Abstract
The implementation of the 2013 World Health Organization Option B+ recommendations for HIV treatment during pregnancy has helped drive significant progress in achieving universal treatment for pregnant and postpartum women in sub-Saharan Africa (SSA). Yet, critical research and implementation gaps exist in achieving the UNAIDS 90-90-90 targets. To help guide researchers, programmers and policymakers in prioritising these areas, we undertook a comprehensive review of the progress, gaps and research needs to achieve the 90-90-90 targets for this population in the Option B+ era, including early infant HIV diagnosis (EID) for HIV-exposed infants. Salient areas where progress has been achieved or where gaps remain include: (1) knowledge of HIV status is higher among people with HIV in southern and eastern Africa compared to western and central Africa (81% versus 48%, UNAIDS); (2) access to antiretroviral therapy (ART) for pregnant women has doubled in 22 of 42 SSA countries, but only six have achieved the second 90, and nearly a quarter of pregnant women initiating ART become lost to follow-up; (3) viral suppression data for this population are sparse (estimates range from 30% to 98% peripartum), with only half of women maintaining suppression through 12 months postpartum; and (4) EID rates range from 15% to 62%, with only three of 21 high-burden SSA countries testing >50% HIV-exposed infants within the first 2 months of life. We have identified and outlined promising innovations and research designed to address these gaps and improve the health of pregnant and postpartum women living with HIV and their infants.
Keywords: prevention of mother-to-child transmission, prevention of vertical transmission, HIV, pregnancy, postpartum, sub-Saharan Africa
Introduction
The expansion of HIV care and treatment for pregnant and postpartum women in sub-Saharan Africa (SSA) exemplifies a significant achievement in the global HIV response. In southern and eastern Africa, home to 50% of the HIV population globally, antiretroviral therapy (ART) coverage for pregnant women has increased from 47% in 2010 to 93% in 2017 [1]. A variety of tools and evidence-based strategies have emerged to improve the health of pregnant and postpartum women with HIV, encouraging optimism towards achieving the ambitious targets established by the Joint United Nations Programme on HIV/AIDS (UNAIDS): that 90% of people living with HIV (PLHIV) know their status, 90% of diagnosed people are on ART, and 90% of those on ART are virally suppressed by 2020 [2,3]. Progress in the prevention of mother-to-child transmission (PMTCT) of HIV has also been substantial. With adequate adherence, ART carries the potential to virtually eliminate the risk of vertical HIV transmission for pregnant and breastfeeding women. At the country level, achieving ≥95% population-level ART coverage for pregnant women is a process indicator for national elimination of MTCT (defined as MTCT rates <5% in breastfeeding populations or <2% in non-breastfeeding populations) [4-8].
However, progress across SSA has not been uniform, and as our understanding of the HIV epidemic has deepened, critical gaps have been identified, which will need to be addressed in order to achieve the 90-90-90 goals for pregnant and postpartum women. Examples of these gaps include: (1) facility-based HIV testing has been broadly implemented for pregnant women in much of southern and eastern Africa, but central and western Africa are lagging behind [9]; (2) from country to country, the proportions of pregnant women accessing ART vary considerably, with many still far from reaching the second 90, even as others have surpassed it [10]; (3) achieving and sustaining viral suppression throughout pregnancy, delivery and the breastfeeding period remains a challenge in multiple countries [11,12], and as a result, there were still an estimated 180,000 new HIV infections in children in 2017 [1]; and (4) for HIV-exposed infants, gaps in early infant diagnosis remain substantial [13]. In this review, we summarise recent progress, remaining knowledge gaps, and identify the future research needed to achieve and eventually surpass the 90-90-90 targets for pregnant and postpartum women in SSA (Table 1).
Table 1.
Research priorities to achieve 90-90-90 for pregnant and postpartum women in sub-Saharan Africa
| First 90 |
|---|
|
| Second 90 |
|---|
|
| Third 90 |
|---|
|
| Early infant diagnosis of HIV for HIV-exposed infants |
|---|
|
First 90: knowledge of HIV status
Globally, an estimated 75% of all PLHIV knew their status in 2017, and across many SSA countries, knowledge of HIV status is higher among women of reproductive age (15–49 years) as compared to men [1]. Universal HIV testing for pregnant women initiating facility-based antenatal care (ANC) has been broadly implemented in many regions in southern and eastern Africa, yet central and western Africa, where the healthcare delivery systems are weaker, have been slower to implement this approach [9]. Further, general knowledge of HIV status among PLHIV in western and central Africa is considerably lower than other African regions – at 48%, as compared to 81% in southern and eastern Africa [1]. Stigma and discrimination, test kits stock outs, healthcare worker shortages and user fees all contribute to undermining achievement of the first 90 for pregnant and postpartum women [14]. In addition, as many as 20% of women in SSA are estimated to have no antenatal care, and thus miss antenatal HIV testing entirely [15]. To complicate matters further, pregnancy and the perinatal period are associated with an increased risk of HIV acquisition [16,17]. In a cohort of over 1300 pregnant women in western Kenya, followed through 9 months postpartum, HIV incidence was 2.31 per 100 person-years [18]. Studies from Tanzania and Zambia [19] demonstrated similar results [20]. A critical testing gap also exists for sexually active adolescent girls, particularly in western and central African countries. For example, in Nigeria, 33% of adolescents (ages 10–19 years), as compared to 52% of adult women, reported being tested for HIV during their last pregnancy [21,22].
Several evidence-based strategies exist to improve HIV testing uptake among pregnant women, with fewer strategies specifically focused in the postpartum or breastfeeding periods. Universal, opt-out testing in ANC clinics has been highly successful in eastern and southern Africa. Repeat testing during pregnancy, and at least once during breastfeeding, is cost-effective, although it has been implemented with varying success [23,24]. Door-to-door testing by lay healthcare workers, and recent approaches integrating immediate ART initiation with community testing, may improve knowledge of HIV status pre-pregnancy, and identify pregnant women who have not enrolled in ANC [25,26]. Testing as part of home-based couples counselling may be particularly beneficial for women who are afraid of knowing their status and disclosing to their partner(s) [26]. Self-testing is an emerging strategy that may be particularly beneficial for sexually active adolescent girls [27-29]. Increased knowledge of personal HIV status among the general population using promising approaches, such as integration of HIV testing within multi-disease health campaigns, is likely to increase the proportion of women who know their HIV status prior to pregnancy and breastfeeding [30,31].
Important research gaps exist that need to be filled in order to improve knowledge of HIV status in pregnant and postpartum women. Identification and assessment of innovative HIV testing strategies that consider the lower HIV prevalence are needed for west and central Africa. Furthermore, approaches to improving uptake of antenatal care in women with low ANC attendance, including community-based peer navigators or thoughtful engagement of traditional birth attendants to promote HIV testing, should be explored. Further work is needed to identify optimal timing and implementation of repeat HIV testing for women, and to better understand structural and individual level barriers to repeat testing [32]. Testing strategies that take into account the unique developmental stage and challenges facing pregnant adolescents need to be identified and evaluated in order to increase testing uptake in this population.
Second 90: uptake of ART
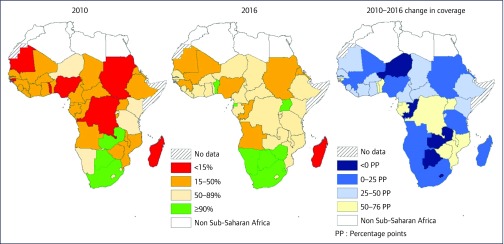
The scale-up of World Health Organization (WHO) recommendations, including Option B (ART for all women through pregnancy and breastfeeding) and Option B+ (lifelong ART for pregnant women), has yielded remarkable progress in ART coverage for pregnant and postpartum women in SSA (Figure 1). ART access for PMTCT more than doubled in 22 of 42 SSA countries with available UNAIDS Global AIDS Monitoring (GAM) data, and six countries (Botswana, Namibia, South Africa, Swaziland, Uganda and Zimbabwe) have surpassed the second 90 target for pregnant women as of 2016 [4,10]. Yet, wide variations in ART coverage exist within the continent, with fewer than 40% of HIV-positive pregnant women accessing ART in Nigeria and Mali compared to over 95% in Botswana, South Africa and Uganda in 2016 [10]. Furthermore, barriers to retention in care and maintenance on ART have surfaced as key obstacles to achieving the second 90 in SSA [33-36]. Since the implementation of Option B+, pooled retention estimates across SSA suggest that one-in-four women become lost to follow-up within 6 months of ART initiation [37], with women initiating ART during pregnancy up to five times more likely to become disengaged compared to women starting ART because of advanced HIV disease [35]. Adverse birth outcomes as a result of in utero ART exposure are persistent concerns, as various studies have reported increased risk of preterm birth and low birth weight, which are themselves associated with increased newborn morbidity and mortality in resource-limited settings [5,38,39]. Protease inhibitors, and to a lesser extent nucleoside and non-nucleoside transcriptase inhibitors, are most often associated with these adverse birth outcomes. Of particular concern is a preliminary report of increased neural tube defects in women who conceive on the integrase inhibitor dolutegravir, which is a potent and well-tolerated antiretroviral agent, whose use is rapidly growing in SSA [40,41].
Figure 1.
Antiretroviral therapy coverage for pregnant women living with HIV in sub-Saharan Africa, 2010–2016 (excluding single-dose nevirapine). Data from [10]
A variety of evidence-based, scalable interventions have been described that could help reach the second 90 in SSA [42]. These interventions span the social, behavioural and structural aspects of PMTCT care at both the facility and community levels [43]. Improved ART uptake and retention has been demonstrated with expert peer support and ‘mentor mothers’ [44,45], individual or group counselling sessions [45], and community health workers who provide supportive supervision, ART counselling and tracing of women who default from care [46-48]. Male partner involvement has also been shown to improve maternal ART uptake [49-51]. Behavioural interventions by way of text message or phone call appointment reminders (mHealth) have been effective in improving ART uptake and retention across multiple HIV populations, and there is promising evidence of their feasibility and potential efficacy in the PMTCT population as well. Economic incentives, such as conditional cash transfer [52] and disclosure support to partners [53] have been less well studied, but may provide additional retention benefits. Structurally, the integration of HIV services into maternal and child health and postnatal services has played an important role in expanding capacity for ART coverage, and may improve retention in care compared to non-integrated services [54,55]. Combination interventions are also increasingly being examined to maximise gains in retention and maintenance on ART [42,56,57]. Finally, although many SSA countries have yet to implement national surveillance for ART-related adverse birth outcomes, researchers in Botswana, through the Botswana-Harvard partnership (Tsepamo study), have established a unique surveillance programme that has contributed key data to inform our understanding about the safety of ART for the mother and fetus [40,41].
Despite important progress, knowledge gaps persist concerning the optimal implementation strategies to achieve the second 90. Overall, the evidence for many of the aforementioned interventions is weak and heterogeneous, with limited duration of effect. Further research is needed to: (1) identify the barriers to care; (2) study the impact of interventions on long-term retention; (3) determine successful integration and differentiated care models for PMTCT; and (4) evaluate strategies to optimise ART uptake while maximising safety. Failure to engage in antenatal and HIV care threatens not only HIV-exposed infants, but the health of women with HIV and their partners.
Identifying barriers to care is critical, including their relative significance, and the optimal social and behavioural interventions to address them, particularly in terms of their cost-effectiveness and scalability for care programmes. Many studies have looked at the impact of interventions to improve adherence and retention for the period up to 12 months post-delivery; however, breastfeeding and PMTCT follow-up can last 18 months or longer in many cultures. Studying the impact of interventions through the conclusion of PMTCT follow-up, and through the transition back to an HIV clinic for postpartum women, is vital to understanding their durability and true effect sizes.
On a structural level, integrated service delivery models that maximise efficiency and efficacy for stable PMTCT clients, including models of differentiated and decentralised ART delivery, and patients’ preferences for such models, need to be rigorously evaluated [58]. High-quality, large, population-based studies to better understand the factors that drive regional variations in ART coverage for pregnant and postpartum women in SSA will also help achieve the second 90 goals for them. Finally, questions remain concerning the timing of ART initiation, and the effects of established and novel ART regimens (e.g. dolutegravir) on adverse birth outcomes (e.g. stillbirth, preterm birth, growth restriction, congenital anomalies) and infant development [41,59-61].
Third 90: viral suppression
Achieving and maintaining viral suppression is urgent for pregnant and postpartum women in order to minimise vertical HIV transmission and maximise women's health outcomes [62]. While routine viral load monitoring among PLHIV on ART is now recommended by WHO, scale-up has been slow. In a study focused on seven of the SSA countries, only one was performing at least one viral load on more than 85% of patients on ART (South Africa [91%]); two countries (Kenya [40%] and Namibia [23%]) tested fewer than 50% of patients on ART, and four countries (Côte d’Ivoire [11%], Malawi [19%], Tanzania [9%], and Uganda [22%]) tested fewer than 25% [63]. Further, recognition of pregnant and postpartum women as a priority population for monitoring and rapid intervention in the setting of virological failure is lacking [63-65]. As a result, literature on viral suppression among pregnant and breastfeeding women in SSA is sparse and frequently comes from research studies rather than routine care programmes. Additionally, several of these studies were conducted in settings and during time periods when universal ART was not the standard of care, and thus have a mixed population of women who are on ART as well as those on PMTCT prophylaxis [66].
Available data show viral suppression rates at delivery or immediately postpartum have ranged from 30% to 98% in different SSA settings, and are dependent on the viral load threshold used (Table 2). Keeping women engaged in care and optimally adherent to ART following delivery is a well-known challenge, and undermines sustained viral suppression[35,48]. In Malawi, although 70% of women starting ART during pregnancy and breastfeeding had adequate adherence (defined as having ART drugs available ≥90% of days between clinic visits), only one-third of them maintained adequate adherence over 2 years of treatment [67]. Similarly, among women who became pregnant after initiating ART in South Africa, the risk of non-adherence was nearly 50% higher during the postpartum period compared to the non-pregnant period [68,69]. Further, even among those who are retained in care, optimal viral control (>90% with VL <50 copies/mL) in the postpartum period is rarely achieved. In Nigeria, only 58% of women attained suppression (<20 copies/mL) at 6 months postpartum [70]. While in a follow-up study of 523 previously virally suppressed (<50 copies/mL) women in Cape Town, only 70% were able to maintain viral suppression through 12 months postpartum [11]. The most promising and longest follow-up comes from the PROMOTE trial in Uganda, in which approximately 90% of women had VL <400 copies/mL at 24 months postpartum [71]; and 5 years later, among a random sample of 200 participants, 90% had maintained viral suppression [72].
Table 2.
Summary of studies on the prevalence of viral suppression in sub-Saharan Africa
| Author | Enrolment period | Region | Design | ART eligibility and naivety status | Timing of enrolment | Analytical sample | Viral load threshold (copies/mL) | Timing of VL sampling | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chagomerana et al. [73] | Jun 2015– Nov 2016 | Lilongwe, Malawi | Prospective cohort | ART-naïve and experienced | First ANC of current pregnancy | 252 | <1000 | Delivery | 84 |
| <40 | Delivery | 69 | |||||||
| Chetty et al. [12] | 2010–2015 | Rural KwaZulu-Natal, South Africa | Prospective cohort | ART-experienced (at least 6 months) | Pregnancy, during first ANC visit | 1425 | <1000 | Pre-pregnancy | 89 |
| Pregnancy | 85 | ||||||||
| 6 months postpartum | 84 | ||||||||
| 12 months postpartum | 82 | ||||||||
| 25 months postpartum | 83 | ||||||||
| <50 | Pre-pregnancy | 82 | |||||||
| Pregnancy | 77 | ||||||||
| 6 months postpartum | 77 | ||||||||
| 12 months postpartum | 76 | ||||||||
| 25 months postpartum | 76 | ||||||||
| Cohan et al.[71] | Dec 2009– Mar 2013 | District Tororo, Uganda | Randomized control trial (test efficacy of efavirenz vs lopinavir/ritonavir) | ART naïve-eligible | Pregnant, 12-28 weeks | 389 | <400 | Delivery (efavirenz arm) | 98 |
| Delivery (lopinavir/ritonavir arm) | 86 | ||||||||
| 48 weeks postpartum (efavirenz arm) | 91 | ||||||||
| 48 weeks postpartum (lopinavir/ritonavir arm) | 88 | ||||||||
| Gill et al. [74] | Apr 2013– Jan 2014 | Kigali, Rwanda | Prospective cohort | ART-naïve and experienced | Third trimester of pregnancy and up to 2 weeks post-partum | 608 | <20 | ≤4 months under ART | 30 |
| <20 | >4 months under ART | 66 | |||||||
| <20 | >12 months under ART | 66 | |||||||
| <1000 | >12 months under ART | 90 | |||||||
| Hosseinipour et al. [75] | Jan 2003– Mar 2017 | Central and southern Malawi | Randomized control trial | ART naïve-eligible | Pregnancy or post- partum | 1269 | <1000 | 6 months post enrollment | 84 |
| Koss et al. [72] | Mar 2015– Sept 2015 | District Tororo, Uganda | Cross-sectional | ART naïve-eligible | Pregnancy, 12-28 weeks | 150 | <400 | Postpartum | 90 |
| Myer et al. [76] | Apr 2013– May 2014 | Cape Town, South Africa | Retrospective cohort | ART naïve-eligible | First ANC of current pregnancy | 620 | ≤1000 | Delivery | 91 |
| ≤50 | Delivery | 73 | |||||||
| Myer et al. [11] | Apr 2013– May 2014 | Cape Town, South Africa | Prospective cohort | Women who initiated ART during pregnancy and achieved initial viral suppression during follow-up | First ANC of current pregnancy | 523 | sustained viral load <50 during follow-up | Across follow-up (median 322 days) during the postpartum period: 7 days, 6 weeks, 3 months, 6 months, 9 months, and 12 months | 70 |
| Sam-Agudu et al. [45] | Apr 2014– Sept 2015 | Federal Capital Territory and Nasarawa states, North-central Nigeria | Prospective cohort | ART-naïve and experienced | Pregnancy, mixed gestational ages | 497 | <20 | Postpartum | 58 |
ANC: antenatal clinic; cp: copies; VL: viral load.
Evidenced-based approaches to address the main drivers of virological failure are being elucidated. While in many settings, data are lacking or not well utilised to address gaps in the third 90, South Africa's rapid and successful scale-up of PMTCT has been attributed to the use of data-driven continuous quality improvement interventions that identify problems as they emerge, and empowers front-line staff to create local solutions [77,78]. This strategy is currently being fully evaluated in a cluster randomised trial focused on women initiating ART in pregnancy or during breastfeeding in the Democratic Republic of Congo [57]. A large number of studies have identified that non-adherence in pregnancy and the postpartum period is multifactorial, and may be driven by individual-level and biomedical issues, such as ART toxicity, stigma, mental health, ART toxicity and comorbidities, and structural issues related to healthcare worker attitudes and the availability of supportive services [11,66,72-74,79-84]. Thus, combination strategies that address these challenges are needed. Several studies are currently evaluating behavioural, peer navigator and mHealth interventions that may prove successful in attaining and maintaining viral suppression for pregnant and postpartum women [85,86].
In order to reach the third 90 for pregnant and postpartum women, the lack of data highlighted above, especially data from routine PMTCT implementation at programme and country level, will need to be addressed. Additionally, there is need for evidence-based interventions that improve engagement in care and adherence on ART among pregnant and breastfeeding women, and target previously identified modifiable predictors of viral suppression in this population. In order to achieve the third 90 for pregnant and postpartum women, a research agenda targeted on addressing sustained adherence is vital. Additional research should address the optimal timing and frequency of VL monitoring in pregnant and postpartum women and biomedical and behavioural interventions that rapidly achieve suppression in women with viremia.
Early infant diagnosis for HIV-exposed infants
Over 80% of newly infected children acquire their HIV infection through MTCT, yet fewer than 50% of infants exposed to HIV are tested at 6 weeks of age, and up to 45% are lost after initial testing [14,48]. EID is the crucial first step in addressing the alarmingly high mortality rate for infants infected with HIV, and is intricately linked with 90-90-90 goals for pregnant and postpartum women [87]. Some countries in SSA have made significant progress scaling-up EID testing; in a report on six SSA countries (Cote d’Ivoire, Democratic Republic of Congo, Malawi, South Africa, Uganda and Zambia) it was noted that the total number of EID tests performed on HIV-exposed infants significantly increased between 2011 and 2015, but testing before 6 weeks of age was poor, ranging between 15% and 62% [88]. In 2016, among the 21 UNAIDS-designated high-burden countries (all of which are in SSA) (90), only three – South Africa, Swaziland, and Zimbabwe – managed to test over 50% of exposed infants within the first 2 months of life [14,89]. Even when infants are tested, turn-around time for test results is so exceptionally long that caregivers may never receive the results [90].
Multiple strategies are gaining evidence of effectiveness in improving the first 90 for exposed infants in SSA. South Africa is the first high-burden country to introduce routine birth testing for exposed infants, and has achieved greater than 90% coverage. Complexities of neonatal HIV treatment, and high rates of early losses to follow-up after birth testing, remain concerns for the introduction of birth testing on a wide scale [91,92]. Point-of-care diagnostics may also be a promising new strategy that avoids the challenges associated with sample transport to centralised laboratories, and facilitates early intervention for positive results [93,94]. mHealth innovations, such as returning test results via short text message (SMS), electronic tracking systems and national web-based result dashboards, such as those being used in Kenya and South Africa, may support improved uptake as well as efficient return of results to providers and caregivers [95-97]. Integration of maternal and child health services, and psychosocial support through peer or community interventions, supports EID and retention of both mothers and exposed infants [49,54,98-101].
Further research into testing strategies that maximise uptake and facilitate early ART for infected infants are needed. How and where to integrate accurate point-of-care technologies into national EID algorithms, including as testing at birth, need to be determined. Strategies for integrating surveillance for acute HIV infection in pregnant and breastfeeding women with EID for infants should be explored. Further evidence is needed for cost-effective psychosocial support models that encourage HIV testing among exposed infants.
Conclusion
Despite considerable expansion of quality PMTCT services, and widespread uptake of Option B+, most countries in SSA have not achieved UNAIDS 90-90-90 targets for pregnant and postpartum women. A targeted research agenda as outlined in this paper is an important next step in reaching these critical goals, in order to achieve the elimination of vertical HIV transmission and ensure the health and well-being of women and their families.
References
- 1. World Health Organization Global guidance on criteria and processes for validation: Elimination of mother-to child transmission of HIV and syphilis . Geneva, Switzerland: WHO; 2017. Available at: www.who.int/reproductivehealth/publications/emtct-hiv-syphilis/en ( accessed September 2018).
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014. Available at: www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/90-90-90_en.pdf ( accessed September 2018).
- 3. UNAIDS Global AIDS Update 2016. 2016. Available at: www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf ( accessed September 2018).
- 4. UNAIDS 2015 Progress Report on the Global Plan. 2015. Available at: www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf ( accessed September 2018).
- 5. Fowler MG, Qin M, Fiscus SA et al. . Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375: 1726– 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kesho Bora Study Group Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 2011; 11: 171– 180. [DOI] [PubMed] [Google Scholar]
- 7. Jamieson DJ, Chasela CS, Hudgens MG et al. . Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet 2012; 379: 2449– 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bispo S, Chikhungu L, Rollins N et al. . Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis. J Int AIDS Soc 2017; 20: 1– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Staveteig S, Croft TN, Kampa KT, Head SK.. Reaching the ‘first 90’: gaps in coverage of HIV testing among people living with HIV in 16 African countries. PLoS One 2017; 12: e0186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. UNAIDS UNAIDS Data 2017. 2017. Available at: www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf ( accessed September 2018).
- 11. Myer L, Dunning L, Lesosky M et al. . Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis 2017; 64: 422– 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chetty T, Newell ML, Thorne C, Coutsoudis A.. Viraemia before, during and after pregnancy in HIV-infected women on antiretroviral therapy in rural KwaZulu-Natal, South Africa, 2010–2015. Trop Med Int Health 2018; 23: 79– 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Essajee S, Vojnov L, Penazzato M et al. . Reducing mortality in HIV-infected infants and achieving the 90-90-90 target through innovative diagnosis approaches. J Int AIDS Soc 2015; 18: 20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. UNAIDS Ending AIDS: Progress Towards the 90-90-90 Targets. Geneva: 2017. Available at: www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017 ( accessed September 2018).
- 15. UNICEF UNICEF Data: Monitoring the Situation of Children and Women. 2018. Available at: https://data.unicef.org/topic/maternal-health/antenatal-care/ ( accessed September 2018).
- 16. Brubaker SG, Bukusi EA, Odoyo J et al. . Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Med 2011; 12: 316– 321. [DOI] [PubMed] [Google Scholar]
- 17. Thomson KA, Hughes J, Baeten JM et al. . Increased risk of female HIV-1 acquisition throughout pregnancy and postpartum: a prospective per-coital act analysis among women with HIV-1 infected partners. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kinuthia J, Drake AL, Matemo D et al. . HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS 2015; 29: 2025– 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heemelaar S, Habets N, Makukula Z et al. . Repeat HIV testing during pregnancy and delivery: missed opportunities in a rural district hospital in Zambia. Trop Med Int Health 2015; 20: 277– 283. [DOI] [PubMed] [Google Scholar]
- 20. Lawi JD, Mirambo MM, Magoma M et al. . Sero-conversion rate of syphilis and HIV among pregnant women attending antenatal clinic in Tanzania: a need for re-screening at delivery. BMC Pregnancy Childbirth 2015; 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helleringer S. Understanding the adolescent gap in HIV testing among clients of antenatal care services in West and Central African Countries. AIDS Behav 2017; 21: 2760– 2773. [DOI] [PubMed] [Google Scholar]
- 22. Ronen K, McGrath CJ, Langat AC et al. . Gaps in adolescent engagement in antenatal care and prevention of mother-to-child HIV transmission services in Kenya. J Acquir Immune Defic Syndr 2017; 74: 30– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim LH, Cohan DL, Sparks TN et al. . The cost-effectiveness of repeat HIV testing during pregnancy in a resource-limited setting. J Acquir Immune Defic Syndr 2013; 63: 195– 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soorapanth S, Sansom S, Bulterys M et al. . Cost-effectiveness of HIV rescreening during late pregnancy to prevent mother-to-child HIV transmission in South Africa and other resource-limited settings. J Acquir Immune Defic Syndr 2006; 42: 213– 221. [DOI] [PubMed] [Google Scholar]
- 25. Labhardt N, Ringera I, Lejone T et al. . Same-day ART initiation after home-based HIV testing: a randomized controlled trial. Conference on Opportunistic Infections and Retroviruses. March 2018. Boston, MA, USA. Abstract 94.
- 26. Turan JM, Darbes LA, Musoke PL et al. . Development and piloting of a home-based couples intervention during pregnancy and postpartum in Southwestern Kenya. AIDS Patient Care STDS 2018; 32: 92– 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kojima N, Klausner JD.. Accelerating epidemic control: the role of HIV self-testing. Lancet HIV 2018. [DOI] [PubMed] [Google Scholar]
- 28. Hector J, Davies MA, Dekker-Boersema J et al. . Acceptability and performance of a directly assisted oral HIV self-testing intervention in adolescents in rural Mozambique. PLoS One 2018; 13: e0195391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inwani I, Chhun N, Agot K et al. . High-yield HIV testing, facilitated linkage to care, and prevention for female youth in Kenya (GIRLS Study): implementation science protocol for a priority population. JMIR Res Protoc 2017; 6: e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen M, Balzer L, Kwarsiima D et al. . Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA 2017; 317: 2196– 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayes R, Floyd S, Schaap A et al. . A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med 2017; 14: e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogers AJ, Weke E, Kwena Z et al. . Implementation of repeat HIV testing during pregnancy in Kenya: a qualitative study. BMC Pregnancy Childbirth 2016; 16: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abrams EJ, Langwenya N, Gachuhi A et al. . Impact of Option B+ on ART uptake and retention in Swaziland: a stepped-wedge trial. Conference on Retroviruses and Opportunistic Infections. February, 2016. Boston, MA, USA. Abstract 34.
- 34. Llenas-Garcia J, Wikman-Jorgensen P, Hobbins M et al. . Retention in care of HIV-infected pregnant and lactating women starting ART under Option B+ in rural Mozambique. Trop Med Int Health 2016; 21: 1003– 1012. [DOI] [PubMed] [Google Scholar]
- 35. Tenthani L, Haas AD, Tweya H et al. . Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS 2014; 28: 589– 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woelk GB, Ndatimana D, Behan S et al. . Retention of mothers and infants in the prevention of mother-to-child transmission of HIV programme is associated with individual and facility-level factors in Rwanda. J Int AIDS Soc 2016; 19: 20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knettel BA, Cichowitz C, Ngocho JS et al. . Retention in HIV care during pregnancy and the postpartum period in the Option B+ era: systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr 2018; 77: 427– 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saleska JL, Turner AN, Maierhofer C et al. . Use of antiretroviral therapy during pregnancy and adverse birth outcomes among women living with HIV-1 in low and middle-income countries: a systematic review. J Acquir Immune Defic Syndr 2018. [DOI] [PubMed] [Google Scholar]
- 39. Katz J, Lee AC, Kozuki N et al. . Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013; 382: 417– 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zash R, Jacobson DL, Diseko M et al. . Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171: e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization Potential safety issue affecting women living with HIV using dolutegravir at the time of conception. Geneva: WHO; 2018. Available at: www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf?ua=1 (accessed [Google Scholar]
- 42. Vrazo AC, Firth J, Amzel A et al. . Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health 2018; 23: 136– 148. [DOI] [PubMed] [Google Scholar]
- 43. Ambia J, Mandala J.. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J Int AIDS Soc 2016; 19: 20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Phiri S, Tweya H, Lettow M et al. . Impact of facility- and community-based peer support models on maternal uptake and retention in Malawi's Option B+ HIV prevention of mother-to-child transmission program: a 3-arm cluster randomized controlled trial (PURE Malawi). J Acquir Immune Defic Syndr 2017; 75 Suppl 2: S140– S148. [DOI] [PubMed] [Google Scholar]
- 45. Sam-Agudu NA, Ramadhani HO, Isah C et al. . The impact of structured mentor mother programs on 6-month postpartum retention and viral suppression among HIV-positive women in rural Nigeria: a prospective paired cohort study. J Acquir Immune Defic Syndr 2017; 75: S173– S181. [DOI] [PubMed] [Google Scholar]
- 46. Geldsetzer P, Yapa HM, Vaikath M et al. . A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc 2016; 19: 20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nance N, Pendo P, Masanja J et al. . Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in Tanzania to improve treatment adherence and retention in care: a cluster-randomized trial. PLoS One 2017; 12: e0181919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sibanda EL, Weller IVD, Hakim JG, Cowan FM.. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS 2013; 27: 2787– 2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aliyu MH, Blevins M, Audet CM et al. . Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV 2016; 3: e202– 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Audet CM, Blevins M, Chire YM et al. . Engagement of men in antenatal care services: increased HIV testing and treatment uptake in a community participatory action program in Mozambique. AIDS Behav 2016; 20: 2090– 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takah NF, Kennedy ITR, Johnman C.. The impact of approaches in improving male partner involvement in the prevention of mother-to-child transmission of HIV on the uptake of maternal antiretroviral therapy among HIV-seropositive pregnant women in sub-Saharan Africa: a systematic review and meta-analysis. BMJ Open 2017; 7: e018207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yotebieng M, Thirumurthy H, Moracco KE et al. . Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV 2016; 3: e85– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sarko KA, Blevins M, Ahonkhai AA et al. . HIV status disclosure, facility-based delivery and postpartum retention of mothers in a prevention clinical trial in rural Nigeria. Int Health 2017; 9: 243– 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Myer L, Phillips TK, Zerbe A et al. . Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Med 2018; 15: e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Washington S, Owuor K, Turan JM et al. . Implementation and operational research: effect of integration of HIV care and treatment into antenatal care clinics on mother-to-child hiv transmission and maternal outcomes in Nyanza, Kenya: results From the SHAIP cluster randomized controlled trial. J Acquir Immune Defic Syndr 2015; 69: e164– 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fayorsey RN, Chege D, Wang C et al. . Mother Infant Retention for Health (MIR4Health): study design, adaptations, and challenges with PMTCT implementation science research. J Acquir Immune Defic Syndr 2016; 72 Suppl 2: S137– 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yotebieng M, Behets F, Kawende B et al. . Continuous quality improvement interventions to improve long-term outcomes of antiretroviral therapy in women who initiated therapy during pregnancy or breastfeeding in the Democratic Republic of Congo: design of an open-label, parallel, group randomized trial. BMC Health Serv Res 2017; 17: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Myer L, Iyun V, Zerbe A et al. . Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: a cohort study. J Int AIDS Soc 2017; 20: 21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li N, Sando MM, Spiegelman D et al. . Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis 2016; 213: 1057– 1064. [DOI] [PubMed] [Google Scholar]
- 60. Uthman OA, Nachega JB, Anderson J et al. . Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017; 4: e21– e30. [DOI] [PubMed] [Google Scholar]
- 61. Hill A, Clayden P, Thorne C et al. . Safety and pharmacokinetics of dolutegravir in HIV-positive pregnant women: a systematic review. J Virus Erad 2018; 4: 66– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis NL, Miller WC, Hudgens MG et al. . Maternal and breastmilk viral load: impacts of adherence on peripartum HIV infections averted: the Breastfeeding, Antiretrovirals, and Nutrition Study. J Acquir Immune Defic Syndr 2016; 73: 572– 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lecher S, Williams J, Fonjungo PN et al. . Progress with scale-up of HIV viral load monitoring - seven sub-Saharan African countries, January 2015–June 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1332– 1335. [DOI] [PubMed] [Google Scholar]
- 64. Myer L, Essajee S, Broyles LN et al. . Pregnant and breastfeeding women: a priority population for HIV viral load monitoring. PLoS Med 2017; 14: e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. Available at: www.who.int/hiv/pub/arv/arv-2016/en ( accessed September 2018). [PubMed] [Google Scholar]
- 66. Ngarina M, Kilewo C, Karlsson K et al. . Virologic and immunologic failure, drug resistance and mortality during the first 24 months postpartum among HIV-infected women initiated on antiretroviral therapy for life in the Mitra plus Study, Dar es Salaam, Tanzania. 2015; 15: 175. [DOI] [PMC free article] [PubMed]
- 67. Haas AD, Msukwa MT, Egger M et al. . Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi's Option B+ Program. Clin Infect Dis 2016; 63: 1227– 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Henegar CE, Westreich DJ, Maskew M et al. . Effect of pregnancy and the postpartum period on adherence to antiretroviral therapy among HIV-infected women established on treatment. J Acquir Immune Defic Syndr 2015; 68: 477– 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nachega JB, Uthman OA, Anderson J et al. . Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS 2012; 26: 2039– 2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sam-Agudu NA, Ramadhani HO, Isah C et al. . The Impact of Structured Mentor Mother Programs on 6-Month Postpartum Retention and Viral Suppression among HIV-Positive Women in Rural Nigeria: A Prospective Paired Cohort Study. J Acquir Immune Defic Syndr 2017; 75 Suppl 2: S173- S181. [DOI] [PubMed] [Google Scholar]
- 71. Cohan D, Natureeba P, Koss CA et al. . Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS 2015; 29: 183– 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koss CA, Natureeba P, Kwarisiima D et al. . Viral Suppression and retention in care up to 5 years after initiation of lifelong ART during pregnancy (Option B plus ) in rural Uganda. J Acquir Immune Defic Syndr 2017; 74: 279– 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chagomerana MB, Miller WC, Tang JH et al. . Optimizing prevention of HIV mother to child transmission: duration of antiretroviral therapy and viral suppression at delivery among pregnant Malawian women. PLoS One 2018; 13: e0195033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gill MM, Hoffman HJ, Bobrow EA et al. . Detectable viral load in late pregnancy among women in the Rwanda Option B+ PMTCT Program: enrollment results from the Kabeho study. PLoS One 2016; 11: e0168671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hosseinipour M, Nelson JAE, Trapence C et al. . Viral suppression and HIV drug resistance at 6 months among women in Malawi's Option B+ program: results from the PURE Malawi study. J Acquir Immune Defic Syndr 2017; 75 ( Suppl 2): S149– S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Myer L, Phillips TK, McIntyre JA et al. . HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med 2017; 18: 80- 88. [DOI] [PubMed] [Google Scholar]
- 77. Mate KS, Ngubane G, Barker PM.. A quality improvement model for the rapid scale-up of a program to prevent mother-to-child HIV transmission in South Africa. Int J Qual Health Care 2013; 25: 373– 380. [DOI] [PubMed] [Google Scholar]
- 78. Bhardwaj S, Barron P, Pillay Y et al. . Elimination of mother-to-child transmission of HIV in South Africa: rapid scale-up using quality improvement. S Afr Med J 2014; 104: 239– 243. [DOI] [PubMed] [Google Scholar]
- 79. Koss CA, Natureeba P, Nyafwono D et al. . Brief report: food insufficiency is associated with lack of sustained viral suppression among HIV-infected pregnant and breastfeeding Ugandan women. J Acquir Immune Defic Syndr 2016; 71: 310– 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Denoeud-Ndam L, Fourcade C, Ogouyemi-Hounto A et al. . Predictive factors of plasma HIV suppression during pregnancy: a prospective cohort study in Benin. PLoS One 2013; 8: e59446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. El-Khatib Z, Ekstrom AM, Coovadia A et al. . Adherence and virologic suppression during the first 24 weeks on antiretroviral therapy among women in Johannesburg, South Africa - a prospective cohort study. BMC Public Health 2011; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Koss CA, Natureeba P, Mwesigwa J et al. . Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 2015; 29: 825– 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hoffmann CJ, Cohn S, Mashabela F et al. . Treatment failure, drug resistance, and CD4 T-cell count decline among postpartum women on antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2016; 71: 31– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shapiro RL, Hughes MD, Ogwu A et al. . Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010; 362: 2282– 2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Odeny TA, Onono M, Owuor K et al. . Maximizing adherence and retention for women living with HIV and their infants in Kenya (MOTIVATE! study): study protocol for a randomized controlled trial. Trials 2018; 19: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rollins NC, Essajee SM, Bellare N et al. . Improving retention in care among pregnant women and mothers living with HIV: lessons from INSPIRE and implications for future WHO guidance and monitoring. J Acquir Immune Defic Syndr 2017; 75 Suppl 2: S111– S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Violari A, Cotton MF, Gibb DM et al. . Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359: 2233– 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Diallo K, Kim AA, Lecher S et al. . Early diagnosis of HIV Infection in infants: one Caribbean and six sub-Saharan African countries, 2011–2015. MMWR Morb Mortal Wkly Rep 2016; 65: 1285– 1290. [DOI] [PubMed] [Google Scholar]
- 89. UNAIDS Countdown to zero: global plan towards the elimination of new hiv infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland: 2011. Available at: www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en.pdf ( accessed September 2018). [Google Scholar]
- 90. Ciaranello AL, Park JE, Ramirez-Avila L et al. . Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med 2011; 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Technau KG, Mazanderani AH, Kuhn L et al. . Prevalence and outcomes of HIV-1 diagnostic challenges during universal birth testing: an urban South African observational cohort. J Int AIDS Soc 2017; 20: 21761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jean-Philippe P, Spiegel H, Gnanashanmugam D et al. . HIV birth testing and linkage to care for HIV-infected infants. AIDS 2017; 31: 1797– 1807. [DOI] [PubMed] [Google Scholar]
- 93. Jani IV, Meggi B, Mabunda N et al. . Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr 2014; 67: e1– 4. [DOI] [PubMed] [Google Scholar]
- 94. Diallo K, Modi S, Hurlston M et al. . A proposed framework for the implementation of early infant diagnosis point-of-care. AIDS Res Hum Retroviruses 2017; 33: 203– 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Finocchario-Kessler S, Odera I, Okoth V et al. . Lessons learned from implementing the HIV infant tracking system (HITSystem): A web-based intervention to improve early infant diagnosis in Kenya. Healthc (Amst) 2015; 3: 190– 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Odeny TA, Bukusi EA, Cohen CR et al. . Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS 2014; 28: 2307– 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Finocchario-Kessler S, Gautney BJ, Khamadi S et al. . If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS 2014; 28 Suppl 3: S313– 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sam-Agudu NA, Cornelius LJ, Okundaye JN et al. . The impact of mentor mother programs on PMTCT service uptake and retention-in-care at primary health care facilities in Nigeria: a prospective cohort study (MoMent Nigeria). J Acquir Immune Defic Syndr 2014; 67 Suppl 2: S132– 138. [DOI] [PubMed] [Google Scholar]
- 99. McColl K. Mentor mothers to prevent mother-to-child transmission of HIV. BMJ 2012; 344. [DOI] [PubMed] [Google Scholar]
- 100. Rotheram-Borus MJ, le Roux IM, Tomlinson M et al. . Philani Plus (+): a Mentor Mother community health worker home visiting program to improve maternal and infants’ outcomes. Prev Sci 2011; 12: 372– 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim MH, Ahmed S, Buck WC et al. . The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc 2012; 15 Suppl 2: 17389. [DOI] [PMC free article] [PubMed] [Google Scholar]