all’ era. Robust estimates of substance use and characterisation of substance use treatment services are lacking, particularly at the country level, within SSA [119,120]. Although substance use and harm-reduction services are expanding across SSA, a number of structural barriers remain, including a lack of trained providers and an absence of policy for expansion [83-86,120]. Population estimates and identification of evidence-based substance use disorder prevention and treatment services will provide essential data for informing future programme development and research.
Longitudinal studies are needed on the time-varying changes in patterns of substance use, as well as the effect of substance use, on HIV outcomes and co-infections. Substance use is not widely captured in HIV clinical settings, particularly within SSA. This lack of regularity and standardisation in assessing substance use within clinical cohorts leads to a reliance on small convenience samples for characterising substance use among PLWH [121,122]. While these studies enhance our understanding of the impact of substance use on HIV outcomes and co-infections, the estimates are susceptible to bias, limiting the potential for guiding HIV treatment policies [123-126].
Conclusion
As the rollout of ART expands in SSA, substance use will remain an ongoing challenge for achieving universal testing and treatment. Evidence from other settings overwhelmingly emphasises the negative impact of substance use on HIV care and treatment outcomes. Within SSA, the evidence is limited for understanding and addressing substance use and HIV. Participating clinics within IeDEA can serve as a representative sample of HIV treatment sites and provide critical insights on the implementation and operational management of service delivery for substance use screening, referral and treatment. Furthermore, this platform has successfully collected core information on ART exposure and has strong potential to contribute to more robust and standardised estimations of substance use among PLWH, especially in the context of ‘treat all’.
Acknowledgements
We would like to thank the members of the International Epidemiology Databases to Evaluate AIDS (IeDEA) Substance Use Working Group for their support during the preparation of the manuscript ( www.iedea.org/working-groups/substance-use/)
Declaration of interests
The authors declare no competing interests.
References
Note: Sources marked with ‘*’ indicate countrywide prevalence data used in Figure 1. All other sources are smaller studies within SSA countries.
- 1. Kasirye R. Diffusion of evidence-based interventions in HIV and substance user programs: flaws and lessons from the sub-Saharan African region. Subst Use Misuse 2015; 50: 1110– 1116. [DOI] [PubMed] [Google Scholar]
- 2. El-Bassel N, Shaw SA, Dasgupta A, Strathdee SA.. Drug use as a driver of HIV risks: re-emerging and emerging issues. Curr Opin HIV AIDS 2014; 9: 150– 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathers BM, Degenhardt L, Phillips B et al. . Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008; 372: 1733– 1745. [DOI] [PubMed] [Google Scholar]
- 4. Raguin G, Lepretre A, Ba I et al. . Drug use and HIV in West Africa: a neglected epidemic. Trop Med Int Health 2011; 16: 1131– 1133. [DOI] [PubMed] [Google Scholar]
- 5. * United Nations Office on Drugs and Crime World Drug Report 2016. (United Nations publication, Sales No. E.16.XI.7); Available at: www.unodc.org/wdr2016 ( accessed September 2018).
- 6. * World Health Organization Management of Substance Abuse Unit Global status report on alcohol and health, 2014. Geneva: World Health Organization; 2014. Available at: www.who.int/substance_abuse/publications/global_alcohol_report/en ( accessed September 2018).
- 7. Azar P, Wood E, Nguyen P et al. . Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infect Dis 2015; 15: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strathdee SA, Stockman JK.. Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep 2010; 7: 99– 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spire B, Lucas GM, Carrieri MP.. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST). Int J Drug Policy 2007; 18: 262– 270. [DOI] [PubMed] [Google Scholar]
- 10. Knudsen HK, Cook J, Lofwall MR et al. . A mixed methods study of HIV-related services in buprenorphine treatment. Subst Abuse Treat Prev Policy 2017; 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korthuis PT, Edelman EJ.. Substance use and the HIV care continuum: important advances. Addict Sci Clin Pract 2018; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gouse H, Joska JA, Lion RR et al. . HIV testing and sero-prevalence among methamphetamine users seeking substance abuse treatment in Cape Town. Drug Alcohol Rev 2016; 35: 580– 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization WHO Guidelines Approved by the Guidelines Review Committee. Geneva: World Health Organization; 2015. Available at: www.who.int/publications/guidelines/en ( accessed September 2018).
- 14. Eholie SP, Badje A, Kouame GM et al. . Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther 2016; 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen MS, Chen YQ, McCauley M et al. . Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375: 830– 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardner EM, McLees MP, Steiner JF et al. . The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52: 793– 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization Treat All: policy adoption and implementation status in countries. Geneva: Available at: http://apps.who.int/iris/bitstream/ 10665/259532/1/WHO-HIV-2017.58-eng.pdf ( accessed September 2018).
- 18. Shubber Z, Mills EJ, Nachega JB et al. . Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13: e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kresina T, Kaplowitz L, Ahadpour M, Johnson K.. Test and start for people living with HIV and who use drugs in low and middle income countries. HIV/AIDS Res Treat Open J 2016; 3: 15– 21. [Google Scholar]
- 20. * World Health Organization Country Profiles. Geneva: World Health Organization; 2014. Available at: www.who.int/substance_abuse/publications/global_alcohol_report/profiles/en ( accessed September 2018). [Google Scholar]
- 21. Hahn JA, Woolf-King SE, Muyindike W. Adding fuel to the fire: alcohol's effect on the HIV epidemic in Sub-Saharan Africa. Curr HIV/AIDS Rep 2011; 8: 172– 180. [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Li X, Stanton B.. Alcohol use among female sex workers and male clients: an integrative review of global literature. Alcohol Alcoholism 2010; 45: 188– 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lancaster KE, Cernigliaro D, Zulliger R, Fleming PF.. HIV care and treatment experiences among female sex workers living with HIV in sub-Saharan Africa: A systematic review. Afr J AIDS Res 2016; 15: 377– 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandfort TGM, Knox JR, Alcala C et al. . Substance use and HIV risk among men who have sex with men in africa: a systematic review. J Acquir Immune Defic Syndr 2017; 76: e34– e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bryant KJ, Nelson S, Braithwaite RS, Roach D.. Integrating HIV/AIDS and alcohol research. Alcohol Res Health 2010; 33: 167– 178. [PMC free article] [PubMed] [Google Scholar]
- 26. Korthuis PT, Fiellin DA, McGinnis KA et al. . Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J Acquir Immune Defic Syndr 2012; 61: 171– 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azar MM, Springer SA, Meyer JP, Altice FL.. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010; 112: 178– 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiene SM, Subramanian SV.. Event-level association between alcohol use and unprotected sex during last sex: evidence from population-based surveys in sub-Saharan Africa. BMC Public Health 2013; 13: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalichman SC, Simbayi LC, Kaufman M et al. . Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci 2007; 8: 141– 151. [DOI] [PubMed] [Google Scholar]
- 30. Kiene SM, Simbayi LC, Abrams A et al. . High rates of unprotected sex occurring among HIV-positive individuals in a daily diary study in South Africa: the role of alcohol use. J Acquir Immune Defic Syndr 2008; 49: 219– 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mbonye M, Rutakumwa R, Weiss H, Seeley J.. Alcohol consumption and high risk sexual behaviour among female sex workers in Uganda. Afr J AIDS Res 2014; 13: 145– 151. [DOI] [PubMed] [Google Scholar]
- 32. Bazargan-Hejazi S, Gaines T, Bazargan M et al. . Alcohol misuse and multiple sexual partners. West J Emerg Med 2012; 13: 151– 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalichman SC, Pinkerton SD, Carey MP et al. . Heterosexual anal intercourse and HIV infection risks in the context of alcohol serving venues, Cape Town, South Africa. BMC Public Health 2011; 11: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morojele NK, Kachieng’ a MA, Mokoko E et al. . Alcohol use and sexual behaviour among risky drinkers and bar and shebeen patrons in Gauteng province, South Africa. Social Science & Medicine 2006; 62: 217– 227. [DOI] [PubMed] [Google Scholar]
- 35. Chersich MF, Luchters SM, Malonza IM et al. . Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. Int J STD AIDS 2007; 18: 764– 769. [DOI] [PubMed] [Google Scholar]
- 36. Pandrea I, Happel KI, Amedee AM et al. . Alcohol's role in HIV transmission and disease progression. Alcohol Health Res World 2010; 33: 203. [PMC free article] [PubMed] [Google Scholar]
- 37. Loganantharaj N, Nichols WA, Bagby GJ et al. . The effects of chronic binge alcohol on the genital microenvironment of simian immunodeficiency virus-infected female rhesus macaques. AIDS Res Human Retrovir 2014; 30: 783– 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hendershot CS, Stoner SA, Pantalone DW, Simoni JM.. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr 2009; 52: 180– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lancaster KE, Lungu T, Mmodzi P et al. . The association between substance use and sub-optimal HIV treatment engagement among HIV-infected female sex workers in Lilongwe, Malawi. AIDS care 2017; 29: 197– 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nkosi S, Rich EP, Kekwaletswe CT, Morojele NK.. Experiences of alcohol consumption and taking antiretroviral medication among men living with HIV in Tshwane, South Africa. Afr J AIDS Res 2016; 15: 367– 376. [DOI] [PubMed] [Google Scholar]
- 41. McCance-Katz EF, Gruber VA, Beatty G et al. . Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. J Addict Med 2013; 7: 264– 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. * Degenhardt L, Peacock A, Colledge S et al. . Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5: e1192– e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tiberio J, Laurent YI, Ndayongeje J et al. . Context and characteristics of illicit drug use in coastal and interior Tanzania. Int J Drug Policy 2018; 51: 20– 26. [DOI] [PubMed] [Google Scholar]
- 44. Amosun SL, Mutimura E, Frantz JM.. Health promotion needs of physically disabled individuals with lower limb amputation in Rwanda. Disabil Rehabilitat 2005; 27: 837– 847. [DOI] [PubMed] [Google Scholar]
- 45. Leprêtre A, Ba I, Lacombe K et al. . Prevalence and behavioural risks for HIV and HCV infections in a population of drug users of Dakar, Senegal: the ANRS 12243 UDSEN study. J Int AIDS Soc 2015; 18: 19888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. * Degenhardt L, Bucello C, Calabria B et al. . What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug Alcohol Depend 2011; 117: 85– 101. [DOI] [PubMed] [Google Scholar]
- 47. Kayembe PK, Mapatano MA, Busangu AF et al. . Determinants of consistent condom use among female commercial sex workers in the Democratic Republic of Congo: implications for interventions. Sex Transm Infect 2007. [DOI] [PubMed] [Google Scholar]
- 48. Letamo G, Bowelo M, Majelantle RG.. Prevalence of substance use and correlates of multiple substance use among school-going adolescents in Botswana. Afr J Drug Alcohol Stud 2016; 15: 75– 89. [Google Scholar]
- 49. Mafuta C. Prevalence of moderate and high risk substance use and service needs among psychiatric inpatients at Zomba Mental Hospital in Malawi. University of Cape Town; 2015. Available at: https://open.uct.ac.za/handle/11427/16700 ( accessed September 2018). [Google Scholar]
- 50. Mbwambo J, McCurdy SA, Myers B et al. . Drug trafficking, use, and HIV risk: the need for comprehensive interventions. Sahara J 2012; 9: 154– 159. [DOI] [PubMed] [Google Scholar]
- 51. Mhlongo GT. Drug abuse in adolescents in Swaziland. 2009. Available at: uir.unisa.ac.za/bitstream/handle/10500/2363/dissertation.pdf ( accessed September 2018).
- 52. Osman T, Victor C, Abdulmoneim A et al. . Epidemiology of substance use among university students in Sudan. J Addict 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. International Narcotics Control Board Report of the International Narcotics Control Board: 2012. 2013. Available at: www.incb.org/documents/Publications/AnnualReports/AR2012/AR_2012_E_Chapter_III_Africa.pdf ( accessed September 2018).
- 54. Drug and Alcohol Council of Seychelles Seychelles National Drug Control Plan 2014-2018. Victoria, Seychelles: 2015. Available at: www.aho.afro.who.int/networks/sites/default/files/national_drug_control_master_plan_2014-2018.pdf ( accessed September 2018).
- 55. * United Nations Office on Drugs and Crime UNODC Statistics Online. Vienna, Austria: Available at: https://data.unodc.org/ (accessed
- 56. Amakali K, Haoses-Gorases L, Taukuheke L. Tobacco smoking among University of Namibia students: Behaviors, reasons, attitudes, awareness and knowledge of associated health risks. 2013. Available at: repository.unam.na/handle/11070/1641 ( accessed September 2018).
- 57. Awuzu EA, Kaye E, Vudriko P. Prevalence of cannabis residues in psychiatric patients: a case study of two mental health referral hospitals in Uganda. Subst Abuse Res Treat 2014; 8: SART. S13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Beckerleg S, Telfer M, Sadiq A.. A rapid assessment of heroin use in Mombasa, Kenya. Subst Use Misuse 2006; 41: 1029– 1044. [DOI] [PubMed] [Google Scholar]
- 59. Bøås M, Hatløy A.. Alcohol and drug consumption in post war Sierra Leone: an exploration. 2005. Available at: www.add-resources.org/getfile.php/ 949686.994.vqbqxqawaf/496final.pdf ( accessed September 2018).
- 60. Chapman J, Koleros A, Delmont Y et al. . High HIV risk behavior among men who have sex with men in Kigali, Rwanda: making the case for supportive prevention policy. AIDS Care 2011; 23: 449– 455. [DOI] [PubMed] [Google Scholar]
- 61. Ekouevi DK, Coffie PA, Salou M et al. . HIV seroprevalence among drug users in Togo. Sante Publique 2013; 25: 491– 498. [PubMed] [Google Scholar]
- 62. International Narcotics Control Board Report of the International Narcotics Control Board: 2004. United Nations Publications; 2005. Available at: www.incb.org/documents/Publications/AnnualReports/AR2004/AR_04_English.pdf ( accessed September 2018).
- 63. Lippitt MW. Risk factors and consequences of substance use among youth in post-conflict Liberia: A qualitative study. 2013. Available at: elischolar.library.yale.edu/ysphtdl/1175/ ( accessed September 2018).
- 64. Asante KO, Meyer-Weitz A, Petersen I.. Substance use and risky sexual behaviours among street connected children and youth in Accra, Ghana. Subst Abuse Treat Prev Policy 2014; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peltzer K, Ramlagan S, Johnson BD, Phaswana-Mafuya N.. Illicit drug use and treatment in South Africa: a review. Subst Use Misuse 2010; 45: 2221– 2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Senterre C, Levêque A, Piette D, Dramaix M.. Injury-related profiles among school-aged children in Cameroon: analysis based on the ‘First Survey—Health Young People’. Open J Epidemiol 2014; 4: 89. [Google Scholar]
- 67. Tesfaye G, Derese A, Hambisa MT. Substance use and associated factors among university students in Ethiopia: a cross-sectional study. J Addict 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cox G, Rampes H.. Adverse effects of khat: a review. Adv Psychiatr Treatment 2003; 9: 456– 463. [Google Scholar]
- 69. Swaziland Department of Youth Affairs Swaziland National Youth Policy. 2009. Available at: www.youthpolicy.org/national/Swaziland_2009_National_Youth_Policy.pdf ( accessed September 2018).
- 70. * Degenhardt L, Mathers B, Guarinieri M et al. . Meth/amphetamine use and associated HIV: implications for global policy and public health. Int J Drug Policy 2010; 21: 347– 358. [DOI] [PubMed] [Google Scholar]
- 71. Bhadain R. Mauritius: Analysis – the rise of synthetic drugs in Paradise Island (Part 2). 2018. Available at: allafrica.com/stories/201802280832.html ( accessed September 2018).
- 72. Bureau of International Narcotics and Law Enforcement Affairs 2016 International Narcotics Control Strategy Report (INCSR). Washington, D.C., United States of America: 2016. Available at: www.state.gov/j/inl/rls/nrcrpt/2016/vol1/253312.htm (accessed
- 73. Scheibe A, Makapela D, Brown B et al. . HIV prevalence and risk among people who inject drugs in five South African cities. Int J Drug Policy 2016; 30: 107– 115. [DOI] [PubMed] [Google Scholar]
- 74. Stimson G. Drug Injecting and HIV Infection. Routledge; 1998. [Google Scholar]
- 75. McCurdy SA, Ross MW, Williams ML et al. . Flashblood: blood sharing among female injecting drug users in Tanzania. Addiction 2010; 105: 1062– 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Syvertsen JL, Agot K, Ohaga S et al. . Evidence of injection drug use in Kisumu, Kenya: Implications for HIV prevention. Drug Alcohol Depend 2015; 151: 262– 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Malta M, Strathdee SA, Magnanini MM, Bastos FI.. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction 2008; 103: 1242– 1257. [DOI] [PubMed] [Google Scholar]
- 78. Palepu A, Tyndall MW, Joy R et al. . Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend 2006; 84: 188– 194. [DOI] [PubMed] [Google Scholar]
- 79. Parry CD, Carney T, Petersen P et al. . HIV-risk behavior among injecting or non-injecting drug users in Cape Town, Pretoria, and Durban, South Africa. Subst Use Misuse 2009; 44: 886– 904. [DOI] [PubMed] [Google Scholar]
- 80. Asher AK, Hahn JA, Couture MC et al. . People who inject drugs, HIV risk, and HIV testing uptake in sub-Saharan Africa. J Assoc Nurses AIDS Care 2013; 24: e35– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Joint United Nations Programme on HIV/AIDS 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014. Available at: www.unaids. org/en/resources/909090 ( accessed September 2018).
- 82. Mathers BM, Degenhardt L, Ali H et al. . HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet 2010; 375: 1014– 1028. [DOI] [PubMed] [Google Scholar]
- 83. Ndimbii J, Guise A, Ayon S et al. . Implementing needle and syringe programmes in Kenya: Changes, opportunities and challenges in HIV prevention. Afr J Drug Alcohol Stud 2015; 14: 95– 103. [Google Scholar]
- 84. Tran OC, Bruce RD, Masao F et al. . Linkage to Care among methadone clients living with HIV in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2015; 69: e43– e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rhodes T, Guise A, Ndimbii J et al. . Is the promise of methadone Kenya's solution to managing HIV and addiction? A mixed-method mathematical modelling and qualitative study. BMJ Open 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rhodes T, Closson EF, Paparini S et al. . Towards ‘evidence-making intervention’ approaches in the social science of implementation science: The making of methadone in East Africa. Int J Drug Policy 2016; 30: 17– 26. [DOI] [PubMed] [Google Scholar]
- 87. Lambdin BH, Bruce RD, Chang O et al. . Identifying programmatic gaps: inequities in harm reduction service utilization among male and female drug users in Dar es Salaam, Tanzania. PLoS One 2013; 8: e67062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ratliff EA, Kaduri P, Masao F et al. . Harm reduction as a complex adaptive system: a dynamic framework for analyzing Tanzanian policies concerning heroin use. Int J Drug Policy 2016; 30: 7– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Parry C, Petersen P, Carney T et al. . Rapid assessment of drug use and sexual HIV risk patterns among vulnerable drug-using populations in Cape Town, Durban and Pretoria, South Africa. Sahara J 2008; 5: 113– 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kurth AE, Cleland CM, Des Jarlais DC et al. . HIV prevalence, estimated incidence, and risk behaviors among people who inject drugs in Kenya. J Acquir Immune Defic Syndr 2015; 70: 420– 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Parry C, Petersen P, Dewing S et al. . Rapid assessment of drug-related HIV risk among men who have sex with men in three South African cities. Drug Alcohol Depend 2008; 95: 45– 53. [DOI] [PubMed] [Google Scholar]
- 92. Needle R, Kroeger K, Belani H, Hegle J.. Substance abuse and HIV/AIDS in sub-Saharan Africa: Introduction to the special issue. AfrJ Drug Alcohol Stud 2006; 5: 83– 94. [Google Scholar]
- 93. Parcesepe AM, Mugglin C, Nalugoda F et al. . Screening and management of mental health and substance use disorders in HIV treatment settings in low- and middle-income countries within the global IeDEA consortium. J Int AIDS Soc 2018; 21: e25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kader R, Seedat S, Koch JR, Parry CD.. A preliminary investigation of the AUDIT and DUDIT in comparison to biomarkers for alcohol and drug use among HIV-infected clinic attendees in Cape Town, South Africa. Afr J Psychiatry (Johannesbg) 2012; 15: 346– 351. [DOI] [PubMed] [Google Scholar]
- 95. WHO ASSIST Working Group The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002; 97: 1183– 1194. [DOI] [PubMed] [Google Scholar]
- 96. World Health Organization Guide to drug abuse: epidemiology. Geneva: World Health Organization; 2000. Available at: apps.who.int/iris/handle/10665/63850 ( accessed September 2018). [Google Scholar]
- 97. Kuria MW. Drug abuse among urban as compared to rural secondary schools students in Kenya: a short communication. East Afr Med J 1996; 73: 339. [PubMed] [Google Scholar]
- 98. Kinyanjui DW, Atwoli L. Substance use among inmates at the Eldoret prison in Western Kenya. BMC Psychiatry 2013; 13: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Atwoli L, Mungla PA, Ndung’ u MN et al. . Prevalence of substance use among college students in Eldoret, western Kenya. BMC Psychiatry 2011; 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chibanda D, Benjamin L, Weiss HA, Abas M.. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low- and middle-income countries. J Acquir Immune Defic Syndr 2014; 67 Suppl 1: S54– 67. [DOI] [PubMed] [Google Scholar]
- 101. Syvertsen JL, Ohaga S, Agot K et al. . An ethnographic exploration of drug markets in Kisumu, Kenya. Int J Drug Policy 2016; 30: 82– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Morojele NK, Nkosi S, Kekwaletswe CT et al. . Utility of brief versions of the alcohol use disorders identification test (AUDIT) to identify excessive drinking among patients in HIV care in South Africa. J Stud Alcohol Drugs 2017; 78: 88– 96. [DOI] [PubMed] [Google Scholar]
- 103. Papas RK, Gakinya BN, Mwaniki MM et al. . Associations between the phosphatidylethanol alcohol biomarker and self-reported alcohol use in a sample of HIV-infected outpatient drinkers in Western Kenya. Alcohol Clin Exp Res 2016; 40: 1779– 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hahn JA, Emenyonu NI, Fatch R et al. . Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction 2016; 111: 272– 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jaquet A, Wandeler G, Nouaman M et al. . Alcohol use, viral hepatitis and liver fibrosis among HIV-positive persons in West Africa: a cross-sectional study. J Int AIDS Soc 2017; 19: 21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jaquet A, Ekouevi DK, Bashi J et al. . Alcohol use and non-adherence to antiretroviral therapy in HIV-infected patients in West Africa. Addiction 2010; 105: 1416– 1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vinikoor MJ, Zyambo Z, Muyoyeta M et al. . Point-of-care urine ethyl glucuronide testing to detect alcohol use among hiv-hepatitis B virus coinfected adults in Zambia. AIDS Behav 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. World Health Organization mhGAP Intervention Guide for mental, neurological and substance use disorders in non-specialized settings: Version 2.0. Geneva: 2016. Available at: www.who.int/mental_health/publications/mhGAP_intervention_guide/en ( accessed September 2018). [PubMed]
- 109. Parry CD, Petersen P, Carney T, Needle R.. Opportunities for enhancing and integrating HIV and drug services for drug using vulnerable populations in South Africa. Int J Drug Policy 2010; 21: 289– 295. [DOI] [PubMed] [Google Scholar]
- 110. Haldane V, Cervero-Liceras F, Chuah FL et al. . Integrating HIV and substance use services: a systematic review. J Int AIDS Soc 2017; 20: 21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Affinnih YH. A review of literature on drug use in sub-Saharan Africa countries and its economic and social implications. Subst Use Misuse 1999; 34: 443– 454. [DOI] [PubMed] [Google Scholar]
- 112. Volkow ND, Montaner J.. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff (Millwood) 2011; 30: 1411– 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rhodes T, Abdool R.. Drug harms and drug policies in sub-Saharan Africa: implementation science and HIV epidemics. Int J Drug Policy 2016; 30: 1– 6. [DOI] [PubMed] [Google Scholar]
- 114. Becker SJ, Kelly LM, Kang AW et al. . Factors associated with contingency management adoption among opioid treatment providers receiving a comprehensive implementation strategy. Subst Abuse 2018; 1– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Acuda W, Othieno CJ, Obondo A, Crome IB.. The epidemiology of addiction in sub-Saharan Africa: a synthesis of reports, reviews, and original articles. Am J Addict 2011; 20: 87– 99. [DOI] [PubMed] [Google Scholar]
- 116. Charlson FJ, Diminic S, Lund C et al. . Mental and substance use disorders in sub-Saharan Africa: predictions of epidemiological changes and mental health workforce requirements for the next 40 years. PLoS One 2014; 9: e110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Odeny TA, Padian N, Doherty MC et al. . Definitions of implementation science in HIV/AIDS. Lancet HIV 2015; 2: e178– e180. [DOI] [PubMed] [Google Scholar]
- 118. Cunningham SD, Card JJ.. Realities of replication: implementation of evidence-based interventions for HIV prevention in real-world settings. Implement Sci 2014; 9: 5– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Odejide AO. Status of drug use/abuse in Africa: a review. Int J Mental Health Addict 2006; 4: 87– 102. [Google Scholar]
- 120. Springer SA, Larney S, Alam-mehrjerdi Z et al. . Drug treatment as HIV prevention among women and girls who inject drugs from a global perspective: progress, gaps, and future directions. J Acquir Immune Defic Syndr 2015; 69: S155– S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lancaster KE, Lungu T, Mmodzi P et al. . The association between substance use and sub-optimal HIV treatment engagement among HIV-infected female sex workers in Lilongwe, Malawi. AIDS Care 2017; 29: 197- 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kerrigan D, Mbwambo J, Likindikoki S et al. . Project Shikamana: baseline findings from a community empowerment-based combination HIV prevention trial among female sex workers in Iringa, Tanzania. J Acquir Immune Defic Syndr 2017; 74 Suppl 1: S60– s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lesko CR, Keil AP, Moore RD et al. . Measurement of current substance use in a cohort of HIV-infected persons in continuity HIV care, 2007–2015. Am J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hernan MA, Hernandez-Diaz S, Robins JM.. A structural approach to selection bias. Epidemiology 2004; 15: 615– 625. [DOI] [PubMed] [Google Scholar]
- 125. Westreich D. Berkson's bias, selection bias, and missing data. Epidemiology 2012; 23: 159– 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kurth AE, Cleland CM, Des Jarlais DC et al. . HIV Prevalence, Estimated Incidence, and Risk Behaviors Among People Who Inject Drugs in Kenya. J Acquir Immune Defic Syndr 2015; 70: 420- 427. [DOI] [PMC free article] [PubMed] [Google Scholar]