Abstract
The prevalence of metabolic diseases including obesity, diabetes, cardiovascular diseases, hypertension and cancer has evolved into a global epidemic over the last century. The rate of these disorders is continuously rising due to the lack of effective preventative and therapeutic strategies. This warrants for the development of novel strategies that could help in the prevention, treatment and/ or better management of such disorders. Although the complex pathophysiology of these metabolic diseases is one of the major hurdles in the development of preventive and/or therapeutic strategies, there are some factors that are or can speculated to be more effective to target than others. Recently, gut microbiome has emerged as one of the major contributing factors in metabolic diseases, and developing positive modulators of gut microbiota is being considered to be of significant interest. Natural non-digestible polysaccharides from plants and food sources are considered potent modulators of gut microbiome that can feed certain beneficial microbes in the gut. This has led to an increased interest in the isolation of novel bioactive polysaccharides from different plants and food sources and their application as functional components to modulate the gut microbiome composition to improve host’s health including metabolism. Therefore, polysaccharides, as prebiotics components, are being speculated to confer positive effects in managing metabolic diseases like obesity and diabetes. In this review article, we summarize some of the most common polysaccharides from plants and food that impact metabolic health and discuss why and how these could be helpful in preventing or ameliorating metabolic diseases such as obesity, type 2 diabetes, hypertension and dyslipidemia.
Keywords: Polysaccharides, Prebiotic, Gut, Microbiome, Microbiota, Metabolic syndrome, Diabetes, Obesity, Probiotics, Hypertension, Dyslipidemia
Introduction
Polysaccharides are a major group of organic macromolecules that are formed by the polymerization of simple sugar units. They are produced in plants with the primary purpose of storing energy and forming structural components. Starch, which is found mainly in two forms, amylose and amylopectin, is one such example of an energy storing polysaccharide. Cellulose is another most abundant polysaccharide in nature that acts as a structural component of the plant cell wall [1].
The characteristics and metabolic behavior of polysaccharides through the mammalian digestive process explains their valuable nutritional and health effects [2]. The digestion of plant polysaccharides starts right away in the mouth from mechanical stress and with the release of salivary enzymes such as amylase. Then, through the esophagus, these polysaccharides make it to the stomach where these polysaccharides absorb water, swell and get solubilized either completely or partially in the digestive fluid. The kind and magnitude of the process of acid hydrolysis in the stomach and enzyme digestion in the intestine depend on the constitution of the monosaccharide units, the array of covalent bonds and their position within the polymer, anomeric forms and substitutions on the sugar molecules of the polysaccharide. A major proportion of the sugar subunits originating from these digested polysaccharides are then absorbed in the small intestine. However, some polysaccharides (i.e. dietary fibers) resist hydrolysis in the stomach and the small intestine of humans. These polysaccharides are classified into two groups: fermentable and non-fermentable. Non-fermentable polysaccharides pass to the large intestine and are eventually excreted out as waste/ feces. However, the indigestible but fermentable polysaccharides are consumed (i.e., metabolized) by the microflora of the large intestine and are fermented to produce diverse products or metabolites that act as an additional energy source for the host. Short Chain Fatty Acids (SCFA), the major microbial metabolites found in the human gut, are produced mainly as a result of this microbial fermentation of such polysaccharides [3,4].
Given the well-established importance and beneficial roles of SCFAs in human gut physiology, it is suggested that the host gut microbiota is positively modulated by the fermentable polysaccharides wherein the growth and population of specific beneficial bacterial groups is promoted in the gut; and this phenomenon is called the prebiotic effect [5]. Since the prebiotic activity of these complex polysaccharides influences the host metabolism, the polysaccharide consumption is suggested to be able to beneficially enhance the host gut physiology and metabolic health by influencing the metabolic functions [6]. It is well known that perturbations in the gut microbiome composition (gut dysbiosis) and intestinal SCFAs levels can negatively impact the host metabolism and physiology and hence could also play a role in the pathology of metabolic diseases like obesity and diabetes [7–9]. Increasing the production of SCFAs in the gut by manipulating the gut microbiome using probiotics and prebiotics enhances metabolic functions and significantly reduces obesity- and diabetes-related metabolic derangements [7,10–12]. Herein, we review and summarize some of the major and recent evidences about the types of polysaccharides present in various edible plants, and how these polysaccharides could be exploited to improve host metabolic health and ameliorate the pathophysiology of metabolic diseases such as obesity, type 2 diabetes, hypertension and dyslipidemia.
Structural Features of Polysaccharides in Edible Plants
Distribution and structural diversity of polysaccharides in nature is highly complex and wide-ranging. Starch, cellulose, pectin, chitosan, xyloglucan, β-glucan, xanthan, arabinoxylan, carrageenan, inulin, agar and plant gums are common polysaccharides found in our daily food ingredients and around the nature. The molecular structures of food polysaccharides are shown in Table 1 and their molecular structure and chemical bonding and formula are depicted in Figure 1. Classification of polysaccharides is highly diverse; they are classified in different ways eg. on the basis of composition, function and origin. Depending on the single sugar moieties (i.e. glucose, galactose, fructose, mannose, etc.), polysaccharides are classified in two groups: 1) homo-polysaccharides, i.e., containing only one kind of polymerized sugar unit like starch, xylan, galactan and froctan, and 2) hetero-polysaccharides, i.e., containing two or more kinds of sugar units like pectin. Although, several hetero-polysaccharides are common; however, the abundance of homo-polysaccharides is found to be higher in the food forms [1,13]. These polysaccharides are extensively used in the manufacturing of food products such as confectionery, dairy, desserts, meat products, ready-to-eat foods, food dressings and also as fat replacers to produce low-fat products [14,15]. The digestion, metabolism and end-products produced from diverse polysaccharides depend on the sugar moieties as well as the covalent bonding between these units to form a complex polysaccharide chain. Thereby, as mentioned above, some of the polysaccharides gets digested and absorbed by human digestive enzymes with in the upper intestine; however, many complex polysaccharides are resistant to acidic as well as enzymatic digestion of human gastric and intestinal juices and thus reach to the complex world of microbes constituting the colonic microbiome. Several microbial communities are able to digest these non-digestible complex polysaccharides and produce metabolites that can interact with host cells and impact cellular and whole body metabolism. However, due to highly complex chemical structures, certain polysaccharides are still resistant to be digested by human gastrointestinal enzymes and microbiome and hence are eventually discarded as fecal waste at the end of digestion process.
Table 1:
Plant polysaccharides and their chemical composition, covalent linkage, source, abundance and degree of digestion
| Polysaccharides | Composition and linkage | Source | Abundance in compartment | Degree of digestion or fermentation |
|---|---|---|---|---|
| Cellulose | Glucose , Unbranched, β 1→4 | Plants | Cell walls | Non-or poorly fermentable |
| Starch (Amylose) | Glucose , Unbranched, α: 1→4 | Plants | Fruits, seeds, tubers | Two types: 1- Non digestible readily fermentable 2- Gelatinization making it more accessible to digestion |
| Starch (Amypectin) | Glucose , Branched α: 1→4 and 1→6 | Plants | Cell walls | Digestible |
| Inulin | Fructose Unbranched, β2→1 | Artichokes | Tuber | Readily fermentable |
| β- glucan | Glucose , Branched β1→3 , 1→6 | Cereal, bacteria, and fungi |
Cell walls | Readily fermentable |
| Pectins | Linear chains of α-(1–4)-linked D-galacturonic acid |
Terrestrial plants |
Non-woody parts specially fruits |
Readily fermentable |
| Xyloglucans | β1→4- Linked glucose 1–6 xylose followed by galactose or fucose | Vascular plants |
Primary cell wall | Readily fermentable |
| Arabinoxylan | D-xylosyl monomeric units linked β−1,4 and extensively modified with α-L- arabinofuranosyl residues on positions 2 and 3 | Rye and wheat |
Bran | |
| Arabic gum | Branched chains of (1–3)-linked D-galactopyranosyl units containing L-arabinofuranosyl, L- rhamnopyranosyl, D - glucuronopyranosyl and 4-O-methyl- D-glucuronopyranosyl units |
Acacia senegal |
Exudated gum from stems and branches |
Readily fermentable |
Figure 1: Structure of basic units of sugar moieties and their covalent bonding involved in formation of polysaccharide chains.
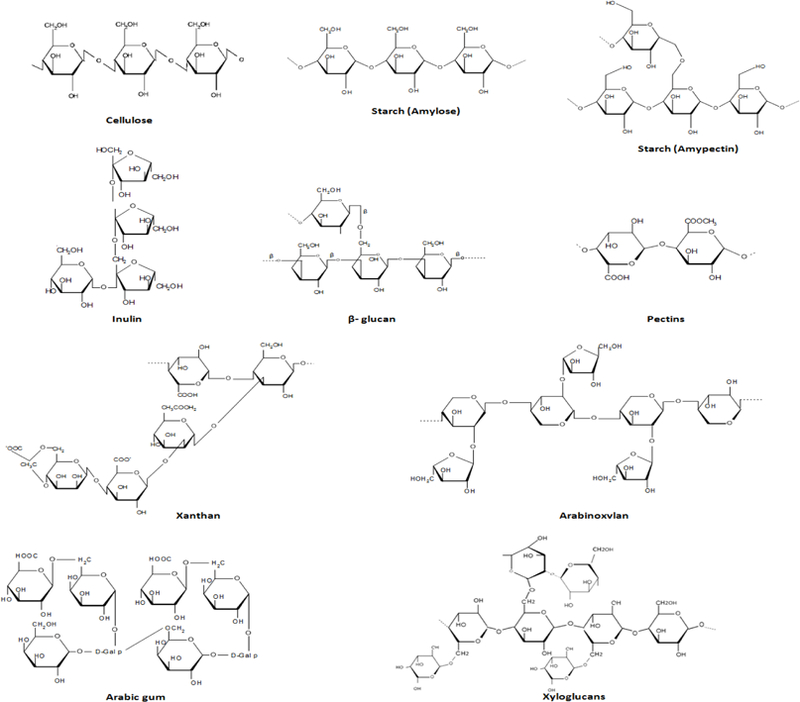
Cellulose consists a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Starch (Amylose) has α(1→4) glycosidic bonds connecting α-D-glucose units, while Starch (Amylpectin) consists glucose units linked in a linear manner with α(1→4) glycosidic bonds and α(1→6) bonds on branches which occur every 24 to 30 glucose units. Inulin possess β (2,1) bonds that connects terminating glucosyl moieties and a repetitive fructosyl moieties. β-Glucan composes β-D-glucose moieties that form a linear backbone with 1–3 β-glycosidic bonds. Pectins, prefer-entially form linear chains of α-(1–4)-linked D-galacturonic acid, however other saccharide residues i.e. D-xylose, D-apiose, rhamnose, D-galactose, L-arabinose and D-xylose also presents in the branching and linear chains inserted as random sequences. Xanthan has the monosaccharides i.e. β-D-glucose, α-D-mannose and α-D-glucoronic acid that are found in a ratio of 2:2:1 and linked with β-(1- > 4) glycosidic linkage. Arabinoxylan consist big chains of 1,4-linked xylose units. Arabic gum contains a complex chemical structure comprising with contiguous hydroxyprolines that are attached to oligo-α−1,3-L-arabinofurans and non-contiguous hydroxyprolines attached to galactose residues of oligo-arabinogalactans. Structure also consists β−1,3-D-galactopyran core with side chains of β-D-uronic acids, β-D-galactose, α-L-arabinose and α-L-rhamnose that are branched to the main chain by 1,6-linkages and single termini of α-L-rhamnopyranose, α-L-arabinofuranose and β-D-uronic acids via 1,2- and 1–4-linkages. Xyloglucan contains a core chain of β1→4-linked glucose moieties, in which of are substituted with 1–6 linked xylose sidechains.
Metabolic Diseases and Diet
The interaction of diet and metabolic health related to metabolic diseases like obesity, diabetes and cardiovascular diseases is well known and reviewed [16–20]. Common metabolic abnormalities are obesity and type 2 diabetes that are associated with hyperglycemia, hyperinsulinemia, dyslipidemia and hypertension. These abnormalities, collectively called as syndrome X or metabolic syndrome, are considered a strong risk factor for cardiovascular diseases. Metabolic syndrome is also associated with several other disorders like non-alcoholic fatty liver disease, reproductive disorders and sleep disorders. Low-grade inflammation and insulin resistance are common pathologies causing the obesity and type 2 diabetes. Growing prevalence of these metabolic diseases is due to over-nutrition and sedentary lifestyles [21–24]. Some of the common pathologies associated with these metabolic diseases that can be impacted by dietary polysaccharides are summarized in Figure 2 and described in brief below:
Figure 2: Pathological mechanisms/pathways involved in pathology of metabolic diseases like obesity and diabetes.
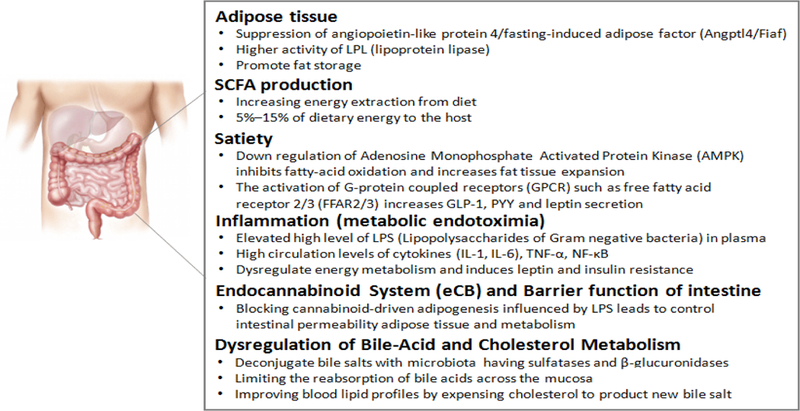
In obesity and type 2 diabetes, Adipose Tissue pathology is known to be mediated Suppression of angiopoietin-like protein 4/fasting-induced adi-pose factor (Angptl4/Fiaf) and increased LPL (lipoprotein lipase) activity that ultimately promote fat storage. SCFA production is contributing in increasing energy extraction from diet that may contribute in extra supply to the host to promote obesity, however, the biological function of SCFA is highly important as they contribute in 5%–15% of dietary energy to the host. Hunger versus Satiety remains one of the important mechanism to regulate energy homeostasis. Down regulation of Adenosine Monophosphate Activated Protein Kinase (AMPK) inhibits fatty-acid oxidation and increases fat tissue expansion. In addition, activation of certain G-protein coupled receptors (GPCRs) i.e. free fatty acid receptor 2/3 (FFAR2/3) increases GLP-1, PYY and leptin secretions. Inflammation (metabolic endotoximia) characterized with elevated high level of LPS (Lipopolysaccharides of Gram negative bacteria) in plasma, high circulation levels of cytokines (IL-1, IL-6), TNF-α and NF-κB, is another critical factor contribute in pathology of obesity/diabetes that contribute in dysregulation of energy metabolism and induces leptin and insulin resistance. Endocannabinoid system (eCB) and gut barrier function is crucial as blocking of cannabinoid-driven adipogenesis influenced by LPS leads to control intestinal permeability adipose tissue and metabolism. Dysregulation in bile-acid and cholesterol metabolism ie. deconjugation of bile salts with microbiota having sulfatases and β-glucuronidases, limiting the reabsorption of bile acids across the mucosa and improving blood lipid profiles by expensing cholesterol to product new bile salt are also prominent mechanism to contribute in obesity and diabetes pathologies.
Insulin Resistance:
Insulin resistance leads to a rise in insulin levels during fasting and after meals in order to maintain blood glucose at appropriate levels [25,26]. Increased insulin levels promote fat storage into the adipose tissues and other metabolic organs like liver and skeletal muscles [27,28]. On the other hand, insulin resistance leads to an increase in the amount of fatty acids in the blood circulation due to the loss of insulin’s ability to suppress lipolysis via Lipoprotein Lipase (LPL) activity in the adipose tissue [29]. Increased fatty acids from the adipose tissue disturb the lipid production function in the liver and result in more free fatty acids released into the bloodstream that cause high VLDL (very low-density lipoproteins) and low HDL (high-density lipoprotein) levels and dense LDL particles [30–32].
Some studies have also hinted at mechanisms explaining how insulin resistance is associated with blood pressure (hypertension) [33,34]. Insulin also acts as a vasodilator, and hence, in insulin resistant state, although insulin sodium re-absorption function remains normal but insulin vasodilatory actions become non-functional that causes blood pressure increase [35,36]. In addition, fatty acids also act as vasoconstrictor, and hence accompanied by the increased activity of the sympathetic system by insulin, these are collectively affected during the insulin resistance state [37,38].
Low-Grade Inflammation:
The relationship of metabolic diseases i.e., obesity and diabetes with low-grade inflammation has been previously documented and reviewed [39,40]. In brief, the levels of pro-inflammatory cytokines such as IL-6, tumor necrosis factor α (TNFα), resistin and C-Reactive Protein (CRP), all known to be released from the adipose tissues, are found to be significantly higher in obese and diabetic individuals [39,41]. In addition, mucosal immune system and the macrophages stimulated by dietary and microbial ingredients across the intestinal epithelial layer plays a crucial role in the regulation of metabolic function. However, the precise role of inflammation in the progression of obesity and diabetes still remains unclear. The presence of inflammatory cytokines remains higher in the blood circulation as well as in the local tissues i.e. adipose tissues and the vascular tissues of metabolic organs, thereby increasing the risk of cardiovascular diseases [42,43]. The level of endotoxin, called Lipopolysaccharides (LPS), is also commonly found to be increased in the circulation of obese and diabetic individuals and this condition is termed as Endotoxemia [44,45]. Increase LPS levels are another indicator of inflammatory state in obese and diabetic conditions, and provide evidence that dysbiotic gut microbiome may be the source of LPS [46]. Increased low-grade inflammation is further known to induce insulin resistance in different animal models and human studies [39,47].
Satiety Dysregulation:
Increased energy/food intake with reduced energy expenditure remains one of the biggest factors in inducing obesity and diabetes rate [48,49]. Food intake is tightly regulated by gut-brain axis, where gut hormones influence the hypothalamic area of brain to regulate hunger and satiety signals [50–52]. Ghrelin, a gut hormone, is released from an empty state of stomach, while others like Cholecystokinin (CCK), Glucagon-Like Protein-1 (GLP-1), Peptide YY (PYY), and Gastric Inhibitory Polypeptide (GIP) are decreased during hungry stage [53]. Ghrelin activates neuronal activity of hunger related neurons i.e. Agouti-related peptide (AgRP) and Neuropeptide Y (NPY) expressing cells and suppresses activity of satiety related neurons i.e. proopiomelanocortin (POMC) expressing cells in the hypothalamus [54]. This results to induce a response to search and seek the food. However, in full stomach, ghrelin levels reduces significantly and CCK, GLP-1, PYY and GIP levels increases that cause to reverse the hypothalamic signals to stop food search and seeking behavior [55]. Obese individuals show abnormalities in gut-hormones-hypothalamic axis to regulate energy sensing that results to increased food intake and fat accumulation. Dietary fibers in combination of microbiome may influence the gut-brain axis to regulate pathophysiology of metabolic diseases [56,57].
Endocannabinoids System and Tight Junctions in the Intestine:
The endocannabinoid system (ECS) is widely available in different metabolically active tissues and cells, that exert regulatory control on diverse aspects of metabolic functions including storage and burning of calories [58,59]. Therefore, this system represents a potential pharmaco-therapeutic target for obesity, diabetes and other eating disorders. Cannabinoid type 1 (CB-1) receptor blockers have been known for beneficial effects against obesity, diabetes and cardiovascular diseases; however, their clinical use has been paused due to the occasionally occurring psychiatric side effects upon their use [60,61]. Although, recent research in animals and humans has provided new knowledge on the mechanisms of actions of the ECS in the regulation of metabolism, detailed mechanisms underlying the pathology of metabolic diseases still remains to be illustrated completely. Wide spectrum studies have shown that the ECS is widely distributed throughout the gut. Impact of ECS in the regulation of food intake, nausea and emesis, gastric secretion and gastro-protection, GI motility, ion transport, visceral sensation, intestinal inflammation and cell proliferation in the gut is well known [62–64]. Molecular and cellular targets for ECS that include cannabinoid receptors, transient receptor potential vanilloid 1 receptors, peroxisome proliferator-activated receptor alpha receptors and the orphan G-protein coupled receptors, GPR55 and GPR119, which are widely expressed in the enteric nervous system, epithelial and immune cells, are also becoming clear [65]. ECS is also known to control the tight junctions of the intestinal tissues that regulate the integrity of the intestinal epithelial barrier and also prevent the abnormal permeability (leaky gut) conditions which is otherwise often found to occur in obesity and diabetic conditions [66].
Bile Acids and Cholesterol Metabolism:
Dietary fat is solubilized by the emulsification process mediated by bile acids that allows dietary fat to intestinal absorption. In liver tissues, cholesterol is converted into bile acids, thus the synthesis of bile acids regulate the whole body cholesterol metabolism [67]. Chenodeoxycholic acid and cholic acid that are the primary bile acids conjugate with taurine or glycine in human liver, and this conjugated form of bile acids enable the solubilization of fat that results in their intestinal absorption[68,69]. During digestion process, the absorption of primary bile acids happen actively in the distal ileum and results in their recycling; however, non-absorbed bile acids encounter colonic bacteria – mostly the bacteroides species – and are de-conjugated to deoxycholic acid and lithocholic acid that are further reabsorbed through the portal system [70]. Treatment of bile acids and their derivatives are strongly correlated with the improvement in glucose and lipid metabolism in rodent and human studies. Bile acids are known to activate intestinal L-cells and increase the release of GLP-1, that exhibit several beneficial effects in obese and diabetic individuals [71–74].
Microbiome Dysbiosis and SCFA Production:
Although the pathophysiology of metabolic diseases remains complex; the contribution of environmental and dietary factors, however, remain significant in the progression and management of these disorders. Weight loss, exercise and physical activity contribute in controlling the blood glucose, insulin sensitivity, lipid levels, and blood pressure in obese and diabetic individuals [75]. However, recent research has shown that gut microbiome is an important factor in the pathophysiology of glucose hemostasis and incidence of obesity and diabetes [76]. Consequently, the gut microbiome has recently been viewed as a potential target for the development of preventive and therapeutic strategies against metabolic diseases like obesity, diabetes and cardiovascular diseases [77].
There are numerous convincing evidences about the involvement of gut microbiota in glucose homeostasis and hence in obesity and diabetes [78,79]. Dietary changes can cause diversion in both quantitative and qualitative microbial population of the gut, where Gram-negative bacteria are significantly increased In the study on germ-free or gnotobiotic mice models, high-fat and high-sugar diets changed the intestinal microbial ecosystem, metabolic pathways, and gene expression only after two days and doubled the host body weight after two weeks, as compared to healthy diet. This indicates that changes in the gut microbiota appear earlier than expected in response to obesogenic diets that cause progression of obesity. In addition, fecal microbiome transplantation studies further evidence that the germ-free mice receiving microflora from obese mice gain twice fat as compared to the mice receiving microflora from lean mice, while eating the same diet, clearly suggesting that the gut microflora contribute in the progression of obesity and diabetes regardless the type of diets [81–83].
Role of Polysaccharides in Metabolic Health:
Biological activities of polysaccharides such as antibacterial, anti-cancer, antithrombotic, antioxidant, antiangiogenic and antiviral are known and reviewed in several independent articles [84,85]. Complex polysaccharides also help in regulating and lowering blood glucose levels, cholesterol levels, lipid levels, inhibition of fat accumulation, enhancing the absorption of minerals, regulating gastric emptying and bowel movement, increasing satiety and modulating the gut microbiota [15,85]. A number of in-vivo studies using different model systems (e.g. mice, rats, rabbits and humans) have reported that complex polysaccharides show positive effects in decreasing the risk of metabolic diseases like diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia and hypercholesterolemia as well as help in better management of these disorders [86,87]. Diverse metabolic effects of polysaccharides and their derived metabolites i.e., SCFAs on host health are summarized in Figure 3.
Figure 3: Fermentation of indigestible polysaccharides in colon and production of SCFA, that can further impact diverse mechanisms to maintain human health.
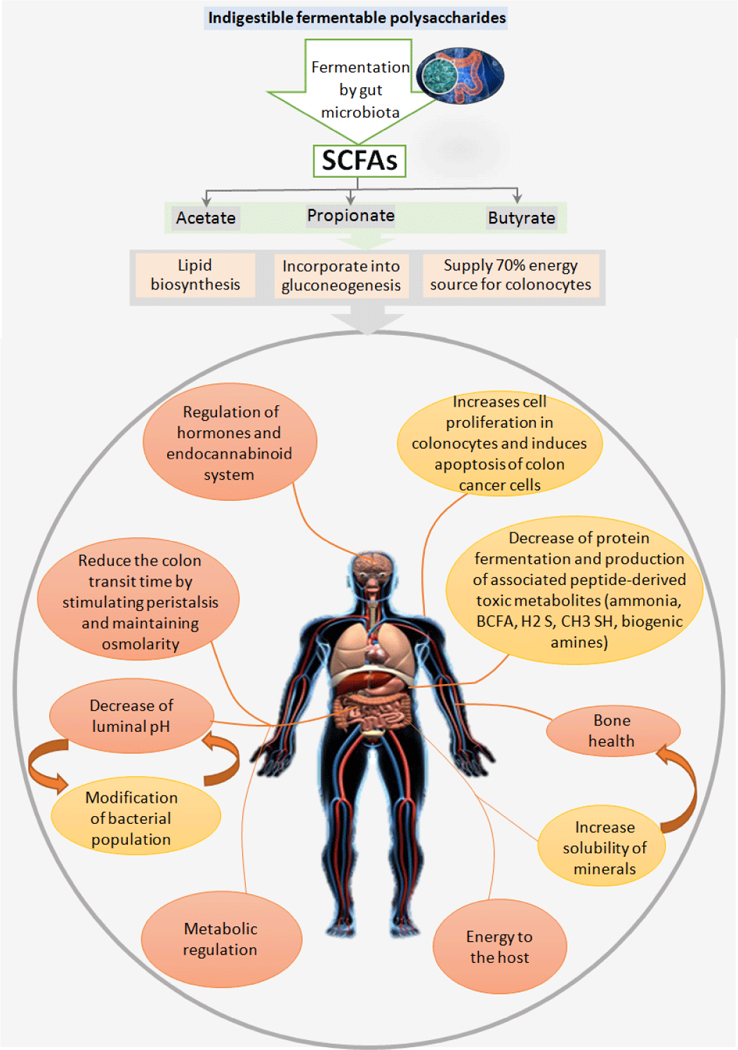
SCFA i.e. acetate, propionate and butyrate as fermented end products of oligosaccharides contributes in lipid biosynthesis, gluconeogenesis and en-ergy source for colonocytes. In addition, SCFA are also known impact several biological pathways in the host i.e. hormones, eCB system, cell prolifera-tion, cell death, bone health, mineral absorption, gut motility, intestinal pH and back to impact gut microbiome composition and its metabolic function, all these system manipulations contribute in changing metabolic health and energy homeostasis of the host.
Studies reporting the effect of polysaccharides from different plant sources on metabolic diseases are summarized in Table 2. Along with the beneficial effects on host metabolism including prevention or reduction of diabetes and obesity, it is known that polysaccharide like inulin, β- glucan oligosaccharides and soy fibers induce a shift in the gut microbiome to favor advantageous effects [88–90]. Certain natural dietary supplements (eg. green tea) that are traditionally known and used for their antidiabetic and anti-obesity effects are believed to be exhibiting these effects via alkaloids and flavonoids. However, recent studies show that the polysaccharide contents of green tea play a significant role in beneficial effects against obesity and diabetes [91,92]. Resistant starch from maize and Yacon syrup has also been reported to decrease food intake and appetite while increasing the plasma levels of hunger reducing and satiety promoting gut hormones like PYY and GLP-1 [93]. Considering these evidences and current paradigm about the beneficial effects of dietary fibers and polysaccharides, it is reasonable to speculate that polysaccharides could be explored and exploited for their use for both preventative and therapeutic measures against metabolic diseases like obesity, diabetes and cardiovascular events, as most of the studies have been performed on both healthy models as well as in the models of metabolic diseases.
Table 2:
Summary of findings about the biological efficacies of polysaccharides against various metabolic diseases
| S# | Source | Polysaccharides | Biological activity | Major findings | Ref. |
|---|---|---|---|---|---|
| 1 | Pistachio | Water-soluble polysaccharides |
Antioxidant and antihypertensive |
Pistachio polysaccharide have the potential to inhibit angiotensin converting enzyme (ACE; a blood pressure regulator) in-vitro and it is a promising candidate for future application for natural ACE inhibitory and antioxidant activities. | [116] |
| 2 | Almond | Water-soluble polysaccharides |
Antioxidant and antihypertensive |
Almond polysaccharide also have the potential to inhibit ACE and antioxidant activities in-vitro. | [116] |
| 3 | Tea | Polysaccharide | Antidiabetic | Tea polysaccharides exhibit high α-glucosidase inhibitory and antioxidant activity in vitro and also in vivo (mice), that can be beneficial for hyperglycemia treatment in diabetes. | [117] |
| Antioxidant | Antioxidant properties of tea polysaccharides can be exploited for chronic diseases including diabetes, obesity and oxidative stress related disorders. | ||||
| Anti-obesity | Polysaccharides and polyphenols suppressed body weight increase and fat accumulation and also improved blood lipid and antioxidant levels, along with effectively reducing serum leptin levels, inhibiting the absorption of fatty acids and markedly reducing the expression levels of the IL-6 and TNF-α gene in white adipose tissue of rats. Furthermore, polysaccharides and polyphenols were synergistic in reduction of serum leptin levels and in anti- inflammatory activity. | [91] | |||
| 4 |
Psyllium (Plantago psyllium) |
Husk fiber | Anti-diabetic and hypocholesterolemic |
Plantago psyllium husk fiber feeding to Albino rats for 7 weeks reduced blood glucose and cholesterol levels. | [118] |
| 5 | Basil seed (Ocimum basilicum Linn) |
AEOBS: aqueous extraction (gum) |
Antidiabetic | AEOBS reduces blood glucose levels in diabetic rats, with improvements in body weight, serum electrolytes, and hematological indices, along with increased pancreatic islets. | [119] |
| 6 | Fenugreek seeds (Trigonella foenum graecum L.) |
Sub-fraction rich in fibers |
Antidiabetic | The addition of fiber rich sub fraction of fenugreek seeds to insulin treatment decreases hyperglycemia, glycosuria, plasma glucagon, somatostatin levels and hyperglycemic response to the oral glucose tolerance test in diabetic dogs. | [120] |
| 7 | Jerusalem artichokes and chicory |
Inulin type fructans (commercially available) Oligofructose |
Anti-dyslipidemia | Prebiotic supplementation reduces plasma total cholesterol, LDL-cholesterol, triglycerides and increased HDL-cholesterol concentrations in diabetic human. | [121] |
| Anti-diabetic | Inulin and oligofructose reduces plasma cholesterol and triglycerides and decreases fat accumulation in liver and reduced hepatic steatosis in rodents. | [89] | |||
| Anti-obese/diabetes | Daily intake of oligofructose decreases energy intake, epididymal fat mass, body-weight gain, glycaemia, endotoxemia, adipose tissue pro-inflammatory cytokines, glucose tolerance and glucose-induced insulin secretion and intestinal permeability in humans and rodents. | [122] | |||
| 8 | Pumpkin | Protein-bound polysaccharide (PBPP) |
Antidiabetic | PBPP increases serum insulin, reduced the blood glucose with improvements in glucose tolerance of glucose in diabetic rats in dose dependent manner. | [123] |
| 9 | Yacon syrup | Fructo- oligosaccharide |
Anti-obesity/ diabetes |
Daily intake of Yacon syrup by obese and slightly dyslipidemic pre-menopausal women produced a significant decrease in body weight, waist circumference, body mass index and fasting serum insulin, by increasing defecation frequency, satiety sensation and serum LDL- cholesterol levels. | [124] |
| Galacto- oligossacharide (GOS) |
Microbiome dysbiosis and metabolism |
12-week supplementation of Yacon’s GOS to overweight or obese prediabetic men and women selectively increased fecal Bifidobacterium species abundance, without significant changes in insulin sensitivity and energy metabolism. | [125] | ||
| 10 | Maize | High amylose resistant starch (RS) |
Anti-obese/diabetes | The consumption of 15–30 g/d high-amylose maize resistant starch improves insulin sensitivity in men. | [126] |
| Body fat patterning and central appetite regulation |
Dietary RS significantly impacts on adipose tissue patterning by decreasing adipocyte size, glucose and insulin metabolism, as well as affecting hypothalamic neuronal appetite regulation in mice. | [93] | |||
| Anti-obese and energy metabolism |
RS potentially reduced adiposity and weight gain in obesity-prone (OP) and obesity-resistant (OR) male rats, by reducing energy intake, and changes in gut hormones and large bowel carbohydrate fermentation. | [127] | |||
| Insulin sensitivity and metabolic syndrome |
Consumption of resistant starch improves insulin sensitivity in human subjects with the metabolic syndrome. | [128] | |||
| Anti-obese/diabetes | Rats fed fermentable RS had increased cecal weights and plasma gut hormones e.g. peptide YY (PYY) and glucagon like protein-1 (GLP-1), gene transcription of PYY and proglucagon, short-chain fatty acids in cecal contents of rats. | [129] | |||
| 11 | Oatrim | β-Glucan | Anti-hyperglycemic | Consumption of foods containing moderate amounts of Oatrim fibers improves postprandial insulin release and glucose levels in normal and overweight women. | [130] |
| 12 | Oat and barley grains |
β-Glucan | Preventive and therapeutic against metabolic syndrome |
β-glucan improves postprandial glucose and insulin responses, and improve insulin sensitivity both in diabetic and nondiabetic humans. | [90] |
| 13 | Fruit of Lycium barbarum L. (Wolfberry) |
Acidic polysaccharide (LBP-s-1) |
Hypoglycemic | LBP-s-1 exhibit hypoglycemic and insulin-sensitizing activities via increasing glucose metabolism and insulin secretion and promoting pancreatic β cell proliferation in mice. | [92] |
| 14 | Apple | Thinned young apple polysaccharide (TYAP) |
Anti-obese/diabetes | TYAP attenuates high fat diet induced obesity and associated hepatic metabolic disorder by activating the hepatic mitochondrial respiratory function in mice. | [131] |
| 15 | Artemisia Iwayomogi |
Oligosaccharide | Anti-obese/ dyslipidemia |
Orally feeding of Artemisia Iwayomogi oligosaccharide not only has triglyceride and cholesterol reducing effects, but also reduces body weight and abdominal adipose tissue weights in obese rats. | [132] |
| 16 |
Physalis alkekengi L. |
A water-soluble polysaccharide (PPSB) |
Hypoglycemic activity in diabetic rats |
PPSB oral administration to alloxan-induced diabetic rats reduces blood glucose levels, and increase the body weight of diabetic mice compared with alloxan-induced diabetic control group. | [133] |
| 17 |
Solanum lycocarpum fruits or fruta-de-lobo (wolf-fruit) |
Polysaccharides | Anti-obese/diabetes | The main component of Solanum lycocarpum St.-Hil fruit as a traditional antidiabetic and cholesterol lowering medicine is polysaccharides. Therefore,I t is concluded that the anti-obesity and antidiabetic activity of the this medicine is related to its polysaccharides contents. | [134] |
| 18 | Wheat | Arabinoxylan | Hypoglycemic | Postprandial glucose and insulin responses improves upon ingestion of Arabinoxylan-rich fiber in human subjects. | [135] |
| 19 | Anti-obese/diabetes | Arabinoxylan feeding increased circulating satiety inducing gut hormones (peptide YY and glucagon-like peptide-1), decreased insulin resistance, body weight gain and fat mass with improved gut barrier function in mice. | [136] | ||
| 20 | Soy pods | Activated soy pod fiber (ASPF) |
Absorption of dietary fat in mice |
Mice fed high fat diet with 15% ASPF did not gain body fat, with decreased absorption of calories into the gut, with decreased plasma concentrations of the anti- inflammatory cytokine e.g. IL-10 and fecal excretion of triglycerides increased, which was associated to decreased bile acid secretion. A shift in abundances of microbiota in 10 genera was observed. Flavonifractor, Barne-siella, Bacteroides, Oscillibactor and Alistipes, were significantly increased but Parabacteroides, Ruminococcus, Hydrogenoanaerobacterium, Akkermansia and Lactococcus were significantly decreased in feces from ‘HFD-fed group. | [88] |
| 21 | Acacia senegal | Arabic gum | Anti-obese/diabetes | The total body weight gain, insulin level, fasting blood glucose concentrations were significantly decreased in Arabic gum treated mice in comparison with high fat diet or glucose treated mice. | [137] |
| Anti-nephropathic | Arabic gum treatment decreased blood pressure and proteinuria in diabetic mice. | [138] | |||
| Anti-obesity in healthy adult females |
Significant reduction in BMI by 0.32 and body fat percentage by 2.18% following regular intake of 30 g/day Arabic gum for 6 weeks was observed in adult female. | [139] |
Mechanisms of Polysaccharides to Modulate Gut Microbiome and Benefit Metabolic Health
Indigestibility of carbohydrates or polysaccharides in foods is one of the most important parameters determining their health effect on the host. Some polysaccharides are completely or partly broken down during the gastrointestinal tract digestion process; however, the ones that are not digested by human digestive enzymes are considered bulking agents and pass through the gut. These bulking agents dilute or bind to toxins and carcinogens in the intestine and physically remove them from the body as they move down the large intestine, and exhibit significant benefiting effects on the host. Indigestible but fermentable carbohydrates entering the large intestine are broken down and fermented (metabolized) by the residing micro-flora and the metabolites (eg. SCFAs) produced from this process confer a direct effect on colon function and the host health [2,3]. Polysaccharide fermentation by the gut microbiota yields energy for microbial growth as well as for intestinal cells including colonocytes mainly by producing metabolites like SCFA [4,94]. In addition, mixed gases (CO2, methane and hydrogen), lactate, formate, ethanol and some heat are also produced as a by-product of this fermentation process [95]. The ratio of fecal SCFAs produced upon polysaccharide fermentation into the human gut are in the order of: acetate (CH3COOH) > propionate (CH3CH2COOH) ≥ butyrate (CH3CH2CH2COOH) [96–98]. The concentration and proportion of these SCFAs is closely proportional to the amount of non-digestible polysaccharides available in the diet that reaches the colon [4]. The health benefits of a polysaccharides generally depends on the type and concentration of SCFAs enhanced in the region of the intestinal tissues where fermentation and SCFAs production takes place [2,4]. The major fraction of acetate is known to be included in lipid biosynthesis and hepatic gluconeogenesis, whereas propionate is primarily known to enter in hepatic gluconeogenesis flux. Butyrate is the major energy source for colonocytes, which provides approximately 70% of daily energy. The free form of these SCFAs exhibit several biological functions that can alter several physiological mechanism(s) in human body (Figure 3).
Selected indigestible but fermentable oligosaccharides or polysaccharides function as prebiotics. Prebiotics are defined as “a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a specific number of bacteria in the colon, and thus improving host health” [5]. Fermentation and the prebiotic effect of a fiber/ polysaccharides depends on its chemical composition of sugar moieties, solubility, chain length, degree of branching, particle size, porosity and presence of other dietary components like proteins and lipids [2]. For example, SCFA-producing bacterial population increases upon dietary fibers/ polysaccharides that help in reducing obesity and diabetes [99,100].
Gut microbiome and its fermentative properties control several nutrient sensing and enteric neuroendocrine mechanism(s) that regulate whole body metabolism. Gut microbiome metabolite like butyrate is known to enhance GLP-1 production that results to decrease obesity and diabetes in several rodent models [101]. On the same time, gut microbiome metabolites induced upon polysaccharide consumption are known to decrease ghrelin levels and ultimately reduce food intake via manipulating gut-brain axis [102,103]. Microbiome and its metabolites impact adipose tissue to reduce lipolysis by suppressing Fiaf and LPL and ultimately result in decreased fat storage [104,105]. Dietary fibers/ polysaccharide feeding lowers adiposity via lipolysis due to the gene expression pattern changing in white adipose tissue (by acting on PPARγ and G-coupled receptors protein), decreasing adipogenesis and increasing metabolic response to hormones such as leptin [72].
The microbiome changes are explained by the modulation of microbial communities that are over grown in favorable conditions (i.e., feeding of dietary fibers) versus other communities are suppressed in identical conditions [106,107]. Suppression of certain bacterial communities i.e., Bacteriodetes and increase in Firmicutes are known to be contributing in the pathology of obesity and diabetes in animal models and humans. However, this gives an opportunity to target these microbial species to reverse their proportion that can help in improving host metabolic function. Dietary fibers/polysaccharides are one of the efficient modulators of gut microbiome communities to help host metabolic health. In addition, certain bacteria species are also known to modulate immune function; for example: Bifidobacteria, Faecalibacterium and Akkermansia are interesting bacteria known for their anti-inflammatory properties and benefits in diabetes and weight gain [71,108–112]. Polysaccharide feeding dramatically increases the population of these bacterial species in several animal models and humans.
A plethora of literature is coming recently suggesting several parallel mechanisms connecting gut microbiota and metabolic diseases, and how dietary fibers/polysaccharides feeding can modulate gut microbiome-metabolic axis to reduce insulin resistance and adiposity [12,113,114]. These mechanisms include regulation of satiety, inflammation (metabolic endotoxemia), endocannabinoid system (eCB) and barrier function of intestine, regulation of bile-acid and cholesterol metabolism, effect on adipose tissue, production of SCFA and metabolism of choline [76,82,115]. Dietary polysaccharides are known to impact these pathways via modulation of gut microbiome via suggested pathways and indicate that the consumption of dietary polysaccharides can ameliorate metabolic diseases by diverse mechanisms (Figure 4).
Figure 4: Mechanisms link gut microbiome-prebiotic interactions to modulate host metabolism and their effects on miscellaneous mecha-nisms of metabolic regulation.
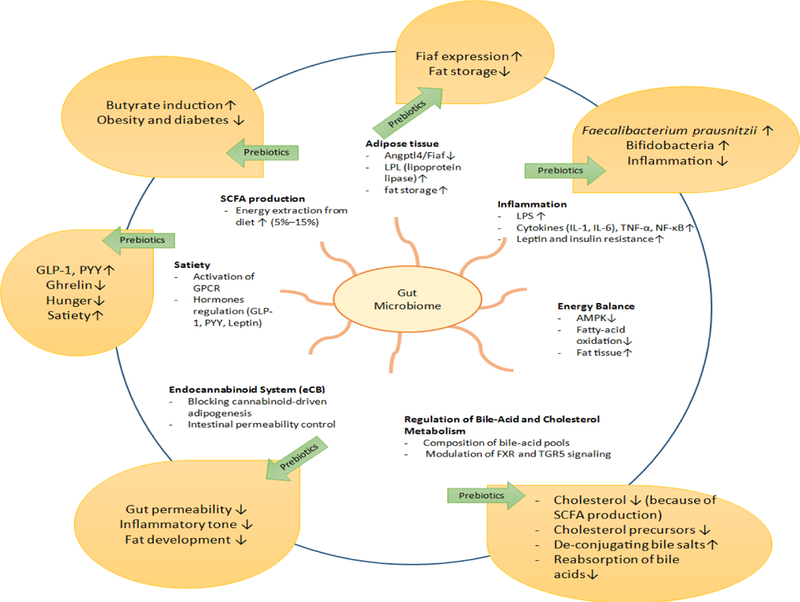
Non-digestible oligosaccharides (prebiotics) manipulation gut microbiome than involves in modulation of pathological pathways of obesity and dia-betes i.e. adipose tissue, inflammation, energy balance, bile acid and cholesterol metabolism, eCB, satiety and SCFA production (details in Figure 2) in beneficial manner, that further manipulate the gene expression, bacterial colonization and physiological function to reduce metabolic burden into the host body. Overall these changes contribute in reduction of obesity and diabetes.
Conclusions and Future Perspectives
Growing epidemic of metabolic diseases warrants development of novel and effective preventive and therapeutic strategies. Recent research findings are approving the relationship between gut microbiota and metabolic diseases beside other important factors such as diets and lifestyle. Dietary polysaccharides have received significant attention as functional biomaterials for manipulating the gut microbiome. Indigestible but fermentable polysaccharides, defined as prebiotics, can stimulate the growth and activity of beneficial bacteria in the colon. Therefore, inclusion of polysaccharides in diet influences the host metabolism, fat accumulation and insulin resistance. There are a lot of scientific studies confirming the positive effects of polysaccharides from various plant sources, as value-added products, on metabolic syndrome. In spite of multiple suggested mechanisms and accumulating data, a clear pathway of actions and the relationship between dietary polysaccharides consumption and human metabolism is not well established. Therefore, more research in this area is needed to determine the structural features of functional polysaccharides, accurate dosage for therapeutic applications, gut microbiota and metabolites changes in the intestine as well as to decipher the mechanism-of-action occurring in the gut upon the consumption of these polysaccharides.
Acknowledgments
Authors greatly acknowledge the funding support from Intramural Program of Wake Forest School of Medicine, Winston-Salem, NC, USA and Iran National Science Foundation (INSF), Iran.
References
- 1.Wrolstad RE. Food carbohydrate chemistry 2012.
- 2.Capuano E The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Critical reviews in food science and nutrition 2017;57(16):3543–3564. [DOI] [PubMed] [Google Scholar]
- 3.Slavin J Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5(4):1417–1435. 10.3390/nu5041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81(3):1031–1064. [DOI] [PubMed] [Google Scholar]
- 5.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017;8(2):172–184. 10.1080/19490976.2017.1290756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr 2013;109 Suppl 2:S81–85. 10.1017/S0007114512004047 [DOI] [PubMed] [Google Scholar]
- 7.He M, Shi B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci 2017;7:54 10.1186/s13578-017-0183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubinkina Veronika B, Tyakht Alexander V, Odintsova Vera Y, Yarygin Konstantin S, Kovarsky Boris A, Pavlenko Alexander V, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017. 5(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab 2017;26(4):672–685.10.1016/j.cmet.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, Webb M, Buch A, Keidar A, et al. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes (Lond) 2017. 10.1038/ijo.2017.210 [DOI] [PubMed] [Google Scholar]
- 11.Rondanelli M, Faliva MA, Perna S, Giacosa A, Peroni G, Castellazzi AM. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017;1–23. 10.1080/19490976.2017.1345414 [DOI] [PMC free article] [PubMed]
- 12.Dahiya DK, Renuka, Puniya M, Shandilya UK, Dhewa T, Kumar N, et al. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front Microbiol 2017;8:563 10.3389/fmicb.2017.00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishinari K, Doi E. Food hydrocolloids: structures, properties, and functions. Springer Science & Business Media 1993.
- 14.Milani J, Maleki G. Hydrocolloids in food industry. InTech Open acess 2012.
- 15.Liu J, Willför S, Xu C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioactive Carbohydrates and Dietary Fibre 20155(1):31–61. [Google Scholar]
- 16.Bernstein Laurie, Burns Casey, Sailer-Hammons Melissa Kurtz Angela, Rohr Frances. Multiclinic Observations on the Simplified Diet in PKU. J Nutr Metab 2017. 2017:4083293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murtaza Nida, Cuiv Paraic O Morrison Mark. Diet and the Microbiome. Gastroenterol Clin 2017;46(1):49–60. [DOI] [PubMed] [Google Scholar]
- 18.De Sousa SMD, Norman RJP. Metabolic syndrome, diet and exercise. Best Pract Res Clin Obstet Gynaecol 2016;37:140–151. [DOI] [PubMed] [Google Scholar]
- 19.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 2015;114(7):999–1012. 10.1017/S0007114515002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diet Marcadenti A., Cardiometabolic Factors and Type-2 Diabetes Mellitus: The Role of Genetics. Curr Diabetes Rev 2016;12(4):322–330. [DOI] [PubMed] [Google Scholar]
- 21.Hata Y, Nakajima K. Life-style and serum lipids and lipoproteins. J Atheroscler Thromb 2000;7(4):177–197. [DOI] [PubMed] [Google Scholar]
- 22.De Toro-Martin J, Arsenault BJ, Despres JP, Vohl MC. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017;9(8). 10.3390/nu9080913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol 2017;18(8):843–850. 10.1038/ni.3754 [DOI] [PubMed] [Google Scholar]
- 24.Heianza Y, Qi L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int J Mol Sci 2017;18(4).10.3390/ijms18040787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloomgarden ZT. Concepts of insulin resistance. Metab Syndr Relat Disord 2005;3(4):284–293. [DOI] [PubMed] [Google Scholar]
- 26.Liu CM, Tung TH, Tsai ST, Liu JH, Tsai YK, Chen VT, et al. Serum insulin, insulin resistance, beta-cell dysfunction, and gallstone disease among type 2 diabetics in Chinese population: a community-based study in Kinmen, Taiwan. World J Gastroenterol 2005;11(45):7159–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000106(4):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, et al. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 2000;105(12):1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panarotto D, Remillard P, Bouffard L, Maheux P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Invest 2002;32(2):84–92. [DOI] [PubMed] [Google Scholar]
- 30.Brar PC, Patel P, Katz S. The relationship between insulin resistance and endothelial dysfunction in obese adolescents. J Pediatr Endocrinol Metab 2017;30(6):635–642. 10.1515/jpem-2016-0404 [DOI] [PubMed] [Google Scholar]
- 31.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011;22(9):353–363. 10.1016/j.tem.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med 2005;47(4):201–210. [PubMed] [Google Scholar]
- 33.Geijselaers SLC, Sep SJS, Claessens D, Schram MT, van Boxtel MPJ, Henry RMA, et al. The Role of Hyperglycemia, Insulin Resistance, and Blood Pressure in Diabetes-Associated Differences in Cognitive Performance-The Maastricht Study. Diabetes Care 2017;40(11):1537–1547. 10.2337/dc17-0330 [DOI] [PubMed] [Google Scholar]
- 34.Jung CH, Jung SH, Lee B, Rosenberg M, Reaven GM, Kim SH. Relationship among age, insulin resistance, and blood pressure. J Am Soc Hypertens 2017;11(6):359–365.10.1016/j.jash.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentile MT, Vecchione C, Marino G, Aretini A, Di Pardo A, Antenucci G, et al. Resistin impairs insulin-evoked vasodilation. Diabetes 2008;57(3):577–583. [DOI] [PubMed] [Google Scholar]
- 36.Feldman RD, Bierbrier GS. Insulin-mediated vasodilation: impairment with increased blood pressure and body mass. Lancet 1993;342(8873):707–709. [DOI] [PubMed] [Google Scholar]
- 37.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 2009;4(2):113–119. 10.1111/j.1559-4572.2008.00044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur J A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014:943162 10.1155/2014/943162 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Pereira SS, Alvarez-Leite JI. Low-Grade Inflammation, Obesity, and Diabetes. Curr Obes Rep 2014;3(4):422–431. 10.1007/s13679-014-0124-9 [DOI] [PubMed] [Google Scholar]
- 40.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012;3(4):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahlen EM, Tengblad A, Lanne T, Clinchy B, Ernerudh J, Nystrom FH, et al. Abdominal obesity and low-grade systemic inflammation as markers of subclinical organ damage in type 2 diabetes. Diabetes Metab 2014;40(1):76–81. 10.1016/j.diabet.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 42.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58(8):1091–1103. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet 2005;366(9491):1059–1062. [DOI] [PubMed] [Google Scholar]
- 44.Monte SV, Caruana JA, Ghanim H, Sia CL, Korzeniewski K, Schentag JJ, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 2012;151(4):587–593. 10.1016/j.surg.2011.09.038 [DOI] [PubMed] [Google Scholar]
- 45.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57(6):1470–1481. 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 46.Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292(3):E740–747. [DOI] [PubMed] [Google Scholar]
- 47.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105(2):141–150. 10.1016/j.diabres.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 48.Sone H, Yoshimura Y, Ito H, Ohashi Y, Yamada N. Energy intake and obesity in Japanese patients with type 2 diabetes. Lancet 2004;363(9404):248–249. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz C, King NA, Perreira B, Blundell JE, Thivel D. A systematic review and meta-analysis of energy and macronutrient intake responses to physical activity interventions in children and adolescents with obesity. Pediatr Obes 2017;12(3):179–194. 10.1111/ijpo.12124 [DOI] [PubMed] [Google Scholar]
- 50.Buhmann H, le Roux CW, Bueter M. The gut-brain axis in obesity. Best Pract Res Clin Gastroenterol 2014;28(4):559–571. 10.1016/j.bpg.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 51.Papastamataki M, Papassotiriou I, Bartzeliotou A, Vazeou A, Roma E, Chrousos GP, et al. Incretins, amylin and other gut-brain axis hormones in children with coeliac disease. Eur J Clin Invest 2014;44(1):74–82. 10.1111/eci.12193 [DOI] [PubMed] [Google Scholar]
- 52.Sanger GJ, Lee K. Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat Rev Drug Discov 2008;7(3):241–254. 10.1038/nrd2444 [DOI] [PubMed] [Google Scholar]
- 53.Troke RC, Tan TM, Bloom SR. The future role of gut hormones in the treatment of obesity. Ther Adv Chronic Dis 2014;5(1):4–14. 10.1177/2040622313506730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakurazawa N, Mano-Otagiri A, Nemoto T, Shibasaki T. Effects of intracerebroventricular ghrelin on food intake and Fos expression in the arcuate nucleus of the hypothalamus in female rats vary with estrous cycle phase. Neurosci Lett 2013;541:204–208. 10.1016/j.neulet.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 55.Maric T, Sedki F, Ronfard B, Chafetz D, Shalev U. A limited role for ghrelin in heroin self-administration and food deprivation-induced reinstatement of heroin seeking in rats. Addict Biol 2012;17(3):613–622. 10.1111/j.1369-1600.2011.00396.x [DOI] [PubMed] [Google Scholar]
- 56.de Clercq NC, Frissen MN, Groen AK, Nieuwdorp M. Gut Microbiota and the Gut-Brain Axis: New Insights in the Pathophysiology of Metabolic Syndrome. Psychosom Med 2017;79(8):874–879. 10.1097/PSY.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 57.Clemmensen C, Muller TD, Woods SC, Berthoud HR, Seeley RJ, Tschop MH. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017;168(5):758–774. 10.1016/j.cell.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazier W, Saucisse N, Gatta-Cherifi B, Cota D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol Metab 2015;26(10):524–537. 10.1016/j.tem.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 59.Di Marzo V The endocannabinoid system in metabolic control: a preface. Best Pract Res Clin Endocrinol Metab 2009;23(1):vii–ix. 10.1016/j.beem.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 60.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005;365(9468):1389–1397. [DOI] [PubMed] [Google Scholar]
- 61.Roussel R [Three studies in type 2 diabetes: SERENADE the rimonabant (a blocker of the CB 1 receptors) in monotherapy in type 2 diabetes]. Ann Endocrinol (Paris) 2007;68 Suppl 1:33–35. [DOI] [PubMed] [Google Scholar]
- 62.Acharya N, Penukonda S, Shcheglova T, Hagymasi AT, Basu S, Srivastava PK. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci U S A 2017;114(19):5005–5010. 10.1073/pnas.1612177114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharkey KA, Wiley JW. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016;151(2):252–266. 10.1053/j.gastro.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Marzo V, Piscitelli F. Gut feelings about the endocannabinoid system. Neurogastroenterol Motil 2011;23(5):391–398. 10.1111/j.1365-2982.2011.01689.x [DOI] [PubMed] [Google Scholar]
- 65.Storr MA, Sharkey KA. The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol 2007;7(6):575–582. [DOI] [PubMed] [Google Scholar]
- 66.Cani PD. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clin Microbiol Infect 2012;18 Suppl 4:50–53. 10.1111/j.1469-0691.2012.03866.x [DOI] [PubMed] [Google Scholar]
- 67.Miettinen TA. Lipid absorption, bile acids, and cholesterol metabolism in patients with chronic liver disease. Gut 1972;13(9):682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap--bile acids in metabolic control. Nat Rev Endocrinol 2014;10(8):488–498. 10.1038/nrendo.2014.60 [DOI] [PubMed] [Google Scholar]
- 69.Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol 2009;15(7):804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pean N, Doignon I, Tordjmann T. Gut microbiota and bile acids: an old story revisited (again). Clin Res Hepatol Gastroenterol 2014;38(2):129–131. 10.1016/j.clinre.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 71.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut micro biome and implications for human health. J Transl Med 2017;15(1):73 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Druart C, Alligier M, Salazar N, Neyrinck AM, Delzenne NM. Modulation of the gut microbiota by nutrients with prebiotic and probiotic properties. Adv Nutr 2014;5(5):624S–633S. 10.3945/an.114.005835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biemann Ronald, Penner Marina, Borucki Katrin, Westphal Sabine, Luley Claus, Ronicke Raik, et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: results of a randomized controlled trial. Sci Rep 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, et al. Bile Acids Trigger GLP-1 Release Predominantly by Accessing Basolaterally Located G Protein-Coupled Bile Acid Receptors. Endocrinology 2015;156(11):3961–3970. 10.1210/en.2015-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol 2013;3(1):1–58. 10.1002/cphy.c110062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 2016;8(1):42 10.1186/s13073-016-0303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jayasinghe TN, Chiavaroli V, Holland DJ, Cutfield WS, O’Sullivan JM. The New Era of Treatment for Obesity and Metabolic Disorders: Evidence and Expectations for Gut Microbiome Transplantation. Front Cell Infect Microbiol 2016;6:15 10.3389/fcimb.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J 2016;92(1087):286–300. 10.1136/postgradmedj-2015-133285 [DOI] [PubMed] [Google Scholar]
- 79.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013;27(1):73–83. 10.1016/j.bpg.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 80.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 81.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328(5975):228–231. 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 83.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 2015;26(9):493–501. 10.1016/j.tem.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misurcova L, Skrovankova S, Samek D, Ambrozova J, Machu L. Health benefits of algal polysaccharides in human nutrition. Adv Food Nutr Res 2012;66:75–145. 10.1016/B978-0-12-394597-6.00003-3 [DOI] [PubMed] [Google Scholar]
- 85.Livesey G Implications of plant polysaccharides for human health, especially obesity. Food Hydrocolloids 1991;5(1–2):23–29. [Google Scholar]
- 86.Moreno Franco B, Leon Latre M, Andres Esteban EM, Ordovas JM, Casasnovas JA, Penalvo JL. Soluble and insoluble dietary fibre intake and risk factors for metabolic syndrome and cardiovascular disease in middle-aged adults: the AWHS cohort. Nutr Hosp 2014;30(6):1279–1288. 10.3305/nh.2014.30.6.7778 [DOI] [PubMed] [Google Scholar]
- 87.Rivellese AA, Maffettone A. Dietary Fiber in the Treatment of Metabolic Diseases. European Journal of Clinical Nutrition 1995; 49:S110–S112. [PubMed] [Google Scholar]
- 88.Boue S, Fortgang I, Levy RJ Jr, Bhatnagar D, Burow M, Fahey G, et al. A novel gastrointestinal microbiome modulator from soy pods reduces absorption of dietary fat in mice. Obesity (Silver Spring) 2016;24(1):87–95. 10.1002/oby.21197 [DOI] [PubMed] [Google Scholar]
- 89.Beylot M Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br J Nutr 2005;93 Suppl 1:S163–168. [DOI] [PubMed] [Google Scholar]
- 90.El Khoury D, Cuda C, Luhovyy BL, Anderson GH. Beta glucan: health benefits in obesity and metabolic syndrome. Journal of nutrition and metabolism 2011; 2012:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Y, Zhang M, Wu T, Dai S, Xu J, Zhou Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct 2015;6(1):297–304. 10.1039/c4fo00970c [DOI] [PubMed] [Google Scholar]
- 92.Zhu J, Liu W, Yu J, Zou S, Wang J, Yao W, et al. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr Polym 2013;98(1):8–16. 10.1016/j.carbpol.2013.04.057 [DOI] [PubMed] [Google Scholar]
- 93.So PW, Yu WS, Kuo YT, Wasserfall C, Goldstone AP, Bell JD, et al. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS One 2007;2(12):e1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mortensen PB, et al. The Production of Short Chain Fatty-Acids from Dietary-Fibers, Polysaccharides and Monosacchardies in a Fecal Incubation System. Gastroenterology, 1987;92(5):1328–1328. [Google Scholar]
- 95.Tian G, Wu X, Chen D, Yu B, He J. Adaptation of gut microbiome to different dietary nonstarch polysaccharide fractions in a porcine model. Mol Nutr Food Res 2017;61(10). 10.1002/mnfr.201700012 [DOI] [PubMed] [Google Scholar]
- 96.Siigur U, Norin KE, Allgood G, Schlagheck T, Midtvedt T. Concentrations and Correlations of Fecal Short-Chain Fatty-Acids and Fecal Water-Content in Man. Microbial Ecology in Health and Disease 1994;7(6):287–294. [Google Scholar]
- 97.Goffredo M, Mass K, Parks EJ, Wagner DA, McClure EA, Graf J, et al. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J Clin Endocrinol Metab 2016;101(11):4367–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang P, Chen Y, Zhou Q, Zheng X, Zhu X, Zhao Y. Understanding short-chain fatty acids accumulation enhanced in waste activated sludge alkaline fermentation: kinetics and microbiology. Environ Sci Technol 2010;44(24):9343–9348. 10.1021/es102878m [DOI] [PubMed] [Google Scholar]
- 99.Krogh U, Bruun TS, Poulsen J, Theil PK. Impact of fat source and dietary fibers on feed intake, plasma metabolites, litter gain and the yield and composition of milk in sows. Animal 2017;11(6):975–983. 10.1017/S1751731116002585 [DOI] [PubMed] [Google Scholar]
- 100.Ehle FR, Robertson JB, Van Soest PJ. Influence of dietary fibers on fermentation in the human large intestine. J Nutr 1982;112(1):158–166. [DOI] [PubMed] [Google Scholar]
- 101.Duca FA, Lam TK. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab 2014;16 Suppl 1:68–76. 10.1111/dom.12340 [DOI] [PubMed] [Google Scholar]
- 102.Schroeder N, Marquart LF, Gallaher DD. The role of viscosity and fermentability of dietary fibers on satiety- and adiposity-related hormones in rats. Nutrients 2013;5(6):2093–2113. 10.3390/nu5062093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang ZQ, Zuberi AR, Zhang XH, Macgowan J, Qin J, Ye X, et al. Effects of dietary fibers on weight gain, carbohydrate metabolism, and gastric ghrelin gene expression in mice fed a high-fat diet. Metabolism 2007;56(12):1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Camp JG, Jazwa AL, Trent CM, Rawls JF. Intronic cis-regulatory modules mediate tissue-specific and microbial control of angptl4/ fiaf transcription. PLoS Genet 2012;8(3):e1002585 10.1371/journal.pgen.1002585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paßlack N, Zentek J, Larsen JA, Westropp JL, Fascetti AJ. Impact of hyperlipidaemia on intermediary metabolism, faecal microbial metabolites and urinary characteristics of lipoprotein lipase deficient vs. normal cats. J Anim Physiol Anim Nutr (Berl) 2017. 10.1111/jpn.12721 [DOI] [PubMed]
- 106.Luo Y, Zhang L, Li H, Smidt H, Wright AG, Zhang K, et al. Different Types of Dietary Fibers Trigger Specific Alterations in Composition and Predicted Functions of Colonic Bacterial Communities in BALB/c Mice. Front Microbiol 2017;8:966 10.3389/fmicb.2017.00966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trifonova R, Postma J, Ketelaars JJ, van Elsas JD. Thermally treated grass fibers as colonizable substrate for beneficial bacterial inoculum. Microb Ecol 2008;56(3):561–571. 10.1007/s00248-008-9376-9 [DOI] [PubMed] [Google Scholar]
- 108.Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. Comparing three preparation methods of standard substances for intestinal bifidobacteria of childhood obesity. Acta Paediatr 2016;105(10):e496–498. 10.1111/apa.13464 [DOI] [PubMed] [Google Scholar]
- 109.Yin YN, Yu QF, Fu N, Liu XW, Lu FG. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol 2010;16(27):3394–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng J, Tang H, Li M, Pang X, Wang L, Zhang M, et al. The abundance of fecal Faecalibacterium prausnitzii in relation to obesity and gender in Chinese adults. Arch Microbiol 2014;196(1):73–77. 10.1007/s00203-013-0942-2 [DOI] [PubMed] [Google Scholar]
- 111.Shen W, Shen M, Zhao X, Zhu H, Yang Y, Lu S, et al. , Anti-obesity Effect of Capsaicin in Mice Fed with High-Fat Diet Is Associated with an Increase in Population of the Gut Bacterium Akkermansia muciniphila. Front Microbiol 2017;8:272 10.3389/fmicb.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Everarda Amandine, Belzerb Clara, Geurtsa Lucie, Ouwerkerkb Janneke P., Druarta Celine, Bindelsa Laure B., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013. 110(22):9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohiti-Asli M, Shivazad M, Zaghari M, Aminzadeh S, Rezaian M, Mateos GG. Dietary fibers and crude protein content alleviate hepatic fat deposition and obesity in broiler breeder hens. Poult Sci 2012;91(12):3107–3114. 10.3382/ps.2011-02040 [DOI] [PubMed] [Google Scholar]
- 114.Qi L, Meigs JB, Liu S, Manson JE, Mantzoros C, Hu FB. Dietary fibers and glycemic load, obesity, and plasma adiponectin levels in women with type 2 diabetes. Diabetes Care 2006;29(7):1501–1505. [DOI] [PubMed] [Google Scholar]
- 115.Aguirre M, Venema K. Does the gut microbiota contribute to obesity? Going beyond the gut feeling. Microorganisms 2015;3(2):213–235. 10.3390/microorganisms3020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sila A, Bayar N, Ghazala I, Bougatef A, Ellouz-Ghorbel R, Ellouz-Chaabouni S. Water-soluble polysaccharides from agro-industrial by-products: functional and biological properties. Int J Biol Macromol 2014;69:236–243. 10.1016/j.ijbiomac.2014.05.052 [DOI] [PubMed] [Google Scholar]
- 117.Chen H, Qu Z, Fu L, Dong P, Zhang X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J Food Sci 2009;74(6):C469–74. 10.1111/j.1750-3841.2009.01231.x [DOI] [PubMed] [Google Scholar]
- 118.Ahmed Ishtiaq, Naeem Muhammad, Shakoor Abdul, Ahmed Zaheer Zameer. Investigation of anti-diabetic and hypocholesterolemic potential of psyllium husk fiber (Plantago psyllium) in diabetic and hypercholesterolemic albino rats. International Journal of Medical and Health Sciences 2010;4(1):30–34. [Google Scholar]
- 119.Chaudhary S, Semwal A, Kumar H, Verma HC, Kumar A. In-vivo study for anti-hyperglycemic potential of aqueous extract of Basil seeds (Ocimum basilicum Linn) and its influence on biochemical parameters, serum electrolytes and haematological indices. Biomed Pharmacother 2016;84:2008–2013. 10.1016/j.biopha.2016.11.020 [DOI] [PubMed] [Google Scholar]
- 120.Ribes G, Sauvaire Y, Da Costa C, Baccou JC, Loubatieres-Mariani MM. Antidiabetic effects of subtractions from fenugreek seeds in diabetic dogs. Proc Soc Exp Biol Med 1986;182(2):159–166. [DOI] [PubMed] [Google Scholar]
- 121.Beserra BT, Fernandes R, do Rosario VA, Mocellin MC, Kuntz MG, Trindade EB. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr 2015;34(5):845–858. 10.1016/j.clnu.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 122.Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc 2010;69(3):434–441. 10.1017/S0029665110001813 [DOI] [PubMed] [Google Scholar]
- 123.Quanhong L, Caili F, Yukui R, Guanghui H, Tongyi C. Effects of protein-bound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum Nutr 2005;60(1):13–16. [DOI] [PubMed] [Google Scholar]
- 124.Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, et al. Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clin Nutr 2009;28(2):182–187. 10.1016/j.clnu.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 125.Canfora EE, van der Beek CM, Hermes GDA, Goossens GH, Jocken JWE, Holst JJ, et al. Supplementation of Diet With Galacto-oligosaccharides Increases Bifidobacteria, but Not Insulin Sensitivity, in Obese Prediabetic Individuals. Gastroenterology 2017;153(1):87–97.10.1053/j.gastro.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 126.Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL, et al. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 2012;142(4):717–723. 10.3945/jn.111.152975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belobrajdic DP, King RA, Christophersen CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab (Lond) 2012;9(1):93 10.1186/1743-7075-9-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med 2010;27(4):391–397. 10.1111/j.1464-5491.2010.02923.x [DOI] [PubMed] [Google Scholar]
- 129.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, et al. Effects of resistant starch, a non‐digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring) 2006;14(9):1523–1534. [DOI] [PubMed] [Google Scholar]
- 130.Behall KM, Scholfield DJ, Hallfrisch JG, Liljeberg-Elmstahl HG. Consumption of both resistant starch and β-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 2006;29(5):976–981. [DOI] [PubMed] [Google Scholar]
- 131.Chen Lei, Yang Xi, Liu Run, Liu Lei, Zhao Daina, Liu Jiankang, et al. Thinned young apple polysaccharide improves hepatic metabolic disorder in high-fat diet-induced obese mice by activating mitochondrial respiratory functions. Journal of Functional Foods 2017;33:396–407. [Google Scholar]
- 132.Jang JY, Choi HJ. Effects of Artemisia iwayomogi oligosaccharide on the blood lipids, abdominal adipose tissues and leptin levels in the obese rats. Korean Journal of Nutrition, 2003;36(5):437–445. [Google Scholar]
- 133.Tong Haibin, Liang Zhongyan, Wang Guiyun. Structural characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Physalis alkekengi L. Carbohydrate polymers 2008;71(2):316–323. [Google Scholar]
- 134.Dall Agnol R, Lino von Poser G. The use of complex polysaccharides in the management of metabolic diseases: the case of Solanum lycocarpum fruits. J Ethnopharmacol 2000;71(1–2):337–341. [DOI] [PubMed] [Google Scholar]
- 135.Lu ZX, Walker KZ, Muir JG, Mascara T, O Dea K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am J Clin Nutr 2000;71(5):1123–1128. [DOI] [PubMed] [Google Scholar]
- 136.Neyrinck AM, Van Hee VF, Piront N, De Backer F, Toussaint O, Cani PD, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes 2012;2:e28 10.1038/nutd.2011.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nasir O Effect of Gum Arabic (Acacia Senegal) on Glucose Metabolism and Body Weight Gain in Mice. Journal of Biology, Agriculture and Healthcare 2011;4(9):34–41. [Google Scholar]
- 138.Nasir O, Umbach AT, Rexhepaj R, Ackermann TF, Bhandaru M, Ebrahim A, et al. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res 2012;35(5):365–372. 10.1159/000336359 [DOI] [PubMed] [Google Scholar]
- 139.Babiker R, Merghani TH, Elmusharaf K, Badi RM, Lang F, Saeed AM. Effects of Gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: two-arm randomized, placebo controlled, double-blind trial. Nutr J 2012;11:111 10.1186/1475-2891-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]


