Abstract
Purpose:
There is considerable interest in very short (ultrahypofractionated) radiation therapy regimens to treat prostate cancer based on potential radiobiological advantages, patient convenience, and resource allocation benefits. Our objective is to demonstrate that detectable changes in health-related quality of life measured by the bowel and urinary domains of the Expanded Prostate Cancer Index Composite (EPIC-50) were not substantially worse than baseline scores.
Methods and Materials:
NRG Oncology’s RTOG 0938 is a nonblinded randomized phase 2 study of National Comprehensive Cancer Network low-risk prostate cancer in which each arm is compared with a historical control. Patients were randomized to 5 fractions (7.25 Gy in 2 weeks) or 12 fractions (4.3 Gy in 2.5 weeks). The coprimary endpoints were the proportion of patients with a change in EPIC-50 bowel score at 1 year (baseline to 1 year) >5 points and in EPIC-50 urinary score >2 points tested with a 1-sample binomial test.
Results:
The study enrolled 127 patients to 5 fractions (121 analyzed) and 128 patients to 12 fractions (125 analyzed). Median follow-up for all patients at the time of analysis was 3.8 years. The 1-year frequency for >5 point change in bowel score were 29.8% (P < .001) and 28.4% (P < .001) for 5 and 12 fractions, respectively. The 1-year frequencies for >2 point change in urinary score were 45.7% (P < .001) and 42.2% (P < .001) for 5 and 12 fractions, respectively. For 5 fractions, 32.9% of patients had a drop in 1-year EPIC-50 sexual score of ≥11 points (P = .34); for 12 fractions, 30.9% of patients had a drop in 1-year EPIC-50 sexual score of ≥ 11 points (P = .20). Disease-free survival at 2 years is 99.2% (95% confidence interval: 97.5-100) in the 5-fraction arm and 97.5% (95% confidence interval: 94.6-100) in the 12-fraction arm. There was no late grade 4 or 5 treatment-related urinary or bowel toxicity.
Conclusions:
This study confirms that, based on changes in bowel and urinary domains and toxicity (acute and late), the 5- and 12-fraction regimens are well tolerated. These ultrahypofractionated approaches need to be compared with current standard radiation therapy regimens.
Summary
The urinary and rectal quality of life outcomes reported by patients with prostate cancer undergoing 5- and 12-fraction prostate radiation therapy treatments are comparable to those with current standard 38-to 44-fraction radiation therapy treatments. These shorter radiation therapy treatments need to be compared with the current standard radiation therapy treatments in a larger study.
Introduction
Radiation therapy over 7.5 to 9 weeks is a common treatment option in the management of localized prostate cancer. Randomized clinical trials (RCTs) have confirmed that higher doses (2 Gy fractions) and high-precision radiation therapy techniques result in improved control of prostate-specific antigen (PSA) (1-3). There is considerable interest in treating patients with localized prostate cancer with hypofractionated regimens (HypoRT). Hypofractionation offers the advantage of fewer fractions being more convenient for patients and an economic benefit because more patients could be treated with the available resources. Subsequently, the possibility that hypofractionation may confer a therapeutic advantage was raised because of the low α/β ratio (1/4) of prostate cancer (4-10) relative to late tissue. Some clinicians use the term moderate HypoRT in reference to radiation therapy treatments in 20 to 28 fractions using 2.5 to 3 Gy fractions. To date, similar efficacy and toxicity data are available from 3 RCTs using moderate HypoRT (11-13). One of these studies showed slightly higher late grade 2 gastrointestinal and genitourinary toxicity (11).
Recently, investigators have evaluated shorter HypoRT (5-12 fractions) and have considered ultrahypofractionated regimens (UHRT). The reported UHRT results suggest that the acute and late side effects are acceptable, although the toxicity and efficacy outcomes of these regimens have not been compared with those of standard radiation therapy regimens (SRT) in an RCT (14-19). The availability of modern high-precision radiation therapy techniques enables UHRT to be given with a low incidence of acute and late side effects.
Patient-reported outcomes (PROs) using the Expanded Prostate Index-Cancer (EPIC-50) bowel and urinary scores have been reported by several investigators (20-22). The 50-item EPIC questionnaire is a robust validated prostate cancer patient-reported (PRO) questionnaire (22). Throughout this article, the term EPIC refers to the utilization of the EPIC-50 quality of life instrument. Some case series have reported on PROs using UHRT (23-26); this moderately sized multiinstitution study collected PRO data prospectively as per the study protocol. Based on published data, rectal and bladder scores decline during and for a few weeks after radiation therapy, but by 1 to 2 years, the scores return to near pretreatment levels. This study assessing bowel and urinary PROs was undertaken as a prelude to an RCT comparing UHRT (5-12 fractions) with SRT. If UHRT are found to be noninferior for important efficacy and toxicity endpoints, PROs become an important and relevant endpoint in determining whether UHRT will be used in clinical practice.
Methods and Materials
Randomization and masking
NRG Oncology’s RTOG 0938 (ClinicalTrials.gov #NCT01434290) is a nonblinded randomized phase 2 study of National Comprehensive Cancer Network low-risk localized prostate cancer in which each arm was compared with a historical control. Patients were randomized by the NRG Oncology Statistics and Data Management Center using an automated permuted block randomization scheme to receive UHRT to a dose of 36.25 Gy (5 fractions of 7.25 Gy in 2 weeks) or 51.6 Gy (12 fractions of 4.3 Gy in 2.5 weeks) (27). Patients were stratified according to radiation therapy treatment technique (Cyberknife vs intensity modulated radiation therapy [IMRT]/volumetric modulated arc therapy [VMAT] vs protons). Although this is a randomized phase 2 study, it is essentially 2 single-arm phase 2 studies accruing patients in parallel.
Study patients
Eligibility criteria included patients with prostate adenocarcinoma, Gleason scores of 2 to 6, cT1-2a, and PSA <10 ng/mL. Patients undergoing active surveillance who were rebiopsied and confirmed still to have low-risk disease were eligible for enrollment within 1 year of the repeat biopsy. Patients were only randomized if they were willing and able to complete the EPIC questionnaire. The institutional review board of each participating institution approved the study protocol. All patients were required to read and sign an informed consent document. Ineligibility criteria included prior or concurrent invasive malignancy (except nonmelanomatous skin cancer) or lymphomatous or hematogenous malignancy, unless continually disease-free for a minimum of 5 years). Patients with distant metastases; regional lymph node involvement; previous prostatectomy; cryosurgery; high-intensity focused ultrasound treatment; pelvic irradiation; prostate brachytherapy; bilateral orchiectomy or hormonal therapy, such as luteinizing hormonereleasing hormone agonists or antagonists; antiandrogens; estrogens; or previous or concurrent cytotoxic chemotherapy (for prostate cancer) were ineligible. Patients were also ineligible if they had used finasteride within 30 days or dutasteride within 30 to 90 days before registration. Patients with severe active comorbidities were ineligible.
Study treatment
Patients were treated using stereotactic body radiation therapy (SBRT) in 5 or 12 fractions (UHRT) and could be treated using CyberKnife, IMRT/VMAT techniques, or protons, as long as the protocol-specified dosimetry criteria were met. The dosimetry criteria included planning target volume coverage, normal tissue constraints for rectum, bladder, urethra, penile bulb, and femoral heads. The urethral dose was ≤107% of the prescription dose. The dosimetry criteria were identical in each arm regardless of treatment technique except for maximum dose within the planning target volume, which was 120% for CyberKnife technique and 107% for the other techniques (in recognition of achievable dose distributions with CyberKnife). The first 5 patients accrued from each institution were reviewed for quality assurance of protocol-defined dosimetry parameters. The dosimetry constraints were derived from published case series using high-precision techniques and UHRT regimens. Image guidance was required and is detailed in the study protocol.
Patient assessments
Pretreatment assessments included patient history and physical examination, performance status, PSA measurement, completion of EPIC and health-related quality-of-life questionnaires, and baseline toxicity assessment. Performance status and adverse events were captured weekly during radiation therapy. Performance status, physical examination (including digital rectal examination), PSA measurement, and adverse event evaluation were performed every 3 months for 2 years after randomization and then every 6 months until 5 years. The EPIC and health-related quality-of-life questionnaires were collected at baseline and at 1, 2, and 5 years from the end of radiation therapy.
Endpoints
The primary hypothesis of the study was that UHRT (5-12 fractions) would show bowel and urinary PROs not significantly worse than those with SRT in the treatment of low-risk prostatic carcinoma. The co-primary endpoints of this study were (1) the percentage of patients with >5 point reduction in the EPIC bowel domain at 1 year compared with baseline and (2) the percentage of patients with >2 point reduction in EPIC urinary domain at 1 year compared with baseline.
Secondary endpoints reported here include the sexual and hormonal EPIC scores; acute and late genitourinary and gastrointestinal toxicity as measured by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0); PSA failure rates, using the Phoenix definition (28) of a PSA increase of >2 ng/mL above nadir; and DFS, measured from the date of randomization to the date of documentation of recurrence (based on physical examination, PSA, bone scans, computed tomography/magnetic resonance imaging, and biopsies), the date of death, or the patient’s last known follow-up.
Statistical analysis
It is generally accepted that a half standard deviation (SD) change in the EPIC score is minimally significant difference (29). Analysis of the EPIC scores in 108 patients treated on the standard arm of NRG Oncology’s RTOG 0415 study was undertaken to provide effect size data for the current study. RTOG 0415 was a large RCT in patients with low-risk prostate cancer; the RCT randomized patients to SRT (73.8 Gy in 41 fractions over 8 weeks) or a hypofractioned regimen (70 Gy in 28 fractions over 5.5 weeks). Based on this study, 35% of patients had a change in EPIC bowel score, calculated as baseline subtracted from 1 year >5 points (half of an SD), and 39% of patients had a change in EPIC urinary score >2 points (half of an SD).
The null hypothesis is H0: p ≤ p0, and the alternative is HA : p > pa, where p0 = 35%, pa = 55% for bowel and p0 = 40%, pa = 60% for urinary. In calculating the sample size, if the percentage of patients with a >5 point reduction in bowel score were ≤35%, the radiation therapy regimen would be deemed acceptable; if ≥55%, the regimen would be deemed unacceptable. Similarly, if the percentage of patients with a >2 point reduction in urinary score were ≤40%, the regimen would be deemed acceptable; if found to be ≥60%, it would be deemed unacceptable. Using a 1-sample binomial test, 156 patients were required for 95% power at a 1-sided significance level of 0.025 for each co-primary endpoint, providing an overall significance level of 0.05. The target accrual was 240 patients. For a given arm, if H0 is rejected for either EPIC domain, we will conclude that the regimen given on that arm is unacceptable in terms of PRO.
A drop in EPIC sexual score of ≥11 and a drop of ≥3 in EPIC hormonal score was thougth to be significant. A rate of <35% was acceptable for EPIC sexual score, anda rate of >55% was unacceptable. For EPIC hormonal domain, a rate of <38% was deemed acceptable, and a rate of >58% was unacceptable. Using the required sample size of 156 patients, there is 95% power to detect both differences.
All eligible patients who received protocol treatment were included in the analysis. A 1-sided 1-sample binomial test was used to compare the rate of patients with a change greater than the specified value for EPIC bowel, urinary, and sexual domains. Logistic regression was used to assess the effect of radiation therapy method (IMRT/VMAT vs CyberKnife vs. protons) while adjusting for baseline PSA, age (<65 vs ≥65 years), and race (white vs nonwhite) on each of the EPIC domains (30). DFS was analyzed using the Kaplan Meier method (31), and PSA failure was estimated using cumulative incidence (32). Adverse events, measured by Common Terminology Criteria for Adverse Events version 4, were categorized as acute (≤30 days after radiation therapy completion) or late (>30 days after radiation therapy completion). All analyses were conducted using SAS Version 9.4 of the SAS System for Windows.
Results
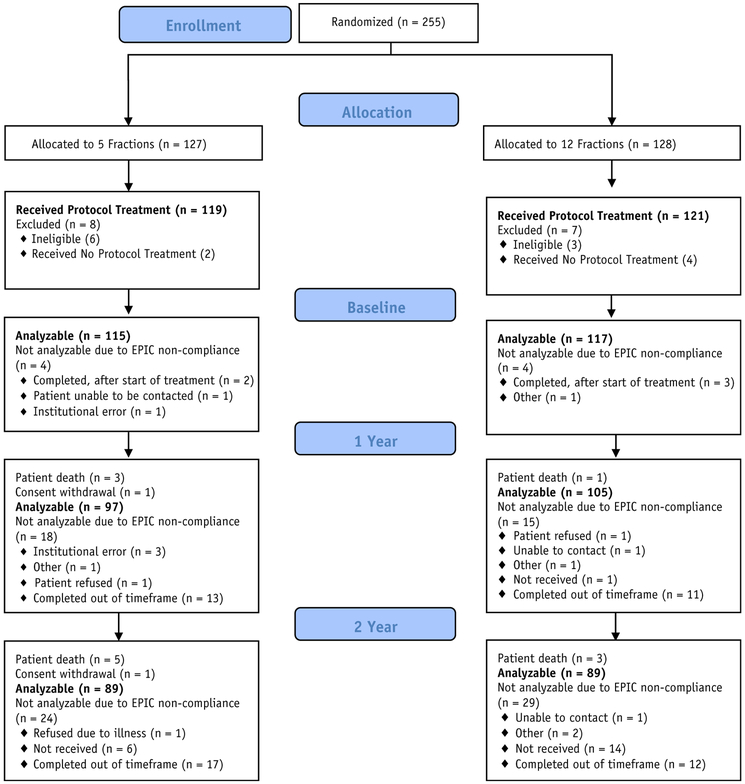
From September 2011 to February 2014, 255 patients were randomized, with 127 patients on the 5-fraction arm of the study and 128 patients on the 12-fraction arm (Fig. 1), from 52 NRG Oncology members. Nine patients were found ineligible (7 with baseline PSA and 2 with history and physical examination out of the time window), and 6 patients did not receive protocol treatment. As a result, 119 and 121 patients were analyzable for the 5- and 12-fraction arms, respectively. The patient characteristics are shown in Table 1. The median age was 65 years. The majority of patients in the 5-fraction and 12-fraction arms had T1c disease (80.7% and 82.6%) and a median PSA of 5.6 and 5.5, respectively. Median follow-up for all patients at the time of analysis was 3.8 years. The study closed to accrual once the target accrual was met; patients are followed until death or study termination.
Fig. 1.
Consolidated Standards of Reporting Trials diagram.
Table 1.
Pretreatment characteristics
| Patient characteristics | 5 fractions (n = 119) |
12 fractions (n = 121) |
|---|---|---|
| Age (y) | ||
| Median | 64 | 66 |
| Range | 48-77 | 50-79 |
| Q1-Q3 | 59-69 | 60-70 |
| Race | ||
| Asian | 1 (1%) | 3 (3%) |
| Black | 11 (9%) | 10 (8%) |
| White | 106 (89%) | 105 (87%) |
| Unknown | 1 (1%) | 3 (3%) |
| Ethnicity | ||
| Hispanic or Latino | 4 (3%) | 3 (3%) |
| Not Hispanic or Latino | 113 (95%) | 115 (95%) |
| Unknown | 2 (2%) | 3 (3%) |
| Zubrod performance status | ||
| 0 | 112 (94%) | 117 (97%) |
| 1 | 7 (6%) | 4 (3%) |
| Clinical N stage | ||
| N0 | 84 (70.6%) | 92 (76%) |
| NX | 35 (29.4%) | 29 (24%) |
| Clinical T stage | ||
| T1a | 2 (2%) | 0 (0%) |
| T1c | 96 (81%) | 100 (83%) |
| T2 | 1 (1%) | 0 (0%) |
| T2a | 20 (17%) | 21 (17%) |
| Treatment techniques/machine* | ||
| All linear accelerator–based treatment (excluding Cyberknife) | 92 (77%) | 95 (79%) |
| Cyberknife | 27 (23%) | 26 (22%) |
| PSA | ||
| Median | 5.6 | 5.5 |
| Range | 0.71-9.9 | 1.69-9.99 |
| Q1-Q3 | 4.5-7.3 | 4.23-6.93 |
| Serum testosterone (ng/mL) | (n = 108) | (n = 110) |
| Median | 257.5 | 265.5 |
| Range | 3.11-667.4 | 0-788 |
| Q1-Q3 | 14.6-390.5 | 12.9-410 |
Abbreviations: PSA = prostate-specific antigen; Q1 = first quartile; Q3 = third quartile.
Stratification factor.
The EPIC questionnaire completion compliance (based on completion of the bowel and urinary domains) was 96.7% before radiation therapy (see Fig. 1) and 84.2% at 1 year—higher than the anticipated rate of 75% and 74.2% at 2 years. Given the median follow-up of 3.8 years, the 5-year EPIC results will not be presented here.
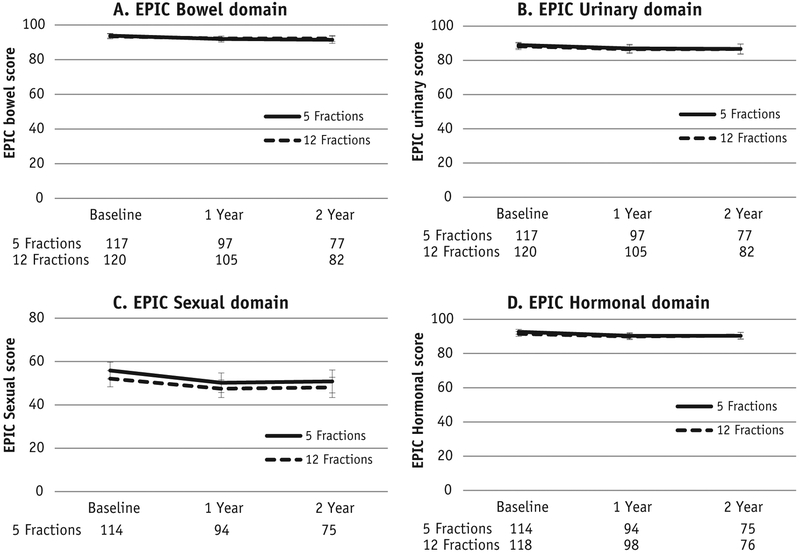
Twenty-eight patients (29.8%) experienced a >5 point reduction in EPIC bowel score at 1 year compared with baseline (P = .14) and 43 (45.7%) had a >2 point reduction in 1-year EPIC urinary score compared with baseline (P = .13) in the 5-fraction arm of the study (Table 2). In the 12-fraction arm, 29 patients (28.4%) had a >5 point reduction in 1-year EPIC bowel score compared with baseline (P = .082) and 43 (42.2%) had a >2 point reduction in 1-year EPIC urinary score compared with baseline (P = .33). In the 5-fraction arm, 32.9% of patients had a drop in 1-year EPIC sexual score of ≥11 points (P = .34); in the 12-fraction arm, 30.9% of patients had a drop in 1-year EPIC sexual score of ≥11 points (P = .20). In the 5-fraction arm, 37.4% of patients had a ≥3 point reduction in 1-year EPIC hormonal score (P = .45); in the 12-fraction arm, 37.4% experienced this reduction (P = .45). Two-year results are presented in Table 2, and a graphical display of EPIC scores at baseline, 1 year, and 2 years is presented in Fig. 2.
Table 2.
EPIC domain reductions
| 5-fraction regimen | P value* | 12-fraction regimen | P value* | |
|---|---|---|---|---|
| 1-year reductions | ||||
| Change >5 in EPIC bowel score† | (n = 94) | (n = 102) | ||
| Yes | 28 (29.8%) | 0.14 | 29 (28.4%) | 0.082 |
| No | 66 (70.2%) | 73 (71.6%) | ||
| Change >2 in EPIC urinary score‡ | (n = 94) | (n = 102) | ||
| Yes | 43 (45.7%) | 0.13 | 43 (42.2%) | 0.33 |
| No | 51 (54.3%) | 59 (57.8%) | ||
| Change > 11 in EPIC sexual score§ | (n = 88) | (n = 94) | ||
| Yes | 29 (32.9%) | 0.39 | 29 (30.9%) | 0.20 |
| No | 59 (67.1%) | 65 (69.7%) | ||
| Change >3 in EPIC hormonal score∥ | (n = 91) | (n = 99) | ||
| Yes | 34 (37.4%) | 0.45 | 37 (37.4%) | 0.45 |
| No | 57 (62.6%) | 62 (62.6%) | ||
| 2-year reductions | ||||
| Change >5 in EPIC bowel score† | (n = 74) | (n = 81) | ||
| Yes | 21 (28.4%) | 0.12 | 26 (32.1%) | 0.29 |
| No | 53 (71.6%) | 55 (67.9%) | ||
| Change >2 in EPIC urinary score‡ | (n = 74) | (n = 81) | ||
| Yes | 35 (47.3%) | 0.10 | 35 (43.2%) | 0.28 |
| No | 39 (52.7%) | 46 (56.8%) | ||
| Change > 11 in EPIC sexual score§ | (n = 71) | (n = 74) | ||
| Yes | 21 (29.6%) | 0.17 | 24 (32.4%) | 0.32 |
| No | 50 (70.4%) | 50 (67.6%) | ||
| Change >3 in EPIC hormonal score∥ | (n = 71) | (n = 79) | ||
| Yes | 26 (36.6%) | 0.41 | 31 (39.2%) | 0.41 |
| No | 45 (63.4%) | 48 (60.8%) |
Abbreviation: EPIC = Expanded Prostate Cancer Index Composite.
P-value from one-sided one-sample z-test.
Rate up to 35% is acceptable; a rate ≥55% is unacceptable.
Rate up to 40% is acceptable; a rate ≥60% is unacceptable.
Rate upto 38% is acceptable; a rate ≥58% is unacceptable.
Rate upto 38% is acceptable; a rate ≥58% is unacceptable.
Fig. 2.
Expanded Prostate Cancer Index Composite-50 (EPIC) domain scores across time. Error bars represent the 95% confidence intervals at each time point for each arm.
A total of 78.5% of patients were treated with IMRT/VMAT technique and 21.5% with Cyberknife. No patients were treated with protons. For both the 5-fraction and 12-fraction arms, there was no significant effect of radiation therapy technique on the EPIC bowel, urinary, sexual, or first hormonal scores (Table E1; available online at www.redjournal.org).
Only 2 patients (2%) had grade 3 acute treatment-related toxicity in the 5-fraction arm (grade 3 diarrhea and urinary retention) (Table 3). In this arm, 2 patients (1.7%) had late grade 3 treatment-related bladder or bowel toxicity (1 patient with proctitis and another with cystitis noninfective, renal, and urinary disorders [other, urinary incontinence, and urinary tract obstruction]). There were no grade 4 or 5 treatment-related acute or late urinary or bowel toxicities. In the 12-fraction arm, 2 patients (2%) had grade 3 acute treatment-related toxicity (both proctitis). Two patients (2%) had late grade 3 treatment-related toxicities (proctitis, urinary retention, and colonic fistula). One of these 2 patients developed a colonic fistula, which was considered a grade 3 late toxicity; further details of the patient’s course and management are not available. No patient had grade 4 or 5 late treatment-related urinary or bowel toxicity.
Table 3.
Number of patients with an adverse event by system organ class, term, and grade
| System organ class | Acute |
Late |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 fractions (n = 119) |
12 fractions (n = 121) |
5 fractions (n = 119) |
12 fractions (n = 121) |
|||||||||
| Grade |
Grade |
Grade |
Grade |
|||||||||
| 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | |
| Gastrointestinal disorders | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Colonic fistula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proctitis | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Renal and urinary disorders | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Cystitis noninfective | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Renal and urinary disorders, other | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Urinary incontinence | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Urinary retention | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Urinary tract obstruction | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
Includes adverse events for which relationship to protocol treatment is missing. Adverse events were graded with Common Terminology Criteria for Adverse Events version 4. Gastrointestinal/genitourinary grade 3+ treatment-related toxicity definitely, probably, or possibly related to protocol treatment.
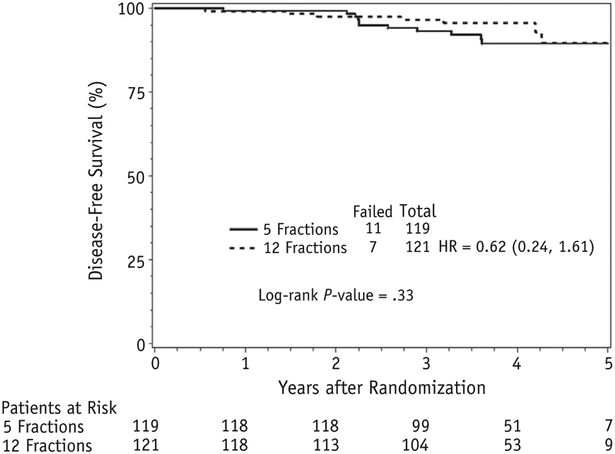
Given the limited follow-up, the rate of PSA failures and DFS events is low. No patients had a PSA failure at two years in either arm (Fig. 3).
Fig. 3.
Disease-free survival.
All evaluable cases had treatment plans reviewed, with most being per protocol. On the 5-fractions arm, 99.2% of patients were per protocol or with an acceptable variation for tumor volume contouring score; on the 12-fractions arm, 99.1% of patients were per protocol or with an acceptable variation. For the organs at risk contouring score, 93.3% and 90.1% were per protocol on the 5-fractions and 12-fractions arms, respectively.
Discussion
Although patients with National Comprehensive Cancer Network low-risk prostate cancer now often choose to undergo active surveillance, some choose to be treated. Treatment of localized prostate cancer using modern high-precision techniques to a dose of 76 to 79.2 Gy over 7.5 to 9 weeks is now established (1-3). Recently presented RCT data using moderate HypoRT have shown bNED (no evidence of biochemical failure) rates and toxicity outcomes comparable to those with SRT (7.5-8 weeks) (11-13). HypoRT is more convenient for patients and enables a greater number of patients to be treated with the available resources. Radiobiological data would also suggest that if the α/β ratio of prostate cancer is low, there may be the potential for therapeutic gain.
High-precision radiation therapy techniques have enabled UHRT involving 5 to 12 fractions to be evaluated (14-19). Several series have reported the bNED rates and toxicity results using such regimens. To date. no RCT data have been published comparing these regimens to SRT. This study was undertaken as a multi-institutional study to examine 2 UHRTs before embarking on an RCT that compared UHRT to SRT in treating localized prostate cancer.
This is one of the first studies to use bowel and urinary PROs in a hypofractionated trial to inform future RCTs. It is generally recognized that when assessing PROs, a change in these functions exceeding half of a standard deviation is a minimal important difference (29). Other investigators have suggested using other measures, such as 2 times the minimal important difference (33, 34). In this study, as per the National Cancer Institute (NCI)-approved protocol, we have reported on the results based on a change in PROs of greater than half of a standard deviation. In this study we assessed the percentage of patients with greater than a half standard deviation change in urinary and bowel function compared with baseline. This EPIC standard deviation data were obtained from the standard arm of the RTOG 0415 study to inform this study. The half standard deviation value for bowel and urinary EPIC score from this study was 5 and 2, respectively. The urinary EPIC score half standard deviation of 2 is small and in keeping with clinical experience of minimal impact on urinary function with prostate radiation therapy. In the standard arm of the RTOG 0415, 39% of patients experienced a change in urinary score >2, whereas in RTOG 0938 45.7% experienced a >2 point change in urinary score. Although this is higher than in RTOG 0415, it is well below the unacceptable 60% figure specified in the protocol. In addition, given that the change of >2 points was relatively small, and although 45.7% is higher than the 39% in RTOG 0415, it was not thought to be clinically meaningful.
The PROs for urinary, bowel, and sexual function for the 5- and 12-fraction radiation therapy regimens of this study are comparable and acceptable compared with the SRT arm of the RTOG 0415 study. The results of this study are in keeping with other case series reporting on PROs using prostate SBRT (23-26). Secondary outcomes included acute and late toxicity. The acute and late toxicity rates of these regimens are low and comparable to those with SRT, although longer follow up is required. Comparisons between SBRT and current standard radiation therapy with regard to sexual function (as a secondary endpoint) are best made in the context of an RCT comparing the 2 treatments, given the variability in sexual functioning with age and other medical conditions. PSA failures required further follow-up to make meaningful assessment of treatment efficacy.
In this study patients could be treated by any radiation therapy technique—CyberKnife, VMAT, IMRT, or protons—as long as the dosimetry parameters (especially normal tissue constraints) could be met. A strength of this study is that real-time radiation therapy and plan review were undertaken for quality assurance of protocol-defined dosimetry parameters. The PROs for patients treated with IMRT/VMAT technique and CyberKnife were comparable.
Conclusions
The 5- and 12-fraction UHRTs in this study are well tolerated. The bowel, urinary, and sexual PROs are comparable to those for SRT. The acute and late toxicity rates for the 5-fraction and 12-fraction regimens were low, but longer follow-up is required. Ongoing and maturing randomized trials are comparing UHRT to moderate HypoRT and conventional fractionation regimens, and we await their mature results.
Supplementary Material
Acknowledgments
This project was supported by the National Cancer Institute, grant nos. UG1CA189867 (National Cancer Institute Community Oncology Research Program), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology Statistics and Data Management Center), and U24CA180803 (Imaging and Radiation Oncology Core).
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005;294:1233–1239. [DOI] [PubMed] [Google Scholar]
- 3.Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer 2015;121:2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner D, Armour E, Corry P, et al. Sublethal damage repair times for a late-responding tissue relevant to brachytherapy (and external-beam radiotherapy): Implications for new brachytherapy protocols. Int J Radiat Oncol Biol Phys 1998;41:135–138. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–1101. [DOI] [PubMed] [Google Scholar]
- 6.Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093–1104. [DOI] [PubMed] [Google Scholar]
- 7.Thames HD, Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys 1986;12:687–691. [DOI] [PubMed] [Google Scholar]
- 8.Wang JZ, Guerrero M, Li XA. How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys 2003;55:194–203. [DOI] [PubMed] [Google Scholar]
- 9.Wang JZ, Li XA, Yu CX, et al. The low alpha/beta ratio for prostate cancer: What does the clinical outcome of HDR brachytherapy tell us? Int J Radiat Oncol Biol Phys 2003;57:1101–1108. [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13. [DOI] [PubMed] [Google Scholar]
- 11.Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III non-inferiority study comparing two radiotherapy fractionation scheduled in patients with low-risk prostate cancer. J Clin Oncol 2016;34:2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high dose intensity modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority phase 3 CHHiP trial. Lancet Oncol 2006;17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017;35:1884–1890. [DOI] [PubMed] [Google Scholar]
- 14.King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043–1048. [DOI] [PubMed] [Google Scholar]
- 15.King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013; 109:217–221. [DOI] [PubMed] [Google Scholar]
- 16.Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: First clinical trial results. Int J Radiat Oncol Biol Phys 2007;67:1099–1105. [DOI] [PubMed] [Google Scholar]
- 17.Menkarios C, Nguyen DHA, Vigneault E, et al. Hypofractionated radiotherapy for low risk prostate cancer: Preliminary results of a phase I/II trials. Radiother Oncol 2009;92(Suppl 2):S43. [Google Scholar]
- 18.Ritter MA, Forman JD, Kupelian PA, et al. A phase I/II trial of increasingly hypofractionated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2009;75(Suppl):S80–S81. [Google Scholar]
- 19.Tang CI, Loblaw DA, Cheung P, et al. Phase I/II study of a five-fraction hypofractionated accelerated radiotherapy treatment for low-risk localised prostate cancer: Early results of pHART3. Clin Oncol (R Coll Radiol) 2008;20:729–737. [DOI] [PubMed] [Google Scholar]
- 20.Wu AW, Jacobson KD, Frick DL, et al. Validity and responsiveness of the EQ5D as a measure of health-related quality of life in people enrolled in an AIDS clinical trial. Qual Life Res 2002;11:273–282. [DOI] [PubMed] [Google Scholar]
- 21.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–1261. [DOI] [PubMed] [Google Scholar]
- 22.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2000;20:557–566. [DOI] [PubMed] [Google Scholar]
- 23.Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat Oncol 2013;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer MJ, Papagikos MA, Kiteley R, et al. Toxicity and quality of life report of phase II study of stereotactic body radiotherapy (SBRT) for low and intermediate risk prostate cancer. Radiat Oncol 2017;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LN, Suy S, Wang H, et al. Patient-reported urinary incontinence following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol 2014;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson WC, Dess RT, Litzenberg DW, et al. A multi-institutional phase 2 trial of prostate stereotactic body radiation therapy (SBRT) using continuous real-time evaluation of prostate motion with patient-reported quality of life. Practical Radiation Oncology 2018;8:40–47. [DOI] [PubMed] [Google Scholar]
- 27.Zelen M The randomization and stratification of patients to clinical trials. J Chron Dis 1974;27:365–375. [DOI] [PubMed] [Google Scholar]
- 28.Roach M 3rd, Hanks G, Thames H Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 29.Brundage M, Osoba D, Bezjak A, et al. National Cancer Institute of Canada Clinical Trials Group. Lessons learned in the assessment of health-related quality of life: Selected examples from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:5078–5081. [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 31.lan EL. Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 32.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 33.Evans JR, Zhao S, Daignault S, et al. Patient-reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol 2015;116:179–184. [DOI] [PubMed] [Google Scholar]
- 34.Dess RT, Jackson WC, Suy S, et al. Predictors of multidomain decline in health-related quality of life after stereotactic body radiation therapy (SBRT) for prostate cancer. Cancer 2017;123:1635–1642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.