Abstract
Men diagnosed with early stage prostate cancer face multiple treatment options, each with distinctive side effects that have significant implications for post-treatment quality of life. Healing Choices for Men with Prostate Cancer is a multimedia educational and decision aid program. This nation-wide randomized controlled trial evaluated the impact of Healing Choices on reducing decisional conflict and distress. Eligible prostate cancer patients who called the National Cancer Institute's Cancer Information Service (CIS) were invited to participate. After a baseline interview, participants were randomized to usual personalized consultation with a CIS specialist (comparison condition) or CIS personalized consultation plus the Healing Choices program (intervention condition). The Decision Conflict Scale and Impact of Event Scale assessed decisional conflict about prostate cancer treatment and cancer-related distress, respectively. Analyses evaluated group differences at 2 months postenrollment. Hypothesized moderation of intervention effects by demographic and clinical characteristics were evaluated. The final sample consisted of N = 349 participants (intervention: n = 181; comparison n = 168). Men were on average 64 years old, primarily White, and well educated. The difference in total decisional conflict was not significant (DCS total score; F[1,311] = .99, p = .32). The difference in cancer-related distress at 2 months between the intervention and the comparison groups was not significant (F[1,337] = .01, p = .93). Evaluation of specific decision processes indicated a significant effect on levels of perceived decisional support (intervention, M = 34.8, SD = 15.7; comparison, M = 38.3, SD = 16.1; F[1,337] = 3.74, p = .05). The intervention effect was greatest for nonwhite minority participants (b = −9.65, SE = 4.67) and those with lower educational attainment (b = 3.87, SE = 2.21). This interactive, comprehensive education and decision aid program may be most effective for a subset of prostate cancer patients in need of educational and decisional support.
Keywords: Prostate cancer, Decision-making intervention, Decision-making software
Results from this nation-wide randomized clinical trial evaluating an electronic education and decision support system for prostate cancer treatment decision making, supported the hypothesis that non-White minority patients, and those with lower educational attainment are best served by comprehensive education and decisional support.
Implications
Practice: Efficient and effective screening methodologies need to be developed and integrated into clinical practice to help identify patients in greatest need for additional support.
Policy: One-size-fits-all approaches to patient information and support are not successful and therefore it is necessary to direct policies and funds toward the development, maintenance, and support of various information channels to aid an increasingly diverse patient population.
Research: Further research is needed to identify prostate cancer patients who would most benefit from supportive interventions and how to best integrate such programs within existing clinical resources.
INTRODUCTION
Treatment options for men diagnosed with localized prostate cancer, defined as a tumor that is confined to the prostate without nodal involvement or distant metastases (T1,T2,N0,M0), include surgery, radiation therapy (i.e. external beam radiation, brachytherapy), and active surveillance [1,2]. The 5-year survival rate with active treatment approaches 100%, although often at the cost of significant, potentially long-lasting urinary, sexual, and bowel dysfunction [3–6]. Because of the various treatment choices available to patients and the risk to future quality of life, treatment decisions for prostate cancer are considered preference sensitive [7]. Preference sensitive decisions are informed by clinical parameters as well as patients’ values and goals for future outcomes and are ideally achieved in an information exchange and values clarification process between providers and patients. In practice, such a decision situation is rarely achieved and patients routinely have to resolve contradictory medical opinions from physicians representing different medical subspecialties [8]. The decision is further complicated by disease and treatment information that is often presented in medical and probabilistic terms [8–10], often leading to a challenging decision-making process, elevated levels of distress, and, for some patients, long-term decision regret [7].
To facilitate patient education and decision-making, researchers have begun to develop evidence-based interactive, multimedia educational programs as an adjunct to cancer care [11–14]. These programs have been used to help prepare patients for medical procedures, provide health information, teach coping strategies, and facilitate patient–physician communication [15]. A growing literature has demonstrated that these educational approaches can be very effective, leading to improved knowledge about treatment options, reduced anxiety and cancer-related worries, and increased confidence in patients’ interaction with their healthcare providers [15–17]. However, patients access information from multiple sources (e.g. advice/guidance from family and friends, media) [8,18] and bring unique cognitive and affective experiences (e.g. risk and treatment-related perceptions, expectations, fears) to the decision-making process. These factors will inevitably influence patients’ assessment of information and increase their need for decision support. Even well-informed patients may require support to integrate personal values and preferences into their decision-making, especially when faced with a lack of consensus among medical providers about the best treatment approach.
Education and decision aids are often implemented at the point of care. Healthcare providers introduce the tool, help with completion, and ideally discuss treatment preferences with their patients. However, this places providers as a gateway to implementation of such tools and limits the reach of decision support intervention. To expand our reach and to target patients across the USA, we partnered with the National Cancer Institute’s Cancer Information Service (CIS) with the goal to augment their existing telephone-based information service. Our newly developed education and decision aid, the Healing Choices program, was designed to complement this telephone-based information service and included video-based information from physicians and survivors, an interactive value clarification module, as well as strategies to deal with distress and to enhance patient–physician communication [11]. Our partnership with the CIS afforded us the opportunity to evaluate the utility of the tool in a pragmatic fashion in the context of an established life-telephone service.
Prostate cancer patients who call the CIS’s toll-free number (1-800-4-Cancer) can receive detailed information from a trained cancer information specialist about their cancer, its treatment options, potential treatment side effects, and ongoing clinical trials. This standard consultation was the comparison condition in the present study. The study intervention condition received the standard consultation and services plus access to the Healing Choices program.
Theoretical framework
Self-regulation theory [19] and social cognitive theory [20] guided the development of the intervention and the selection of study measures. Self-regulation theory postulates that decision-making for health behaviors is influenced by cognitive and affective processes. Cognitive processes consist of representations, such as beliefs about illness-related causes, consequences, duration, controllability, and overall understanding (i.e. illness cohesion). Affective processes are emotional reactions to the illness, such as worry about the disease and its treatment. Concepts postulated by the self-regulation theory were translated into the content and the design of the Healing Choices program in the following ways: providing cancer-relevant information using a virtual library that provides information about treatment options and side effects; addressing individual beliefs and expectations about cancer treatment and disease outcomes through patient testimonials and physicians answering questions; providing emotional support through normalizing statements and a distress-lowering exercise; and modeling skills for decision-making (role models) to enhance self-efficacy in patient–physician communication and for generating and maintaining goal-oriented health-protective behaviors [21–24].
The impact of the Healing Choices program in facilitating treatment decision-making and reducing decisional conflict and cancer-related distress was evaluated in a nation-wide randomized controlled pragmatic trial. Eligible callers were randomized to receive standard consultation with a CIS information specialist via one phone call contact versus standard consultation plus access to the Healing Choices program. We hypothesized that patients in the intervention group would report lower levels of decisional conflict compared with patients in the comparison group (i.e. those receiving consultation with a cancer information specialist and NCI materials only). We also hypothesized that patients in the intervention group would report lower levels of cancer-related distress compared with patients in the comparison condition. The study examined whether the impact of the intervention on decisional conflict and cancer-related distress would be moderated by patients’ demographic and clinical characteristics (e.g. age, race, education, and comorbidity). Based on the literature [25–28], we hypothesized that older, nonWhite patients with lower education and more comorbid diseases would benefit more from the intervention than younger, White patients with higher education and less illness burden.
METHODS
All research protocols and materials were approved by the Institutional Review Board (IRB) of the University of Colorado Denver, Anschutz Medical Campus. IRB approval was also obtained from the collaborating research institutions (i.e. University of California, Los Angeles and Fox Chase Cancer Center) and from parent institutions of the three CIS contact centers (i.e. University of Miami, Fred Hutchinson Cancer Research Center, and Memorial Sloan-Kettering Cancer Center). The trial was registered on www.clinicaltrials.gov (NCT00830635). The study was conducted between 2009 and the end of 2014.
Inclusion/exclusion criteria and recruitment procedures
Recruitment procedures and challenges are described elsewhere [29]. In short, men who called the CIS about prostate cancer or its treatment were recruited at the end of their standard service telephone call by cancer information specialists. Eligibility was ascertained and verbal consent was obtained over the phone. Patients were eligible if they were diagnosed with localized prostate cancer (T1,T2,N0,M0), had not made a treatment decision, had access to a computer, and were English speaking. Exclusion criteria were completion of prostate cancer treatment, presence of other primary cancer, or cancer recurrence. Enrollment and a brief baseline assessment were also conducted over the phone at this time.
The majority of patients were recruited through the CIS. However, a slower than expected recruitment pace exacerbated by CIS internal reorganization made it necessary to establish additional recruitment approaches. This resulted in the establishment of a new call center from the CIS research consortium and a new collaboration with the American Cancer Society’s (ACS) call center [29]. Cancer education specialists from the ACS were trained by the study personnel following a train-the-trainer model. Other accrual sources included recruitment flyers/print materials, CIS Research Consortium Websites, community outreach and word-of-mouth efforts, and NCIS-related information on the radio; none of which accounted for a substantial number of participants alone.
Randomization procedure
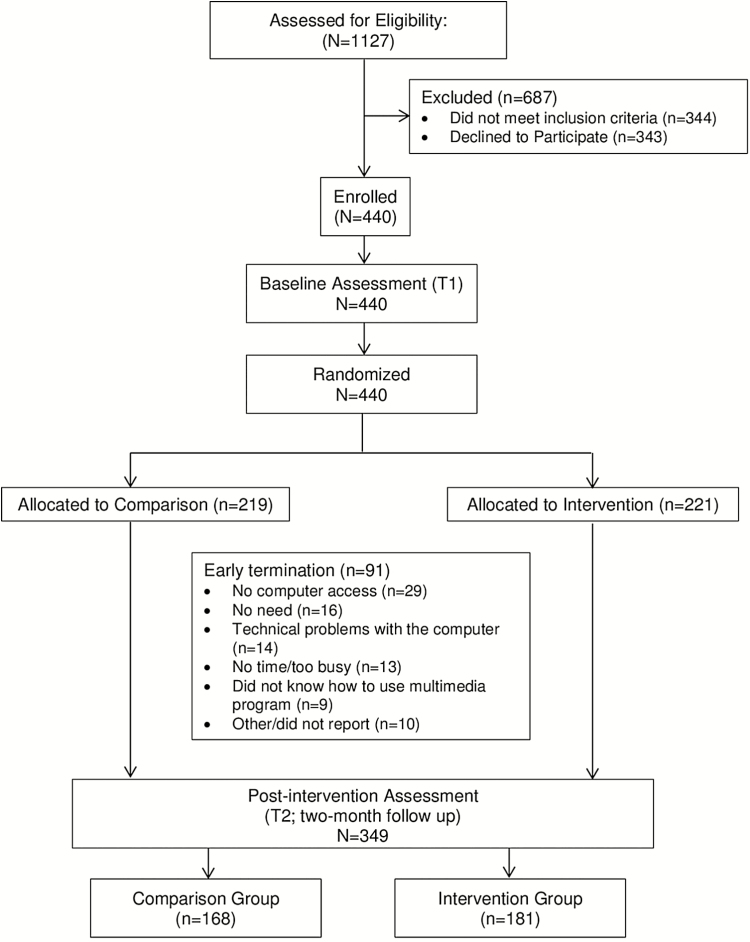
Immediately following the completion of the baseline interview, eligible callers who agreed to participate in the study were randomized to either usual service with a standard consultation (comparison condition; Group 1) or standard consultation plus the Healing Choices program (intervention condition; Group 2; see Figure 1). Group 1 received cancer information by telephone and standard print NCI materials. Group 2 received information by telephone, the NCI print materials, plus access to the multimedia Healing Choices software. All print materials, including an introductory letter, were shipped via express mail and received by study participants within 48 hours of the patient’s call to the CIS. In addition, participants assigned to Group 2 subsequently received a CD-ROM version of the multimedia program, as well as information on how to access the Healing Choices program on the Internet. All Group 2 participants received a second letter 14 days after study enrollment to encourage use of the program. Telephone follow-up interviews were conducted by blinded research staff at 2 months to assess decisional conflict and cancer-related distress and at 6 months postenrollment to assess decisional regret and distress. Because the primary focus of the project was to facilitate decision-making and reduce cancer-related distress with regard to treatment decision-making, only data from the 2-month evaluation were analyzed based on its temporal proximity to the decision-making process [12]. It was not possible to conceal allocation to study group.
Fig. 1.
Overview of research design
Usual care comparison condition
All men who were randomized into the comparison usual service condition spoke with a cancer information specialist from the CIS to have their specific questions answered and to receive personalized information about prostate cancer, treatment options, potential side effects, and existing clinical trials. As part of this standardized service, they also received the CIS standard NCI print materials, which included What You Need to Know about Prostate Cancer (NIH publication No. 12–1576) and Treatment Choices for Men with Prostate Cancer (NIH publication No. 11–4659).
Intervention condition
Men who were randomized to the intervention condition received the usual service provided by the CIS and had the opportunity to participate in the Healing Choices program.
Healing Choices for men with prostate cancer
The development of the software was described by Marcus et al. [30]. In short, the selection of content for the software was guided by our theoretical framework, based on a comprehensive literature review, and supplemented with disease- and treatment-relevant information. All information was vetted by the CIS to meet NCI standards. Content was organized in four different areas that addressed the major identified needs of prostate cancer patients as guided by the conceptual framework. They were The Library, Patient Stories, Doctor’s Office, and the Notebook [11]. The Library, contained factual information (written at a 7th grade reading level) about prostate cancer and its treatment options; Patient Stories contained videos of disease and treatment experiences recounted by a group of ethnically diverse patients who had undergone different treatments; Doctor’s Office provided the physician’s view of treatment and recovery; and finally, the Notebook provided the user with the opportunity to determine his values and preferences regarding treatment and future quality of life as well as provides distress management skills training and exercises. The software program underwent extensive User Testing and Usability Testing before it was released for study purposes.
STUDY MEASURES
Baseline demographic characteristics
Baseline measures included demographic (e.g. age, education, race/ethnicity, income, medical insurance) and clinical characteristics (e.g. cancer stage, comorbidity). Comorbidity was assessed by the Charlson Co-Morbidity scale [31]. This widely used measure is a weighted index that takes into account the number and seriousness of comorbid diseases (e.g. liver disease, diabetes) with higher scores indicating higher comorbidity and illness burden.
Decisional conflict
At 2 months after baseline, participants completed the Decisional Conflict Scale (DCS) [32,33], which consists of five subscales; Decisional Support (3 items); Feeling Informed (3 items); Values Clarity (3 items); Decisional Uncertainty (3 items); and Effective Decision (3 items); one of the four questions from the published version of the DCS Effective Decision subscale (“I expect to stick to my decision”) was not included. All subscales employ a five point Likert response scale from “0 – Strongly Agree” to “4 – Strongly Disagree.” Exploratory factor analyses on the study data confirmed the structure of the five subscales. The full scale and the five subscales have strong psychometric properties with a mean Cronbach’s α of .84 (i.e. Cronbach’s α: Total scale = .93; Feeling Informed = .70; Values Clarity = .81; Decisional Support = .78; Decisional Uncertainty = .79; Effective Decision = .83). A mean score was used to indicate the level of decisional conflict with higher scores indicating higher levels of conflict.
Cancer-related distress
Cancer-related distress was assessed at the baseline and 2-month follow-up time points using the Intrusion subscale of the Impact of Event Scale (IES) [34]. The subscale referred to the experience of being diagnosed with prostate cancer. It is composed of seven items that are answered on a four point Likert scale (0–5) with labels of “Not at all” (0), “Rarely” (1), “Sometimes” (3), and “Often” (5). It has been widely used and has well-established psychometric properties (Cronbach’s α in this study = .82). A higher score indicates higher levels of intrusive thoughts and feelings about prostate cancer, signifying higher distress. Subscale scores of 20 and above on the IES have been defined as clinically significant distress reactions [35–37].
Statistical analysis
Data were analyzed with the SPSS statistical software package, version 19.0. Descriptive statistics, t-test, chi-square test, and analyses of variance (ANOVA) were used to examine attrition from baseline (t1) to 2-month assessment (t2). Analyses examining the differences between the intervention and the comparison groups in decisional conflict and cancer-related distress included those participants that completed the baseline and 2-month assessments. Intervention effects on decisional conflict and cancer-related distress were evaluated using analysis of covariance (ANCOVAs) to test for group differences, controlling for age. The percentage of patients reporting clinically significant levels of cancer-related distress was also compared between groups as a test of the intervention using chi-square test, based on the validated IES cut-off score (scores ≥ 20) [35]. Following standard procedures [38], potential moderation effects of patients’ demographic and clinical characteristics on postintervention outcomes that demonstrated significant main effects were explored. Separate regression models were specified to test the effects of four moderator variables (age, ethnicity, education, and comorbidity) on levels of decision support across the two intervention groups (Group 1, comparison condition vs. Group 2, intervention condition). Each model included intervention group assignment as the independent variable, a moderator variable (dummy coded for categorical variables), and an interaction between the independent and moderator variables. Following statistical conventions, the coefficient of the interaction term was evaluated for significance, above and beyond main effects. The R2 change determined the amount of variance in decision support that was explained with the addition of the interaction term.
RESULTS
Presentation of results is divided into four sections which describe: (1) enrollment and attrition; (2) sample demographic and clinical characteristics; (3) the impact of the intervention on study outcomes; and (4) the moderators of the impact of the intervention on study outcomes.
Enrollment and attrition analyses
The CIS standard service program provided the majority of study participants (60%; n = 262). Only one of the CIS outreach efforts, the collaboration with the ACS (n = 58), provided a noticeable increase in accrual. All other referral sources produced only small or no gains in accrual each and were combined for analyses (e.g. Recruitment Flyers/ Print Materials, CIS Research Consortium Websites, community organization, or group). Final enrollment was N = 440 across all sources. For analyses, recruitment source was collapsed into three categories: CIS, ACS, and other. A comparison of the baseline characteristics of participants enrolled showed no significant differences by recruitment source in any demographic or medical variables. Recruitment source did not vary by group assignment.
Of the 440 eligible patients who completed the baseline assessment, 349 (80%) patients completed the 2-month assessment. The most common reasons for attrition (N = 91) were no computer access (30% of nonusers), no need (17%), technical problems with the computer (15%), no time or too busy (13%), and did not know how to use the multimedia program (9%). The overall rate of attrition did not vary by group condition (x2 = 1.81, p = .20) and reasons for attrition were similar across groups with the exception that the intervention group reported computer-related difficulties, which was not applicable to the standard care comparison group. To examine any potential bias introduced through selective attrition, we compared patients who completed both assessments with patients who only completed the baseline assessment on demographic (e.g. age, race/ethnicity, employment, education levels, comorbid disease/conditions) and psychological variables (e.g. baseline cancer-related distress). Patients who dropped out were more likely to be younger, White, highly educated, more emotionally distressed, with comorbid disease/conditions than those who completed the 2-month assessment.
Demographic characteristics
The majority of the sample was White (76%) and most had completed college or graduate school (54%). The average age of participants was 64.73 (SD = 8.39) years old. At baseline, 23% of patients reported clinically significant levels of prostate cancer-related distress based on the validated IES cut-off score (scores ≥ 20) (see Table 1). There were no significant differences between the two study groups in baseline demographic or clinical variables, or cancer-related distress, with the exception that the intervention group was slightly younger than the standard care comparison group (mean difference of 1.9 years, t[347] = 2.06, p = .04; see Table 1). Therefore, age was included as a covariate in comparative and predictive analyses. Subsequent comparative analyses included data only from patients who completed both the baseline and postintervention assessments (N = 349).
Table 1.
Baseline demographic and clinical characteristics
| Baseline covariates | Full sample at baseline (N = 349) | Study groups | |||
|---|---|---|---|---|---|
| Intervention (n = 181) | Comparison (n = 168) | ||||
| M ± SD or % | M ± SD or % | M ± SD or % | t or x2 | p | |
| Recruitment Source | |||||
| National Cancer Institute’s Cancer Information Service (CIS) | 60% | 61% | 58% | 2.87 | .24 |
| American Cancer Society (ACS) call center | 14% | 11% | 17% | ||
| Othera | 27% | 28% | 26% | ||
| Age | 64.73 ± 8.39 | 63.85 ± 8.59 | 65.69 ± 8.08 | 2.06 | .04 |
| Educational level | |||||
| High school graduate or less | 18.9% | 20.0% | 17.9% | .40 | .82 |
| Some college | 26.6% | 25.6% | 28.0% | ||
| College graduate or more | 54.2% | 54.4% | 54.2% | ||
| Income (USD) | 2.15 | .54 | |||
| <$30,000 | 21.2% | 25.1% | 20.1% | ||
| $30,000–$59,000 | 23.5% | 23.4% | 27.3% | ||
| $60,000–$79,000 | 17.8% | 20.5% | 17.5% | ||
| ≥ $80,000 | 30.7% | 31.0% | 35.1% | ||
| Ethnicity | |||||
| Other | 2.6% | 2.3% | 3.1% | .21 | .90 |
| African-American | 16.9% | 18.1% | 17.2% | ||
| White | 76.2% | 79.5% | 79.8% | ||
| BMI | 27.5 ± 4.4 | 27.6 ± 4.0 | 27.4 ± 4.7 | −.56 | .58 |
| Comorbidity | 82.8% | 80.1% | 85.7% | 1.92 | .17 |
| Medical insurance | 91.6% | 90.2% | 93.1% | .73 | .39 |
| Baseline cancer-related distress | |||||
| Intrusion subscale | 13.33 ± 8.24 | 14.2 ± 8.5 | 12.4 ± 7.8 | −1.94 | .053 |
| Clinically significant levels of distress (scores≥20)b | 23.2%b | 26.1%b | 21.0%b | 1.24b | .27b |
aOther accrual sources included recruitment flyers/ print materials, CIS Research Consortium Websites, community outreach and word-of-mouth efforts, or NCIS-related information on the radio.
bBased on validated case rule of IES scores ≥20 indicating clinically significant or elevated levels of distress [35].
Intervention effects
See Table 2 for analyses of intervention effects. We compared decisional process variables (i.e. DCS total score and subscales) between the intervention and standard care comparison groups at the 2-month follow-up visit (i.e. postintervention), controlling for age. The difference in total decisional conflict was not significant (DCS total score; F[1,311] = .99, p = .32). Evaluation of decisional process subscales indicated that patients with access to the Healing Choices program reported higher levels of decisional support compared to patients who received standard consultation (intervention, M = 34.8, SD = 15.7; comparison, M = 38.3, SD = 16.1; F[1,337] = 3.74, p = .05; covariate adjusted means: intervention, M = 34.9, SE = 1.2; comparison, M = 38.2, SE = 1.2). The intervention and comparison groups reported comparable levels of all other decisional processes including uncertainty (F[1,335] = .03, p = .86), feeling informed (F[1,333] = .09, p = .76), clarity of personal values related to the decision (F[1,330] = 1.92, p = .17), and effective decision-making (F[1,334] = .73, p = .40).
Table 2.
Group differences by study outcomes
| Two-month outcomes | Full sample (N = 349) | Intervention group (n = 181) | Comparison group (n = 168) | F | p |
|---|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | |||
| Decision conflict | |||||
| Total score | 36.72 ± 12.31 | 36.0 ± 12.03 | 37.50 ± 12.60 | .99 | .32 |
| Subscale scores: | |||||
| Uncertainty | 45.70 ± 19.26 | 45.46 ± 18.49 | 45.97 ± 20.13 | .03 | .86 |
| Informed | 35.54 ± 15.79 | 35.77 ± 15.35 | 35.27 ± 16.30 | .09 | .76 |
| Value Clarity | 35.18 ± 15.55 | 33.98 ± 14.75 | 36.52 ± 16.34 | 1.92 | .17 |
| Support | 36.49 ± 15.98 | 34.82 ± 15.76 | 38.26 ± 16.08 | 3.74 | .05 |
| Effective Decision | 33.45 ± 13.25 | 32.84 ± 13.20 | 34.13 ± 13.32 | .73 | .40 |
| Cancer-related distress | |||||
| Intrusion subscale | 11.64 ± 9.02 | 11.86 ± 9.44 | 11.39 ± 8.54 | .01 | .93 |
| % | % | % | x 2 | p | |
| Clinically significant levels of distress (scores ≥ 20)a | 19.2% | 19.7% | 18.5% | .07 | .79 |
Higher DCS scores indicate higher levels of decisional conflict and higher IES scores indicate higher levels of cancer-related distress.
aBased on validated case rule of IES scores ≥20 indicating clinically significant or elevated levels of distress.
The difference in cancer-related distress at 2 months between the intervention and the comparison groups was not significant (F[1,337] = .01, p = .93). In both groups, a smaller percentage of patients reported clinically significant levels of distress at the 2-month follow-up compared with baseline (8% reduction in both groups). Based on a validated case rule indicating clinical levels of cancer-related distress, 30% of the comparison group reported clinically significant distress at baseline and 19% at follow-up. Similarly, within the intervention group, the percent reporting clinically significant distress decreased from 33% at baseline to 20% at follow-up.
Exploring moderating effects
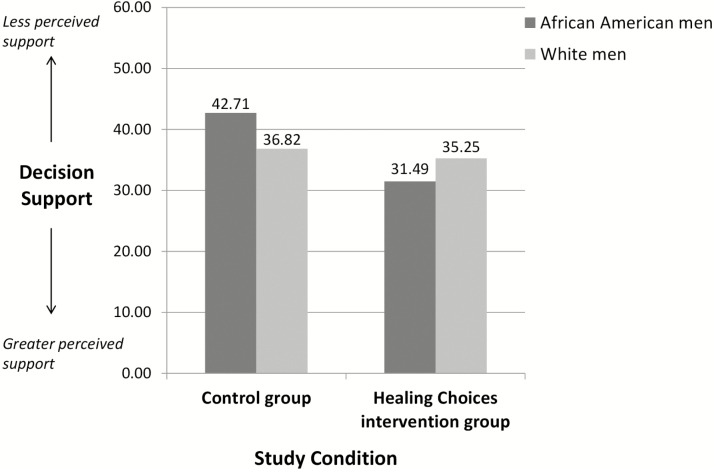
Regression analyses were conducted to examine whether the impact of the intervention on decisional support was moderated by patients’ age, race, educational level, and comorbidity (Table 3). Consistent with hypothesis, race (tested as a two-group variable; White vs. African American) emerged as the only significant moderator (β = −.18; b = −9.65, SE = 4.67, p = .04; F[3,312] = 2.60, p = .05). African-American participants, compared with White participants, reported greater decisional support after having access to the Healing Choices program than individuals who received standard consultation alone (Figure 2). Although at the trend level, findings also suggested that the Healing Choices program may have varying effects for participants based on their education level. Patients with lower levels of education appeared to benefit more from the intervention with respect to perceived decisional support, compared with patients with higher levels of education (β = 0.21; b = 3.87, SE = 2.21; F[3,335] = 2.67, p = .05). No other significant moderation effects emerged indicating that perceptions of decisional support did not vary by age (β = −.45, b = −.22, SE = .21; F[3,336] = 1.78, p = .15) or comorbidity (β = −.08, b = −2.62, SE = 4.72; F[3,336] = 1.72, p = .16).
Table 3.
Multiple regression analyses predicting the moderation effect
| Decisional support at 2 months | ||||
|---|---|---|---|---|
| Model 1: Moderation effect of age | Model 2: Moderation effect of racea | Model 3: Moderation effect of education | Model 4: Moderation effect of comorbidity | |
| Baseline predictor variable | β (b, SE b) | β (b, SE b) | β (b, SE b) | β (b, SE b) |
| Moderator | 0.09 (0.17, 0.16) | .14 (5.89, 3.36)‡ | −0.15 (−3.13, 1.61)* | 0.09 (3.71, 3.58) |
| Intervention | 0.35 (11.06, 13.64) | −.05 (−1.57, 1.97) | −0.27 (−8.68, 3.46)* | −0.04 (−1.15, 4.30) |
| Intervention × Moderator | −0.45 (−.22, 0.21) | −.18 (−9.65, 4.67)* | 0.21 (3.87, 2.2146)‡ | −.08 (−2.61, 4.694) |
| R 2 change | .003 | 0.013* | .009 | .001 |
| R 2 Total | 0.016 | 0.024* | 0.023‡ | 0.015 |
The results displayed are the second steps of hierarchical regression analyses. R2 change refers to the variance in Decision Support explained by the Intervention x Moderator interaction term.
‡ p < .10, *p < .05, **p < .01, ***p < .001.
aRace was tested as a two-group variable comparing Whites (coded 1) with African-Americans (reference group; coded 0).
Fig. 2.
Intervention effect on decision support moderated by race.
DISCUSSION
Based on our pilot data and studies in the published literature, we collaborated with the National Cancer Institute’s Cancer Information Service (CIS) to develop a patient education and decision aid and to evaluate its effectiveness compared to standard information service consultation. The resulting Healing Choices for Men with Prostate Cancer was randomly offered to half of the callers who agreed to be part of the present study. Results from this pragmatic trial were unexpected: there were no significant intervention effects on total decisional conflict or lowering cancer-related distress. Further evaluation of decisional processes indicated that patients with access to the Healing Choices program reported higher levels of decision support at follow-up compared with patients who received standard consultation alone, irrespective of their age. This intervention effect was greatest for African-American participants and those with lower educational attainment.
There are several possible explanations as to why the Healing Choices intervention did not lead to significant differences in overall decisional conflict or cancer-related distress compared with the standard comparison condition. Chief among those explanations is the excellent service the CIS provides to its callers, which was our main reason to partner with them. The CIS’ powerful standard of care consists of time-unlimited personalized consultations to any disease and treatment-related question, in addition to receiving printed NCI information booklets. In this context, it is possible that the vast majority of patients had their questions answered and the Healing Choices program did not improve their overall information needs.
The results of the moderator analyses deserve further mentioning however, as they have important implications for future program development and evaluation. African-American callers reported that Healing Choices provided increased decisional support compared with their White counterparts. It is unclear whether the higher incidence rate and perceived severity of prostate cancer among African-Americans might be responsible for this effect. However, it is clear that despite extensive information tailoring, some African-American men were in need of more decisional support than the CIS standard services provided.
Another potential explanation for the null findings is that baseline levels and specific sources of decisional conflict were not assessed due to concerns in the CIS about participant burden with a telephone-based questionnaire. It may be that some participants had low decisional conflict from the start or that unmet informational needs was their only source of decisional conflict, which was addressed by the standard information consultation (received in both the intervention and comparison conditions). Although participants in the Healing Choices program had access to interactive videos, preference clarification exercises and detailed, visually appealing disease and treatment information, it is unknown whether participants had unmet needs specific to these resources, aside from information seeking. Alternatively, we also observed a higher attrition rate in men who were more distressed and had more comorbidities at baseline. It is unknown whether attrition in this subset of men was due to a lack of more intensive support services, such as extensive one-on-one counseling. Findings suggest that this interactive, comprehensive education and decision aid program is most effective for a subset of patients who might be at risk for unmet support needs and decisional distress related to prostate cancer treatment decision-making.
Given the results, it is critical to be able to identify at-risk patients in need of decision support services as well as when and how to deliver these services. The benefit of the Healing Choices program was reflected in patients’ perceptions of having received better advice, guidance, and decision-making support compared to the standard consultation alone. This was particularly true for African-American men and limited evidence suggested that this effect also applied to men with lower education. Minority patients and those from lower socioeconomic background receive less information and emotional support from providers than nonminority patients from higher socioeconomic background [39]. Providers may also change their counseling practices and clinical management based on patients’ socioeconomic status [40] in part due to misperceptions about patients’ desire and need for information and ability to take part in the care process [41].
Poor prostate cancer knowledge may also stem from low health literacy, particularly in patients of disadvantaged socioeconomic backgrounds, which relates to lower self-efficacy in making prostate cancer treatment decisions and greater decisional conflict [27,28]. The Healing Choice program may have filled these gaps in care or added additional support to those who struggled to understand information provided in the clinical setting.
These results provide context for the ways in which decision aids may be used in real-world contexts in conjunction with other available resources and support. It may be that certain subgroups of patients would benefit from additional support provided by a decision aid, whereas others’ needs are adequately met by publicly available services such as the CIS. Early identification of patients that may benefit from enhanced support services will help to mitigate or avoid distress related to decision uncertainty for patients. Further investigation of ways to assess and triage patients into “stepped up” decision support and how this may be seamlessly integrated into existing, population-based services is warranted.
Interventions also need to be refined to target underserved populations and tailored to meet the specific decision support needs of patient subgroups. Future work is needed to determine the best ways to leverage technology to provide tailored interventions and how to best integrate them at multiple levels of care. The CIS program is a population-based resource in which callers may benefit from being referred to additional decision aid resources when needed. Clinical resources, such as patient portals, may also integrate decision aids for patients. Integration of evidence-based decision support resources into existing real-world services and settings will increase the reach of these interventions, while also conserving clinical resources.
Of note, patients do have varying levels of preferred involvement in treatment decision-making. Older age, being male, lower educational attainment, and poorer socioeconomic status correlate with a preference for less active roles in the decision-making process [42–44]. Men who find it difficult to access or understand medical information about risks may experience anxiety about taking responsibility for treatment outcomes [45]. Some men also may be reluctant to challenge providers recommendations and do not want to be seen as a difficult or disrespectful patient [45], which would naturally limit their involvement and opportunity to ask questions about their specific concerns. Indeed, it is possible that African-American men and those with lower education who benefitted most from the Healing Choices program had, at baseline, the expectancy that understandable information would not be readily available from the medical team; an assertion justified by the literature [39]. To the extent that these biases were operating on the part of patients or medical professionals, the intervention may have improved the degree to which men felt they had enough guidance, advice, and support to make treatment decisions, potentially leading to more active decision-making roles. Passive decision-making has been associated with later regret and retrospective criticism of provider interactions [44–46]. Decision aids for prostate cancer treatment have resulted in a greater proportion of patients wanting and assuming an active role in decision-making [47,48]. Research is needed to develop targeted decision support interventions that address patients’ preferences for involvement, thereby promoting a model of collaborative, shared decision-making between patients and providers that fits patients’ desired decision-making role and treatment outcomes.
There are several limitations that should be considered and that might be associated with conducting research in a service environment within a pragmatic trial. Due to concerns about subject burden, the assessment of decisional conflict was limited. It is therefore unknown whether participants were experiencing decisional conflict before, during, or after their CIS standard consultation with an information specialist. Although eligibility criteria excluded men who had already made a treatment decision, men did not have to be experiencing decision uncertainty or distress to participate. It is also unknown how baseline levels of decisional conflict compared across study groups. Analyses were unable to determine whether the Healing Choices program was more or less effective for subgroups of men with varying levels of decisional conflict at different points in the decision-making process, which may have also explained varying rates of attrition across socio-demographic characteristics. There was also limited baseline assessment of other relevant factors such as information processing and comprehension skills, illness cognitions, preferred role in decision-making, and/or alternative sources of support. We did not collect process data to evaluate the extent to which men accessed Healing Choices and specific program components that may have been more or less beneficial due to strict limitations from the CIS about how many questions could be asked of callers and the associated time burden to callers. However, process-level data would inform our understanding of decision support needs and be important for future intervention development. Clinical disease characteristics such as cancer stage, Gleason score, prognosis, and level of health literacy may also be related to decision-making and the type or extent of decision support needs that patients have. Power analysis was conducted for main effects and follow-up tests of interactions were exploratory.
CONCLUSION
Men diagnosed with early stage prostate cancer face multiple treatment options with distinctive side effect profiles and significant implications for post-treatment quality of life. Healing Choices for Men with Prostate Cancer is an educational and decision aid program designed to support patients’ treatment decision-making. Within the framework of an effectiveness study, patients with access to the program reported significantly higher levels of decision support, compared with those who received standard consultation through the CIS alone, and this intervention effect was greatest for African-American men and those with lower educational attainment. Future work is needed to evaluate potential reasons for null findings regarding other aspects of the decision-making process and cancer-related distress and to develop targeted strategies for addressing decision support needs in at-risk patient subgroups.
Acknowledgments
This research was funded by the National Cancer Institute under Grants No. NCI 2P01-CA057586-09A2, 1R01 CA158019-01, P30 CA006927, and R21 CA176551, and the American Cancer Society Research Scholar Grant No. RSG-15-021-01-CPPB.
Compliance with Ethical Standards
Conflict of Interest: None of the authors have any conflict of interest to disclose.
Primary Data: The content of this manuscript has neither been published nor is simultaneously being considered for publication elsewhere. The data has not been previously reported. The authors have full control of all primary data.
Ethical Approval: This study is in full compliance with institutional review board guidelines for protection of human subjects and animals; however, no animals were used in this study.
Informed consent: Informed consent was obtained from all patients included in the study.
References
- 1. NCCN Guidelines for Patients. Prostate Cancer, Version 1.2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015. [Google Scholar]
- 2. Mohler JL, Armstrong AJ, Bahnson RR, et al. . Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. [DOI] [PubMed] [Google Scholar]
- 3. Fullá J, Rosenfeld R, Sanchez C, et al. . Quality of life evaluation in patients with prostate cancer treated with radical prostatectomy: prospective study and results of eighteen months of follow-up. J Mens Health. 2014;11:88–92. [Google Scholar]
- 4. Capogrosso P, Salonia A, Briganti A, Montorsi F. Postprostatectomy erectile dysfunction: a review. World J Mens Health. 2016;34:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lehto US, Tenhola H, Taari K, Aromaa A. Patients’ perceptions of the negative effects following different prostate cancer treatments and the impact on psychological well-being: a nationwide survey. Br J Cancer. 2017;116:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barocas DA, Alvarez J, Resnick MJ, et al. . Association between radiation Therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317:1126–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knight SJ. Decision making and prostate cancer screening. Urol Clin North Am. 2014;41:257–266. [DOI] [PubMed] [Google Scholar]
- 8. Johnson DC, Mueller DE, Deal AM, et al. . Integrating patient preference into treatment decisions for men with prostate cancer at the point of care. J Urol. 2016;196:1640–1644. [DOI] [PubMed] [Google Scholar]
- 9. Diefenbach MA, Dorsey J, Uzzo RG, et al. . Decision-making strategies for patients with localized prostate cancer. Semin Urol Oncol. 2002;20:55–62. [DOI] [PubMed] [Google Scholar]
- 10. Orom H, Underwood W III, Biddle C. Emotional distress increases the likelihood of undergoing surgery among men with localized prostate cancer. J Urol. 2017;197:350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diefenbach MA, Butz BP. A multimedia interactive education system for prostate cancer patients: development and preliminary evaluation. J Med Internet Res. 2004;6:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diefenbach MA, Mohamed NE, Butz BP, et al. . Acceptability and preliminary feasibility of an internet/CD-ROM-based education and decision program for early-stage prostate cancer patients: randomized pilot study. J Med Internet Res. 2012;14:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diefenbach M, Mohamed NE, Butz BP, et al. . A pilot study to examine the feasibility and preliminary outcomes of an interactive, multimedia education and decision program for early-stage prostate cancer patients. J Med Internet Res. 2012;14:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller SM, Hudson SV, Hui SK, et al. . Development and preliminary testing of progress: a web-based education program for prostate cancer survivors transitioning from active treatment. J Cancer Surviv. 2015;9:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salonen A, Ryhänen AM, Leino-Kilpi H. Educational benefits of internet and computer-based programmes for prostate cancer patients: a systematic review. Patient Educ Couns. 2014;94:10–19. [DOI] [PubMed] [Google Scholar]
- 16. McDermott MS, While AE. Maximizing the healthcare environment: a systematic review exploring the potential of computer technology to promote self-management of chronic illness in healthcare settings. Patient Educ Couns. 2013;92:13–22. [DOI] [PubMed] [Google Scholar]
- 17. Huber J, Ihrig A, Yass M, et al. . Multimedia support for improving preoperative patient education: a randomized controlled trial using the example of radical prostatectomy. Ann Surg Oncol. 2013;20:15–23. [DOI] [PubMed] [Google Scholar]
- 18. Owens OL, Jackson DD, Thomas TL, Friedman DB, Hébert JR. Prostate cancer knowledge and decision making among African-American men and women in the Southeastern United States. Int J Mens Health. 2015;14:55–70. [PMC free article] [PubMed] [Google Scholar]
- 19. Leventhal H, Diefenbach MA, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cognit Ther Res. 1992;16(2):143–163. [Google Scholar]
- 20. Miller S, Diefenbach MA. C-SHIP: a cognitive-social health information processing approach to cancer. In: Krantz DS, Baum A, eds. Perspectives in Behavioral Medicine: Technology and Methods in Behavioral Medicine. Mahwah, NJ: Lawrence Erlbaum Associates; 1998:219–244. [Google Scholar]
- 21. Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–1184. [DOI] [PubMed] [Google Scholar]
- 22. Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q. 1984;11:1–47. [DOI] [PubMed] [Google Scholar]
- 23. Miller SM, Shoda Y, Hurley K. Applying cognitive-social theory to health-protective behavior: breast self-examination in cancer screening. Psychol Bull. 1996;119:70–94. [DOI] [PubMed] [Google Scholar]
- 24. Leventhal H. Findings and theory in the study of fear communications. Adv Exp Soc Psychol. 1970;5:119–186. [Google Scholar]
- 25. Diefenbach M, Mohamed NE, Horwitz E, Pollack A. Longitudinal associations among quality of life and its predictors in patients treated for prostate cancer: the moderating role of age. Psychol Health Med. 2008;13:146–161. [DOI] [PubMed] [Google Scholar]
- 26. Sidana A, Hernandez DJ, Feng Z, et al. . Treatment decision-making for localized prostate cancer: what younger men choose and why. Prostate. 2012;72:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaplan AL, Crespi CM, Saucedo JD, Connor SE, Litwin MS, Saigal CS. Decisional conflict in economically disadvantaged men with newly diagnosed prostate cancer: baseline results from a shared decision-making trial. Cancer. 2014;120:2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Truglio-Londrigan M, Slyer JT, Singleton JK, Worral P. A qualitative systematic review of internal and external influences on shared decision-making in all health care settings. JBI Libr Syst Rev. 2012;10:4633–4646. [DOI] [PubMed] [Google Scholar]
- 29. Stanton AL, Morra ME, Diefenbach MA, et al. . Responding to a significant recruitment challenge within three nationwide psychoeducational trials for cancer patients. J Cancer Surviv. 2013;7:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marcus AC, Diefenbach MA, Stanton AL, et al. ; CISRC Research Team. Cancer patient and survivor research from the cancer information service research consortium: a preview of three large randomized trials and initial lessons learned. J Health Commun. 2013;18:543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 32. O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 33. Song MK, Sereika SM. An evaluation of the decisional conflict scale for measuring the quality of end-of-life decision making. Patient Educ Couns. 2006;61:397–404. [DOI] [PubMed] [Google Scholar]
- 34. Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. [DOI] [PubMed] [Google Scholar]
- 35. Wenzel LB, Fairclough DL, Brady MJ, et al. . Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–1774. [PubMed] [Google Scholar]
- 36. Kaasa S, Malt U, Hagen S, Wist E, Moum T, Kvikstad A. Psychological distress in cancer patients with advanced disease. Radiother Oncol. 1993;27:193–197. [DOI] [PubMed] [Google Scholar]
- 37. Horowitz MJ. Stress response syndromes and their treatment. In: Goldberger L, Breznitz S, eds. Handbook of Stress: Theoretical and Clinical Aspects. New York: The Free Press; 1982:711–732. [Google Scholar]
- 38. Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296:1286–1289. [DOI] [PubMed] [Google Scholar]
- 39. Verlinde E, De Laender N, De Maesschalck S, Deveugele M, Willems S. The social gradient in doctor-patient communication. Int J Equity Health. 2012;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hajjaj FM, Salek MS, Basra MK, Finlay AY. Non-clinical influences on clinical decision-making: a major challenge to evidence-based practice. J R Soc Med. 2010;103:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aelbrecht K, Rimondini M, Bensing J, et al. . Quality of doctor-patient communication through the eyes of the patient: variation according to the patient’s educational level. Adv Health Sci Educ Theory Pract. 2015;20:873–884. [DOI] [PubMed] [Google Scholar]
- 42. Lechner S, Herzog W, Boehlen F, et al. . Control preferences in treatment decisions among older adults - results of a large population-based study. J Psychosom Res. 2016;86:28–33. [DOI] [PubMed] [Google Scholar]
- 43. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song L, Chen RC, Bensen JT, et al. . Who makes the decision regarding the treatment of clinically localized prostate cancer–the patient or physician?: results from a population-based study. Cancer. 2013;119:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chien CH, Chuang CK, Liu KL, Li CL, Liu HE. Changes in decisional conflict and decisional regret in patients with localised prostate cancer. J Clin Nurs. 2014;23:1959–1969. [DOI] [PubMed] [Google Scholar]
- 46. Christie DR, Sharpley CF, Bitsika V. Why do patients regret their prostate cancer treatment? a systematic review of regret after treatment for localized prostate cancer. Psychooncology. 2015;24:1002–1011. [DOI] [PubMed] [Google Scholar]
- 47. Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59:379–390. [DOI] [PubMed] [Google Scholar]
- 48. Song L, Toles MP, Bai J, et al. . Patient participation in communication about treatment decision-making for localized prostate cancer during consultation visits. Health. 2015;7:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]