Abstract
Much of the current research concerning autism spectrum disorder (ASD) focuses on early identification of behaviors that may indicate future deficits or higher risk for a later diagnosis. Additionally, there exists a strong claim regarding the dimensional nature of ASD, such that even among non-diagnosed individuals, a continuous distribution of symptom severity can be observed. Executive function (EF) has been widely studied in children, adolescents, and adults with ASD, with a robust body of research supporting widespread EF deficits in diagnosed individuals. However, it remains unclear how the degree of ASD symptomatology, outside of the presence of a diagnosis, affects EF abilities in a community sample. The First Year Inventory 2.0 (FYI 2.0), a parent-report measure, was designed to identify infants at 12 months who are at risk for an eventual ASD diagnosis. In the current study, a continuous scoring scale was used to examine risk (overall, Social-Communication, and Sensory-Regulatory) from a dimensional perspective. Parents also completed the Behavior Rating Inventory of Executive Function – Preschool Version (BRIEF-P) and the Social Responsiveness Scale – 2nd edition (SRS-2.0) when their children were 42 months (3.5 years) old. Each FYI 2.0 risk variable significantly predicted scores on an overall EF composite and specific EF subscales. When controlling for general ASD symptomatology, Sensory-Regulatory risk still significantly predicted EF deficits. This research provides additional support for a quantitative consideration of risk for ASD and presents novel findings regarding the relation between infant behaviors indicative of ASD risk and EF in early childhood.
Keywords: autism spectrum disorder, executive function, sensory, quantitative measure, infant, early childhood
Lay Summary:
Children with autism spectrum disorder (ASD) often have difficulty with executive function (EF) tasks that require a set of mental processes involved in goal-directed behaviors. Studying children without ASD who may have symptoms affecting EF is also important. This study demonstrates that certain infant behaviors related to ASD are linked to early childhood EF difficulties. These results support looking at a range of ASD symptoms to better understand children who struggle with EF and potentially design tools to help them.
Much of the current research concerning autism spectrum disorder (ASD) focuses on early identification of behavioral or biological markers that may indicate higher levels of risk or predict later diagnosis. The benefits of early identification are widely studied (Boyd et al., 2010; Koegel et al., 2014; Rogers, 1996), especially with regard to the value of early intervention (Dawson, 2008; Landa & Kalb, 2012; Rogers, 1996). The earlier the intervention, the higher likelihood that an individual’s developmental trajectory may show less severe long-term deficits in a wide range of areas (Ben Itzchak & Zachor, 2011; Dawson et al., 2010; Estes et al., 2015). Additionally, there exists a strong claim regarding the dimensional nature of ASD, such that even among non-diagnosed individuals, a continuous distribution of symptom severity can be observed (Ruzich et al., 2015). Although the concept of an “autistic continuum” of deficits in social/communicative behaviors was hypothesized many years ago (Wing, 1988), the extent of its use in empirical research is limited to a few specific measures (Baron-Cohen et al., 2001; Constantino & Gruber, 2002).
A number of behaviors known to show ASD-related deficits have been studied from this quantitative perspective. For example, social deficits that are characteristic of ASD have been found to be common even in non-diagnosed individuals (Constantino, 2011; Constantino & Todd, 2003). Additionally, there is also a strong correlation between ASD characteristics and sensory processing in the general population (Robertson & Simmons, 2013). The dimensional approach to studying behaviors associated with ASD aligns well with research initiatives aimed at quantifying psychopathology across many levels of symptom severity, as opposed to focusing solely on the presence of a diagnosis. However, this dimensional approach to ASD has rarely been used to examine non-social deficits associated with the disorder. Additionally, little is known regarding the significance of quantitative ASD symptomatology in infancy, a critical age for early identification and intervention. Given that ASD symptoms vary extensively and that multiple criteria must be met for a diagnosis, it is worth exploring how the extent of symptomatology, whether in the presence of a diagnosis or not, relates to a range of behaviors important for development. The current study aims to further this area of research by examining relations between quantitative ASD symptomatology and one such area of functioning: executive function (EF) abilities. To our knowledge, this link has not been explored in previous research.
Executive function (EF) is broadly defined as the higher-order cognitive processes that underlie goal-directed behavior (Hughes & Ensor, 2005). These processes are controlled by frontal areas of the brain and neural networks that inhibit automatic responses for efficiently executing a goal-directed action or task (Miller & Cohen, 2001). A number of models exist to explain the structure and purpose of EF in early childhood. For example, some researchers argue that EF in this age range represents a single executive control factor that spans multiple domains (Wiebe et al., 2011; Wiebe et al., 2008). In contrast, a prominent framework of EF across the lifespan divides the construct into three distinct components or factors: working memory, response inhibition, and set shifting (Garon et al., 2008; Miyake et al., 2000). Research has confirmed these factors in both older children and adults (Fisk & Sharp, 2004; Friedman et al., 2008; Lehto et al., 2003). The three-factor model has been applied to young children (Garon et al., 2008), and studies have confirmed factor loading of EF measures on these constructs (e.g., Müller et al., 2012).
Despite differences in perspectives on the structure of EF, researchers agree on the importance of the preschool years in the development of these skills along with the development of language, social competence, self-regulation, symbolic thought, and more (e.g., Carlson, 2005; Liebermann et al., 2007; Müller et al., 2012; Riggs et al., 2006). In fact, such rapid increases in a wide range of cognitive abilities only exacerbate the complexity of defining EF and the difficulty of measuring it. There are a number of tasks assessing aspects of EF during toddlerhood, but these tasks have shown mixed results, especially in the younger ages, due to the higher cognitive demands associated with many EF assessments (see, for example, Carlson, 2005). Difficulty arises from achieving the delicate balance of finding tasks that not only interest (and entertain) toddlers but also uniquely tap individual aspects of EF. As such, any task attempting to measure EF as either a single factor or multiple factors inherently measures individual differences in a range of other cognitive abilities, thus suffering from a problem of “task impurity” (Miyake et al., 2000). Further complicating the research on EF is the lack of a truly developmental understanding of the nature and early trajectory of these skills. Researchers have examined EF abilities during early childhood (i.e., ages 2 through 5 years), through the school years, and into adulthood, but we remain in the dark about the processes underlying the developmental gains in EF.
EF is a commonly studied construct in samples of children and adults diagnosed with ASD. In fact, an entire branch of the literature has suggested that all of the cognitive and social deficits evident in individuals with ASD are due to overarching deficits in EF (Hill, 2004; Hughes et al., 1994). This theory focuses on the rigidity and invariance of many of the behaviors typically associated with ASD and explains these behaviors as an inability to execute higher-order cognitive functions. For example, the tendency for individuals with ASD to become “stuck” while performing an action and the repetitive and stereotypical behaviors associated with ASD are viewed as an inability to flexibly shift attention between stimuli (Hill, 2004; Hughes et al., 1994; Pennington & Ozonoff, 1996; Pennington et al., 1997). Although most researchers now agree that ASD is far more complex than can be explained by this notion of “executive dysfunction,” the close ties between ASD and EF present an intriguing set of questions for exploring this relationship as early as possible.
A number of studies have linked ASD to deficits in EF, looking specifically at prefrontal cortex development (Bishop, 1993; Gilbert et al., 2008; Just et al., 2007; Ozonoff et al., 1991). Because prefrontal cortex maturation occurs around 12 months of age or later, it stands to reason that many manifestations of ASD related to deficits in EF cannot be recognized until after this age. Although there is a considerable literature on infant siblings of children with ASD, with results based on later diagnoses, little research has demonstrated concrete relations between ASD (or high risk for a diagnosis) in infants or toddlers and early EF abilities. Further, it remains unclear how the degree of ASD symptomatology, outside of the presence of a diagnosis, affects EF abilities in a community-based sample. This link is especially critical to establish in younger ages, given the importance of early detection and intervention.
The First Year Inventory 2.0 (FYI 2.0; Baranek et al., 2003; Reznick et al., 2007), a parent-report tool, was designed to identify infants at 12 months who are at risk for an eventual diagnosis of ASD. Researchers obtained completed FYIs from the parents of more than 8,700 children over the course of a multiyear study. Infants who scored above a certain criteria on two domains of risk (Social-Communication and Sensory-Regulatory) were flagged as “at risk” for an eventual diagnosis of ASD and were invited to participate in the Early Development Project-2, a randomized controlled trial of an early intervention (Watson et al., 2017). Infants whose FYI 2.0 scores did not meet the “at risk” criteria were available for other research projects, and a subset of parents of these children constituted the sample of the present study. In this community sample study, we examined the relation between infant risk for ASD and early childhood EF abilities. We hypothesized that this link would be at least partially mediated by parent-reported ASD symptomatology at preschool age, but that there would be unique features of early risk behaviors specifically tied to later EF deficits.
Methods
Participants
Parents were recruited from the EDP-2 FYI database (i.e., all returned FYIs for the Watson et al., 2017 study) when their children were within one month of 42 months (3.5 years) of age. The database included contact information for parents who filled out the FYI 2.0 when their children were 12 months and who agreed to be contacted for follow-up studies. Prior to recruitment for the present study, infants who met the dual-domain risk criteria for invitation to the EDP-2 intervention study (whether or not they participated in the intervention) were removed from the database. Parents and their children were recruited via phone call by trained research assistants and then emailed a link to our online surveys. Reminder emails were sent up to two times as necessary. Of the 618 parents contacted (i.e., with whom we were able to speak), most agreed to complete the online surveys (N = 585).
At least partially completed survey data were obtained from 82% of parents who agreed to participate (N = 479), with 79.3% of these containing complete survey data (N =380). All surveys were completed within one month of children turning 42 months (3.5 years) of age. The surveys included data for approximately the same number of males and females (n = 246 males, 51.4%), and the majority of the sample was White (n = 423, 88.7%). The vast majority of respondents were the mothers of the target children. Most mothers had at least a 4-year college degree (n = 421, 90%). Almost half of the overall sample had completed some post-graduate education (n = 227, 48.5%). The sample included families of relatively high socioeconomic status, with over half of the parents reporting annual incomes of more than $90,000 (n = 270, 57.1%). A number of parents reported that their child had received a diagnosis of ASD (n = 4), a sensory processing disorder (n = 5), a communication disorder (n = 17), and/or something else (n = 11).
Measures
The First Year Inventory 2.0 (FYI 2.0; Baranek et al., 2003):
The FYI 2.0 is a 63-item parent-report questionnaire measuring 12-month-olds’ behaviors representing two domains of behaviors relevant to ASD: Social-Communication (S-C) and Sensory-Regulatory (S-R). Each of the domains was subdivided into four constructs (Reznick et al., 2007): S-C included Social Orienting and Receptive Communication, Social-Affective Engagement, Imitation, and Expressive Communication; S-R included Sensory Processing, Regulatory Patterns, Reactivity, and Repetitive Behavior. Most items (n = 46) are rated on a 4-point scale (never, seldom, sometimes, often), and there are also 14 multiple choice items, two open-ended questions inquiring about concerns and physical/medical characteristics of the child, and one item asking about consonant sounds produced by the child. The FYI 2.0 generated risk scores for the following outcomes: S-C, S-R, total risk, and risk percentile. Risk scores were based on points (0, 1, or 2) assigned for specific answers to questions, derived from the rarity with which parents endorsed a response associated with higher risk for ASD. For example, 1% of parents of 12-month-olds said that their infant “never” looked at the parent’s face for comfort, and only 6% of parents said that their infant “seldom” did so; thus, 2 risk points were assigned to the very rare response of “never” and 1 risk point was assigned to the relatively rare response of “seldom” for this item. However, 40% of parents said that their infant “sometimes” imitated body movements and 53% said that their child “often” imitated body movements; due to the frequency with which parents endorsed both of these responses, neither was assigned any risk points. For detailed information regarding the creation and scoring of the FYI 2.0, along with recruitment for the samples used to design the measure, refer to Reznick et al. (2007), Turner-Brown et al. (2013), and Watson et al. (2007).
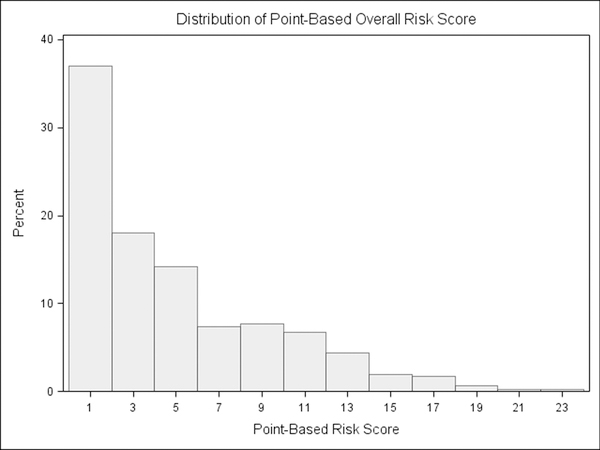
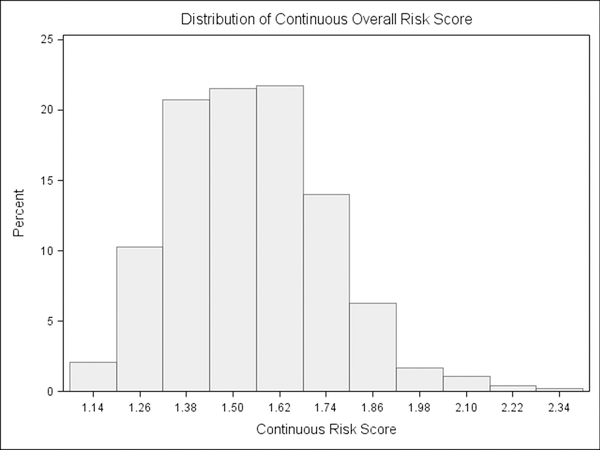
For the current study, the scoring of FYI 2.0 risk was adjusted to establish variables in line with a dimensional approach to ASD symptomatology. Instead of assigning risk points, a continuous scale (scores of 1 through 4 for each item) was used, such that each parent response was assigned a value, and risk scores were calculated as the mean of items in each domain. This method resulted in risk variables showing a range of variability (see Table 1) and distributions better suited for a dimensional approach (see Figure 1a & 1b). Whereas the original scoring reflected the degree to which a parent described a child’s behavior using a rare or relatively rare response, the continuous scoring accounts for the full range of potential ratings (1 through 4) for each item. This scoring algorithm was previously used in establishing a new set of constructs from the FYI 2.0 (Stephens et al., 2017). Similar to original scoring valence, higher continuous scores represent greater risk of a later ASD diagnosis.
Table 1.
Psychometric properties of original and dimensional FYI 2.0 scoring distributions
| Original scoring | Continuous scoring | |
|---|---|---|
| Total Risk | ||
| Mean, SD | 4.68, 4.65 | 1.54, 0.20 |
| Median | 3.63 | 1.53 |
| Skewness, Kurtosis | 1.13, 0.81 | 0.59, 0.68 |
| Range | 0–23.75 | 1.10–2.33 |
| Social-Communication Risk | ||
| Mean, SD | 5.56, 7.10 | 1.39, 0.28 |
| Median | 3.25 | 1.33 |
| Skewness, Kurtosis | 1.78, 3.72 | 1.18, 2.35 |
| Range | 0–45.50 | 1.00–2.76 |
| Sensory-Regulatory Risk | ||
| Mean, SD | 3.80, 5.12 | 1.70, 0.24 |
| Median | 2.00 | 1.69 |
| Skewness, Kurtosis | 1.46, 1.59 | 0.11, −0.12 |
| Range | 0–24.25 | 1.06–2.48 |
Note. FYI 2.0 = First Year Inventory 2.0
Figure 1a.
Example distribution of First Year Inventory 2.0 original point-based risk scoring
Figure 1b.
Example distribution of First Year Inventory 2.0 continuous risk scoring
Behavior Rating Inventory of Executive Function – Preschool Version (BRIEF-P; Gioia et al., 2003):
This measure is a 63-item inventory that assesses aspects of EF in preschool-aged children. Parents in this study completed the BRIEF-P when their children were 42 months of age. Parents rate their child on a 3-point Likert scale (never, sometimes, often), with higher scores on each scale representing more severe deficits in EF. The BRIEF-P reports a Global Executive Composite (GEC) score, which sums responses for all items in the measure. Additionally, the BRIEF-P includes subscales for Inhibit, Shift, Emotional Control, Working Memory, and Plan/Organize, as well as an Emergent Metacognition Index (EMI: Plan/Organize + Working Memory), an Inhibitory Self-Control Index (ISCI: Inhibit + Emotional Control), and a Flexibility Index (FI: Shift + Emotional Control). The composite score (GEC) and indices (EMI, ISCI, and FI) were the primary outcomes of interest for this study, whereas individual subscales were considered for exploratory purposes in bivariate correlation analyses.
Social Responsiveness Scale, Second Edition (SRS-2.0; Constantino & Gruber, 2012):
This scale is designed to measure social deficits and can be used with children as young as 2.5 years. In this study, parents completed the SRS when their children were 42 months of age. The SRS-2.0 yields a total raw score, with higher values indicating more severe social impairment (consistent with ASD symptomatology). This measure includes 65 items scored on a 4-point Likert scale ranging from ‘Not True’ to ‘Almost Always True.’ The SRS-2.0 was used in the current study as a dimensional proxy of ASD symptomatology to look at the consistency of symptoms over time and to determine whether infant risk uniquely predicted EF, above and beyond later ASD symptomatology.
Data Analysis
First, bivariate correlations were calculated to explore relations among all FYI 2.0 variables and outcome variables from the BRIEF-P and the SRS-2.0. Next, we examined all variables in relation to demographic variables of gender, household education, and maternal education. Independent samples t-tests were run to examine gender differences, and general linear models were used to determine relations with maternal education and income. Both maternal education and income were coded as categorical variables. Mothers’ highest level of education was selected from a list of increasing educational attainment (1=less than 8th grade through 9=professional degree (MD, PhD, JD)). Total household income was also rated on an increasing scale (1=less than $5,000 through 11=more than $250,000). Child gender, maternal education, and household income were subsequently included as covariates in all additional analyses. Linear regression models were fit to estimate relations between FYI 2.0 variables and BRIEF-P outcomes (including covariates). In order to determine the relation between infant ASD risk and EF above and beyond preschool ASD symptomatology, another set of regression models were fit, controlling for SRS-2.0 total score. To further probe significant results, additional linear regression models were fit using individual FYI 2.0 subscales as predictors. Given the number of children with at least one diagnosis (n = 30), all analyses were repeated, excluding all children with reported diagnoses. There were no differences in the magnitude or significance of results; therefore, results presented here include all children, in order to maintain a larger sample with an increased range of abilities and behaviors. All analyses were conducted using SAS 9.4.
Results
As a first step, we calculated correlations among FYI 2.0 risk domains and 42-month outcome scores. All three measures (the FYI 2.0, BRIEF-P, and SRS-2.0) have the same scoring valence: higher scores represent greater levels of deficits or risk. Therefore, we expected to see strong positive correlations among scales due to the established relation among behaviors and the consistency in measurement (parent-report). This hypothesis was supported, with correlations ranging in strength (r’s = .10-.33) but all being positive and statistically significant (p < .05; see Table 2).
Table 2.
Bivariate correlations among variables from the FYI 2.0, the BRIEF-P, and the SRS-2.0
| FYI 2.0 | Total Risk | S-C Risk | S-R Risk |
|---|---|---|---|
| BRIEF-P Composite & Indices | |||
| Global Executive Composite | .33 | .28 | .28 |
| Flexibility Index | .19 | .13** | .13** |
| Inhibitory Self-Control Index | .33 | .31 | .31 |
| Emergent Metacognition Index | .31 | .21 | .27 |
| BRIEF-P Subscales | |||
| Inhibit | .25 | .13** | .27 |
| Shift | .25 | .13** | .26 |
| Emotional Control | .24 | .10* | .30 |
| Working Memory | .30 | .22 | .24 |
| Plan/Organize | .29 | .17 | .28 |
| SRS-2.0 Total Raw Score | .39 | .31 | .29 |
Note.
p < .05
p < .01; values in bold p < .001, FYI 2.0 = First Year Inventory; BRIEF-P = Behavior Rating Inventory of Executive Function – Preschool version; SRS-2.0 = Social Responsiveness Scale – 2nd edition; S-C Risk = Social-Communication Risk; S-R Risk = Sensory-Regulatory Risk
Predictors and outcomes of interest were analyzed for differences based on child gender, household income, and maternal education. Both the FYI 2.0 overall risk score and the S-C risk score differed significantly by gender, t(475) = 3.23, p < .01 and t(475) = 3.50, p < .01, respectively. No other variables from the FYI 2.0, BRIEF-P, or SRS-2.0 significantly differed between genders. There were no significant differences in scores at either 12 or 42 months based on income or maternal education.
Next, linear regression models were run to establish the predictive value of FYI 2.0 continuous risk scores on BRIEF-P variables controlling for child gender, maternal education, and household income (see Table 3). All models were statistically significant, p < .05. Since both FYI 2.0 risk domains (S-C and S-R) significantly predicted all BRIEF-P outcomes individually, we analyzed the relative predictive value of these scores by including both in regression models. Both S-C and S-R remained significant in the models predicting the Global Executive Composite (GEC) and Emergent Metacognition Index (EMI). Only S-R significantly predicted the Inhibitory Self-Control Index (ISCI) and the Flexibility Index (FI).
Table 3.
Regression models predicting 42-month BRIEF-P scores from continuous FYI 2.0 risk
| Global Executive Composite B, SE |
Inhibitory Self-Control Index B, SE |
Flexibility Index B, SE |
Emergent Metacognition Index B, SE |
|
|---|---|---|---|---|
| Overall Risk | 25.85*, 3.56 | 10.30*, 1.70 | 8.26*, 1.33 | 11.54*, 1.68 |
| Social-Communication Risk | 9.85*, 2.62 | 2.91, 1.24 | 2.44, 0.97 | 5.57*, 1.22 |
| Sensory-Regulatory Risk | 21.88*, 2.94 | 10.11*, 1.39 | 7.97*, 1.08 | 8.17*, 1.41 |
Note. All models statistically significant, p <.05
p < .001. All models include covariates of child gender, maternal education, and household income. FYI 2.0 = First Year Inventory; BRIEF-P = Behavior Rating Inventory of Executive Function – Preschool version
We were interested in the predictive value of FYI 2.0 scores on 42-month EF above and beyond the level of SRS-2.0 scores. SRS-2.0 scores were significantly predicted by FYI 2.0 overall risk (β = 29.69, SE = 3.29) and both risk domains (S-C Risk: β = 16.20, SE = 2.40, sr2 = .095; S-R Risk: β = 18.26, SE = 2.83, sr2 = .089), all p < .0001, while controlling for gender, household income, and maternal education. These results suggest consistency between ASD risk at 12 months and symptom severity at 42 months. We then calculated regression models predicting 42-month EF from FYI 2.0 risk, controlling again for gender, income, and maternal education and this time also controlling for SRS-2.0 raw score (see Table 4). The relation between overall FYI 2.0 risk and EMI trended toward significance (p = .08); however, no other outcomes of overall risk remained significant. S-C risk significantly predicted ISCI and moderately predicted FI; however, these relations were in the opposite direction from expected, and after Bonferroni correction for multiple comparisons, were no longer significant. S-R risk significantly predicted all of the BRIEF-P outcomes, above and beyond the effect of SRS-2.0 scores. All models predicting BRIEF-P scores from S-R risk with the exception of the EMI outcome remained significant after Bonferroni correction. These results suggest that the S-R aspects of dimensional ASD risk may be directly linked to EF deficits beyond primary deficits as measured by the SRS-2.0.
Table 4.
Regression models predicting 42-month BRIEF-P scores from continuous FYI 2.0 risk, controlling for 42-month SRS-2.0 scores
| Global Executive Composite B, SE |
Inhibitory Self-Control Index B, SE |
Flexibility Index B, SE |
Emergent Metacognition Index B, SE |
|
|---|---|---|---|---|
| Overall Risk | 4.38, 3.08 | 1.27, 1.59 | 1.47, 1.26 | 2.73+, 1.57 |
| Social-Communication Risk | −2.24, 2.09 | −2.26*, 1.08 | −1.50+, 0.85 | 0.71, 1.07 |
| Sensory-Regulatory Risk | 8.57**, 2.41 | 4.69**, 1.24 | 3.90**, 0.98 | 2.46*, 1.25 |
Note.
p < .10,
p < .05
p < .01. All models include covariates of child gender, maternal education, and household income (in addition to 42-month SRS-2.0 scores). FYI 2.0 = First Year Inventory; BRIEF-P = Behavior Rating Inventory of Executive Function – Preschool version; SRS-2.0 = Social Responsiveness Scale – 2nd edition
To further explore the relation of the 12-month S-R domain scores with 42-month EF, we analyzed each of the S-R subscales: Sensory Processing, Regulatory Patterns, Reactivity, and Repetitive Behavior. Bivariate correlation analyses suggest that all of these variables, aside from Regulatory Patterns, are significantly associated with the outcome variables, but to different degrees (see Table 5). Additional regression analyses controlling for selected covariates (gender, maternal education, and income) and SRS-2.0 scores were calculated. When individually entered into the regression models, Sensory Processing and Reactivity significantly predicted GEC. When all four FYI 2.0 S-R subscales were included in the same model, only Reactivity remained significant for all outcome variables except for EMI. See Table 6 for detailed analyses of S-R subscales and BRIEF-P indices.
Table 5.
Bivariate correlations between FYI 2.0 Sensory-Regulatory subscales and BRIEF-P outcomes
| Global Executive Composite | Inhibitory Self-Control Index | Flexibility Index | Emergent Metacognition Index | |
|---|---|---|---|---|
| Sensory Processing | .30*** | .28*** | .26*** | .26*** |
| Regulatory Patterns | .05 | .06 | .07 | .02 |
| Reactivity | .18*** | .21*** | .25*** | .11* |
| Repetitive Behavior | .27*** | .23*** | .18*** | .28*** |
Note
p < .05
p < .001; FYI 2.0 = First Year Inventory; BRIEF-P = Behavior Rating Inventory of Executive Function – Preschool version
Table 6.
Regression models predicting 42-month BRIEF-P scores from continuous FYI 2.0 Sensory-Regulatory risk scales, controlling for 42-month SRS-2.0 scores
| Global Executive Composite B, SE |
Inhibitory Self-Control Index B, SE |
Flexibility Index B, SE |
Emergent Metacognition Index B, SE |
|
|---|---|---|---|---|
| Sensory Processing | 4.46*, 1.67 | 2.11*, 0.86 | 1.77*, 0.68 | 1.57+, 0.86 |
| Reactivity | 4.48***, 1.23 | 2.79***, 0.63 | 2.58***, 0.50 | 0.89, 0.64 |
| Repetitive Behaviors | 2.32, 1.59 | 0.74, 0.82 | −0.09, 0.65 | 1.61*, 0.81 |
| Regulatory Patterns | 0.89, 1.55 | 0.73, 0.80 | 0.86, 0.63 | −0.27, 0.79 |
Note.
p < .10
p < .05
p < .001; values in italics represent those variables that remained significant in regression models including all four FYI S-R subscales. All models include covariates of child gender, maternal education, and household income (in addition to 42-month SRS-2.0 scores). FYI 2.0 = First Year Inventory; BRIEF-P = Behavior Rating Inventory of Executive Function – Preschool version; SRS-2.0 = Social Responsiveness Scale – 2nd edition
Discussion
The goal of the current study was to analyze the relation between 12-month behaviors associated with risk for an eventual diagnosis of ASD and EF abilities, measured by parent-report, at 42-months (3.5 years). Although overall risk as well as individual risk domains were significantly related to executive dysfunction, when controlling for scores on a widely used measure of ASD symptomatology at 42 months, only 12-month sensory-regulatory risk was associated with 42-month EF. This finding may be partially explained by the overlap in content between the SRS and the S-C scale (i.e., social behaviors); however, it should be noted that the correlations between each FYI 2.0 scale and the SRS-2.0 scores were very similar (.31 and .29 for S-C and S-R, respectively). Squared semi-partial correlation values for these risk variables in models predicting SRS-2.0 scores were also similar in magnitude (.095 and .088 for S-C and S-R, respectively), and these moderate values suggest that there is a great deal of variance in SRS-2.0 scores that cannot be accounted for solely by the overlap with infant ASD risk in the social domain. These results suggest that even for a community-based sample of children, infant behaviors related to risk for ASD, especially those related to sensory experiences, may be indicative of later struggles with EF. The link between an ASD diagnosis and difficulties with EF is well-established, and the current study results 1) indicate that the link between ASD symptomatology and EF deficits may exist even earlier than previously established, and 2) provide additional support for researching ASD as a dimensional, as opposed to a binary, construct.
This research establishes specific links between early sensory abnormalities, which are a fundamental aspect of ASD and thought to develop very early, and later cognitive abilities that develop later in early childhood. These results emphasize the importance of looking at sensory and regulatory behaviors related to ASD in addition to (not instead of) social or communication difficulties. By distinguishing different types of early patterns of behaviors, interventions targeting specific areas of difficulty are much more likely to result in improved outcomes.
The S-R risk domain is composed of scores from four separate FYI 2.0 subscales: Sensory Processing, Regulatory Patterns, Reactivity, and Repetitive Behavior. By looking at these variables individually in relation to later EF, Sensory Processing and Reactivity significantly predicted parent-reported EF deficits even when controlling for autism symptom severity at 42 months. Although bivariate correlations also suggest that infant repetitive behaviors are related to EF, early repetitive behaviors did not significantly predict EF outcomes when including all covariates. A lack of significant findings here is likely due to the SRS-2.0 containing items directly related to repetitive or stereotyped behaviors. Regulatory patterns, as reported by parents, did not seem to relate to later EF. Substantial research has established a link between difficulties in sleep regulation and ASD (Cortesi et al., 2010; Kotagal & Broomall, 2012; Souders et al., 2009); however, these problems may not be directly related to cognitive deficits also associated with ASD (Krakowiak et al., 2008; Richdale & Schreck, 2009). This finding should also be interpreted with caution, as the Regulatory Pattern subscale only contains four items.
Previous research has examined links between sensory processing and later cognition in children with ASD, suggesting that the extent of sensory abnormalities may be directly related to academic achievement (Ashburner et al., 2008). Additional research specifically measuring EF as a primary outcome suggests that the nature and frequency of repetitive behaviors significantly negatively affected EF performance (Boyd et al., 2009; Lopez et al., 2005; South et al., 2007). To our knowledge, however, none of the existing research has examined these patterns in children as young as in infancy or toddlerhood. Further, the vast majority of this research has focused on individuals already diagnosed with ASD. There is limited evidence regarding any links either prior to diagnosis or across a range of development reflected in continuous measures of ASD symptomatology.
A wealth of existing research has explored the link between early emotional reactivity and later cognition, specifically EF development. Although most of this is from a temperament perspective, findings consistently support a strong relationship between a child’s ability to regulate emotional reactivity and EF performance (Ursache et al., 2012). While the FYI 2.0 does not specifically measure self-regulation, various subdomains of sensory regulation, including sensory reactivity, were significantly related to early childhood EF. While the S-R subscales available on the FYI provide interesting deeper exploration of the nature of associations between early sensory regulation and later EF, care should be taken to not over-interpret results, especially since three of the four subscales discussed here contain five or fewer items. These results present a broader picture of the links between infant sensory and regulatory behaviors and early childhood EF, but future research is required to tease out the specific relations among these individual behavioral patterns and developing cognition.
The primary limitation of this study is its reliance on parent-report measures. While this method of measurement allows for larger samples across longer periods of time, results should be interpreted with some caution. Future research should explore EF using laboratory-based assessments to determine that these relations extend beyond parent observation. Another concern is the sample homogeneity. The majority of parents who participated in this study were White and had high levels of education and household income. Previous research with the FYI 2.0 found effects of both race and maternal education on risk scores (Reznick et al., 2007). Additionally, substantial research on EF in childhood has found effects of SES (Lawson et al., 2018); therefore it is likely that a more diverse sample in our study would have yielded similar patterns of association between SES and EF. While the homogenous nature of the sample limits generalizability, the present findings also suggest that a more diverse sample may present with a larger range of behaviors and potentially yield greater magnitudes of association than currently found.
An additional caveat is the use of the SRS-2.0 as a pure measure of ASD symptom severity. Although this particular measure is considered a strong indicator of ASD characteristics across a range of development, its primary focus is on social impairment. Therefore, we would expect to see a great deal of overlap between the SRS-2.0 and the S-C scale of the FYI 2.0. Whereas our findings regarding risk specifically associated with sensory-regulatory deficits and later EF are interesting, it is important to note that our findings may be indicative of an overlap in the behaviors being measured by the SRS-2.0 and the S-C domain. As previously discussed, however, the bivariate correlations and squared semi-partial correlations comparing each of the risk scales with the SRS-2.0 were roughly the same.
Conclusions
The goal of the current study was to explore the relations between quantitative infant risk for ASD and early childhood EF. Both domains of risk defined by the FYI 2.0, Social-Communication and Sensory-Regulatory, significantly predicted parent-reported EF behaviors at 42 months (3.5 years), and infant S-R risk predicted EF deficits even when controlling for overall preschool ASD symptomatology. These results support previously established links between ASD and difficulties in EF. Additionally, we extend previous findings to ASD risk as measured in infancy and to a community-based sample that represents a range of developmental abilities. Our findings suggest that EF deficits in ASD may be more strongly tied to certain characteristics of ASD as opposed to the disorder broadly, and future research should explore these relations across different ages, demographics, and types of measures. These patterns have potential implications for the design and implementation of early intervention services and provide further support for the value of studying behaviors related to ASD from a dimensional perspective.
Acknowledgements:
We’d like to extend our gratitude to the many North Carolina families who participated in this research. Additionally, we are grateful to the Program for Early Autism Research, Leadership & Service (PEARLS) team, including co-investigators Grace Baranek, PhD and Lauren Turner-Brown, PhD, for their role in the EDP-2 study and to the undergraduate researchers at the University of North Carolina at Chapel Hill who assisted with the follow-up study. This research was supported by the National Institutes of Health (T32-MH106440 to Dr. Stephens), and by a grant from the Institute of Education Sciences, U.S. Department of Education (R324A100305) to the University of North Carolina at Chapel Hill. The opinions expressed are those of the authors and do not represent views of the funding agencies.
Footnotes
Conflict of Interest Statement: The authors of this manuscript report no sources of conflict of interest.
References
- Ashburner J, Ziviani J, & Rodger S (2008). Sensory Processing and Educational Outcomes in Children With Autism Spectrum Disorder. American Journal of Occupational Therapy, 62(5), 564–573. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Crais E, & Reznick JS (2003). First-Year Inventory (FYI) 2.0 Chapel Hill, NC: University of North Carolina at Chapel Hill. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, & Clubley E (2001). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Ben Itzchak E, & Zachor DA (2011). Who benefits from early intervention in autism spectrum disorders? Research in Autism Spectrum Disorders, 5(1), 345–350. [Google Scholar]
- Bishop DVM (1993). Annotation: Autism, executive functions and theory of mind: A neuropsychological perspective. Journal of Child Psychology and Psychiatry, 34(3), 279–293. [DOI] [PubMed] [Google Scholar]
- Boyd BA, McBee M, Holtzclaw T, Baranek GT, & Bodfish JW (2009). Relationships among repetitive behaviors, sensory features, and executive functions in high functioning autism. Research in Autism Spectrum Disorders, 3(4), 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, Odom SL, Humphreys BP, & Sam AM (2010). Infants and Toddlers With Autism Spectrum Disorder: Early Identification and Early Intervention. Journal of Early Intervention, 32(2), 75–98. [Google Scholar]
- Carlson SM (2005). Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology, 28(2), 595–616. [DOI] [PubMed] [Google Scholar]
- Constantino JN (2011). The quantitative nature of autistic social impairment. Pediatric Research, 69(5 Pt 2), 55R–62R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2002). The social responsiveness scale Los Angeles: Western Psychological Services. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale, Second Edition (SRS-2) Los Angeles: Western Psychological Services. [Google Scholar]
- Constantino JN, & Todd RD (2003). Autistic Traits in the General Population. Archives of General Psychiatry, 60(5), 524–530. [DOI] [PubMed] [Google Scholar]
- Cortesi F, Giannotti F, Ivanenko A, & Johnson K (2010). Sleep in children with autistic spectrum disorder. Sleep Medicine, 11(7), 659–664. [DOI] [PubMed] [Google Scholar]
- Dawson G (2008). Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology, 20(3), 775–803. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, … Varley J (2010). Randomized, Controlled Trial of an Intervention for Toddlers With Autism: The Early Start Denver Model. Pediatrics, 125(1), e17–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Munson J, Rogers SJ, Greenson J, Winter J, & Dawson G (2015). Long-Term Outcomes of Early Intervention in 6-Year-Old Children With Autism Spectrum Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 54(7), 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JE, & Sharp CA (2004). Age-related impairment in executive functioning: Updating, inhibition, shifting, and access. Journal of Clinical and Experimental Neuropsychology, 26(7), 874–890. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General, 137(2), 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, & Smith IM (2008). Executive function in preschoolers: a review using an integrative framework. Psychological Bulletin, 134(1), 31–60. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Bird G, Brindley R, Frith CD, & Burgess PW (2008). Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia, 46(9), 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, & Isquith PK (2003). BRIEF-P: Behavior Rating Inventory of Executive Function--preschool Version: Professional Manual. Psychological Assessment Resources [Google Scholar]
- Hill EL (2004). Evaluating the theory of executive dysfunction in autism. Developmental Review, 24(2), 189–233. [Google Scholar]
- Hughes C, & Ensor R (2005). Executive function and theory of mind in 2 year olds: A family affair? Developmental Neuropsychology, 28(2), 645–668. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, & Robbins TW (1994). Evidence for executive dysfunction in autism. Neuropsychologia, 32(4), 477–492. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, & Minshew NJ (2007). Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex, 17(4), 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL, Ashbaugh K, & Bradshaw J (2014). The importance of early identification and intervention for children with or at risk for autism spectrum disorders. International Journal of Speech-Language Pathology, 16(1), 50–56. [DOI] [PubMed] [Google Scholar]
- Kotagal S, & Broomall E (2012). Sleep in children with autism spectrum disorder. Pediatric Neurology, 47(4), 242–251. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, & Hansen RL (2008). Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research, 17(2), 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, & Kalb LG (2012). Long-term Outcomes of Toddlers With Autism Spectrum Disorders Exposed to Short-term Intervention. Pediatrics, 130(Supplement 2), S186–S190. [DOI] [PubMed] [Google Scholar]
- Lawson GM, Hook CJ, & Farah MJ (2018). A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Developmental Science, 21(2), e12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto JE, Juujärvi P, Kooistra L, & Pulkkinen L (2003). Dimensions of executive functioning: Evidence from children. British Journal of Developmental Psychology, 21(1), 59–80. [Google Scholar]
- Liebermann D, Giesbrecht GF, & Müller U (2007). Cognitive and emotional aspects of self-regulation in preschoolers. Cognitive Development, 22(4), 511–529. [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, & Lai Z (2005). Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. Journal of Autism and Developmental Disorders, 35(4), 445–460. [DOI] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Müller U, Liebermann-Finestone DP, Carpendale JIM, Hammond SI, & Bibok MB (2012). Knowing minds, controlling actions: The developmental relations between theory of mind and executive function from 2 to 4 years of age. Journal of Experimental Child Psychology, 111(2), 331–348. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, & Rogers SJ (1991). Executive function deficits in high‐functioning autistic individuals: relationship to theory of mind. Journal of Child Psychology and Psychiatry, 32(7), 1081–1105. [DOI] [PubMed] [Google Scholar]
- Pennington BF, & Ozonoff S (1996). Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, 37(1), 51–87. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Rogers SJ, Bennetto L, Griffith EM, Reed DT, & Shyu V (1997). Validity tests of the executive dysfunction hypothesis of autism. In Russell J (Ed.), Autism as an executive disorder New York, NY, US: Oxford University Press, pp. 143–178. [Google Scholar]
- Reznick JS, Baranek GT, Reavis S, Watson LR, & Crais ER (2007). A parent-report instrument for identifying one-year-olds at risk for an eventual diagnosis of autism: the first year inventory. Journal of Autism and Developmental Disorders, 37(9), 1691–1710. [DOI] [PubMed] [Google Scholar]
- Richdale AL, & Schreck KA (2009). Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews, 13(6), 403–411. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Jahromi LB, Razza RP, Dillworth-Bart JE, & Mueller U (2006). Executive function and the promotion of social–emotional competence. Journal of Applied Developmental Psychology, 27(4), 300–309. [Google Scholar]
- Robertson AE, & Simmons DR (2013). The relationship between sensory sensitivity and autistic traits in the general population. Journal of Autism and Developmental Disorders, 43(4), 775–784. [DOI] [PubMed] [Google Scholar]
- Rogers SJ (1996). Brief report: early intervention in autism. Journal of Autism and Developmental Disorders, 26(2), 243–246. [DOI] [PubMed] [Google Scholar]
- Ruzich E, Allison C, Smith P, Watson P, Auyeung B, Ring H, & Baron-Cohen S (2015). Measuring autistic traits in the general population: a systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Molecular Autism, 6(2), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders MC, Mason TBA, Valladares O, Bucan M, Levy SE, Mandell DS, … Pinto-Martin J (2009). Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep, 32(12), 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Ozonoff S, & McMahon WM (2007). The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism : The International Journal of Research and Practice, 11(5), 437–451. [DOI] [PubMed] [Google Scholar]
- Stephens RL, Sabatos-DeVito M, & Reznick JS (2017). The Development and Validation of Attention Constructs From the First Year Inventory. Psychological Assessment, 29(5), 568–581. [DOI] [PubMed] [Google Scholar]
- Turner-Brown LM, Baranek GT, Reznick JS, Watson LR, & Crais ER (2013). The First Year Inventory: a longitudinal follow-up of 12-month-old to 3-year-old children. Autism : The International Journal of Research and Practice, 17(5), 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, Blair C, Stifter C, & Voegtline K (2012). Emotional Reactivity and Regulation in Infancy Interact to Predict Executive Functioning in Early Childhood. Developmental Psychology, 49(1), 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Crais ER, Reznick JS, Dykstra J, & Perryman T (2007). The first year inventory: retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. Journal of Autism and Developmental Disorders, 37(1), 49–61. [DOI] [PubMed] [Google Scholar]
- Watson LR, Crais ER, Baranek GT, Turner-Brown L, Sideris J, Wakeford L, … Nowell SW (2017). Parent-Mediated Intervention for One-Year-Olds Screened as At-Risk for Autism Spectrum Disorder: A Randomized Controlled Trial. Journal of Autism and Developmental Disorders, 47(11), 3520–3540. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, & Charak D (2008). Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Developmental Psychology, 44(2), 575–587. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CAC, Chevalier N, & Espy KA (2011). The structure of executive function in 3-year-olds. Journal of Experimental Child Psychology, 108(3), 436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L (1988). The continuum of autistic characteristics. In: Schopler E, Mesibov GB, editors. Diagnosis and assessment in autism Boston, MA: Springer, pp. 91–110. [Google Scholar]