Abstract
In 2015, the World Health Organization (WHO) recommended that all people living with HIV (PLWH) should start antiretroviral therapy (ART) irrespective of clinical or immune status. This recommendation followed almost 20 years of research into the clinical and population-level benefits and risks of starting ART early compared with deferring treatment. This article summarises the ways in which observational data support the work of WHO, including the support provided by the International epidemiology Databases to Evaluate AIDS (IeDEA), taking the example of ‘treat all’.
Introduction
In 2015, the World Health Organization (WHO) recommended that all people living with HIV (PLWH) should start antiretroviral therapy (ART) irrespective of clinical or immune status [1]. This recommendation followed almost 20 years of research into the clinical and population-level benefits and risks of starting ART early compared with deferring treatment [2].
The WHO ‘treat all’ recommendation was supported by evidence from randomised trials showing significant clinical benefits and a reduced risk of HIV transmission following immediate ART initiation [3-5]. The randomised trials confirmed an association that was reported by prior observational studies [6-9]; however, observational data alone were insufficient for the WHO guidelines panel to make a ‘treat all’ recommendation when this question was first assessed in 2013. Nevertheless, the observational evidence helped to strengthen the rationale for this recommendation. Observational studies have also provided important additional evidence supporting the feasibility of implementing the ‘treat all’ approach, and these studies continue to generate insights into the challenges and benefits of a treat-all policy across different settings and populations.
This article summarises the ways in which observational data support the work of WHO, including the support provided by the International epidemiology Databases to Evaluate AIDS (IeDEA) [10], taking the example of ‘treat all’ (see Table 1).
Table 1.
Analysis of data from the International epidemiology Databases to Evaluate AIDS (IeDEA) to inform WHO guidelines
| Guideline | Evidence contributed | Analyses performed |
|---|---|---|
| WHO 2010 ART guidelines for HIV infection in infants and children | Definition of immunological failure in children on ART | Risk of mortality associated with different ages and CD4 values in children on ART [11] |
| WHO 2013 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection | Support for ART for all children aged
<5 years irrespective of disease severity |
Causal modelling of observational data comparing mortality with immediate versus deferred ART in children aged 1–5 years [12] |
| WHO 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection | Support for ART for all children aged
<15 years irrespective of disease severity |
Causal modelling of observational data comparing mortality and growth with immediate versus deferred ART in children aged 1–15 [13] |
| WHO 2017 guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy | Definition of burden of advanced HIV disease in adults and children | Proportion of adults in IeDEA and COHERE collaborations presenting with advanced HIV disease; proportion of children aged<5 years in IeDEA-SA presenting with advanced HIV disease [14,15] |
Role of observational data in WHO guidelines
The development of high-quality guidelines relies on a systematic review of the evidence and an appraisal of the certainty of the evidence. Guideline development processes have widely adopted the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework [16], which, following a longstanding approach to ranking evidence [17], rates randomised trials as generally providing high-quality evidence for questions of intervention effect, and observational studies as providing low-quality evidence (in certain exceptional situations observational studies can be considered to provide evidence of high quality [18]).
WHO adopted the GRADE approach in 2008, following public criticism that many WHO guidelines at the time relied too heavily or exclusively on expert opinion [19]. In contrast to clinical practice guidelines, WHO guidelines aim to make recommendations from a public health perspective, and are thus primarily intended for ministries of health and programme managers in low- and middle-income settings, rather than individual clinicians. As such, the formulation of WHO recommendations relies not only on information about comparative effectiveness and harms, but also considerations about the feasibility, acceptability and resource requirements for implementing a given intervention or set of interventions as well as complex interventions. While randomised trials remain the gold standard study design for assessing efficacy and safety of clinical interventions, observational studies are often a better way – and in some cases the only way – to assess intervention effectiveness in routine settings.
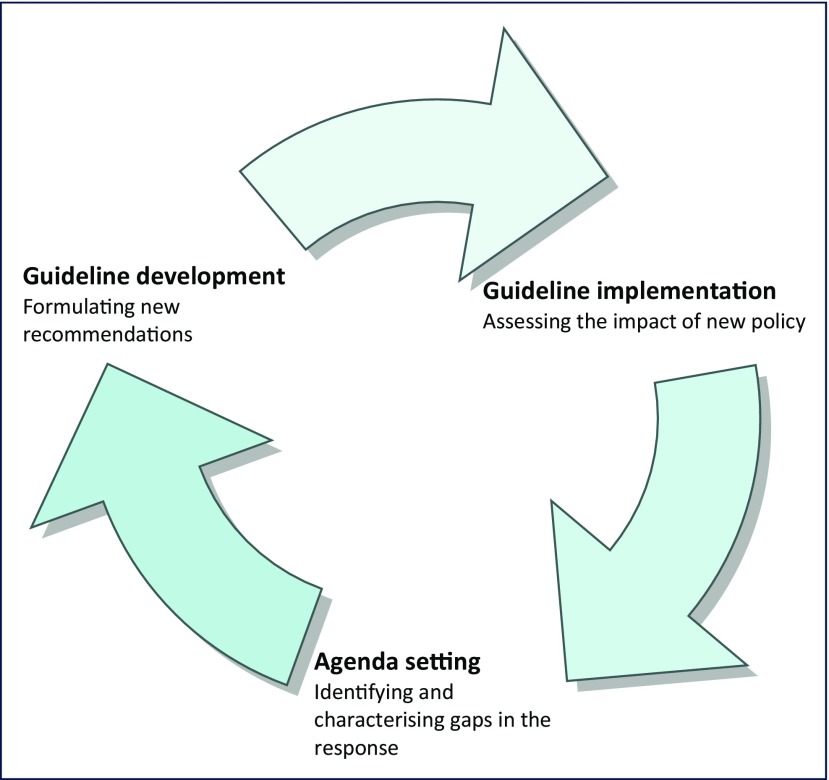
WHO also has a responsibility to evaluate the uptake and impact of the recommendations it makes, and these evaluations rely on observational research from implementation in routine programmes. Lessons from these studies serve to highlight challenges in implementation, which in turn can inform priorities for future guidance (Figure 1).
Figure 1.
The contribution of observational data to guideline development at WHO
From treating the sickest to ‘treat all’: formulating recommendations
WHO first considered making a recommendation to start ART in all people living with HIV irrespective of clinical or immune status in 2013. At the time, there were no available data from randomised trials with respect to clinical benefit, and guideline deliberations were primarily informed by observational data and mathematical modelling. The 2013 WHO Guideline Development Group concluded that there was insufficient evidence to recommend ‘treat all’, and WHO instead recommended that the CD4 cell count threshold for stating ART be raised from ≤350 cells/mm3 to ≤500 cells/mm3 [20].
Randomised controlled trial evidence was available demonstrating the benefit of providing immediate treatment in the context of serodiscordant partnerships to reduce HIV transmission [5], and this recommendation was included in the WHO 2013 guidelines.
For pregnant women with HIV, a recommendation was made in favour of starting ART irrespective of CD4 cell count (PMTCT Option B+) [21]. This recommendation, aimed at increasing ART uptake among pregnant women, was based on a recognition of the need to simplify ART provision during pregnancy and breastfeeding, and to avoid delays in starting ART in pregnant women in settings where CD4 cell count testing was not available or where waiting for results could result in missed opportunities to prevent vertical transmission. Evidence supporting the benefits of this approach came from observational studies that provided outcomes from programmes implementing Option B+; these studies all found that uptake of ART was more timely, and that women experienced health benefits in terms of immunological and clinical parameters [22–24].
Similarly, ART initiation for all children aged under 5 years was recommended to address the low treatment coverage in children; however, at the time, this recommendation was made in the absence of randomised controlled trial evidence of clinical benefit, and primarily on the basis of observed rapid immunological decline in the absence of ART as well as causal modelling analysis of observational data from Southern Africa [13].
While several observational studies also suggested a clinical benefit to providing lifelong ART as soon as possible following an HIV diagnosis [9,25], the WHO recommendation was only made 2 years later, once data from the START and TEMPRANO randomised trials became available [3,4]. However, these trials did not include children, and the ‘treat all’ recommendation across all age groups, including children, was supported by observational data (17 cohort studies) and mathematical modelling [26,27] (Table 1).
The WHO recommendation to treat all people living with HIV also raised questions regarding how quickly ART should be initiated following confirmation of HIV diagnosis. Removing the need to have the results of clinical or laboratory assessments on hand prior to starting ART opens up the possibility to start ART on the same day that an HIV diagnosis is confirmed. In 2017, WHO recommended that ART should be offered within 7 days following a confirmed HIV diagnosis, including the offer of initiating ART on the same day as diagnosis [28]; this recommendation was informed by data from four randomised trials and 11 observational studies [29].
Benefits and challenges of ‘treat all’: assessing implementation
The clinical benefits of ‘treat all’ are no longer disputed, and this recommendation has been adopted by almost all countries worldwide [30]. Questions remain, however, regarding the feasibility of implementation and the extent to which the benefits seen in clinical trials will be realised in routine programme settings [31].
Drawing on both IeDEA data and information retrospectively gathered on the nature and timing of country-specific ART guideline expansion, a recently published analysis from the IeDEA–WHO Collaboration [32] found that ART guideline expansion supporting earlier ART initiation is associated with increased and more timely uptake of ART [33]. This analysis further showed that these improvements did not come at the expense of crowding out sicker patients. This analysis has recently been updated to include settings that have implemented ‘treat all’ and concluded that the greatest improvements in timeliness of ART initiation under successive guideline expansions that included expansion to ‘treat all’ occurred in low-income countries, likely to be due to the simplification of initiation decisions, i.e. opening the possibility to initiate treatment while waiting for baseline CD4 cell count test results. Young people aged 15–25 years also benefited from more timely ART initiation under ‘treat all’, as CD4 cell count-based guidelines disadvantaged this group of patients, who were likely to have been recently infected and therefore more likely to have higher CD4 cell counts.
Experience of implementing ‘treat all’ for pregnant and breastfeeding women (Option B+) has found that, while the approach is feasible across a variety of settings, there is a need to ensure adequate retention in care and medication adherence, particularly during the first year following ART initiation [34]. This may also be a concern for anyone starting ART at higher CD4 cell counts, although the evidence so far is mixed [35,36].
Implementation of recommendations for rapid ART initiation has also been informed by observational data. While the results of several randomised trials all favoured rapid ART initiation, in particular by reducing the risk of loss to follow-up before ART initiation, some observational studies reported increased losses to follow-up after ART initiation. This suggests that different approaches to adherence counselling after starting ART may be needed when ART is started rapidly, as people are still coming to terms with their diagnosis. A recent study by the IeDEA collaboration in Rwanda suggested that patients enrolling in care at sites conducting fewer ART readiness counselling sessions initiated ART more rapidly and had better retention 6 months post initiation [37].
Identifying gaps in policy and practice
Observational cohorts provide valuable insights into the programmatic impact of ART scale-up and, in doing so, can reveal gaps in the response that are a priority for future intervention research and policy guidance.
Several studies from the IeDEA collaboration have highlighted the fact that men with HIV have worse outcomes compared to women with HIV [38,39], and this has contributed to a recognition of the need to identify models of care to improve uptake and outcomes for men [40].
The enduring burden of advanced HIV disease is another challenge that has been highlighted through observational research. Successive studies by the IeDEA collaboration have shown that, despite major progress in ART scale-up, an important proportion of patients continues to present late for care, with advanced HIV disease [14,41,42]. This work directly contributed to the development of WHO guidance on the management of advanced HIV disease in 2017 [28], and continues to drive discussions about how to best promote earlier diagnosis and linkage to care globally.
Rapid introduction of new antiretrovirals for which limited experience has been gathered outside the setting of randomised clinical trials requires increasing attention to longer-term monitoring of treatment outcomes and toxicity profiles across populations. As countries strengthen their pharmaco-vigilance systems to enable active monitoring and high-quality surveillance, cohort collaborations such as IeDEA can play a critical role in addressing this important evidence gap.
Future research will improve our understanding of the challenges faced in implementing the ‘treat all’ policy, in particular whether there are differences in adherence, retention, viral suppression and viral resistance among people starting ART without having developed clinical disease, and the possible need for differential adherence support for different patient populations. Indeed, observational research has the advantage of capturing the experience and patient outcomes that occur outside the controlled environments of research protocols and are critical for policies and guidelines, including large populations of persons who are not typically recruited into, or represented, in randomised trials, but are none the less differentially impacted by the HIV epidemic, such as children, pregnant women, persons with TB, persons with mental health and substance use disorders, and marginalised populations. Observational cohorts, such as IeDEA, also have the advantage of scale and the ability to examine implementation and health outcomes in a variety of diverse settings and care delivery contexts.
Conclusions
Evidence from observational cohorts has made a central contribution to the development of WHO guidelines, and will continue to do so. Observational data can be especially critical for groups of people who have not been enrolled in randomised trials in addressing a given implementation question, for example, pregnant women and children. Randomised trials are also not generally well suited for assessing rare harms (owing to limited sample sizes and rigorous exclusion criteria), which often only become apparent when a drug is being rolled out. In addition, observational studies provide valuable insights into the feasibility and implementation challenges associated with a given intervention or set of interventions. Finally, increasing attention is being paid to the need to evaluate the uptake and impact of WHO guidelines on critical health outcomes in countries (Figure 1). Observational studies are well suited to evaluating the impact of policy change in routine practice.
The continued contribution of observational data to shaping the response to HIV depends on continued investment in data systems by national programmes and international donors. It has been recommended that 5–10% of all HIV programme budgets be directed towards data collection and use [43]. Sustained investment in the generation and analysis of observational data will make an important contribution to programme performance, as well as helping to inform the global response.
Approaches to data interpretation are being continuously updated to improve the reliability of evidence from observational research. Collaboration between cohorts across different countries can enhance the comparability of findings and their generalisability (provided all findings point in the same direction) or point towards important sources of heterogeneity (if they do not). Advances in statistical software have increased the usage of tools such as multiple imputation to analyse incomplete datasets. Increased use of design and analytical approaches, such as regression discontinuity, difference-in-difference, g-estimation and propensity score have helped achieve more analytical rigour for assessing causal associations and impact, provided the most critical confounders have been directly or indirectly controlled.
The IeDEA–WHO partnership [32] is an example of an effective collaboration that can provide valuable insights into whether WHO guidelines are making a difference to outcomes for people living with HIV, and guide how future IeDEA analyses can have more policy relevance. The potential to expand this collaboration to cover other disease areas should be explored.
Acknowledgments
Funding
The International Epidemiology Databases to Evaluate AIDS (IeDEA) is supported by the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse: Asia-Pacific, U01AI069907; CCASAnet, U01AI069923; Central Africa, U01AI096299; East Africa, U01AI069911; NA-ACCORD, U01AI069918; Southern Africa, U01AI069924; West Africa, U01AI069919. This work is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Acknowledgements
Conflicts of interest
The authors declare no conflicts of interests.
References
- 1. World Health Organization 2015. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Available at: www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ ( accessed August 2018). [PubMed]
- 2. Eholie SP, Badje A, Kouame GM et al. . Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther 2016; 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Insight Start Study Group, . Lundgren JD, Babiker AG et al. . Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373: 795– 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Group TAS, Danel C, Moh R et al. . A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med 2015; 373: 808– 822. [DOI] [PubMed] [Google Scholar]
- 5. Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493– 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. HIV-Causal Collaboration , Ray M, Logan R, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24: 123– 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le T, Wright EJ, Smith DM et al. . Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368: 218– 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okulicz JF, Le TD, Agan BK et al. . Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med 2015; 175: 88– 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitahata MM, Gange SJ, Abraham AG et al. . Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360: 1815– 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egger M, Ekouevi DK, Williams C et al. . Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41: 1256– 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies M, Bolton-Moore C, Eley B et al. . Predicting 1-year mortality using current CD4 percent and count in order to guide switching therapy in children on ART in Southern Africa. 3rd International Workshop on HIV Pediatrics. Rome, Italy, 2012. [Google Scholar]
- 12. Schomaker M, Egger M, Ndirangu J et al. . When to start antiretroviral therapy in children aged 2–5 years: a collaborative causal modelling analysis of cohort studies from southern Africa. PLoS Med 2013; 10: e1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schomaker M, Leroy V, Wolfs T et al. . Optimal timing of antiretroviral treatment initiation in HIV-positive children and adolescents: a multiregional analysis from Southern Africa, West Africa and Europe. Int J Epidemiol 2017; 46: 453– 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. IeDea, Cohere Cohort Collaborations Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66: 893– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies MA, Phiri S, Wood R et al. . Temporal trends in the characteristics of children at antiretroviral therapy initiation in southern Africa: the IeDEA-SA Collaboration. PLoS One 2013; 8: e81037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guyatt G, Oxman AD, Akl EA et al. . GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383– 394. [DOI] [PubMed] [Google Scholar]
- 17. Rawlins M. De testimonio: on the evidence for decisions about the use of therapeutic interventions. Lancet 2008; 372: 2152– 2161. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Sultan S et al. . GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011; 64: 1311– 1316. [DOI] [PubMed] [Google Scholar]
- 19. Oxman AD, Lavis JN, Fretheim A.. Use of evidence in WHO recommendations. Lancet 2007; 369: 1883– 1889. [DOI] [PubMed] [Google Scholar]
- 20. Ford N, Vitoria M, Doherty M.. Providing antiretroviral therapy to all who are HIV positive: the clinical, public health and programmatic benefits of Treat All. J Int AIDS Soc 2018; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available at: www.who.int/hiv/pub/guidelines/arv2013/en/ ( accessed August 2018). [PubMed]
- 22. Kim MH, Ahmed S, Hosseinipour MC et al. . Implementation and operational research: the impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr 2015; 68: e77– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamuyango AA, Hirschhorn LR, Wang W et al. . One-year outcomes of women started on antiretroviral therapy during pregnancy before and after the implementation of Option B+ in Malawi: a retrospective chart review. World J AIDS 2014; 4: 332– 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koss CA, Natureeba P, Kwarisiima D et al. . Viral suppression and retention in care up to 5 years after initiation of lifelong ART during pregnancy (Option B+) in rural Uganda. J Acquir Immune Defic Syndr 2017; 74: 279– 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lodi S, Phillips A, Logan R et al. . Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV 2015; 2: e335– 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schomaker M, Davies MA, Malateste K et al. . Growth and mortality outcomes for different antiretroviral therapy initiation criteria in children ages 1–5 years: a causal modeling analysis. Epidemiology 2016; 27: 237– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egger M, Johnson L, Althaus C et al. . Developing WHO guidelines: Time to formally include evidence from mathematical modelling studies. F1000Res 2017; 6: 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization 2017. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Available at: www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/ ( accessed August 2018). [PubMed]
- 29. Ford N, Migone C, Calmy A et al. . Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018; 32: 17– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ford N, Ball A, Baggaley R et al. . The WHO public health approach to HIV treatment and care: looking back and looking ahead. Lancet Infect Dis 2018; 18: e76– e86. [DOI] [PubMed] [Google Scholar]
- 31. Schechter M. Prioritization of antiretroviral therapy in patients with high CD4 counts, and retention in care: lessons from the START and Temprano trials. J Int AIDS Soc 2018; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zaniewski E, Tymejczyk O, Kariminia A et al. . IeDEA–WHO research-policy collaboration: contributing real-world evidence to HIV progress reporting and guideline development. J Virus Erad 2018; 4 ( Suppl 2): 9– 15. [PMC free article] [PubMed] [Google Scholar]
- 33. Tymejczyk O, Brazier E, Yiannoutsos C et al. . HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med 2018; 15: e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tenthani L, Haas AD, Tweya H et al. . Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS 2014; 28: 589– 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bock P, James A, Nikuze A et al. . Baseline CD4 count and adherence to antiretroviral therapy: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2016; 73: 514– 521. [DOI] [PubMed] [Google Scholar]
- 36. Bor J, Fox MP, Rosen S et al. . Treatment eligibility and retention in clinical HIV care: a regression discontinuity study in South Africa. PLoS Med 2017; 14: e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ross J, et al. for the Central Africa IeDEA Collaboration Early outcomes after implementation of treat all in Rwanda: an observational cohort study. In process.
- 38. Cornell M, Myer L, Kaplan R et al. . The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health 2009; 14: 722– 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cornell M, Schomaker M, Garone DB et al. . Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med 2012; 9: e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. UNAIDS 2017. Addressing a blind spot in the response to HIV — reaching out to men and boys. Available at: www.unaids.org/en/resources/documents/2017/blind_spot ( accessed August 2018).
- 41. IeDea ART. Cohort Collaborations , Avila D, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2014; 65: e8– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koller M, Patel K, Chi BH et al. . Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2015; 68: 62– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Low-Beer D, Mahy M, Renaud F, Calleja T.. Sustainable monitoring and surveillance systems to improve HIV programs: review. JMIR Public Health Surveill 2018; 4: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]