Abstract
Background
Early clinical severity assessments during the 2009 influenza A H1N1 pandemic (pH1N1) overestimated clinical severity due to selection bias and other factors. We retrospectively investigated how to use data from the International Network for Strategic Initiatives in Global HIV Trials, a global clinical influenza research network, to make more accurate case fatality ratio (CFR) estimates early in a future pandemic, an essential part of pandemic response.
Methods
We estimated the CFR of medically attended influenza (CFRMA) as the product of probability of hospitalization given confirmed outpatient influenza and the probability of death given hospitalization with confirmed influenza for the pandemic (2009–2011) and post-pandemic (2012–2015) periods. We used literature survey results on health-seeking behavior to convert that estimate to CFR among all infected persons (CFRAR).
Results
During the pandemic period, 5.0% (3.1%–6.9%) of 561 pH1N1-positive outpatients were hospitalized. Of 282 pH1N1-positive inpatients, 8.5% (5.7%–12.6%) died. CFRMA for pH1N1 was 0.4% (0.2%–0.6%) in the pandemic period 2009–2011 but declined 5-fold in young adults during the post-pandemic period compared to the level of seasonal influenza in the post-pandemic period 2012–2015. CFR for influenza-negative patients did not change over time. We estimated the 2009 pandemic CFRAR to be 0.025%, 16-fold lower than CFRMA.
Conclusions
Data from a clinical research network yielded accurate pandemic severity estimates, including increased severity among younger people. Going forward, clinical research networks with a global presence and standardized protocols would substantially aid rapid assessment of clinical severity.
Clinical Trials Registration
NCT01056354 and NCT010561.
Keywords: severity, pandemic influenza, case fatality ratio, clinical research, pandemic preparedness
We demonstrate how to use baseline and prospective data from global clinical research networks to rapidly assess the severity of an emerging influenza pandemic.
In 2009, uncertainty about the emerging pH1N1 virus’ clinical severity hindered the early global response. Although the rapid spread of the virus around the world fulfilled the traditional pandemic definition, its global mortality impact in the end proved to be smaller than any 20th century pandemic [1, 2]. However, its relative mildness was not known in the early months of the outbreak. The earliest estimate of the case fatality ratio (CFR) was on par with the rating for the catastrophic 1918 pandemic, and a June 2009 assessment put it in the 1957 pandemic range (Table 1)[3].
Table 1.
Evolution of the Estimated Case Fatality Ratio Over Time
| Report | Date of Publication | Setting | Estimated Case Fatality Ratio (%) | Severity |
|---|---|---|---|---|
| World Health Organization report [9] | May 2009 | Early outbreaks Mexico | 2 | 1918-like |
| Fraser et al [10] | June 2009 | First wave Mexico | 0.4 | 1957-like |
| Castro-Jiménez et al [11] | July 2009 | First wave Colombia | 3.8 | 1918-like |
| Baker et al [12] | July 2009 | New Zealand first complete season | 0.1 | 1968-like |
| Presanis et al [13] | September 2009 | First wave in 2 US cities | 0.04 | 1968-like |
| Van Kerkhove et al [14] | January 2013 | Global estimate for first season, CONCISE Network | 0.02 | Seasonal |
See also Wong et al [3].
An evaluation of the 2009 pandemic response ordered by the World Health Organization’s (WHO) Director General [4] found that a systematic way to assess both transmissibility and clinical severity—also known as its “seriousness” [5]—is needed in the early phase of a future pandemic to assess the level of threat accurately and to mobilize resources appropriately. CFR is one important measure of clinical severity; others include the risk of admission to the intensive care unit (ICU) and the need for mechanical respiratory support. A WHO task force is currently developing the data inputs and study designs needed to generate timely estimates of clinical severity [6]. The Centers for Disease Control and Prevention has proposed a scheme for comparing pandemic and seasonal influenza graphically, plotting attack rates against clinical severity [7].
In 2009, UK Public Health England spearheaded what has become a standard first-line approach to assessing the clinical severity of a pandemic, known as the “First Few Hundred” (FF100) [8]. These and similar studies gather data on the earliest cases that come to medical attention through outpatient facilities and hospitals and provide important descriptive data about symptoms, risk factors, and risk of progression to severe illness or death [15–21]. These data can in turn be combined with other data on population attack rates to forecast national and global hospitalization and mortality estimates using a pyramid modeling strategy [13, 22].
Standard FF100 studies, however, lack historic controls in the form of a baseline from recent seasonal influenza seasons. They are also subject to selection bias, as the first cases that come to attention are likely to be more severe [23]. Unless an FF100 study is set in an existing surveillance system or ongoing clinical research data collection scheme, there is no obvious seasonal influenza baseline against which to compare the clinical severity of the pandemic virus. Moreover, unless the pandemic is severe, an FF100 study in the outpatient setting alone will not have the statistical power to accurately estimate the CFR unless many thousands of patients are enrolled.
Global clinical research networks that study mild and severely ill influenza patients could be used to overcome many of these problems. Two ongoing clinical cohort studies of influenza are conducted under the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) umbrella, sponsored by the National Institutes of Health. Since 2009, INSIGHT has undertaken 2 cohort studies—1 outpatient (FLU002) and 1 inpatient (FLU003)—specifically to address gaps in clinical research on the emerging influenza pandemic, including factors linked to disease progression and severe outcomes [24]. INSIGHT annually enrolls hundreds of patients with suspected or confirmed influenza, with intake sites in 12 countries. At these sites, experienced teams use a standardized protocol to collect extensive clinical data, perform long-term follow-up (at 28 and 60 days for inpatients, 14 days for outpatients), and bank patient samples for further study. Several articles on influenza have been published using INSIGHT data, including protocol descriptions and preliminary data [24], an exploration of biomarkers of influenza case severity [25], patient outcomes after pH1N1 infection [26], and phylogeography of the pH1N1 virus [27].
We used INSIGHT data collected in the pandemic period (2009–2011) to retrospectively demonstrate how clinical research networks can provide essential early insights into pandemic clinical severity and other epidemiological parameters. To “leverage” the CFR computation, we multiplied the conditional probability of progression from outpatient to hospitalization by that of progression from hospitalization to death. To underscore the importance of having baseline data, we compared the estimated pH1N1 clinical severity to that of seasonal influenza types and subtypes and noninfluenza respiratory patients in the post-pandemic period (2012–2015). Our CFR estimates were in reasonable agreement with final global CFR estimates based on excess mortality estimates from time series of nationwide vital statistics data and seroepidemiology data—final estimates of a type that would only be available several years after the next pandemic emerges [1, 2, 16]. Here, we discuss what it would take to move a clinical research network like INSIGHT from routine research operation into emergency mode to generate timely and robust clinical severity assessments.
METHODS
INSIGHT FLU002 and FLU003 protocols
The National Institute of Allergy and Infectious Diseases (NIAID)–funded INSIGHT network initially focused solely on HIV but expanded first to include pH1N1 and then all influenza types and subtypes and emerging respiratory pathogens such as Middle East respiratory syndrome and severe acute respiratory syndrome. Sites, located in 5 of 6 world regions (Figure 1), consecutively enroll adult patients aged ≥18 years with suspected influenza. FLU002 recruits patients who present at a physician’s office or clinic with influenza-like illness (ILI), defined as fever with either cough or sore throat. FLU003 recruits patients with known or suspected influenza who require hospitalization. At enrollment, patient medical history and demographic information are recorded, and blood and oropharyngeal swabs are analyzed and stored. Testing for influenza is done both locally and at an INSIGHT central laboratory. All patients are followed up, regardless of influenza test result, at 14 days after enrollment in FLU002 and at 28 and 60 days in FLU003.
Figure 1.
Map of International Network for Strategic Initiatives in Global HIV Trials influenza protocol patient intake sites. Blue markers indicate FLU002 outpatient sites and red markers indicate FLU003 inpatient sites.
We extracted INSIGHT data on demographics, illness onset, medical history, and vital status at follow-up visit from the protocol databases. We defined the pandemic period as the first 2 seasons, October 2009 through September 2011, and the post-pandemic influenza period as October 2012 through September 2015 (last 3 complete INSIGHT seasons, skipping the 2011–2012 season as a transition). Patients who were lost to follow-up were treated as missing and removed from the analysis.
We identified 9 relevant case series in the literature reporting data on patients aged >18 years. After excluding studies with fewer than 100 patients or with a specialty population (such as high-risk patients), we chose 2 outpatient studies, 1 set in the United States [28] and 1 in the United Kingdom [8], and 2 inpatient studies [18, 20], both set in the United States, for comparison with FLU002 and FLU003 pH1N1 laboratory-confirmed patients during the pandemic period (Table 2).
Table 2.
Findings on Clinical Symptoms, Demographics, and Underlying Illness from FLU003 and FLU002 Protocols
| Inpatient Studies (Ward and Intensive Care Unit Combined) | Outpatient Studies | ||||||
|---|---|---|---|---|---|---|---|
| Study | Country | United States | Global | United States | United Kingdom | Global | |
| First Author | Jain [18] | Louie [20] | INSIGHT 003 | Dawood [28] | McLean [8] | INSIGHT 002 | |
| (N) adults (unless noted) | 150 | 744 | 282 | 642 (L) | 392 (M) | 559 | |
| Adult median age, y (range) | 41 (18–86) | 39 (18–92) | 48 (19–87) | … | ... | 30 (18–73) | |
| Major symptoms (%) | Fever | 100 | 87 | ... | 94 | 94 | ... |
| Cough or sore throat | 93 | 88 | ... | 92 | 85 | ... | |
| Gastrointestinal symptoms | 26 | 34 | ... | 25 | 28 | ... | |
| Myalgia | 51 | 41 | ... | ... | 80 | ... | |
| Headache | 45 | 22 | ... | ... | 84 | ... | |
| Shortness of breath | 73 | 66 | ... | ... | 44 | ... | |
| Comorbidities (%) | At least 1 comorbidity | 83 | >72 | 55 | 4 | 11 | 16 |
| Pregnant (of women in study) | 11 | 13 | 10 | ... | 1 | 2 | |
| Immunosuppression | 19 | 20 | 11 | 0.4 | 1 | 1 | |
| Human immunodeficiency virus only | ... | 15 | 4 | ... | ... | 8 | |
| Cardiovascular disease | 20 | 19 | 14 | 0.4 | 1.0 | 0.4 | |
| Chronic obstructive pulmonary disease | 15 | 16 | 11 | 2.5 | 8 | 0.7 | |
| Asthma | 27 | 21 | 17 | ||||
| Diabetes | 25 | 15 | 11 | ... | 1.3 | 2 | |
| Other factors (%) | Influenza vaccination | 44 | ... | 23 | ... | 10 | 14 |
| Obesity (BMI >30) | 55 | 58 | 25 | ... | ... | 16 | |
| Morbid obesity (BMI ≥40) | 26 | 25 | 5 | ... | ... | 2 | |
| Smoker (ever) | 24 | ... | 59 | ... | ... | 21 | |
| Progression of illness (%) | Hospitalized | 100 | 100 | 100 | 9 | 6 | 5 |
| Died | 9 | 15 | 9 | 0.5 | 0 | 0.2 | |
| Intensive care unit | 29 | 34 | 26 | 3 | ... | 0.2 | |
| Chest X-ray infiltrate | 39 | 68 | ... | 4 | 0.8 | 0.7 | |
| Mechanical ventilation | 22 | 31 | 22 | 2 | 0.8 | 0.2 | |
| Sepsis | 12 | ... | 6 | ... | ... | 0 | |
| Treatment (%) | Antiviral use | 79 | 81 | 80 | 7 | 92 | 20 |
| Antibiotic use | 82 | ... | 83 | ... | 11 | ... | |
| Corticosteroid use | 39 | ... | 33 | ... | ... | ... | |
Data are for the pandemic period October 2009 through September 2011 and select studies that either presented or allowed extraction of similar findings for adults aged ≥18 years.
Abbreviations: BMI, body mass index; INSIGHT, International Network for Strategic Initiatives in Global HIV Trials.
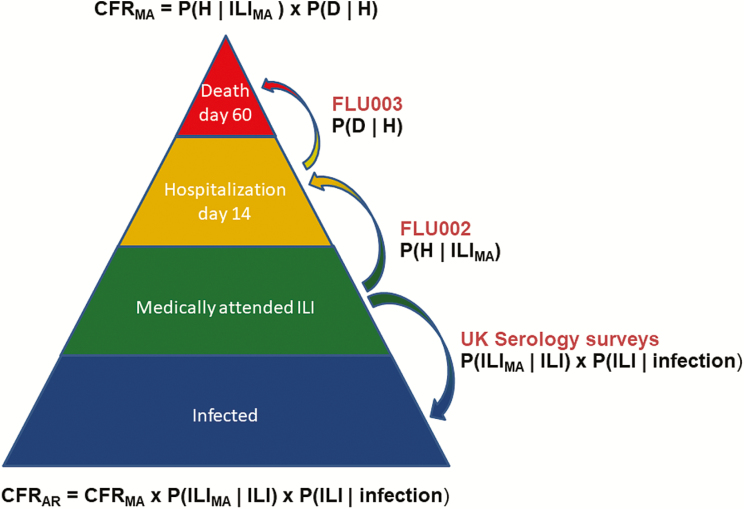
We calculated the medically attended CFR (CFRMA) from the probability that a medically attended ILI (FLU002) patient would progress to hospitalization by day 14 and the probability that a hospitalized (FLU003) patient would die by day 60:
where H = hospitalization and D = death
To estimate CFR among all infected persons (CFRAR), we used findings from a UK health behavior survey that found that 25% of patients aged ≥18 years with ILI sought care for their illness [29] and a UK serology study that found that 25% of influenza-infected adults aged 25–64 years were symptomatic [30]. Assuming that the nonmedically attended and asymptomatic influenza cases would not progress to severe illness, we have:
where “infection” is defined as a person who responded immunologically.
The 95% confidence intervals (CIs) on the CFR estimate were generated from the variance of the product of the 2 proportions, P(H/ILI) × P(D/H), using the delta method or a first-order Taylor series expansion. We assumed the 2 proportions were independent. In small samples with large variability, this may not be a good approximation. In some cases, negative values for the CIs may be obtained.
Data analysis was done using SAS, version 9.4, and Excel. The FLU 002 and FLU 003 protocols were approved by the institutional review boards or institutional ethics committees at the University of Minnesota and at each of the participating clinical sites. All patients (or their proxies) gave signed informed consent prior to enrollment.
RESULTS
Descriptive Comparison of INSIGHT Patient Findings to Findings from Other FF100 Studies
During the pandemic period (October 2009 through September 2011), 559 ILI and 384 hospitalized patients tested influenza pH1N1 positive. Of these, 99.6% of pH1N1-infected FLU002 outpatients were aged 18–64 years compared to only 88% of the FLU003 inpatients. During the post-pandemic period (October 2012 through September 2015), 704 ILI and 245 hospitalized patients were pH1N1 positive; of these, 96% of ILI outpatients and 81% of hospitalized patients were aged 18–64 years. In the pandemic period about 1/2 of outpatients and 2/3 of inpatients were from European sites, while during the post-pandemic period, after the network expanded to sites in 5 world regions, these figures were 1/3 of outpatients and 2/5 of inpatients.
We found that demographic and clinical characteristics of INSIGHT pandemic period pH1N1 patients were similar to those described in published FF100-like studies of adult pH1N1 patients [26] with respect to mean age, prevalence of symptoms and underlying diseases, mortality rates, and other characteristics (Table 2).
CFR Estimates
CFRMA in the Pandemic Period 2009–2011
Five percent of pH1N1-confirmed ILI patients were hospitalized, and 8.7% of pH1N1-positive inpatients died (Table 3, Figure 2). This yielded a pH1N1 CFRMA of 0.4% (0.2%–0.7%) both for all adults and for adults aged 18–64 years. The CFRMA for patients aged ≥65 years could not be established with confidence due to the small number of older outpatients in the study. As a nonhistoric control, the all-ages CFRMA of influenza test–negative patients was 0.1% during the pandemic period, albeit with wide CIs. It was not possible to establish a seasonal influenza comparison for the pandemic period because non-pH1N1 influenza cases (H3N2, B) in the pandemic period were rare.
Table 3.
Estimated Case Fatality Ratio among Medically Attended Cases
| Period | Age | Viral Subtype | N (Outpatient) | N (Inpatient) | P (H|ILI) | P (D|H) | Case Fatality Ratio/% (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|
| Pandemic (2009–2011) | All ages | pH1N1 | 541 | 358 | 0.052 | 0.087 | 0.45 (0.23, 0.67) |
| H3N2 | 273 | 31 | 0.004 | 0.065 | * | ||
| B | 33 | 12 | 0.061 | 0.000 | * | ||
| Negative | 971 | 117 | 0.031 | 0.043 | 0.13 (0.01, 0.25) | ||
| 18–64 | pH1N1 | 539 | 313 | 0.052 | 0.083 | 0.43 (0.21, 0.65) | |
| H3N2 | 254 | 14 | 0.000 | 0.000 | * | ||
| B | 31 | 8 | 0.065 | 0.000 | * | ||
| Negative | 924 | 84 | 0.025 | 0.024 | * | ||
| 65+ | pH1N1 | 2 | 45 | 0.000 | 0.111 | * | |
| H3N2 | 19 | 17 | 0.053 | 0.118 | * | ||
| B | 2 | 4 | 0.000 | 0.000 | * | ||
| Negative | 47 | 33 | 0.149 | 0.091 | * | ||
| Post-pandemic (2012–2015) | All ages | pH1N1 | 667 | 218 | 0.019 | 0.046 | 0.09 (0.02, 0.16) |
| H3N2 | 1345 | 424 | 0.009 | 0.047 | 0.04 (0.01, 0.07) | ||
| B | 639 | 185 | 0.020 | 0.070 | 0.14 (0.04, 0.25) | ||
| Negative | 4089 | 422 | 0.018 | 0.107 | 0.19 (0.12, 0.26) | ||
| 18–64 | pH1N1 | 639 | 174 | 0.019 | 0.046 | 0.09 (0.01, 0.16) | |
| H3N2 | 1248 | 191 | 0.006 | 0.016 | 0.01 (0.00, 0.02) | ||
| B | 602 | 118 | 0.017 | 0.042 | 0.07 (0.00, 0.14) | ||
| Negative | 3778 | 244 | 0.016 | 0.057 | 0.09 (0.04, 0.14) | ||
| 65+ | pH1N1 | 28 | 44 | 0.036 | 0.045 | * | |
| H3N2 | 97 | 233 | 0.041 | 0.073 | * | ||
| B | 37 | 67 | 0.081 | 0.119 | * | ||
| Negative | 311 | 178 | 0.039 | 0.174 | 0.67 (0.24, 1.1) |
Data are for the pandemic and post-pandemic periods, computed as the product of the risk of FLU002 influenza-like illness outpatients getting hospitalized and the FLU003 hospitalized patients having died at day 60.
Abbreviations: P (D|H), probability of death given hospitalization; P (H|ILI) , probability of hospitalization given influenza-like illness.
*Case fatality rate not calculated when fewer than 100 outpatients or inpatients contained in any stratum.
Figure 2.
A schematic representation of the pyramid modeling approach used to estimate the 2009 pandemic case fatality ratio among medically attended cases from probabilities of disease progression from International Network for Strategic Initiatives in Global HIV Trials outpatient (FLU002) and inpatient (FLU003) data. Modeling was also done for 18–64 and 65+ year age groups separately due to known differences in attack rates and preexisting immunity. Abbreviations: AR, all infected persons; CFR, case fatality ratio; ILI, influenza-like illness; MA, medically attended; P (D|H), probability of death given hospitalization; P (H|ILI) , probability of hospitalization given influenza-like illness.
CFRMA in the Post-Pandemic Period 2012–2015
The CFRMA for pH1N1 cases in the post-pandemic period was 0.09% for patients aged 18–64 years, 5-fold lower than the value for the pandemic period and comparable to the influenza-negative patients of the same age. We could not reliably assess pH1N1 CFRMA for the ≥65 years age group due to small numbers in the post-pandemic period; however, CFRMA was 0.4% for seniors aged ≥65 years positive for any influenza virus in the post-pandemic period vs 0.04% for younger adults positive for any influenza virus. For the post-pandemic period (any subtype), we also estimated the conditional probabilities and the CFRMA by region (Table 4).
Table 4.
Numbers of Patients Who Test Positive for Influenza, Probabilities of Progression to Hospitalization and Death, and Medically Attended Case Fatality Ratio by International Network for Strategic Initiatives in Global HIV Trials Geographic Region in the Post-Pandemic Period
| Region | Positive for Any Influenza (N) | Probabilities | Medically Attended Case Fatality Ratio (95% Confidence Interval) | ||
|---|---|---|---|---|---|
| FLU002 | FLU003 | P(H|ILI) | P(D|H) | ||
| Asia | 616 | 116 | 0.010 | 0.009 | 0.01% (−0.01, 0.03) |
| Australia | 10 | 106 | 0.000 | 0.010 | * |
| Europe | 678 | 280 | 0.028 | 0.068 | 0.19% (0.07, 0.31) |
| North America | 183 | 233 | 0.044 | 0.034 | 0.15% (0.01, 0.29) |
| South America | 1164 | 92 | 0.004 | 0.152 | * |
| All regions | 2651 | 827 | 0.014 | 0.052 | 0.07% (0.04, 0.11) |
Abbreviations: P (D|H), probability of death given hospitalization; P (H|ILI) , probability of hospitalization given influenza-like illness
*Case fatality ratio not calculated when fewer than 100 outpatients or inpatients contained in any stratum.
Converting CFRMA to CFRAR
Because the final WHO CFR estimate from the 2009 pandemic was based on attack rates as revealed by serology data, we sought to convert our medically attended CFR to one based on the attack rate. To do so, we used data from a study that indicated that approximately 25% of all cases are asymptomatic [29] and from survey data that indicate that approximately 25% of adult ILI cases sought medical attention [30]. We found the CFRAR to be 0.03% (0.01%–0.04%; Table 5), or 16-fold lower than the CFRMA.
Table 5.
Conversion of Medically Attended Case Fatality Ratio (CFR) to CFR for All Infected Persons Estimates Using 2 UK Studies
| Source | Measure | Parameter | Estimate | Lower Bound | Upper Bound |
|---|---|---|---|---|---|
| This study | CFR based on persons with medically attended ILI | CFRMA | 0.4% | 0.2% | 0.6% |
| Brooks-Pollock et al [28]; UK survey of healthcare-seeking behavior in adults | Probability of seeking medical care given ILI | P(ILIMA|ILI) | 0.25 | 0.25 | 0.25 |
| Hayward et al [29]; serology study | Probability of having ILI symptoms given H1N1pdm infection (based on antibody titers) | P(ILI|Inf) | 0.25 | 0.25 | 0.25 |
| Multiplying the 3 figures | CFR based on persons with influenza infection | CFRAR | 0.03% | 0.01% | 0.04% |
Abbreviations: AR, attack rate; CFR, case fatality ratio; ILI, influenza-like illness; MA, medically attended.
DISCUSSION
WHO has recently expanded its pandemic definition to include clinical severity. This means that rapid and accurate estimates of pandemic clinical severity are needed to characterize the threat level and guide the global response. Our analysis combining data from inpatient and outpatient INSIGHT cohorts demonstrates how preestablished global research networks could immediately begin rigorous studies to estimate the CFR, a key parameter of clinical severity of an emerging pandemic.
Assessments of the clinical severity in the 2009 pandemic became less dire as time passed [3]. The earliest estimate of CFR, an FF100-like case series of hospitalized patients in Mexico, was a disturbing 2% of influenza-positive patients. However, as studies of the first (summer) wave in the United States, the complete southern hemisphere 2009 season in New Zealand, and further studies from Mexico were completed, it became clear that the pandemic would be relatively mild (Table 1).
Several factors contributed to the early confusion in 2009. The most important was probably selection bias toward sicker patients in the earliest FF100-type case series studies [3]. Another factor was simply that studies reported on different types of CFR—either as a proportion of medically attended cases (CFRMA) or as a proportion of all infected individuals (CFRAR). Most early assessments were of the CFRMA type, but these were not directly comparable.
Our method, retroactively applied to INSIGHT databases, yielded a CFRMA estimate of 0.4%. Using literature values that indicated that the probability of symptomatic people seeking medical treatment was 25% [29] and that the probability of infected individuals being asymptomatic was also 25% [30], our CFRMA value would be equivalent to a CFRAR of .025%, which is in reasonable agreement with the final global WHO CFRAR estimate of 0.02% [1, 2, 16].
In addition to an absolute measurement of CFR, data from previous seasons can provide a relative comparison of pandemic to seasonal influenza severity; even if the absolute estimate of CFR is uncertain, it would be useful to know if an emerging pandemic has a CFR far higher than previous seasonal influenza experiences. Thus, we also estimated CFRs for influenza patients from seasonal influenza epidemics 2012–2015, as a surrogate for pre-pandemic baseline seasons.
Age greatly influences both seasonal and pandemic clinical severity estimates. In all 4 influenza pandemics since 1900, mortality was higher than normal in younger people and lower than normal in seniors, sometimes dramatically so [31]. In the post-pandemic period (2012–2015) we found that the CFRMA of pH1N1 for patients aged 18–64 years had fallen 5-fold from the pandemic period value, becoming similar to that of A/H3N2 and B. This suggests that the emerging virus had settled into a seasonal epidemic pattern due to accumulated population immunity. Moreover, in the post-pandemic period patients aged ≥65 years with any influenza virus had a CFRMA approximately 10-fold higher than patients aged <65 years. These results corroborate a previous metaanalysis of FF100 studies that concluded that age is an important confounder of CFR estimates for pH1N1 pandemic influenza [3]. They also show how important it is to take into account both the age group and the type of CFR being calculated when comparing across regions and time.
It is also possible that discrepancies in early assessments of CFR may in fact have reflected true geographical differences. For example, a comprehensive study of 2009 pandemic mortality that applied a uniform methodology to different regions found the mortality impact in Central and South American countries was approximately 20-fold higher than in Europe [1]. This indicates that early reports of higher severity in Mexico than in New Zealand may not solely have been the result of ascertainment bias. Clinical severity can even increase substantially over time, as was seen in the 1918 influenza pandemic when a milder summer wave preceded the severe autumn waves [32].
The best way around the measurement problems that occur early in a pandemic would be to compute the same type of CFR with the same protocol in multiple geographical settings. If possible, estimates should be stratified by risk factors, such as pregnancy and chronic illness, and baseline data should be collected during seasonal epidemics. While some countries have created FF100 protocols since the 2009 pandemic, a global standard along the lines we have outlined here would be helpful.
We recognize limitations to our approach to computing CFR by multiplying conditional probabilities of disease progression. First, we used distinct groups of outpatients and inpatients who were recruited under different circumstances at different sites, often in different countries. It is therefore possible the 2 cohorts differed in age composition, health status, or other important respects that could bias the result. However, we argue that the approach, while not ideal, would nonetheless supply timely and useful data, especially if it could be compared to baseline seasons. We also note that the characteristics of the INSIGHT pH1N1 outpatients and inpatients in the pandemic period 2009–2011 are reassuringly similar in terms of age, symptoms, comorbidities, and outcomes to published UK and US FF100 studies of adult pH1N1 influenza outpatients and inpatients (Table 2). A second possible caveat—that INSIGHT inclusion criteria might have varied over time and explained the drop in CFRMA over time—could be dismissed on the grounds that the influenza-negative patients did not have a significant drop in CFRMA between the pandemic and post-pandemic period. This means that the measured decrease in pH1N1 clinical severity was real and not due to ascertainment or other bias.
CONCLUSIONS
Our retrospective analysis of 2009 pandemic clinical severity indicates that it is possible to use research networks to assess both the absolute magnitude of the clinical severity of a future pandemic and the relative increase compared to a seasonal influenza baseline. Even if the seroepidemiology and health-seeking behavior surveys needed to convert CFRMA to CFRAR could not be done rapidly, comparison of CFRMA to previous seasons would reveal much about the relative magnitude of the emerging threat. To be useful in a prospective scenario, however, it would be necessary to ramp up the network’s pace of operations from routine to emergency mode. For INSIGHT, that would mean, at a minimum, enhancing enrollment in sites located in areas initially affected by the emerging pandemic and increasing the tempo of laboratory processing of specimens and data analysis.
In addition to assessing clinical severity, global research networks could play other key roles in pandemic response including studies of comorbidity patterns, risk factors, hospital and ICU utilization, and mortality risk of hospitalized patients. Moreover, protocols that enroll children could be used to understand the pathogen in this key age group. Once a future pandemic outbreak begins, studies set in these networks could both characterize pathophysiology to optimize clinical management and provide a platform for rigorous clinical trials of new therapeutics. We suggest, therefore, that a specific role for clinical research networks carrying out ongoing rigorous research compliant with international standards be added to the international health regulations that govern international and national responsibilities for public health emergencies of international concern.
Notes
Financial support. International Network for Strategic Initiatives in Global HIV Trials is funded by the National Institutes of Health (grant UOI-AI068641, SAIC-Frederick HHSN261200800001E). This study was funded through subcontracts (13XS134 and 16Q152) under Leidos Biomedical Corporation’s prime contract (HHSN261200800001E and HHSN261201500003I) with the National Cancer Institute and the National Institute of Allergy and Infectious Diseases. The authors also acknowledge grant support from Danish National Research Foundation (grant 126) and the European Research Commission Horizon 2020 Research and Innovation Framework Program Marie Curie (grant 659437).
Acknowledgments. We thank and acknowledge all the patients who participated in this study.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. L. S. and R. J. T. report earning consulting fees from Sage Analytica, LLC, during the conduct of the study. R. L. reports serving as co-editor on a book on infectious disease surveillance published by Wiley Blackwell, with royalties donated to the Minnesota Department of Health. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
The INSIGHT Influenza FLU 002 and FLU 003 Study Groups are as follows:
Community representatives: David Munroe, Claire Rappoport, Siegfried Schwarze. Coordinating centers: Copenhagen: Bitten Aagaard, Dejan Adzic, Jesper Grarup, Patricia Herrero, Per Jansson, Marie Louise Jakobsen, Birgitte Jensen, Karoline B. Jensen, Heidi Juncher, Jesper Kjær, Jens Lundgren, Paco Lopez, Amanda Mocroft, Mary Pearson, Begoña Portas, Caroline Sabin, Klaus Tillmann. London: Abdel Babiker, Nafisah Braimah, Yolanda Collaco-Moraes, Fleur Hudson, Ischa Kummeling, Filippo Pacciarini, Nick Paton. Statistical and Data Management Center–Minneapolis–Alain DuChene, Michelle George, Merrie Harrison, Kathy Herman, Eric Krum, Gregg Larson, Ray Nelson, Kien Quan, Siu-Fun Quan, Cavan Reilly, Terri Schultz, Greg Thompson, Nicole Wyman. Sydney: Anchalee Avihingsanon, Lara Cassar, Kanlaya Charoentonpuban, Sean Emery, Kobkeaw Laohajinda, Thidarat Jupimai, Isabel Lanusse, Alejandra Moricz, Ines Otegui, Kiat Ruxrungtham, Rose Robson. Washington: Elizabeth Finley, Fred Gordin, Adriana Sanchez, Michael Vjecha. Specimen repositories and laboratories: John Baxter, Shawn Brown (SAIC Frederick, Inc.), Elodie Ghedin (JCVI), Rebecca Halpin (JCVI), Marie Hoover (ABML). National Institute of Allergy and Infectious Disease: Beth Baseler (SAIC Frederick Inc.), Julia A. Metcalf. Other experts: Centers for Disease Control: Nancy Cox, Larisa Gubareva, Kathy Hancock, Jackie Katz, Alexander Klimov, Michael Shaw. Department of Health and Human Services: Lewis Rubinson. FLU 002 clinical site investigators: Argentina: Laura Barcan, Jorge Alberto Corral, Daniel Omar David, Hector Enrique Laplume, Maria Beatriz Lasala, Gustavo Daniel Lopardo, Marcelo H. Losso, Sergio Lupo, Eduardo Warley. Australia: Mark Bloch, Dominic E. Dwyer, Richard Moore, Sarah L. Pett, Norman Roth, Tuck Meng Soo, Emanuel Vlahakis. Austria: Heinz Burgmann. Belgium: Nathan Clumeck, Stephan De Wit, Eric Florence, KabambaKabeya, JozefWeckx. Chile: Carlos Perez, Marcelo J. Wolff. Denmark: Jan Gerstoft, Jens D. Lundgren, Lars∅stergaard. Estonia: Kai Zilmer. Germany: Johannes R. Bogner, Norbert H. Brockmeyer, Gerd Faetkenheuer, Hartwig Klinker, Andreas Plettenberg, Juergen Rockstroh, Christoph Stephan. Greece: Anastasia Antoniadou, Georgios Koratzanis, Nikolaos Koulouris, Vlassis Polixronopoulos, Helen Sambatakou, Nikolaos Vasilopoulos. Lithuania: Saulius Caplinskas. Peru: Alberto La Rosa, Fernando Mendo, Raul Salazar, Jorge Valencia. Poland: Elzbieta Bakowska, Andrzej Horban, Brygida Knysz. Portugal: Francisco Antunes, Manuela Doroana. South Africa: Nesri Padayatchi. Spain: David Dalmau, Eduardo Fernandez-Cruz, Jose Maria Gatell, Jesus Sanz Sanz, Vincent Soriano. Thailand: Ploenchan Chetchotisakd, Kiat Ruxrungtham, Gompol Suwanpimolkul. United Kingdom: Clifford L.S. Leen. United States: Calvin Cohen, David L. Cohn, Jack A. DeHovitz, Wafaa El-Sadr, Marshall Glesby, Fred M. Gordin, Sally Hodder, Norman Markowitz, Richard M. Novak, Robert Schooley, Gary L. Simon, Ellen Marie Tedaldi, Zelalem Temesgen, Joseph Timpone, Daniel Z. Uslan, Barbara Heeter Wade. FLU 003 clinical site investigators: Argentina: Laura Barcan, Jorge Alberto Corral, Daniel Omar David, Hector Enrique Laplume, Maria Beatriz Lasala, Gustavo Daniel Lopardo, Marcelo H. Losso, Eduardo Warley. Australia: Dominic E. Dwyer, Julian Elliott, Pam Konecny, John McBride, Sarah L. Pett. Austria: Heinz Burgmann. Belgium: Nathan Clumeck, Stephan De Wit, Philippe Jorens, KabambaKabeya. Chile: Marcelo J. Wolff. China: Tak Chiu Wu. Denmark: Jan Gerstoft, Lars Mathiesen, Henrik Nielsen, Lars Østergaard, Svend Stenvang Pedersen. Germany: Frank Bergmann, Johannes R. Bogner, Norbert H. Brockmeyer, Gerd Faetkenheuer, Hartwig Klinker, Juergen Rockstroh, Christoph Stephan. Greece: Anastasia Antoniadou, Georgios Koratzanis, Nikolaos Koulouris, Vlassis Polixronopoulos, Helen Sambatakou, Nikolaos Vasilopoulos. Norway: Anne Maagaard. Peru: Fernando Mendo, Raul Salazar
Poland: Elzbieta Bakowska, Andrzej Horban. South Africa: Nesri Padayatchi. Spain: David Dalmau, Vicente Estrada, Eduardo Fernandez-Cruz, Hernando Knobel Freud, Rosa M Blazquez Garrido, Jose Maria Gatell, Jose Sanz Moreno, Jose Ramon Pano-Pardo, Jesus Sanz Sanz, Vincent Soriano. Thailand: Ploenchan Chetchotisakd, Kiat Ruxrungtham, Gompol Suwanpimolkul. United Kingdom: Brian J. Angus, David R. Chadwick, David Dockrell, Clifford L.S. Leen, Melanie Newport, Ed Wilkins. United States: Harry Anderson III, Jason V. Baker, David L. Cohn, Jack A. DeHovitz, Wafaa El-Sadr, Matthew S. Freiberg, Fred M. Gordin, Roy Gulick, David Gurka, Sally Hodder, Norman Markowitz, Richard M. Novak, Armando Paez, NamrataPatil, Annette Reboli, Michael Sands, Robert Schooley, Gary L. Simon, Zelalem Temesgen, Joseph Timpone, Daniel Z. Uslan, Barbara Heeter Wade.
Contributor Information
INSIGHT FLU002 and FLU003 Study Groups:
David Munroe, Claire Rappoport, Siegfried Schwarze, Bitten Aagaard, Dejan Adzic, Jesper Grarup, Patricia Herrero, Per Jansson, Marie Louise Jakobsen, Birgitte Jensen, Karoline B Jensen, Heidi Juncher, Jesper Kjær, Jens Lundgren, Paco Lopez, Amanda Mocroft, Mary Pearson, Begoña Portas, Caroline Sabin, Klaus Tillmann, Abdel Babiker, Nafisah Braimah, Yolanda Collaco-Moraes, Fleur Hudson, Ischa Kummeling, Filippo Pacciarini, Nick Paton, Michelle George, Merrie Harrison, Kathy Herman, Eric Krum, Gregg Larson, Ray Nelson, Kien Quan, Siu-Fun Quan, Cavan Reilly, Terri Schultz, Greg Thompson, Nicole Wyman, Anchalee Avihingsanon, Lara Cassar, Kanlaya Charoentonpuban, Sean Emery, Kobkeaw Laohajinda, Thidarat Jupimai, Isabel Lanusse, Alejandra Moricz, Ines Otegui, Kiat Ruxrungtham, Rose Robson, Elizabeth Finley, Fred Gordin, Adriana Sanchez, Michael Vjecha, John Baxter, Shawn Brown, Elodie Ghedin, Rebecca Halpin, Marie Hoover, Julia A Metcalf, Larisa Gubareva, Kathy Hancock, Jackie Katz, Alexander Klimov, Michael Shaw, Laura Barcan, Jorge Alberto Corral, Daniel Omar David, Hector Enrique Laplume, Maria Beatriz Lasala, Gustavo Daniel Lopardo, Marcelo H Losso, Sergio Lupo, Eduardo Warley, Mark Bloch, Dominic E Dwyer, Richard Moore, Sarah L Pett, Norman Roth, Tuck Meng Soo, Emanuel Vlahakis, Heinz Burgmann, Nathan Clumeck, Stephan De Wit, Eric Florence, Kabamba Kabeya, Jozef Weckx, Carlos Perez, Marcelo J Wolff, Jan Gerstoft, Jens D Lundgren, Lars Østergaard, Kai Zilmer, Johannes R Bogner, Norbert H Brockmeyer, Gerd Faetkenheuer, Hartwig Klinker, Andreas Plettenberg, Juergen Rockstroh, Christoph Stephan, Anastasia Antoniadou, Georgios Koratzanis, Nikolaos Koulouris, Vlassis Polixronopoulos, Helen Sambatakou, Nikolaos Vasilopoulos, Saulius Caplinskas, Alberto La Rosa, Fernando Mendo, Raul Salazar, Jorge Valencia, Elzbieta Bakowska, Andrzej Horban, Brygida Knysz, Francisco Antunes, Manuela Doroana, Nesri Padayatchi, David Dalmau, Eduardo Fernandez-Cruz, Jose Maria Gatell, Jesus Sanz Sanz, Vincent Soriano, Ploenchan Chetchotisakd, Kiat Ruxrungtham, Gompol Suwanpimolkul, Clifford L S Leen, Calvin Cohen, David L Cohn, Jack A DeHovitz, Wafaa El-Sadr, Marshall Glesby, Fred M Gordin, Sally Hodder, Norman Markowitz, Richard M Novak, Robert Schooley, Gary L Simon, Ellen Marie Tedaldi, Zelalem Temesgen, Joseph Timpone, Daniel Z Uslan, Barbara Heeter Wade, Laura Barcan, Jorge Alberto Corral, Daniel Omar David, Hector Enrique Laplume, Maria Beatriz Lasala, Gustavo Daniel Lopardo, Marcelo H Losso, Eduardo Warley, Dominic E Dwyer, Julian Elliott, Pam Konecny, John McBride, Sarah L Pett, Heinz Burgmann, Nathan Clumeck, Stephan De Wit, Philippe Jorens, Kabamba Kabeya, Marcelo J Wolff, Tak Chiu Wu, Jan Gerstoft, Lars Mathiesen, Henrik Nielsen, Lars Østergaard, Svend Stenvang Pedersen, Frank Bergmann, Johannes R Bogner, Norbert H Brockmeyer, Gerd Faetkenheuer, Hartwig Klinker, Juergen Rockstroh, Christoph Stephan, Anastasia Antoniadou, Georgios Koratzanis, Nikolaos Koulouris, Vlassis Polixronopoulos, Helen Sambatakou, Nikolaos Vasilopoulos, Anne Maagaard, Fernando Mendo, Raul Salazar, Elzbieta Bakowska, Andrzej Horban, Nesri Padayatchi, David Dalmau, Vicente Estrada, Eduardo Fernandez-Cruz, Hernando Knobel Freud, Rosa M Blazquez Garrido, Jose Maria Gatell, Jose Sanz Moreno, Jose Ramon Pano-Pardo, Jesus Sanz Sanz, Vincent Soriano, Ploenchan Chetchotisakd, Kiat Ruxrungtham, Gompol Suwanpimolkul, Brian J Angus, David R Chadwick, David Dockrell, Clifford L S Leen, Melanie Newport, Ed Wilkins, Harry Anderson III, Jason V Baker, David L Cohn, Jack A DeHovitz, Wafaa El-Sadr, Matthew S Freiberg, Fred M Gordin, Roy Gulick, David Gurka, Sally Hodder, Norman Markowitz, Richard M Novak, Armando Paez, Namrata Patil, Annette Reboli, Michael Sands, Robert Schooley, Gary L Simon, Zelalem Temesgen, Joseph Timpone, Daniel Z Uslan, and Barbara Heeter Wade
References
- 1. Simonsen L, Spreeuwenberg P, Lustig R, et al. . Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dawood FS, Iuliano AD, Reed C, et al. . Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012; 12:687–95. doi: 10.1016/S1473-3099(12)70121–4. [DOI] [PubMed] [Google Scholar]
- 3. Wong JY, Kelly H, Ip DKM, Wu JT, Leung GM, Cowling BJ. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology. 2013; 24:830–41. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24045719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Report of the Review Committee on the Functioning of the International Health Regulations (2005) in relation to Pandemic (H1N1) 2009 2011;1–180. [Google Scholar]
- 5. Wong JY, Wu P, Nishiura H, et al. . Infection fatality risk of the pandemic a(H1N1)2009 virus in Hong Kong. Am J Epidemiol. 2013; 177:834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Pandemic Influenza Severity Assessment (PISA). 2017. Available at: http://www.who.int/influenza/surveillance_monitoring/pisa/en/. [Google Scholar]
- 7. Reed C, Biggerstaff M, Finelli L, et al. . Novel framework for assessing epidemiologic effects of influenza epidemics and pandemics. Emerg Infect Dis 2013; 19:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLean E, Pebody RG, Campbell C, et al. . Pandemic (H1N1) 2009 influenza in the UK: clinical and epidemiological findings from the first few hundred (FF100) cases. Epidemiol Infect. 2010; 138:1531–41. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009; 21:185–90. [PubMed] [Google Scholar]
- 10. Fraser C, Donnelly CA, Cauchemez S, et al. . Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009; 324:1557–61. Available at: http://science.sciencemag.org/content/324/5934/1557.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castro-Jiménez MA, Castillo-Pabón JO, Rey-Benito GJ, et al. ; Virology Group; Communicable Diseases Surveillance Group Epidemiologic analysis of the laboratory-confirmed cases of influenza A(H1N1)v in Colombia. Euro Surveill 2009; 14:19284. [DOI] [PubMed] [Google Scholar]
- 12. Baker M, Wilson N, Huang Q, et al. . Pandemic influenza A(H1N1)v in New Zealand: the experience from April to August 2009. Euro Surveill. 2009; 14:1–6. [DOI] [PubMed] [Google Scholar]
- 13. Presanis AM, De Angelis D, Hagy A, et al. . The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Kerkhove MD, Hirve S, Koukounari A, et al. . Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respir Viruses. 2013; 7:872–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AlMazroa MA, Memish ZA, AlWadey AM. Pandemic influenza A (H1N1) in Saudi Arabia: description of the first one hundred cases. Ann Saudi Med 2010; 30:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. . Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009; 302:1880–7. Available at: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2009.1536. Accessed 8 March 2018. [DOI] [PubMed] [Google Scholar]
- 17. Davey RT, Markowitz N, Beigel J, et al. . INSIGHT FLU005: an anti-influenza virus hyperimmune intravenous immunoglobulin pilot study. J Infect Dis. 2016; 213:574–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain S, Kamimoto L, Bramley AM, et al. . Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009; 361: 1935–44. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19815859. [DOI] [PubMed] [Google Scholar]
- 19. Khandaker G, Dierig A, Rashid H, King C, Heron L, Booy R. Systematic review of clinical and epidemiological features of the pandemic influenza A (H1N1) 2009. Influenza Other Respir Viruses 2011; 5:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louie JK, Winter K, Jean C, et al. . Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. 2009; 302. [DOI] [PubMed] [Google Scholar]
- 21. van Gageldonk-Lafeber AB, van der Sande MA, Meijer A, et al. . Utility of the first few100 approach during the 2009 influenza A(H1N1) pandemic in the Netherlands. Antimicrob Resist Infect Control. 2012; 1: 30 Available at: http://www.ncbi.nlm.nih.gov/pubmed/22995284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pelat C, Ferguson NM, White PJ, et al. . Optimizing the precision of case fatality ratio estimates under the surveillance pyramid approach. Am J Epidemiol 2014; 180:1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipsitch M, Donnelly CA, Fraser C, et al. . Potential biases in estimating absolute and relative case-fatality risks during outbreaks. PLoS Negl Trop Dis. 2015; 9:e0003846 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4504518&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dwyer DE. Surveillance of illness associated with pandemic (h1n1) 2009 virus infection among adults using a global clinical site network approach: the insight flu 002 and flu 003 studies. Vaccine. 2011; 29:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davey RT, Lynfield R, Dwyer DE, et al. . The association between serum biomarkers and disease outcome in influenza A(H1N1)pdm09 virus infection: results of two international observational cohort studies. PLoS One. 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynfield R, Davey R, Dwyer DE, et al. . Outcomes of influenza A(H1N1)pdm09 virus infection: results from two international cohort studies. PLoS One. 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmes EC, Ghedin E, Halpin R a, et al. . Extensive geographical mixing of 2009 human H1N1 influenza A virus in a single university community. J Virol. 2011; 85:6923–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawood F, Jain S, Finelli L, et al. . Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009; 360:2605–15. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa0903810. Accessed 7 March 2018. [DOI] [PubMed] [Google Scholar]
- 29. Brooks-Pollock E, Tilston N, Edmunds WJ, Eames KTD. Using an online survey of healthcare-seeking behaviour to estimate the magnitude and severity of the 2009 H1N1v influenza epidemic in England. BMC Infect Dis. 2011; 11:68 Available at: http://www.scopus.com/inward/record.url?eid=2-s2.0-79952706157&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayward AC, Fragaszy EB, Bermingham A, et al. . Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014; 2:445–54. doi:10.1016/S2213-2600(14)70034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010; 2:RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008; 197:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]