Abstract
Background
Lung cancer is a good example of the potential benefit of symptom-based diagnosis, as it is the commonest cancer worldwide, with the highest mortality from late diagnosis and poor symptom recognition. The diagnosis and risk assessment tools currently available have been shown to require further validation. In this study, we determine the symptoms associated with lung cancer prior to diagnosis and demonstrate that by separating prior risk based on factors such as smoking history and age, from presenting symptoms and combining them at the individual patient level, we can make greater use of this knowledge to create a practical framework for the symptomatic diagnosis of individual patients presenting in primary care.
Aim
To provide an evidence-based analysis of symptoms observed in lung cancer patients prior to diagnosis.
Design and setting
Systematic review and meta-analysis of primary and secondary care data.
Method
Seven databases were searched (MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature, Health Management Information Consortium, Web of Science, British Nursing Index and Cochrane Library). Thirteen studies were selected based on predetermined eligibility and quality criteria for diagnostic assessment to establish the value of symptom-based diagnosis using diagnosistic odds ratio (DOR) and summary receiver operating characteristic (SROC) curve. In addition, routinely collated real-time data from primary care electronic health records (EHR), TransHis, was analysed to compare with our findings.
Results
Haemoptysis was found to have the greatest diagnostic value for lung cancer, diagnostic odds ratio (DOR) 6.39 (3.32–12.28), followed by dyspnoea 2.73 (1.54–4.85) then cough 2.64 (1.24–5.64) and lastly chest pain 2.02 (0.88–4.60). The use of symptom-based diagnosis to accurately diagnose lung cancer cases from non-cases was determined using the summary receiver operating characteristic (SROC) curve, the area under the curve (AUC) was consistently above 0.6 for each of the symptoms described, indicating reasonable discriminatory power. The positive predictive value (PPV) of diagnostic symptoms depends on an individual’s prior risk of lung cancer, as well as their presenting symptom pattern. For at risk individuals we calculated prior risk using validated epidemiological models for risk factors such as age and smoking history, then combined with the calculated likelihood ratios for each symptom to establish posterior risk or positive predictive value (PPV).
Conclusion
Our findings show that there is diagnostic value in the clinical symptoms associated with lung cancer and the potential benefit of characterising these symptoms using routine data studies to identify high-risk patients.
Introduction
Lung cancer has the highest mortality rate of any cancer worldwide and constitutes more than 40% of all new cancer diagnoses [1]. Although survival rates in England have improved in the last 40 years, they remain lower than in comparable European countries. Improving early diagnosis is a key component of relieving the cancer burden [2]. It has been estimated that earlier diagnosis of the four commonest cancers in England (lung, breast, prostate and colorectal), would benefit over 11,000 patients each year [3]. The National Institute for Health and Care Excellence (NICE) 2015 urgent referral guidelines for suspected cancer, set the positive predictive value (PPV) threshold of clinical presentations for cancer at 3% [4]. In this study, we aim to determine the validity of symptom-based lung cancer diagnosis, using published studies, routine data from electronic health records and published prior risk models. A recent review of lung cancer diagnosis using ‘Risk Assessment Tools’ (RATs) found that there was insufficient validation, and that the inclusion of ‘epidemiological risk factors’ in the models, along with symptoms, created confounders [5]. In this review, we specifically assess symptoms associated with lung cancer diagnosis without epidemiological factors, to avoid confounding. We can then determine the prior risk using epidemiological models and calculate the posterior probability, or PPV, using Bayes’ theorem.
Methods
Systematic literature search
We performed a systematic review and meta-analysis of studies reporting the sensitivity, specificity, predictive values, odds ratios or likelihood ratios for lung cancer in patients consulting their GP with symptoms prior to diagnosis. Searches were performed on 24th September 2017 of seven databases using search terms specific for lung cancer diagnosis (Fig 1) presented using the prisma flow chart [6]. For prisma checklist and full search terms and outcomes, see S1 and S3 Tables.
Fig 1. Prisma flow chart of database search.
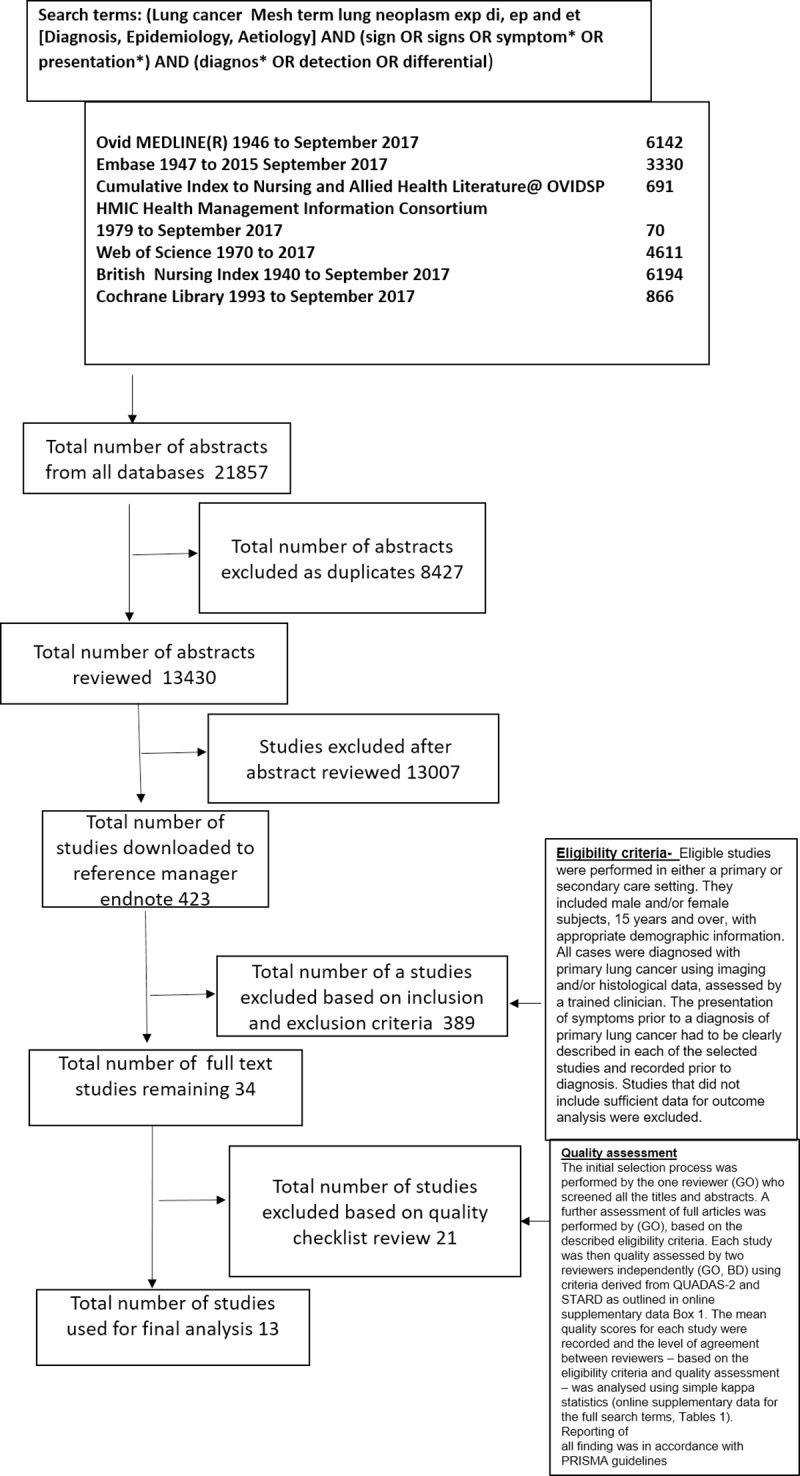
Eligibility criteria
Eligible studies were performed in either a primary or secondary care setting. They included male and/or female subjects, 15 years and over, with appropriate demographic information. All cases were diagnosed with primary lung cancer using imaging and/or histological data, assessed by a trained clinician. The presentation of symptoms prior to a diagnosis of primary lung cancer had to be clearly described in each of the selected studies and recorded prior to diagnosis. Studies that did not include sufficient data for outcome analysis using a 2x2 contigency table were excluded from the meta-analysis.
Data collection from the TransHis primary care electronic health record
The Transition Project “TransHis” is an electronic patient record used by 230 general practices worldwide to collate data in real-time [7]. All patients whose initial consultations were subsequently linked to a diagnosis of lung cancer were assessed [8]. These allowed us to monitor the evolution of an initial presenting symptom to its final diagnosis [9]. Data extraction was performed on 24th September 2017.
Outcome analysis and statistical methods
For diagnostic analysis, we constructed 2x2 contingency tables for each study, using data collated prior to diagnosis [10]. For the meta-analysis, a random effects model for diagnostic accuracy was used to pool the data, as this accounts for differences in index test threshold, based on patient and/or clinical interpretation of presentations. A measure of the discriminatory power of the index test was calculated using diagnostic odds ratios (DOR). Heterogeneity in results across a study was assessed for each presenting symptom as a subgroup using Cochran's Q (Q*) and I-squared (I2) statistics [11, 12]. Summary Receiver Operating Characteristic (SROC) curves for each presenting symptom were plotted from pooled sensitivity against (1-pooled specificity) using Moses’ Model (weighted regression, inverse variance). The area under the curve (AUC) was used to measure diagnostic accuracy. STATA version 13 (STATACorp, USA) was used for the statistical analyses.
Results
The search strategy shown in Fig 1, produced 13,430 unique references. A further review of these titles followed by abstracts and selection of those studies that met the inclusion criteria, resulted in the selection of 34 studies by the first reviewer (GO). A full text review of the 34 studies was performed by the first and second reviewer independently (GO and BD) with good agreement, kappa of 0.85 (0.430–0.938). After discussion, a final thirteen studies were selected. All findings were reported in accordance with PRISMA guidelines.
Study strengths, limitations and bias assessment
The design and protocol used in each of the selected studies were subject to different types of bias (Table 1). The selected studies include six case series, three case-control and four cohort studies, summarised in Tables 2 and 3. Likelihood ratios (LR) are the most clinically useful outcome measures, as the LR is the probability of a cancer patient having the symptom divided by the probability of a non-cancer patient having that symptom. Table 3 details those studies where likelihood ratios could be calculated. Five of the selected studies included sufficient data to assess the diagnostic accuracy of symptoms associated with lung cancer using a dichotomous test approach. This data was compared with LRs from TransHis data.
Table 1. Bias risk in selected studies.
| Type of bias | Case series studies | Case-control studies | Cohort studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Koyi et al. 2002 |
Corner et al. 2005 | Barros et al. 2006 | Cajoto et al. 2009 | Shresthra et al. 2010 | Gonzalez- Baracala et al. 2014 | Kubik et al. 2002 | Hamilton et al. 2005 | Iyen-Omofoman et al. 2013 | Hoppe et al. 1977 | Jones et al. 2007 | Hippisley-Cox et al. 2011 | Walter et al. 2015 | |
| Random sequence generation (selection bias) |
|
|
|
|
|
|
|
|
|
|
|
||
| Allocation concealment (selection bias) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Blinding of participants and personnel (performance bias) | |||||||||||||
| Blinding outcome assessment (detection bias) |
|||||||||||||
| Incomplete outcome data (attrition bias) |
|||||||||||||
| Selective reporting (attrition bias) |
|||||||||||||
| Other bias | |||||||||||||
Indicates high risk of bias
Indicates uncertain risk of bias
Indicates low risk of bias
Table 2. Summary of selected studies.
| Study (year) | Geographic area | Study design | Data source period | Sample demography and use of controls | Period of initial presentation | Characterisation of symptom | Staging or surgical management | Outcome measure |
|---|---|---|---|---|---|---|---|---|
| Koyi et al. 2002 | Gaevleborg, Sweden |
Prospective case series study using patient questio -nnaires completed within a specialist lung clinic | Patient questionnaire Jan 1997 –Dec 1999 |
364 participants–no controls | Not stated | Not characterised | Yes | Percentages |
| Corner et al. 2005 | England, United Kingdom | Retrospective case series study interview triangulated with medical records | Medical Records (< 2 years before diagnosis) |
22 participants (Male 54.5% Female 45.5%)–no controls |
6–24 months prior to diagnosis |
Not characterised | Yes, operability | Percentages |
| Barros et al. 2006 |
Curitian, Brazil | Retrospective case series study | Medical records Jan 1991- Dec 1997 |
268 participants–no controls | Not stated | Not characterised | Yes | Percentages |
| Cajoto et al. 2009 (SPANISH) |
Santiago de composteka, Spain |
Retrospective case series study | Medical records (codes) Jan 1997-Dec 1999 |
481 participants–no controls | Not stated | Not characterised | None | Percentages |
| Shrethra et al. 2010 | Kathmandu Nepal |
Retrospective case series study | Medical records July 2004—July 2008 |
174 participants–no controls | 117.3 days prior to diagnosis | Not characterised | None | Percentages |
| Gonzalez- Barcala et al. 2014 |
Ponteveda Health Area, Spain | Retrospective case series study |
Hospital records 1 June 2005–31 May 2008 |
358 patients–no controls | Unknown | Not characterised | Yes | Percentages |
| Kubik et al. 2002 | Czech Republic |
Case-control study | Patient interview questionnaire (not validated) April 1998—October 2000 |
All female 268 cases and 1076 control participants (not diagnosed with lung cancer), aged 25–89. | < 2 years | Yes, duration of presentation- looked at two presentations. Also, one associated feature, cough +/- phlegm. | None | Odds Ratio (adjusted for age, residence and education) |
| Hamilton et al. 2005 | Exeter, United Kingdom |
Case-control study controls | GP Medical records (codes) 1998–2002 |
247 cases and 1235 control participants no lung cancer with same presentation (GP/age/sex matched, age >40 years) | 180 days to 2 years | Yes, associated symptoms as first and second symptom prior to diagnosis for seven specific symptoms. | None | Positive Predictive Value and Likelihood Ratios |
| Iyen-Omoforman et al. 2013 | United Kingdom |
Case-control study–controls from same general practice | GP Medical records (The Health Improvement Network database) Jan 2000—July 2009 |
12, 074 cases and 120,731 control participants | 4–12 months 13–24 months |
Yes, onset (period prior to diagnose) five specific symptoms | None | Odds Ratio, sensitivity, specificity |
| Hoppe et al. 1977 (GERMAN) |
Hamburg, Germany | Retrospective Cohort study | Hospital records 1967–1974 |
20,000 participants in cohort | Not stated | Yes, duration of symptom prior to diagnosis | None | Percentages |
| Jones et al. 2007 | United Kingdom | Retrospective Cohort study | Medical records (CPRD) Jan 1994—Dec 2000 |
4812 participants (>15 years) in cohort |
6 months– 3 years | Assessed haemoptysis only as a lung cancer symptom. | None | Positive Predictive Value, Likelihood Ratios |
| Hippisley-cox et al. 2011 | England and Wales, United Kingdom | Prospective Cohort study | GP Medical records (QResearch EMIS) Jan 2000- Sept 2010 |
3785 participants in cohort | < 2 years | Not characterised | None | Positive predictive value |
| Walter et al. 2015 | England United Kingdom |
Prospective Cohort study |
Medical records and Questionnaire completed by interviewer Dec 2010 and Dec 2012 |
963 participants in cohort | 28 days– 2 years | Yes, duration and presence of synchronous symptoms | None | Hazard ratios (adjusted for waiting time) and percentages |
Table 3. Likelihood ratios for each presentation where indicted in selected studies.
| Study (year) | Outcome measure | Symptom |
| Hamilton et al. 2005 | LR+ (from raw data obtained from a referenced author) |
Haemoptysis LR+13.2 (7.9–22) LR- 0.8 (0.76–0.86); Loss of weight LR+ 6.2 (4.5–8.6) LR- 0.76 (0.71–0.82); Loss of appetite LR+4.8 (3.3–7.0) LR- 0.84 (0.79–0.9); Dyspnoea LR+3.6 (3.1–4.3) LR- 0.52 (0.45–0.60); Chest or rib pain LR+3.3 (2.7–4.1) LR- 0.68 (0.61–0.75); Fatigue LR+2.3 (1. 9–2.9) LR-0.76 (0.7–0.84). |
| Iyen-Omoforman et al. 2013 | LR+ calculated from published sensitivity and specificity |
Haemoptysis 13.9; Cough 2.5; Chest/shoulder pain 1.9; Dyspnoea 5.4; Weight loss 3.6; Voice hoarseness 1.9. |
| Jones et al. 2007 | LR+ and PPV | PPV 5.8% (5.0%-6.7%) and LR+ 116.7 (99.1–134.3) in men, and PPV 3.3% (2.6%-4.3%) and LR+ 153.1 (115.3–190.8) in women |
| Study (year) | Outcome measure where LR not available | Symptom |
| Kubik et al. 2000 | OR (adjusted for age, residence, education and pack years) |
Chronic cough 2.93 (2.03–4.22); Chronic phlegm 2.44 (1.59–3.76); Chronic phlegm < 2 years 4.74 (2.56–8.76); Chronic phlegm ≥2 years 1.43 (0.80–2.54); Dyspnoea 1.66 (1.18–2.34); Attacks of dyspnoea 1.10 (0.60–2.04). |
| Hippisley-cox et al. 2011 | PPV | Current haemoptysis female 23.9 (20.6–27.6) male 21.5 (19.3–23.9); Current appetite loss female 4.14 (3.15–5.45) male 4.71 (3.69–6.00); Current weight loss female 4.52 (3.80–5.38) male 6.09 (5.33–6.95); New onset cough in last 12 months female 1.90 (1.56–2.32) male 1.47 (1.23–1.75) |
| Walter et al. 2015 | HR (adjusted for waiting time paradox) |
Coughing up blood (not included as less than 10 cases); Cough or worsening cough 43 weeks 1.16 (0.78–1.74) P = 0.46; Breathlessness or worsening 43 weeks 0.70 (0.45–1.08) P = 0.1; Chest/shoulder pain 43 weeks 1.79 (1.08–2.99) P = 0.03; Hoarseness 43 weeks 0.98 (0.48–2.01) P = 0.97; Decreased appetite 1.41 (0.78–2.53) P = 0.25; Unexplained weight loss 0.86 (0.43–1.71) P = 0.66; Fatigue or tiredness ‘unusual for you’ 1.16 (0.75–1.79) P = 0.49; Different ‘in yourself’ 1.52 (0.93–2.46) P = 0.09. |
LR+ = positive likelihood ratio, PPV = positive predictive value, OR = odds ratio, HR = hazard ratio
Cohort studies
Retrospective cohort studies typically use data collected in the electronic health record: they usually exclude data collected in the 6–12 months before diagnosis to address the potential bias from including post-diagnosis symptoms and to minimise the influence of GPs preferentially coding possible lung cancer symptoms when considering this as a potential diagnosis. Cohort studies accounted for 31% of the selected studies. Jones and colleagues (2007), used a symptom-based approach to investigate all diagnoses associated with haemoptysis in a large general practice database (Clinical Practice Research Datalink) of 762,325 UK patients. Of the 4,812 new episodes of haemoptysis, 6.3% were subsequently diagnosed with lung cancer. This study also reported PPVs and positive likelihood ratios (LR+) as shown in Table 3 [13]. Hippisley-Cox and colleagues (2011) determined the hazard ratios for lung cancer in a risk assessment model that considered three clinical predictors (haemoptysis, loss of appetite and weight loss) presenting within 12 months prior to a lung cancer diagnosis. Risk of lung cancer was greatest in patients with haemoptysis: hazard ratio 23.9 (20.6–27.6) in females and 21.5 (19.3–23.9) in males, after adjustment for late-stage diagnosis and the associated shorter time-to-diagnosis, waiting time paradox [14]. Walter and colleagues (2015) used a prospective cohort study design and interviewed patients who had been referred to a specialist respiratory clinic by their GP. Half of the referred patients (49.3%) reported that they had presented to their GP with a single first symptom. Almost 40% (>37.8%) presented with more than one presenting symptom that worsened over time. Haemoptysis had the greatest causative association to lung cancer with an adjusted hazard ratio of 2.17 (1.63–2.89) (P = 0.00) [15].
Case-control studies
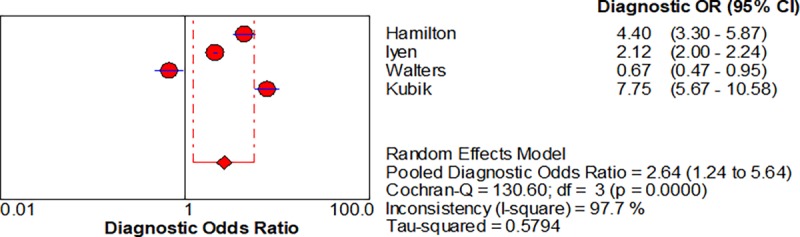
Case-control studies accounted for 23% of the selected studies and are limited in that the outcome measures such as PPVs cannot be generalised beyond the study. They are a product of the selection of cases and controls, not reflecting any natural population, although LRs may be valid for use with prior risk data. Kubik and colleagues (2000) assessed the diagnostic value of dyspnoea, chronic non-productive and productive cough. When adjusted for age, residence, education, and smoking pack-years, non-productive cough had an odds ratio of 2.93 (2.03–4.22), higher than that for productive cough 2.44 (1.59–3.76) and dyspnoea 1.66 (1.18–2.34) [16]. Hamilton and colleagues (2005) used a case-control study design to investigate the clinical features of lung cancer before diagnosis. Cases were identified retrospectively from local general practices and cancer registry databases. Symptoms reported within 180 days to 2 years prior to lung cancer diagnosis were assessed, to avoid bias, and compared with age and sex-matched control groups from the same general practices who did not have lung cancer. Seven specific presentations were assessed with the greatest positive likelihood ratios observed with haemoptysis 13.2 (7.9–22.0), then loss of weight 6.2 (4.5–8.6), loss of appetite 4.8 (3.3–7.0), dyspnoea 3.6 (3.1–4.3) and chest pain 3.3 (2.7–4.1)[17]. Iyen-Omofoman and colleagues (2013) used a routine data source (The Health Improvement Network). Clinical predictors were recorded during two time periods: 4–12 and 13–24 months prior to diagnosis. The highest odds ratio: 20.15 (16.24–25.01) was for haemoptysis presenting 4–12 months before diagnosis[18].
Case series studies
Case series studies accounted for 46% of the selected studies, the most common study design observed in this review but the least informative in relation to diagnostic value. The diagnostic value of these symptoms cannot be assessed because patients without lung cancer were not included in the study.
TransHis data
TransHis is an EHR specifically designed to capture the initial consultation as ‘Reason for Encounter’ (RfE) and maintain the episode of care structure as an ongoing prospective cohort study. The TransHis data were used to determine the relationship between lung cancer diagnosis and RfE, expressed as odds ratios. Cough followed by haemoptysis, dyspnoea, weight loss, chest pain and voice symptoms were the most prevalent RfEs in patients subsequently diagnosed with lung cancer (S5 Table). Constitutional symptoms (tiredness, weight loss, anorexia, fever and sweating) were collectively the third most common. As TransHis data is captured from routine care using a primary-care specific classification (ICPC2) and the odds ratios are relative to ‘all consulting patients’, we compared the outcomes with our selected studies.
When considering all the selected studies, haemoptysis, cough, dyspnoea, chest pain and constitutional symptoms were found to be the most prevalent presentations. In all studies haemoptysis, dyspnoea and cough were consistently the most predictive symptom for lung cancer.
Statistical analysis for diagnostic accuracy of clinical presentations associated with lung cancer
Five studies enabled us to assess the diagnostic accuracy of symptoms associated with lung cancer [16–18]. The pooled diagnostic odds ratios (DOR) for.haemoptysis, dyspnoea, cough and chest pain were 6.39 (3.32–12.28), 2.73 (1.54–4.85), cough 2.64 (1.24–5.64) and chest pain 2.01 (0.88–4.6) respectively, shown in Figs 2–5 respectively.
Fig 2. Forest plots with pooled diagnostic odds ratio (95% confidence interval) and weights calculated using a random effects model for haemoptysis in the diagnosis of lung cancer.
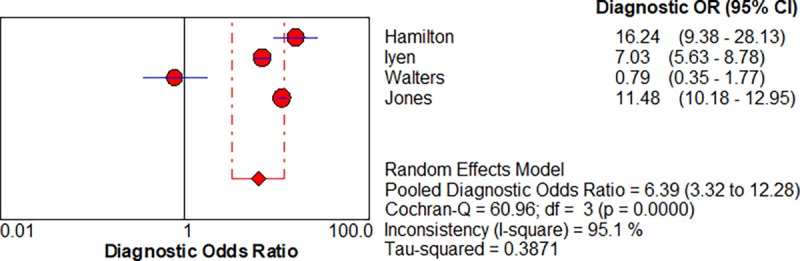
Fig 5. Forest plots with pooled diagnostic odds ratio (95% confidence interval) and weights calculated using a random effects model for Chest Pain in the diagnosis of lung cancer.
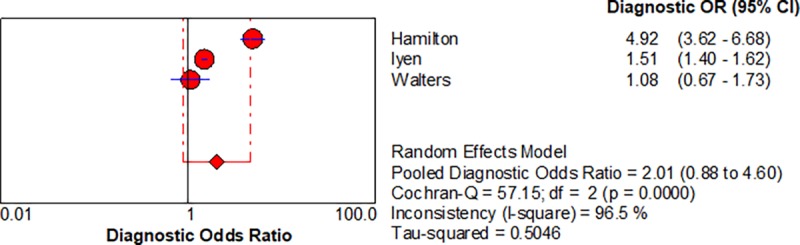
Fig 4. Forest plots with pooled diagnostic odds ratio (95% confidence interval) and weights calculated using a random effects model for Cough in the diagnosis of lung cancer.
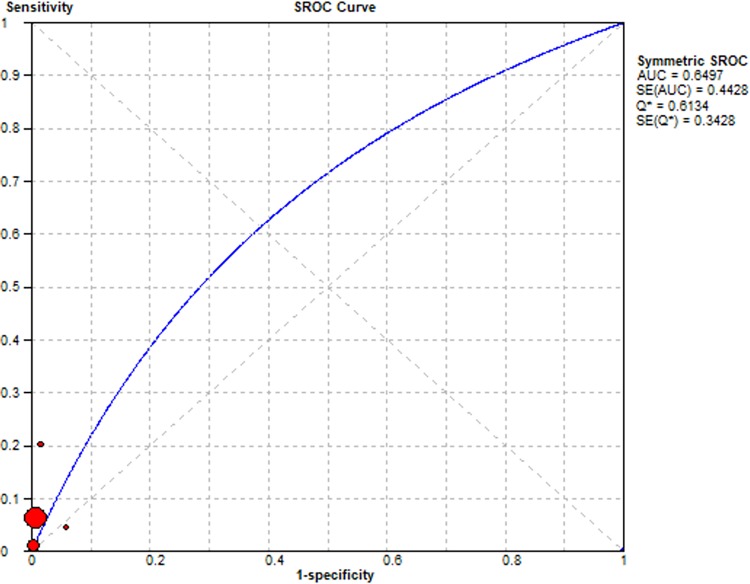
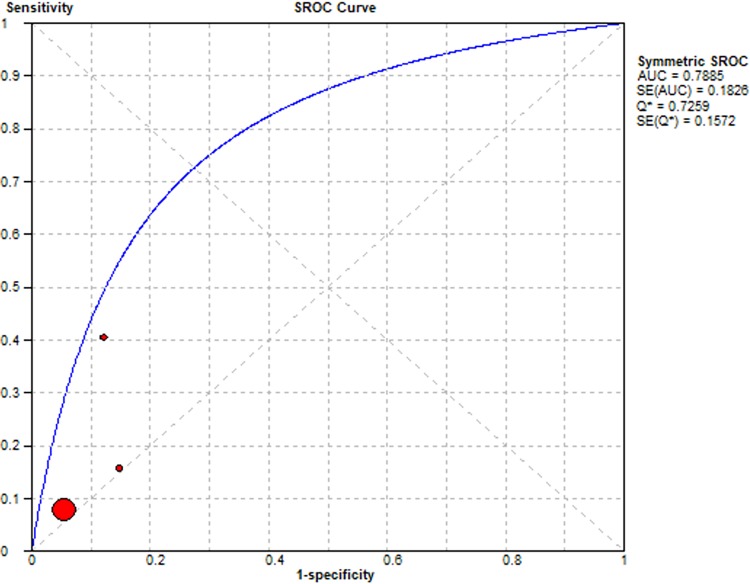
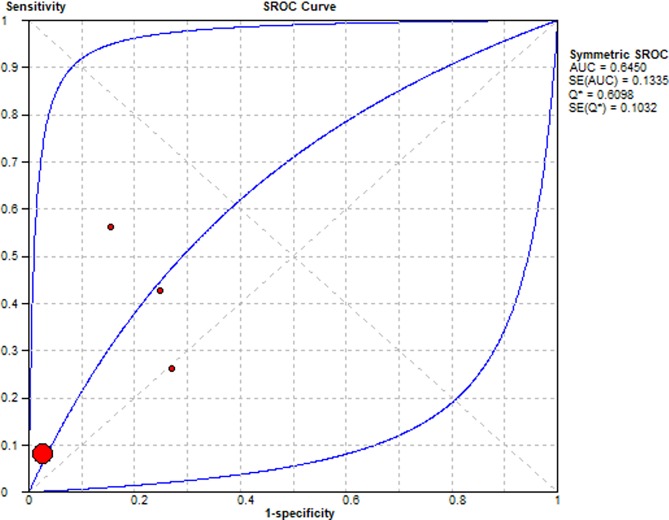
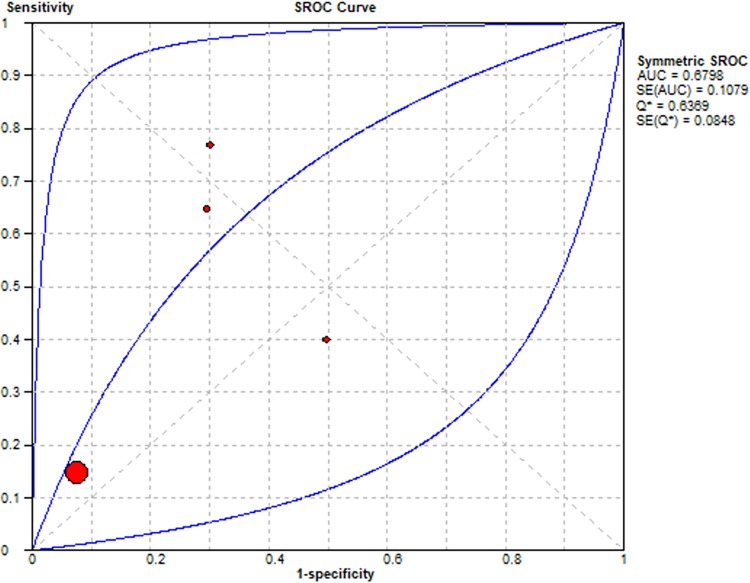
Summary Receiver Operating Characteristic (SROC) curves were used to determine the predictive accuracy of each presentation in the diagnosis of lung cancer, using the area under the curve (AUC). Accuracy for lung cancer diagnosis was confirmed for haemoptysis AUC = 0.65, dyspnoea AUC = 0.65, cough AUC = 0.68 and chest pain AUC = 0.79. The SROC curves are summarised Figs 6–9 respectively.
Fig 6. Summary Receiver Operator Curve for Haemoptysis as a diagnostic symptom in lung cancer.
Fig 9. Summary Receiver Operator Curve for Chest pain as a diagnostic symptom in lung cancer.
Fig 7. Summary Receiver Operator Curve for Dyspnoea as a diagnostic symptom in lung cancer.
Fig 8. Summary Receiver Operator Curve for Cough as a diagnostic symptom in lung cancer.
The limited availability of studies that fit the criteria for diagnostic value, differences in study design and the differing thresholds for recording presence/absence of a symptom, shown in Tables 2 and 3, created the heterogeneity (I2) observed in the SROC curves Fig 3. We compared the overall diagnostic value of each presentation from the selected studies with measurable outcome data and TransHis data using likelihood ratios as shown in Table 4.
Fig 3. Forest plots with pooled diagnostic odds ratio (95% confidence interval) and weights calculated using a random effects model for Dyspnoea in the diagnosis of lung cancer.
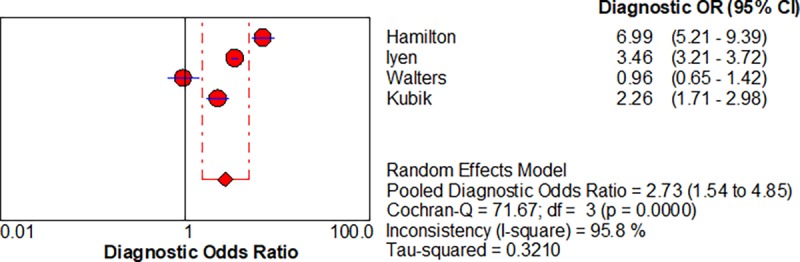
Table 4. Positive Likelihood Ratios (LR) for symptoms in Lung Cancer patients prior to diagnosis.
| Symptom |
Pooled positive likelihood ratio for selected studies (95% confidence intervals) | TransHis positive likelihood ratios (95% confidence intervals) |
|---|---|---|
| Haemoptysis | 5.968 (3.183–11.189) | 51.76 (24.91–107.56) |
| Dyspnoea | 2.138 (1.350–3.385) | 3.02 (1.72–5.32) |
| Cough | 1.748 (1.290–2.369) | 1.09 (0.69–1.73) |
| Chest pain | 1.756 (0.953–3.237) | 0.69 (0.17–2.73) |
The symptom most likely to be observed in lung cancer vs non lung cancer patients is haemoptysis, followed by dyspnoea, cough and finally chest pain.
Staging at diagnosis of lung cancer
The tumour stage at diagnosis, or its operability, was indicated in only four of the thirteen studies and most of the diagnosed cases were inoperable or at stages IIa and above. Hence, 31% of selected studies described the prognostic benefits of symptom-based early diagnosis by including data on disease stage and operability at diagnosis [14, 15, 17–19].
In the most common form of lung cancer, non-small cell, the weighted means as a percentage of all cases in each study was calculated as follows: Stage I 10.7%, Stage II 6.9%, Stage III 43.2% and Stage IV 39.2%. These studies found that less than 8.2% of the lung cancer patients were amenable to surgery at diagnosis [20].
Discussion
Summary of findings
We found haemoptysis, had the greatest diagnostic value in both the selected studies and the TransHis database, followed by dyspnoea, cough and chest pain. The review also indicated that most of cancer patients are diagnosed at a late stage when there are limited surgical management options and less favourable clinical outcomes. More precise coding for symptoms and characterisation of symptoms, such as severity, timing and associated features, in electronic health records such as TransHis may provide sufficient evidence for early symptom-based diagnosis of lung cancer. It is hoped that the introduction of a new and global clinical vocabulary for electronic health records, SNOMED CT (Systematized Nomenclature of Medicine–Clinical Terms), will also contribute to better utilisation of electronic health records to improve evidence-based research. Although, codes will need to be carefully restricted to a classification of symptoms to enable calculation of odds ratios.
Findings within the context of the current literature
To date, this is the only review to include a meta-analysis of clinical symptoms for the diagnosis of lung cancer. A previously published systematic review based on primary care data showed haemoptysis to be a predictor of lung cancer, but there were insufficient data to perform a meta-analysis [21]. We included studies where the index cases were identified in both primary care and secondary care studies as long as patients were referred by their GP. We made this decision on the basis that referral to a clinic for investigation of respiratory symptoms represents a cohort of people in whom the GP is considering cancer, and in the absence of better data on the evolution of symptoms over time, may yield useful LRs (but not PPVs). Our findings are consistent with previous findings that haemoptysis is predictive of lung cancer, but in addition demonstrates the diagnostic value of dyspnoea, cough and chest pain [15, 18, 21, 22].
Previously published studies suggest that efforts to expedite the diagnosis of symptomatic cancer are likely to benefit patients in terms of improved survival, earlier-stage diagnosis and improved quality of life [19, 23–27]. This review clearly identifies a place for symptom-based diagnosis, as the epidemiology of cancer symptoms is becoming better understood. Risk models that assess prior risk factors and then presenting symptoms could identify high-risk patients for early diagnosis [28].
Strengths and weaknesses of the review
All selected studies used routine data sources, a cost-effective and powerful resource for evidence-based research. Though variability in the study designs creates heterogeneity, there was sufficient data to perform a meta-analysis and determine the diagnostic accuracy of clinical presentations associated with lung cancer. Five of the thirteen studies assessed the association of lung cancer with a specific set of symptoms and did not investigate all symptoms reported in lung cancer patients [13, 14, 16–18]. As a result, their findings may have missed other symptoms not already known to be associated with lung cancer. Each study provided demographic data on age, sex and smoking status; male smokers over 40 years were found to have the greatest incidence of lung cancer. However, routine data sources can also be subject to bias, such as missing data, coding inconsistencies, and work-up bias [29, 30] Thus, these studies can miss the complexities of the clinical assessment necessary for cancer diagnosis, for example weight loss was found to be the fifth most prevalent presentation prior to diagnosis and, in one study, it was observed even in operable disease, indicative of a presentation associated with early diagnosis [31]. In 62% of the selected studies, weight loss was grouped with constitutional symptoms, therefore, specific analysis of weight loss as an isolated symptom was not possible. More data are required for diagnostic assessment of weight loss because it may prove to be a cost-effective predictor of high-risk patients. These patients could be identified for further investigations to facilitate early cancer diagnosis.
Implications for clinical practice and research
Case series studies represent a majority of the studies into symptoms associated with lung cancer, but this design has no diagnostic benefit because there are no controls. This highlights the importance of devising a study design that will produce clinically significant outcomes that will be of patient benefit.
Understanding the precise diagnostic value of symptoms is a powerful tool in clinical decision making [28]. Table 5 outlines three case scenarios where symptomology is considered in combination with prior risk [32] to establish indivualised risk and appropriate management. Up to 20% of all chest X-ray requests from primary care in patients subsequently diagnosed with lung cancer are negative [33, 34]. If we consider a high posterior risk of lung cancer, as shown in the Case C, even with a negative chest X-ray this patient still meets the criteria for urgent referral (PPV>3%), based on epidemiological risk factors and symptomology using Bayesian incorporation for posterior risk [17, 23, 35–39].
Table 5. Clinical case analysis using prior risk assessment and Bayesian incorporation of clinical symptoms to determine posterior risk.
| Case | Sex | Age | Smoking status | Age started | Age stopped | Smoking duration | Smoking intensity (cigarettes/day) |
Symptoms | LR+ | Calculated prior risk % | PPV Calculated on the basis of individual posterior risk % |
PPV based on presenting symptoms in the published cohort[17]* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 68 | Smoker | 20 | NA | 48 | 10 | cough + fatigue |
3.45 | 1.86 | 6.14% Moderate risk |
0.63% Low risk |
| Case A represents a low risk patient based on symptoms alone and therefore would not require further investigation or referral. When we take into account prior risk defined by age, sex, smoking status and intensity, this patient is at greater risk then the moderate risk patient in Case B below and does require further investigation (chest X-ray). | ||||||||||||
| B | F | 61 | Never | NA | NA | NA | NA | dyspnoea + haemoptysis | 27.98 | 0.124 | 3.36% Moderate risk |
4.90% Moderate risk |
| Case B represents a patient with moderate risk when considering symptoms alone. Here consideration of prior risk has little effect on the risk status. | ||||||||||||
| C | M | 58 | Ex | 17 | 47 | 30 | 10 | loss of appetite + haemoptysis |
449.74 | 0.2697 | 54.88% High risk |
45.28% High risk |
| Case C represents a high risk patient based on symptoms and even with a negative chest X-ray this patient would require further investigation to exclude lung cancer[17], as 20% of all chest X-ray requests from primary care in confirmed lung cancer patients are negative[33, 34]. The current cut-off of urgent cancer referrals in the UK is PPV>3% so this patient would be considered at high risk and should be investigated further, regardless of the chest X-ray findings. | ||||||||||||
* from raw data provided by one of the referenced authors
Likelihood ratio = LR+ Positive predictive value = PPV
Over-reliance on chest X-ray findings and ignoring the patient’s prior risk could result in a missed diagnosis. This observation is reflected in the most recent NICE guidelines for referral of suspected cancer, it supports better primary care access to high-resolution imaging when indicated for high-risk patients [4].
Hamilton et al., 2005 investigated first and subsequent presenting symptom in lung cancer patients. Raw data from this study was utilised in the Bayesian model for risk of lung cancer described Table 5. Walters et al., 2015 looked at synchronous symptoms that occurred at the same time but did not define the specific symptom only the frequency of a single or synchronous symptom at first presentation.
In this systematic review we provide supporting evidence for four important symptoms for lung cancer diagnosis: haemoptysis, dyspnoea, cough and chest pain. It also highlights the difficulties with evaluating the diagnostic value of constitutional symptoms. For the diagnosis of relatively rare conditions such as cancer, population-based prospective cohort studies may never be feasible, hence, Walter and colleagues (2015) used selected high-risk patients. As we reach the limit of what we can be achieve with routine data in their current form, we must develop more defined and sophisticated criteria for clinical coding of symptoms and routine risk stratification of patients in real-time during clinical decision making [40, 41].
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thanks Dr Jean Karl Soler, Mediterranean Institute of Primary Care, for advice on the use of TRANSHis, and Professor William Hamilton, University of Exeter, for the original data for his study and comments on the manuscript. The authors gratefully acknowledge infrastructure support from the Cancer Research UK Imperial Centre, the Imperial Experimental Cancer Medicine Centre and the National Institute for Health Research Imperial Biomedical Research Centre.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was partly funded by the National Awareness and Early Diagnosis Initiative, grant number C33754/A17871. The Funding Partners relevant to this award are (in alphabetical order): Cancer Research UK; Department of Health England; Economic and Social Research Council; Health & Social Care Research and Development Division, Pubic Health Agency, Northern Ireland; National Institute for Social Care and Health Research, Wales; and the Scottish Government.
References
- 1.GLOBOCON. Lung Cancer Incidence and Mortality Worldwide in 2012 2012 [Available from: http://globocan.iarc.fr/old/FactSheets/cancers/lung-new.asp.
- 2.Walters S, Benitez-Majano S, Muller P, Coleman MP, Allemani C, Butler J, et al. Is England closing the international gap in cancer survival? Br J Cancer. 2015;113(5):848–60. 10.1038/bjc.2015.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saving lives averting costs An analysis of the financial implications of achieving earlier diagnosis of colorectal, lung and ovarian cancer. [press release]. September 22, 2014 2014.
- 4.NICE. Suspected Cancer: Recognition and Referral. London: National Institute for Health and Care Excellence (UK) Copyright (c) National Collaborating Centre for Cancer.; 2015. [Google Scholar]
- 5.Schmidt-Hansen M, Berendse S, Hamilton W, Baldwin DR. Lung cancer in symptomatic patients presenting in primary care: a systematic review of risk prediction tools. The British journal of general practice: the journal of the Royal College of General Practitioners. 2017;67(659):e396–e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 7.Soler JK, Okkes I, Oskam S, van Boven K, Zivotic P, Jevtic M, et al. An international comparative family medicine study of the Transition Project data from the Netherlands, Malta, Japan and Serbia. An analysis of diagnostic odds ratios aggregated across age bands, years of observation and individual practices. Fam Pract. 2012;29(3):315–31. 10.1093/fampra/cmr100 [DOI] [PubMed] [Google Scholar]
- 8.Weed LL. The problem oriented record as a basic tool in medical education, patient care and clinical research. Annals of clinical research. 1971;3(3):131–4. [PubMed] [Google Scholar]
- 9.Soler JK, Corrigan D, Kazienko P, Kajdanowicz T, Danger R, Kulisiewicz M, et al. Evidence-based rules from family practice to inform family practice; the learning healthcare system case study on urinary tract infections. BMC Fam Pract. 2015;16:63 10.1186/s12875-015-0271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90. 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong R, Water E, Doyle J. Cochrane Handbook for Systematic Reviews of Interventions. 1993. ed. [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: Cohort study using General Practice Research Database. British Medical Journal. 2007;334(7602):1040–4. 10.1136/bmj.39171.637106.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hippisley-Cox J, Coupand C. Identifying patients with suspected lung cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2011;61(592):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter FM, Rubin G, Bankhead C, Morris HC, Hall N, Mills K, et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br J Cancer. 2015;112 Suppl 1:S6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubik AK, Zatloukal P, Tomasek L, Petruzelka L. Lung cancer risk among Czech women: a case-control study. Prev Med. 2002;34(4):436–44. 10.1006/pmed.2001.1002 [DOI] [PubMed] [Google Scholar]
- 17.Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made?—A population based case-control study. Thorax. 2005;60(12):1059–65. 10.1136/thx.2005.045880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyen-Omofoman B, Tata LJ, Baldwin DR, Smith CJ, Hubbard RB. Using socio-demographic and early clinical features in general practice to identify people with lung cancer earlier. Thorax. 2013;68(5):451–9. 10.1136/thoraxjnl-2012-202348 [DOI] [PubMed] [Google Scholar]
- 19.Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112 Suppl 1:S92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyi H, Hillerdal G, Branden E. A prospective study of a total material of lung cancer from a county in Sweden 1997–1999: gender, symptoms, type, stage, and smoking habits. Lung Cancer. 2002;36(1):9–14. [DOI] [PubMed] [Google Scholar]
- 21.Shim J, Brindle L, Simon M, George S. A systematic review of symptomatic diagnosis of lung cancer. Family Practice. 2014;31(2):137–48. 10.1093/fampra/cmt076 [DOI] [PubMed] [Google Scholar]
- 22.Hamilton W, Sharp D. Diagnosis of lung cancer in primary care: A structured review. Family Practice. 2004;21(6):605–11. 10.1093/fampra/cmh605 [DOI] [PubMed] [Google Scholar]
- 23.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. Jama. 2012;307(22):2418–29. 10.1001/jama.2012.5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black WC. Computed tomography screening for lung cancer: review of screening principles and update on current status. Cancer. 2007;110(11):2370–84. 10.1002/cncr.23059 [DOI] [PubMed] [Google Scholar]
- 25.Manser R, Lethaby A, Irving LB, Stone C, Byrnes G, Abramson MJ, et al. Screening for lung cancer. The Cochrane database of systematic reviews. 2013(6):Cd001991 10.1002/14651858.CD001991.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ades AE, Biswas M, Welton NJ, Hamilton W. Symptom lead time distribution in lung cancer: natural history and prospects for early diagnosis. International journal of epidemiology. 2014;43(6):1865–73. 10.1093/ije/dyu174 [DOI] [PubMed] [Google Scholar]
- 27.Biswas M, Ades AE, Hamilton W. Symptom lead times in lung and colorectal cancers: What are the benefits of symptom-based approaches to early diagnosis? Br J Cancer. 2015;112(2):271–7. 10.1038/bjc.2014.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emery JD, Shaw K, Williams B, Mazza D, Fallon-Ferguson J, Varlow M, et al. The role of primary care in early detection and follow-up of cancer. Nature reviews Clinical oncology. 2014;11(1):38–48. 10.1038/nrclinonc.2013.212 [DOI] [PubMed] [Google Scholar]
- 29.Kane R WK, Free C GJ. Uses of routine data sets in the evaluation of health promotion interventions: opportunities and limitations. Health Educ Behav. 2000;100(1):33–41. [Google Scholar]
- 30.Price SJ, Stapley SA, Shephard E, Barraclough K, Hamilton WT. Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case-control study. BMJ Open. 2016;6(5):e011664 10.1136/bmjopen-2016-011664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corner J, Hopkinson J, Fitzsimmons D, Barclay S, Muers M. Is late diagnosis of lung cancer inevitable? Interview study of patients' recollections of symptoms before diagnosis. Thorax. 2005;60(4):314–9. 10.1136/thx.2004.029264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus MW, Chen Y, Raji OY, Duffy SW, Field JK. LLPi: Liverpool Lung Project Risk Prediction Model for Lung Cancer Incidence. Cancer prevention research (Philadelphia, Pa). 2015;8(6):570–5. [DOI] [PubMed] [Google Scholar]
- 33.Stapley S, Sharp D, Hamilton W. Negative chest X-rays in primary care patients with lung cancer. The British journal of general practice: the journal of the Royal College of General Practitioners. 2006;56(529):570–3. [PMC free article] [PubMed] [Google Scholar]
- 34.Turkington PM, Kennan N, Greenstone MA. Misinterpretation of the chest x ray as a factor in the delayed diagnosis of lung cancer. Postgrad Med J. 2002;78(917):158–60. 10.1136/pmj.78.917.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bach PB, Elkin EB, Pastorino U, Kattan MW, Mushlin AI, Begg CB, et al. Benchmarking lung cancer mortality rates in current and former smokers. Chest. 2004;126(6):1742–9. 10.1378/chest.126.6.1742 [DOI] [PubMed] [Google Scholar]
- 36.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Annals of internal medicine. 2012;157(8):571–3. 10.7326/0003-4819-157-8-201210160-00524 [DOI] [PubMed] [Google Scholar]
- 37.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–8. [DOI] [PubMed] [Google Scholar]
- 38.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. The New England journal of medicine. 2013;369(3):245–54. 10.1056/NEJMoa1301851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wender R, Fontham ET, Barrera E Jr., Colditz GA, Church TR, Ettinger DS, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–17. 10.3322/caac.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ethier JF, Curcin V, Barton A, McGilchrist MM, Bastiaens H, Andreasson A, et al. Clinical data integration model. Core interoperability ontology for research using primary care data. Methods of information in medicine. 2015;54(1):16–23. 10.3414/ME13-02-0024 [DOI] [PubMed] [Google Scholar]
- 41.Kostopoulou O, Rosen A, Round T, Wright E, Douiri A, Delaney B. Early diagnostic suggestions improve accuracy of GPs: a randomised controlled trial using computer-simulated patients. The British journal of general practice: the journal of the Royal College of General Practitioners. 2015;65(630):e49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.