Abstract
Background:
Swallowing inefficiency is a prevalent but understudied problem in individuals with Amyotrophic Lateral Sclerosis (ALS). Although reduced pharyngeal constriction has been identified as a mechanism contributing to swallowing inefficiency following stroke, this relationship has not been empirically tested in the ALS population. This study sought to characterize profiles of swallowing efficiency in a sample of ALS patients and investigate relationships between pharyngeal constriction and swallowing efficiency.
Methods:
Twenty-six adults with ALS underwent videofluoroscopic swallowing studies, involving 3mL-thin, 20mL-thin, and 3mL-pudding boluses. Full-length recordings were segmented into bolus clips and randomized for analysis. We recorded the total number of swallows per bolus and obtained normalized pixel-based measures of pharyngeal constriction area and post-swallow residue in the vallecular and pyriform sinuses. Linear mixed models with Spearman’s correlations were used to determine relationships between pharyngeal constriction and swallowing efficiency, with added factors of bolus volume and thickness.
Key Results:
Individuals with ALS demonstrated reduced pharyngeal constriction and increased vallecular and pyriform sinus residue, compared to norms. Reduced pharyngeal constriction had a significant effect on the presence of vallecular and pyriform sinus residue, as well as the number of swallows per bolus. Increased bolus thickness was associated with increased vallecular residue, while increased bolus volume was associated with reduced pharyngeal constriction. Results were significant at p<0.05.
Conclusions & Inferences:
Our results suggest that reduced pharyngeal constriction is a significant physiological parameter related to swallow inefficiency in ALS. Future work is needed to corroborate these preliminary results and investigate factors to mitigate such impairments.
Keywords: Amyotrophic Lateral Sclerosis, deglutition disorders, pharyngeal constriction, post-swallow residue, videofluoroscopy
Abbreviated abstract: This study was conducted to 1) characterize swallowing efficiency and pharyngeal constriction in a sample of individuals with ALS and 2) explore reduced pharyngeal constriction as a potential mechanism for swallowing inefficiency. Our results showed a high prevalence of inefficient swallowing in the sample, and identified that reductions in pharyngeal constriction are associated with inefficient swallowing (i.e., post-swallow pharyngeal residue, multiple swallows).
Dysphagia, or swallowing impairment, is a serious consequence of bulbar disease in Amyotrophic Lateral Sclerosis (ALS) and manifests with functional impairments in both swallowing safety and efficiency.1–3 Swallowing inefficiency (i.e., the presence of post-swallow pharyngeal residue and/or the need to take multiple swallows per bolus) is particularly challenging for individuals with ALS, leading to prolonged mealtimes, mealtime fatigue, and reduced quality of life.4,5 Still, it remains unclear which physiological mechanisms contribute to impaired swallowing efficiency in this complex patient population. Further, in individuals with ALS, post-swallow pharyngeal residue has been identified as an independent risk for subsequent aspiration events6–8 and is linked to the development of malnutrition9–11 that is noted to increase the risk of death by 7.7 times.12 Despite these concerns, the majority of ALS dysphagia research has focused on studying swallowing safety with only 29.9% of published literature on dysphagia in ALS addressing problems with swallowing efficiency.1 In order to identify treatments with the potential to improve swallowing efficiency, it is first important to identify the pathophysiological mechanisms behind inefficiency.
Potential mechanisms of impaired swallowing efficiency in ALS can be posited based on findings from manometry data in ALS that document decreased pharyngeal pressures, reduced base of tongue contact to the posterior pharyngeal wall, and overall reduced pharyngeal motility during swallowing.6,13–17 Higo et al. explored pharyngeal pressure generation profiles in patients with ALS,14 grouping patients by disease duration and onset type (i.e., bulbar vs. spinal/”classic”). The authors identified differences in pharyngeal pressure generation during swallowing for patients with a bulbar-onset, within 6 months following the onset of symptoms. Similarly, Tomik et al. identified reduced base of tongue pressure and prolonged bolus transit times in patients with bulbar ALS.18 Using combined manometry and videofluoroscopy, Goeleven and colleagues reported converging evidence of low tongue driving force and reduced pharyngeal contraction amplitudes during swallowing, and noted co-occurrence of these impairments and the presence of post-swallow pharyngeal residue.6 Therefore, physiological impairments in pharyngeal pressure generation have been postulated as mechanisms contributing to swallow inefficiency, thus leading to post-swallow residue in patients with ALS.
Reduced pharyngeal strength and constriction have been implicated as factors contributing to the presence of pharyngeal residue in other clinical populations of individuals with dysphagia.19–23 Research quantifying pharyngeal area during swallowing, using 2-dimensional (2-D) lateral videofluoroscopy in individuals with dysphagia following stroke, identified associations between larger (i.e., less constricted) measures of pharyngeal area at the point of maximum constriction and the amount of post-swallow residue.24 Despite the inherent limitations of using a 2-D measurement to make inferences about 3-dimensional (3-D) anatomical regions, the validity of this analysis method is supported by previous work. Strong correlations have been identified between fluoroscopic measurement of pharyngeal constriction area and concurrent manometric readings of pharyngeal swallowing pressures.25,26 Further, 2-D measures of pharyngeal residue area on lateral videofluoroscopic images have been shown to have a high correlation with 3-D measures of pharyngeal residue volume on computed tomography.27 On this basis, it is likely that a similar relationship can be expected between lateral measures of pharyngeal area and actual pharyngeal volume.
The objectives of the current study were twofold. First, we sought to quantify and characterize various metrics of swallowing efficiency and pharyngeal constriction profiles in a sample of individuals with ALS and explore whether bolus properties (i.e., volume, thickness) modulated measures of pharyngeal constriction and/or swallow efficiency. Second, we aimed to determine whether impaired pharyngeal constriction (i.e., larger unobliterated pharyngeal area during swallowing) is associated with impaired swallowing efficiency in ALS. Based on existing manofluorographic and manometric studies with patients who have ALS, we hypothesized that measures of pharyngeal area during swallowing would show larger unobliterated pharyngeal area at maximum constriction (i.e., less constriction) compared to healthy normative data, correlating with disease severity and presence of bulbar symptoms. Further, we hypothesized that larger or thicker boluses may evoke larger pharyngeal constriction areas (i.e., reflecting less constriction) compared to smaller/thinner boluses,28 due to increased bolus weight and/or an inability for the swallowing mechanism to adapt to such bolus properties.
In terms of residue, we hypothesized that larger and thicker boluses would be associated with increased post-swallow residue and number of swallows required to clear the bolus,29,30 and finally, that the amount of post-swallow residue and the number of swallows needed per bolus would be greater (i.e., less efficient) for individuals with larger pharyngeal constriction area (i.e., less constricted).24,31
Materials and Methods:
Data Collection & Processing
This study involved retrospective analysis of videofluoroscopic swallow studies (VFSS) collected from 26 adults (14 male) with a confirmed diagnosis of ALS (definite or probable), based on revised EI-Escorial criteria. Eight participants in this analysis presented with bulbar-onset ALS, 17 with spinal-onset ALS, and 1 had a mixed-onset ALS profile. The mean age of participants was 63 years (range: 30–75 years), and average symptom duration was 24 months (range: 1–54 months). ALS Functional Rating Scale-Revised (ALSFRS-R) scores were available for a subset of the sample (n=21), with a mean ALSFRS-R total score of 32.7 (range: 16–45) and a mean ALSFRS-R bulbar sub-score of 8.8 (range: 2–12).
Each participant swallowed pre-measured volumes of thin liquid (3mL, 20mL), and pudding-thick liquid (3mL), using 40% weight-to-volume concentration barium preparations (VARIBAR® barium sulfate, Bracco Diagnostics Inc., Monroe Township, NJ). VFSS were recorded on a KayPENTAX Digital Swallowing Workstation at 30 frames per second. Using MatLab (The MathWorks, Inc., Natick, MA), the full-length recordings were segmented into bolus-level clips, stripped of audio and patient identifiers using screen scrubbers, and randomized for blinded rating. All collected and analyzed data were approved by the governing academic institutional review boards.
Videofluoroscopic Rating
Four speech-language pathologists experienced in videofluoroscopic analysis completed blinded ratings of each bolus-level clip using ImageJ software (National Institutes of Health, Bathesda, MD), following a standard protocol. This protocol follows a two-step process: 1) identification of events (frames) and the total number of swallows, and 2) pixel-based tracing of pharyngeal area and residue.
Identification of Events.
Frame-by-frame review of each bolus trial was conducted to count the total number of swallows per bolus and select frames utilized to measure pharyngeal constriction (i.e., Maximum Pharyngeal Constriction Frame, defined as the frame showing the least amount of bolus flow and/or airspace in the pharynx) and post-swallow residue (i.e., Swallow Rest Frame, defined as the frame when the pyriform sinuses are at their lowest position after the swallow, relative to the cervical spine). To obtain estimates of inter-rater reliability for Maximum Pharyngeal Constriction Frame identification, 30% of clips (n=21) in this analysis were rated in duplicate. For Swallow Rest Frame identification, we calculated inter-rater reliability based on a larger set of videos rated in duplicate (n=76 bolus clips; 197 swallows in total), which included 19 videos from the current analysis (i.e., 27% of the dataset). Any differences in frame selection which exceeded >5 frames were reviewed and resolved through consensus with a third rater. For cases where the difference between two raters was ≤5 frames, the later frame between the two was selected as the final tracing frame. Once agreement was achieved on frame selection, pixel-based measures of pharyngeal constriction area and post-swallow residue were taken.
Pixel-Based Tracing.
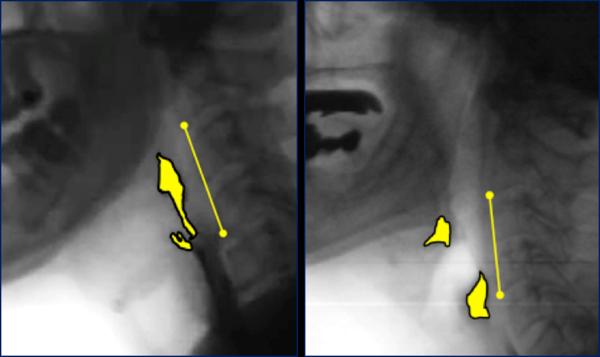
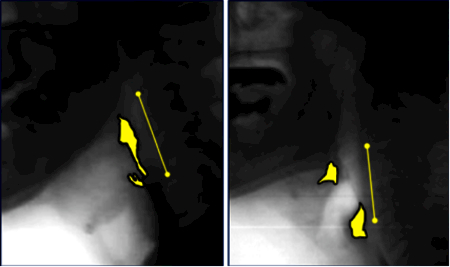
Pharyngeal area was traced (in pixels) at the previously identified Maximum Pharyngeal Constriction Frame, following the procedure outlined by Stokely et al.24 The total number of pixels measured from the unobliterated pharyngeal space was divided by the squared length of an anatomical scalar (i.e., cervical spine: C2–4)32 and multiplied by a factor of 100 to yield a normalized measure of pharyngeal constriction area (MPCAN) in %C2–4 units. The Normalized Residue Ratio Scale33 (NRRS) was used to quantify the amount of residue remaining in the valleculae (NRRSv) and pyriform sinuses (NRRSp) at Swallow Rest Frame. Duplicate ratings were completed for 30% of MPCAN, and NRRSv and NRRSp to calculate inter-rater reliability. Example pixel tracings are shown in Figure 1.
Figure 1.
Example pixel-based measures of pharyngeal constriction (left) and post-swallow residue (right), with cervical C2–C4 scalar.
For each bolus trial, we recorded the total number of swallows as well as MPCAN, NRRSv and NRRSp following the first swallow. All ratings were completed for 3mL thin, 20mL thin, and 3mL pudding bolus trials, yielding up to 78 bolus trials. However, n=7 bolus trials were not available or excluded for the following tasks: 1mL thin (n=1), 20mL thin (n=5), 3mL pudding (n=1), adjusting the total n=71 bolus trials for analysis.
Statistical Analysis
To characterize pharyngeal constriction and swallow efficiency in this sample, we calculated the group mean and 95% confidence intervals (CI) for MPCAN, NRRSv and NRRSp, and average number of swallows for each bolus task. NRRSv and NRRSp scores were compared against published thresholds34 to calculate the proportion of the sample with clinically significant residue. MPCAN values obtained for the 3mL thin task were compared against normative reference data (based on a 5mL thin liquid barium task)24 using independent samples t-tests. Spearman’s correlations were used to illustrate associations between metrics of disease severity (i.e., months since initial symptom, ALSFRS-R total and bulbar sub-scores) and average MPCAN NRRSv and NRRSp values.
To explore associations between pharyngeal area and the total number of swallows per bolus, we tested mean differences in MPCAN using Mann-Whitney U tests for each bolus type, comparing trials with only 1–2 swallows to trials with 3 or more swallows. To investigate relationships between pharyngeal constriction and post-swallow residue, we ran linear mixed models between MPCAN and pharyngeal residue at each anatomical site (NRRSv, NRRSp), with an added factor of bolus type (i.e., 3mL thin, 20mL thin, 3mL pudding). The direction and magnitude of significant associations were illustrated using Spearman’s correlations. All statistical analyses were completed using IBM SPSS Statistics version 24, with statistical significance set at p<0.05. A Bonferroni correction was applied for repeated (3) Mann-Whitney U tests (i.e., p<0.05/3=0.016).
Results:
Inter-rater Reliability
For frame selection, we calculated the proportion of videos rated in duplicate which exceeded the a priori agreement threshold (>5 frames). For the frame of maximum pharyngeal constriction, all ratings fell within the 5-frame threshold; therefore, none of the videos required secondary review through consensus for Maximum Pharyngeal Constriction Frame selection. For Swallow Rest Frame, 27% of all n=197 swallows rated in duplicate exceeded the a priori agreement threshold, requiring resolution through consensus.
Inter-rater reliability for pixel-based measures was evaluated using Intraclass Correlation Coefficients (two-way fixed, consistency). The ICCs for pixel-based videofluoroscopy measures were good-to-excellent for MPCAN (0.843, 95%CI: 0.613–0.936), NRRSv (0.914, 95%CI: 0.801–0.963) and NRRSp (0.928, CI: 0.832–0.929).
Swallowing Efficiency and Pharyngeal Constriction Profiles
Descriptive statistics for all obtained measures are summarized in Table 1. Fifteen participants (i.e., 58% of the sample) presented with significant vallecular residue (NRRSv >0.09) and 35% displayed significant pyriform sinus residue (NRRSp >0.2) on at least one bolus trial. A previous report characterizing pharyngeal residue using NRRS measures identified these cut-off levels (i.e., NRRSv>0.09 and NRRSp>0.2) as being clinically meaningful, due to an increased risk of aspiration on subsequent swallows.34 The median number of swallows per bolus was 2 (range: 1–6).
Table 1.
Mean and 95% confidence intervals (CI) for measures of pharyngeal constriction and swallowing efficiency.
| 3mL thin | 20mL thin | 3mL pudding | ALL | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95%CI | Mean | 95%CI | Mean | 95%CI | |
| MPCAN | 8.78 | 5.22–12.34 | 13.36‡ | 7.79–18.94 | 7.95 | 3.77–12.12 | 9.81 | 7.36–12.26 |
| NRRSv | 0.16 | 0.07–0.25 | 0.34 | 0.13–0.66 | 0.29‡ | 0.11–0.48 | 0.25 | 0.15–0.35 |
| NRRSp | 0.16 | 0.03–0.30 | 0.28 | 0.00–0.57 | 0.12 | 0.01–0.23 | 0.17 | 0.08–0.26 |
| # of swallows | 1.88 | 1.52–2.24 | 2.86 | 2.11–3.61 | 1.92 | 1.48–2.36 | 2.18 | 1.88–2.49 |
MPCAN = Maximum Pharyngeal Constriction Area (normalized to squared length of C2–4), displayed in %C2–42 units; NRRSv = Normalized Residue Ratio Scale (Vallecular Space); NRRSp = Normalized Residue Ratio Scale (Pyriform Sinuses).
Pairwise comparisons revealed statistically significant difference (p<0.05), compared to 3mL thin task.
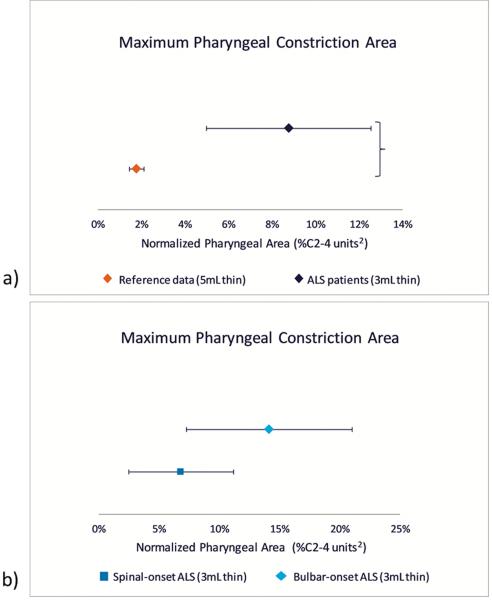
When we compared MPCAN for the 3mL thin task against normative reference data (5mL thin),24 we identified significantly larger pharyngeal area (i.e., less constriction) in patients with ALS, with a large effect size (t=4.03, p<0.001, Cohen’s d=1.141). When stratified by bulbar- versus spinal-onset cases, we identified that individuals with spinal-onset showed smaller pharyngeal constriction areas (i.e., greater constriction) compared to bulbar cases, however this was not statistically significant (p=0.054). MPCAN in spinal-onset cases remained above the upper 95% CI obtained from normative data (i.e., >2.2% C2–42).24 Group comparisons of MPCAN are illustrated in Figure 2.
Figure 2.
95% confidence intervals comparing normalized pharyngeal constriction area in a) patients with ALS (3mL thin) vs. healthy normative reference data (5mL thin), and b) spinal- vs. bulbar-onset ALS cases (3mL thin only).
Spearman’s correlations revealed statistically significant relationships between lower (worse) ALSFRS-R bulbar subscores and higher (less constricted) mean MPCAN (rs= −0.766, p<0.001), NRRSv (rs= −0.650, p=0.001), NRRSp (rs= −0.513, p=0.017) and number of swallows (rs= −0.526, p=0.014). No significant relationships were identified with respect to symptom duration or with ALSFRS-R total score. Linear mixed models exploring bolus factors (volume, thickness) on pharyngeal constriction and residue revealed a main effect of bolus volume on MPCAN (F=10.729, df=1,18.505, p=0.004) and a main effect of thickness on NRRSv (F=4.304, df=1,22.387, p=0.05). Pairwise comparisons illustrated larger pharyngeal constriction area (i.e., less constriction) with the larger 20mL thin liquid task, and greater vallecular residue with the pudding-thick bolus.
Exploration of Relationship between Pharyngeal Constriction and Swallow Efficiency
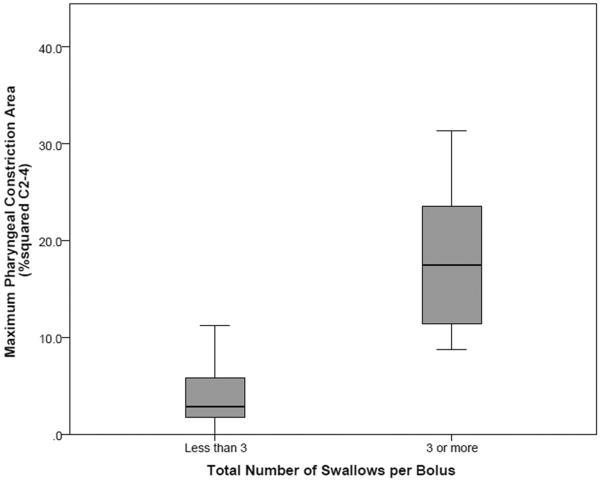
Results of the Mann-Whitney U tests revealed that 3mL thin bolus trials with fewer than 3 swallows per bolus had smaller MPCAN values (mean rank = 8.75) than trials with 3 or more swallows (mean rank = 20.00), shown in Figure 3; this difference was statistically significant (U=4.00, z=−3.674, p<0.001). A similar result was found for the 20mL thin task (mean rank <3 swallows = 7.22; mean rank ≥3 swallows = 12.50); however, once we applied a Bonferroni correction for running multiple tests, this difference did not reach statistical significance (U=20.00, z=−2.041, p=0.041). The relationship between MPCAN and number of swallows was not statistically significant for the 3mL pudding task (U=27.00, z=−1.342, p=0.180).
Figure 3.
Box-and-whisker plots showing increased pharyngeal area at maximum constriction and increased number of swallows per bolus (i.e., ≥3 swallows for a single bolus), for 3mL thin task.
Linear mixed models exploring the influence of pharyngeal constriction on vallecular residue, with a repeated factor of bolus type, revealed main effects of MPCAN (F=92.600, df=1,29.594, p<0.001) and task (F=4.340, df=2,31.881, p=0.022). Increased vallecular residue was seen with larger pharyngeal constriction areas (i.e., less constriction) and with 3mL pudding compared to 3mL and 20mL thin tasks. Similarly, in terms of pyriform sinus residue, a main effect of MPCAN was identified on NRRSp (F=66.878, df=1,33.085, p<0.001), showing increased residue with larger (worse) pharyngeal constriction areas. Significant interaction effects between MPCAN and bolus type were identified for both NRRSv (F=12.669, df=2,32.499, p<0.001) and NRRSp (F=6.139, df=2,31.664, p=0.006), reflecting differences in the magnitude of the association between pharyngeal constriction and residue, related to bolus type (i.e., 3mL thin, 20mL thin, 3mL pudding). Therefore, we ran post-hoc Spearman’s rank correlations stratified by bolus task.
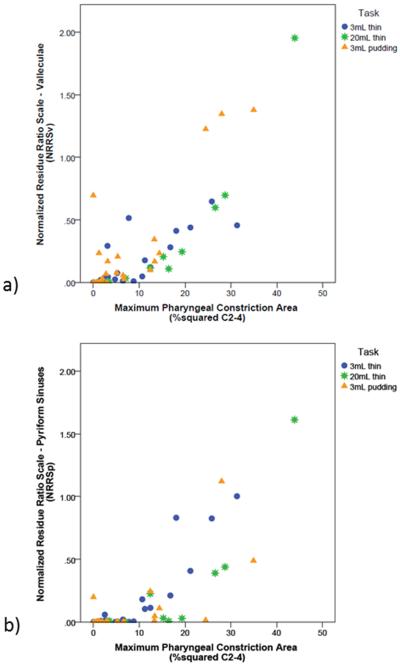
Spearman’s correlations revealed moderate-to-strong associations between MPCAN and NRRSv, by task (3mL thin: rs=0.821, p<0.001; 20mL thin: rs=0.921, p<0.001; 3mL pudding: rs=0.631, p=0.001). Similar associations were observed between MPCAN and NRRSp (3mL thin: rs=0.773, p<0.001; 20mL thin: rs=0.852, p<0.001; 3mL pudding: rs=0.552, p=0.006). These associations are illustrated in Figure 4.
Figure 4.
Associations between normalized pharyngeal constriction area and a) vallecular residue (NRRSv), and b) pyriform sinus residue (NRRSp).
Discussion:
Swallowing inefficiency is often the first clinical sign and patient report of dysphagia in individuals with ALS.16 Still, our understanding of the mechanisms contributing to swallow inefficiency is limited given the lack of focus in this area.1 Extending from previous reports of poor pharyngeal constriction contributing to post-swallow residue in post-stroke patients,24 our study aimed to profile pharyngeal constriction during swallowing in patients with ALS and explore relationships between maximum pharyngeal constriction, disease metrics and bolus flow. Our results demonstrate that measures of pharyngeal constriction area in patients with ALS are markedly larger (i.e., reduced constriction) compared to published data from healthy adults.24 These data support previous descriptions of swallowing inefficiency from videofluoroscopy,16 as well as studies using manometry that have demonstrated reduced pharyngeal swallowing pressures in individuals with ALS.6,13–15,18 Our data suggest that the degree to which pharyngeal constriction is reduced is more pronounced in bulbar-onset ALS patients compared to spinal-onset, and is associated with ALSFRS-R bulbar sub-scores. Although not as severe as bulbar onset ALS patients, we also identified reduced pharyngeal constriction in individuals with spinal-onset ALS. This later finding is in agreement with reports by Murono et al. who identified reductions in pharyngeal constriction in patients with ALS irrespective of the overt presence of other bulbar signs or symptomology.16 Interestingly, a study by Higo et al. failed to find differences in pharyngeal pressures during swallowing between spinal (“classic”) onset patients and healthy controls.17 These differences may reflect the inherent heterogeneity of ALS, differences in study samples and disease duration in spinal onset cases, and methodologic differences in instrumental assessment technique and outcome metrics (i.e., measuring pharyngeal pressure via manometry versus constriction via videofluoroscopy).
In this cohort of ALS patients, 58% of swallows demonstrated inefficiency post swallow at the vallecular anatomical site compared to inefficiency in the pyriform sinus anatomical site on 35% of swallows. Interestingly, upon further inspection of the data, all (100%) of trials with pyriform sinus residue also demonstrated residue in the vallecular anatomical site with no swallows demonstrating residue only at the pyriform sinus site. This finding aligns with a proposed hypothesis of a rostrocaudal pattern of ALS bulbar disease progression, wherein oral and oropharyngeal impairments precede hypopharyngeal impairments.35 Atypical tongue function is commonly reported as the earliest clinical sign of bulbar involvement,(e.g., 27,36) and instrumental assessments of the pharyngeal stage of swallowing have identified differential impairments between the oro- and hypopharyngeal regions.6,14,17 Still, many of these findings – including those from the current study – are based on cross-sectional studies with small samples and require confirmation by future prospective longitudinal studies to determine the evolution and development of swallowing impairment throughout the ALS disease progression.
Our results illustrate a strong relationship between pharyngeal constriction area and the accumulation of post-swallow residue, such that larger unobliterated pharyngeal areas during the swallow coincide with greater post-swallow residue. This finding was true for both vallecular and pyriform sinus residue, across all bolus types. The pudding-thick bolus also led to greater post-swallow residue in the vallecular space. Thicker boluses have been implicated as a potential risk factor for post-swallow residue, as they require greater driving force through the pharynx.29,30,37,38 Still, as thicker liquids flow more slowly and are shown to reduce aspiration in many populations,(e.g., 29,39–41) the prescription of thickened liquids to individuals with ALS who aspirate may still be clinically justified. More work exploring additional liquid viscosities and textural features (e.g., cohesiveness, stickiness) is necessary to determine which bolus types are optimal for patients with ALS who have impaired bolus clearance, in terms of both safety and efficiency.
In this study of individuals with ALS, we identified an inverse relationship between bolus volume on measures of pharyngeal constriction area (i.e., less constriction with 20mL thin, compared to 3mL thin boluses), while no differences in MPCAN were observed when comparing 3mL thin and 3mL pudding-thick boluses. Although Leonard et al. identified a similar effect of bolus volume on pharyngeal constriction area in healthy individuals, this was only true when comparing 1mL vs. 20mL thin boluses (i.e., 3mL vs. 20mL were not statistically different).28 It is possible that the influence of bolus volume on pharyngeal constriction area in ALS may be exacerbated by reduced strength/control of the pharyngeal musculature due to ALS neuropathology (e.g., atrophy/wasting from lower motor neuron degeneration; spasticity related to upper motor neuron degeneration) such that the motor plan and/or pharyngeal muscles are unable to accommodate to larger bolus volumes. Current understanding of the role of bolus volume and thickness on pharyngeal constriction and pharyngeal pressures in healthy swallowing is inconsistent, as some studies identify higher pharyngeal pressures with increasingly larger or thicker boluses,(e.g., 42-44) while other studies show the opposite effect28,45 or no differences based on bolus properties.(e.g., 22,46-48) Although further research is needed to elucidate these physiological patterns, the presence of unexpected variability and/or the absence of known bolus effects in the ALS population may indicate loss of flexibility in swallowing function, which is imperative to accommodate to the range of stimuli we encounter when eating and drinking.
Limitations
There are several limitations to this study. First, it is possible that the degrees of association we have illustrated are inflated due to small sample size and a limited range of disease severity. Future studies with larger sample size and/or additional observations per participant are needed to fully explore these correlations. Further, for the purpose of this preliminary study, we stratified by ALS onset-type when exploring differences in pharyngeal constriction. This poses an important limitation, as many patients with spinal-onset ALS also experience a decline in bulbar function;17,49 this was true to a varying degree for at least 11/17 of the spinal-onset patients included in this study, based on available ALSFRS-R bulbar sub-scores. Unfortunately, due to the retrospective nature of this analysis, further details describing the bulbar involvement in this sample are limited. In an effort to normalize the degree of bulbar involvement across both onset-types, future work investigating swallowing physiology in ALS may consider using the duration of bulbar symptoms17 or articulatory/speaking rates49 to characterize the study sample in greater detail.
Previous work has described piecemeal swallowing as a common behavior in patients with ALS.(e.g., 3,6,15,50) Ertekin and colleagues, and others, have reported on the clinical utility of repeated swallows to detect the presence of dysphagia (e.g., dysphagia limit: the volume of liquid >20mL which evokes repeated swallows).50–53 In the current study, we frequently observed repeated swallows per bolus, even for small 1mL and 3mL volumes. In 55% of all bolus trials, repeated swallows were piecemeal swallows (i.e., the bolus was divided into smaller volumes in the oral cavity, and each division was swallowed separately). As our results indicated an influence of bolus volume on measures of pharyngeal area and bolus volume effects have also been reported on other kinematic variables (e.g., hyolaryngeal movement), published elsewhere,(e.g.,54-56) an important consideration and potential limitation may exist when it comes to the amount swallowed during each piecemeal swallow. It would be difficult to account for variability related to the volume of bolus swallowed when it is piecemealed into smaller (unknown) amounts. By reporting results following only the first swallow per bolus, we aimed to minimize the influence of variability seen in later swallows, with presumably smaller bolus volumes. Further, the proportion of boluses which required piecemeal or multiple swallows was not significantly different by task, leading us to infer that piecemeal swallowing did not influence the overall findings we reported.
For this study, we maintained a narrow focus to explore the specific role of pharyngeal constriction on swallowing efficiency. This decision was made based on previous descriptive links described in ALS dysphagia research,(e.g.,6) and findings from other clinical populations.24 Such a discrete focus does pose some inherent limitations. First, we focused exclusively on the pharyngeal phase of swallowing, without regard to the oral or esophageal phases. Future work may consider exploring the interaction between the various phases of swallowing, in addition to the discrete features within each individual phase of swallowing. Similarly, we concentrated upon one physiological feature (i.e., pharyngeal constriction) and reported on a limited selection of bolus consistencies (thin, pudding) and volumes (3mL, 20mL) which may impact swallowing efficiency. Future work will be needed to incorporate additional physiological mechanisms into the model (e.g., UES function, epiglottic deflection, tongue strength), and include a broader representation of the textures that patients encounter at mealtimes.
Future Research
Although this analysis points to pharyngeal constriction as a key mechanism contributing to the accumulation of post-swallow residue and to overall swallow inefficiency in ALS, it does not present a perfect correlation, suggesting that other parameters are also involved and further research is needed. Additional factors that may be involved include function of the upper esophageal sphincter (i.e., timeliness and degree of relaxation, maximum distension) and bolus properties such as cohesiveness or stickiness. Further, the metric of pharyngeal constriction area in this paper does not capture the true strength of the pharyngeal musculature nor the patterning of pharyngeal contractions. It is possible that either reductions in pharyngeal strength or changes in the rostral-caudal pharyngeal stripping wave may affect the association identified in the current paper. Future research using combined manometry and fluoroscopy may help us further understand the role of the timing patterns, sequence, and strength of pharyngeal musculature as they relate to swallowing inefficiency in ALS.
Conclusions
Swallowing inefficiency is a common concern for individuals with ALS. Results from this preliminary work indicate that impaired pharyngeal constriction is one mechanism associated with impaired swallowing efficiency in ALS, across selected bolus volumes and consistencies. These data support the assumption that reduced pharyngeal constriction contributes to the presence of post-swallow residue and is a key parameter in the pathophysiology of dysphagia in ALS. Future work is needed to explore these relationships in greater detail, across a wider range of bolus textures, considering additional physiological mechanisms which may also affect swallowing efficiency. Longitudinal research studies will also be beneficial to elucidate how these observations change throughout ALS disease progression.
Key Points:
Swallowing inefficiency is a common problem for individuals with ALS, characterized by an increased number of swallows per bolus and post-swallow pharyngeal residue.
In this study, reduced pharyngeal constriction was associated with swallow inefficiency in patients with ALS. Pudding-thick boluses were also associated with increased residue in the valleculae.
These results add to a limited dataset exploring mechanisms of dysphagia in ALS. By understanding such physiological changes, future research can explore ways to modulate and compensate for subsequent impairment.
Acknowledgments, funding, and disclosures:
The authors gratefully acknowledge Stephanie Watts, Joy Gaziano for their role in data collection, as well as Vivian Chak, Melanie Peladeau-Pigeon, Teresa Valenzano, and Talia Wolkin for their contributions to data analysis.
This work was supported in part by doctoral research funding provided by the University of Toronto and the Toronto Rehabilitation Institute, as well as grant funding from the National Institutes of Health (NIDCD Grant 2R01DC011020). The authors have no competing interests to declare.
Abbreviations:
- NRRSv
Normalized Residue Ratio Scale – Vallecular Space
- NRRSp
Normalized Residue Ratio Scale – Pyriform Sinuses
- MPCAN
Normalized Maximum Pharyngeal Constriction Area
- ALS
Amyotrophic Lateral Sclerosis
- VFSS
Videofluoroscopic Swallow Study
References:
- 1.Waito AA, Valenzano TJ, Peladeau-Pigeon M, Steele CM. Trends in Research Literature Describing Dysphagia in Motor Neuron Diseases (MND): A Scoping Review. Dysphagia. December 2017;32(6):734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruoppolo G, Schettino I, Frasca V, et al. Dysphagia in amyotrophic lateral sclerosis: prevalence and clinical findings. Acta Neurologica Scandinavica. December 2013;128(6):397–401. [DOI] [PubMed] [Google Scholar]
- 3.Tabor LC, Plowman EK. (2017) Dysphagia in Amyotrophic Lateral Sclerosis In: Medical Radiology. Springer, Berlin, Heidelberg. [Google Scholar]
- 4.Paris G, Martinaud O, Petit A, et al. Oropharyngeal dysphagia in amyotrophic lateral sclerosis alters quality of life. J Oral Rehabil. March 2013;40(3):199–204. [DOI] [PubMed] [Google Scholar]
- 5.Tabor LC, Gaziano J, Watts S, Robison R, Plowman EK. Defining swallowing-related quality of life profiles in individuals with Amyotrophic Lateral Sclerosis. Dysphagia (0179051X). 2016;31(3):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goeleven A, Robberecht W, Sonies B, Carbonez A, Dejaeger E. Manofluorographic evaluation of swallowing in amyotrophic lateral sclerosis and its relationship with clinical evaluation of swallowing. Amyotroph Lateral Scler. December 2006;7(4):235–240. [DOI] [PubMed] [Google Scholar]
- 7.Lo Re G, Galia M, La Grutta L, et al. Digital cineradiographic study of swallowing in patients with amyotrophic lateral sclerosis. Radiol Med (Torino). December 2007;112(8):1173–1187. [DOI] [PubMed] [Google Scholar]
- 8.Solazzo A, Del Vecchio L, Reginelli A, et al. Search for compensation postures with videofluoromanometric investigation in dysphagic patients affected by amyotrophic lateral sclerosis. Radiol Med (Torino). October 2011;116(7):1083–1094. [DOI] [PubMed] [Google Scholar]
- 9.Carrión S, Cabré M, Monteis R, et al. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clinical nutrition. 2015;34(3):436–442. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira DL, Moreira EAM, de Freitas MB, de A. Gonçalves J, Furkim AM, Clavé P. Pharyngeal residue and aspiration and the relationship with clinical/nutritional status of patients with oropharyngeal dysphagia submitted to videofluoroscopy. The journal of nutrition, health & aging. 2017// 2017;21(3):336–341. [DOI] [PubMed] [Google Scholar]
- 11.Rofes L, Arreola V, Almirall J, et al. Diagnosis and management of oropharyngeal Dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterology research and practice. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53(5):1059. [DOI] [PubMed] [Google Scholar]
- 13.Solazzo A, Monaco L, Vecchio LD, et al. Earliest videofluoromanometric pharyngeal signs of dysphagia in ALS patients. Dysphagia. October 2014;29(5):539–544. [DOI] [PubMed] [Google Scholar]
- 14.Higo R, Tayama N, Watanabe T, Nitou T. Videomanofluorometric study in amyotrophic lateral sclerosis. Laryngoscope. May 2002;112(5):911–917. [DOI] [PubMed] [Google Scholar]
- 15.Kawai S, Tsukuda M, Mochimatsu I, et al. A study of the early stage of Dysphagia in amyotrophic lateral sclerosis. Dysphagia. 2003;18(1):1–8. [DOI] [PubMed] [Google Scholar]
- 16.Murono S, Hamaguchi T, Yoshida H, et al. Evaluation of dysphagia at the initial diagnosis of amyotrophic lateral sclerosis. Auris Nasus Larynx. June 2015;42(3):213–217. [DOI] [PubMed] [Google Scholar]
- 17.Higo R, Tayama N, Nito T. Longitudinal analysis of progression of dysphagia in amyotrophic lateral sclerosis. Auris Nasus Larynx. September 2004;31(3):247–254. [DOI] [PubMed] [Google Scholar]
- 18.Tomik J, Tomik B, Gajec S, et al. The Balloon-Based Manometry Evaluation of Swallowing in Patients with Amyotrophic Lateral Sclerosis. International journal of molecular sciences. March 27 2017;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia. 2001/// 2001;16(4):272–278. [DOI] [PubMed] [Google Scholar]
- 20.Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nature reviews. Neurology. 2014;10(11):661. [DOI] [PubMed] [Google Scholar]
- 21.Dejaeger E, Pelemans W, Ponette E, Joosten E. Mechanisms Involved in Postdeglutition Retention in the Elderly. Dysphagia. 1997;12(2):63–67. [DOI] [PubMed] [Google Scholar]
- 22.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103(1):128. [DOI] [PubMed] [Google Scholar]
- 23.Palmer JB, Tanaka E, Siebens AA. Motions of the posterior pharyngeal wall in swallowing. The Laryngoscope. 1988;98(4):414–417. [DOI] [PubMed] [Google Scholar]
- 24.Stokely SL, Peladeau-Pigeon M, Leigh C, Molfenter SM, Steele CM. The Relationship Between Pharyngeal Constriction and Post-swallow Residue. Dysphagia. June 2015;30(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard R, Rees CJ, Belafsky P, Allen J. Fluoroscopic surrogate for pharyngeal strength: the pharyngeal constriction ratio (PCR). Dysphagia. March 2011;26(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard R, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: the pharyngeal constriction ratio. Ann Otol Rhinol Laryngol. December 2006;115(12):897–901. [DOI] [PubMed] [Google Scholar]
- 27.Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1994;37(1):28–37. [DOI] [PubMed] [Google Scholar]
- 28.Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural Displacements in Normal Swallowing: A Videofluoroscopic Study. Dysphagia. 2000;15(3):146–152. [DOI] [PubMed] [Google Scholar]
- 29.Barata LF, De Carvalho GB, Carrara-De Angelis E, De Faria JCM, Kowalski LP. Swallowing, speech and quality of life in patients undergoing resection of soft palate. European Archives of Oto-Rhino-Laryngology. // 2013;270(1):305–312. [DOI] [PubMed] [Google Scholar]
- 30.Hind J, Divyak E, Zielinski J, et al. Comparison of standardized bariums with varying rheological parameters on swallowing kinematics in males. J Rehabil Res Dev. December 2012;49(9):1399–1404. [DOI] [PubMed] [Google Scholar]
- 31.Steele CM, Peladeau-Pigeon M, Barbon CE, et al. Mechanisms contributing to post-swallow pharyngeal residue in healthy swallowing. Paper presented at: 26th Annual Dysphagia Research Society Meeting2018; Baltimore, MD. [Google Scholar]
- 32.Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res. June 1 2014;57(3):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson WG Jr., Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: the normalized residue ratio scale. Dysphagia. June 2013;28(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molfenter SM, Steele CM. The relationship between residue and aspiration on the subsequent swallow: an application of the normalized residue ratio scale. Dysphagia. December 2013;28(4):494–500. [DOI] [PubMed] [Google Scholar]
- 35.Plowman EK, Tabor LC, Robison R, Wymer J. Delineating Mechanisms of Dysphagia in ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2016;17(sup1):33. [Google Scholar]
- 36.Shellikeri S, Green JR, Kulkarni M, et al. Speech Movement Measures as Markers of Bulbar Disease in Amyotrophic Lateral Sclerosis. Journal of Speech, Language, and Hearing Research. 2016;59(5):887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner MR, Hardiman O, Benatar M, et al. Controversies and priorities in amyotrophic lateral sclerosis. The Lancet. Neurology. 2013;12(3):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele CM, Alsanei WA, Ayanikalath S, et al. The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia. October 25 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration-aspiration in post-stroke patients. Neurology India. // 2010;58(1):42–47. [DOI] [PubMed] [Google Scholar]
- 40.Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson’s disease. Dysphagia. March 2008;23(1):26–32. [DOI] [PubMed] [Google Scholar]
- 41.Rofes L, Arreola V, Mukherjee R, Swanson J, Clave P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment Pharmacol Ther. May 2014;39(10):1169–1179. [DOI] [PubMed] [Google Scholar]
- 42.Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108(5):1418–1426. [DOI] [PubMed] [Google Scholar]
- 43.Omari TI, Dejaeger E, Tack J, Van Beckevoort D, Rommel N. Effect of Bolus Volume and Viscosity on Pharyngeal Automated Impedance Manometry Variables Derived for Broad Dysphagia Patients. Dysphagia. 2013;28(2):146–152. [DOI] [PubMed] [Google Scholar]
- 44.Butler SG, Stuart A, Castell D, Russell GB, Koch K, Kemp S. Effects of Age, Gender, Bolus Condition, Viscosity, and Volume on Pharyngeal and Upper Esophageal Sphincter Pressure and Temporal Measurements During Swallowing. Journal of Speech, Language, and Hearing Research. 2009;52(1):240–253. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. The Laryngoscope. 2010;120(12):2367–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gumbley F, Huckabee ML, Doeltgen SH, Witte U, Moran C. Effects of Bolus Volume on Pharyngeal Contact Pressure During Normal Swallowing. Dysphagia. 2008;23(3):280–285. [DOI] [PubMed] [Google Scholar]
- 47.Lin T, Xu G, Dou Z, Lan Y, Yu F, Jiang L. Effect of bolus volume on pharyngeal swallowing assessed by high-resolution manometry. Physiology & Behavior. 2014;128:46–51. [DOI] [PubMed] [Google Scholar]
- 48.Perlman AL, Schultz JG, VanDaele DJ. Effects of age, gender, bolus volume, and bolus viscosity on oropharyngeal pressure during swallowing. Journal of Applied Physiology. 1993;75(1):33–37. [DOI] [PubMed] [Google Scholar]
- 49.Green JR, Yunusova Y, Kuruvilla MS, et al. Bulbar and speech motor assessment in ALS: challenges and future directions. Amyotroph Lateral Scler Frontotemporal Degener. December 2013;14(7–8):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ertekin C, Aydogdu I, Yuceyar N, Kiylioglu N, Tarlaci S, Uludag B. Pathophysiological mechanisms of oropharyngeal dysphagia in amyotrophic lateral sclerosis. Brain. January 2000;123(Pt 1):125–140. [DOI] [PubMed] [Google Scholar]
- 51.Ertekin C, Aydogdu I, Yuceyar N, et al. Electrodiagnostic methods for neurogenic dysphagia. Electroencephalogr Clin Neurophysiol. August 1998;109(4):331–340. [DOI] [PubMed] [Google Scholar]
- 52.Aydogdu I, Tanriverdi Z, Ertekin C. Dysfunction of bulbar central pattern generator in ALS patients with dysphagia during sequential deglutition. Clinical Neurophysiology. June 2011;122(6):1219–1228. [DOI] [PubMed] [Google Scholar]
- 53.Aydogdu I, Kiylioglu N, Tarlaci S, et al. Diagnostic value of “dysphagia limit” for neurogenic dysphagia: 17 years of experience in 1278 adults. Clin Neurophysiol. March 2015;126(3):634–643. [DOI] [PubMed] [Google Scholar]
- 54.Nascimento WV, Cassiani RA, Santos CM, Dantas RO. Effect of Bolus Volume and Consistency on Swallowing Events Duration in Healthy Subjects. J Neurogastroenterol Motil. 2015;21(1):78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagy A, Molfenter SM, Peladeau-Pigeon M, Stokely S, Steele CM. The effect of bolus volume on hyoid kinematics in healthy swallowing. BioMed research international. 2014;2014:738971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarus CL, Logemann JA, Rademaker AW, et al. Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Archives of Physical Medicine and Rehabilitation. 1993;74(10):1066–1070. [DOI] [PubMed] [Google Scholar]