Abstract
Purpose
To improve the event-free survival (EFS) and overall survival (OS) for patients with clear cell sarcoma of the kidney (CCSK) by incorporating cyclophosphamide and etoposide into treatment on National Wilms Tumor Study (NWTS)-5.
Patients and Methods
Patients less than 16 years of age with a centrally confirmed pathological diagnosis of CCSK were eligible for treatment on this prospective single-arm study conducted between August,1995 and June 2002. Staging consisted of CT scans of chest, abdomen, pelvis, bone scan, skeletal survey, and CT or MRI of head. Treatment consisted of vincristine/doxorubicin/cyclophosphamide alternating with cyclophosphamide/etoposide for 24 weeks and radiation to sites of disease.
Results
108 eligible patients were enrolled on study (69% males, 63% Caucasian) with a median age of 22 months. Stage distribution was: stage I, 12; II, 44; III, 45; IV, 7. Median follow up was 9.7 years. Five-year EFS and OS were 79% (95% CI: 71%−88%) and 90% (95% CI: 84%−96%). Five-year EFS for Stage I-IV was 100%, 88%, 73% and 29%, respectively. Twenty of the 23 disease-related events occurred within three years of initial treatment. The most common site of recurrence was brain (12/23).
Conclusion
The outcome for patients with CCSK treated on NWTS-5 was similar to NWTS-4 and accomplished over a shorter treatment duration. Stage was highly predictive of outcome. Brain metastases occurred more frequently than on NWTS-4. Regimen I showed more benefit for patients with stage I and II disease as compared to higher stages of disease where new therapies are needed.
Keywords: pediatric kidney cancer, treatment, clear cell sarcoma kidney
Clear cell sarcoma of the kidney (CCSK), an uncommon neoplasm of the kidney characterized by its tendency to metastasize to bone and for late recurrences, and distinct from Wilms tumor, has been treated on National Wilms Tumor Study (NWTS) Group protocols.1–4 NWTS 1–3 (conducted between 1969–1986) showed improved event-free survival (EFS) for CCSK patients with the addition of doxorubicin to vincristine and dactinomycin.1 There was no improvement in outcome for these patients when cyclophosphamide was given at a dose of 900 mg/m2 every 6–7 weeks for 10 doses. During this period, abdominal radiation therapy, which all CCSK patients received independent of stage, was modified from 20 Gy to 10 Gy. NWTS-4 (conducted from 1986–1994) showed that single dose (or pulse intensive) administration of dactinomycin and doxorubicin was adequate and that patients with CCSK may benefit from longer duration of three drug therapy (15 months therapy - eight-year relapse-free survival (RFS) 87.8%; 6 months therapy – eight-year RFS 60.6%, P=.08) .5 In NWTS-5, all patients with CCSK were treated with a new regimen that included vincristine, doxorubicin, cyclophosphamide, and etoposide (regimen I). We now report the clinical characteristics and outcome for patients with CCSK treated with regimen I on NWTS-5.
Patients
Any patient less than 16 years of age with newly diagnosed CCSK was eligible. All pathology was confirmed by central review. Patients underwent staging work up at the time of diagnosis consisting of chest, abdominal, and pelvic CT, bone scan, skeletal survey, MRI or CT of the brain and bone marrow aspirations. The protocol was approved by the Institutional Review Board (IRB) at each participating institution and signed IRB-approved consent by the legal guardian was obtained prior to enrollment. Assent was obtained when appropriate for age from the patient. Patients started treatment within five days of surgery. All patients received postoperative radiation therapy to the tumor bed (10 Gy) and other infradiaphragmatic and metastatic sites as necessary. Radiation therapy started within 9 days from surgery. The treatment plan is shown in Figure 1.
Figure 1.
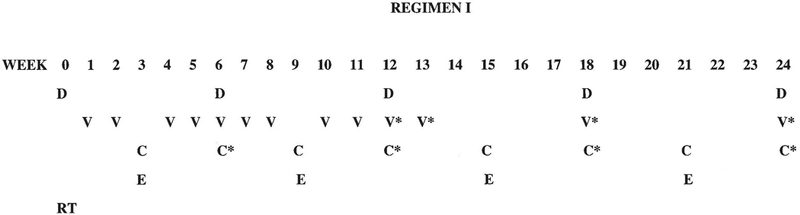
Regimen I treatment schema. D: Doxorubicin 1.5 mg/kg IV weeks 0, 6, 12, 18 and 24 for all patients weighing ≤ 30 kg. Doxorubicin dose administered at week 6 is reduced by 50% (0.75 mg/kg) if whole lung or whole abdomen radiation therapy has been given. Doxorubicin dose at weeks 0, 6, 12, 18 and 24 is 45 mg/m2 IV for all patients who weigh > 30 kg. Doxorubicin dose administered at week 6 is reduced by 50% (22.5 mg/m2) if whole lung or whole abdomen radiation therapy has been given. V: vincristine 0.05 mg/kg IV (maximum dose – 2 mg), beginning day 7 post-nephrectomy (week 1) and then weeks 2, 4, 5, 6, 7, 8, 10 and 11 for patients weighing ≤ 30 kg; The dose of vincristine is 1.5 mg/m2 IV for all patients weighing > 30 kg but no single dose should exceed 2 mg. V*: vincristine 0.067 mg/kg IV push (maximum dose – 2 mg) at week 13, and with doxorubicin at weeks 12, 18, and 24 for all patients weighing ≤ 30 kg. The dose of vincristine is 2 mg/m2 IV for all patients who weigh more than 30 kg, but no single dose should exceed 2 mg. C: cyclophosphamide 14.7 mg/kg/dose IV x 5 days at weeks 6, 12, 18 and 24 weeks for patients weighing ≤ 30 kg. For patients weighing > 30 kg, the dose of cyclophosphamide is 440 mg/m2/dose IV x 5 days. C*: cyclophosphamide 14.7 mg/kg/dose IV x 3 days for patients ≤ 30 kg; for patients weighing > 30 kg, cyclophosphamide 440 mg/m2/dose IV x 3 days; E: etoposide 3.3 mg/kg/dose IV x 5 days, weeks 3, 9, 15 and 21 for patients weighing ≤ 30 kg. For patients weighing >30 kg, etoposide 100 mg/m2/dose IV x 5 days. Infants (< 12 months of age) received 50% of the recommended dose of all chemotherapeutic agents as calculated on the basis of body weight. Radiation therapy (RT): All patients received post-operative RT consisting of 10.8 Gy flank radiation with a 10.8 Gy boost for gross residual disease after surgery. Patients with preoperative tumor rupture, cytology-positive ascites, or diffuse peritoneal seeding were treated with whole-abdomen RT to a dose of 10.8 Gy. Patients with pulmonary nodules received whole lung RT to a dose of 12 Gy.
The definition of “Stage 2” was changed from NWTS-4 to NWTS-5. Patients on NWTS-4 with invasion of the renal sinus vessels within the hilar plane (a line joining the most medial aspects of the upper and lower poles) were classified as Stage I (if there was no other evidence of Stage II disease). On NWTS-5, patients with any invasion of the renal sinus vessels were classified as Stage II. To compare outcome between NWTS-4 and NWTS-5, NWTS-4 patients were classified using NWTS-5-defined stage, and estimates of outcome were made.
Statistical Methods
The association between categorical variables was assessed using Chi-squared test or Fisher’s Exact test when appropriate. Kaplan-Meier plots were generated for EFS and overall survival (OS).6 The Peto-Peto Methods were used to compute the confidence intervals for the EFS and OS.7 The associations between EFS or OS and other covariates were evaluated using log-rank test and Cox Proportional Hazards model. The software SAS and R were used.
Results
There was a total of 123 patients with centrally confirmed CCSK. Eleven patients were excluded from the analysis due to initiating therapy with a regimen other than regimen I. Among the 112 remaining cases, four were excluded (two due to lack of staging information at diagnosis, one was lost to follow up and one was a rhabdoid tumor initially misdiagnosed as CCSK before immunohistochemistry was available for detecting INI-1 loss). The remaining 108 patients form the basis for this analysis. (See Supplemental Figure 1) There were 56 patients (52%) entered who were less than 24 months of age, 33 patients (30%) age 24 to less than 48 months and 19 patients (18%) who were 48 months of age or older. Seventy-four patients (69%) were males. There were 68 Caucasian patients (63%), 18 African American (17%) and the remaining 22 patients (20%) were of other ethnicities.
There were 12 stage I (11%), 44 stage II (41%), 45 stage III (42%) and 7 stage IV (6%) patients. The most common site of metastases in stage IV patients was the lung(s) (3). Other sites included bone (2), bone, brain and lung (1), soft tissue of thigh and other kidney (1). Six patients received pre-nephrectomy chemotherapy.
The five-year EFS and OS for the entire group of patients were 79% (95% CI: 71-%−88%) and 90% (95% CI: 84–96%) respectively with a median follow up of 9.7 years (range 0.7 – 19.1 years). (Figure 2) There were 24 events, 23 of which were related to disease recurrence. These 24 events occurred between 0.4 and 13.6 years (median – 2.0 years) after diagnosis. Twenty of the events occurred within three years after diagnosis. The sites of relapses included brain (12), lung/thorax (5 with 1 in combination with bone), soft tissue (1), tumor bed (1), abdomen/pelvis (1), bone (2), and opposite kidney (1). One event occurred at 13.6 years characterized by recurrence of tumor in the contralateral kidney in a patient originally treated by partial nephrectomy. There were 13 deaths, 12 of which were related to disease. The 13 deaths occurred between 0.8 and 6.0 years (median 3.2 years) after study entry. Ten of the 12 disease-related deaths occurred within four years. One death secondary to complication of intestinal obstruction occurred at 5.8 years. The EFS and OS according to stage are shown in Figures 3 and 4. All 12 stage I patients had EFS longer than five years. Five of the seven patients with stage IV disease relapsed.
Figure 2.
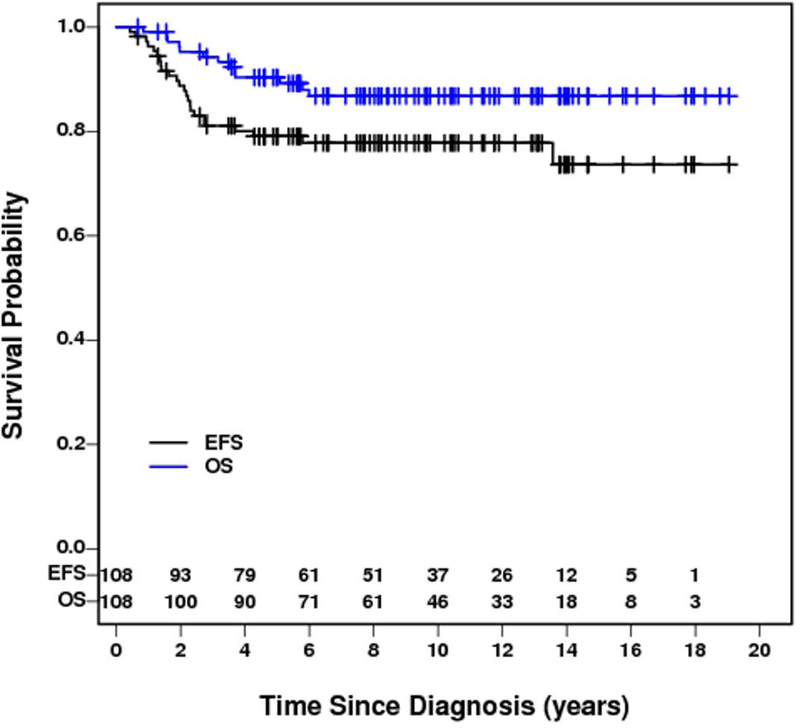
Event-free survival and overall survival for all CCSK patients in NWTS-5
Figure 3.
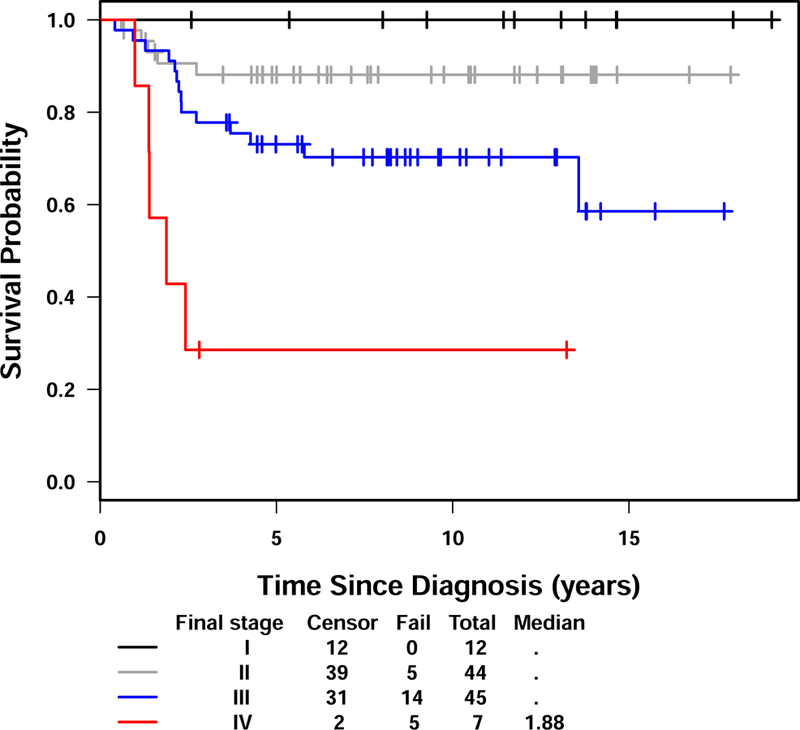
Event-free survival for NWTS-5 CCSK patients by stage
Figure 4.
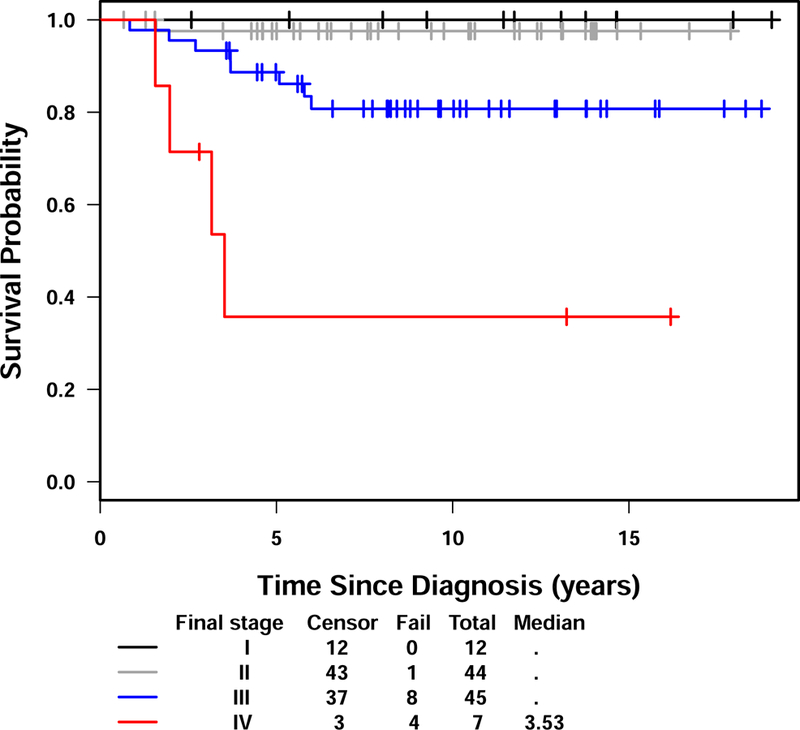
Overall survival for NWTS-5 CCSK patients by stage
The effect of age on outcome was examined for the study population. There was no difference in outcome when patients were compared by age < 24 months and 24 months of age and older. Patients less than 12 months of age (n=18) had a poorer EFS than older patients with a 50% (95% CI: 26%−75%) five-year EFS compared to 85% (95% CI: 77%−93%) for patients 12 months of age and older (P=.003). Because the estimated EFS and OS for the 12 stage I patients was 100% and the estimated five-year EFS and OS for the seven patients with stage IV disease were 29 % (95% CI: 0%−76%) and 36% (95% CI: 0%−75%), respectively, prognostic factors were assessed by focusing only on the remaining 89 patients with stage II or III disease. The five-year EFS and OS for stage II patients were 88% (95% CI: 77%−99%) and 98% (95% CI: 92%−100%) and for stage III were 73% (95% CI: 57%−87%) and 89% (95% CI: 79%−99%). Prognostic factors examined included age, gender, race, stage, sinus vessel invasion, tumor spill, tumor capsule invasion, sinus soft tissue invasion and lymph node involvement. No factor was predictive of OS in the multivariate analysis. Only age equal to or greater than 12 months correlated with better EFS and OS by univariate analysis (P=0.0024) and was borderline significant in the multivariate analysis for EFS (P=0.046).
We examined the possible effect of the staging change on NWTS-4 patients by looking at the differences in outcome between those treated on NWTS-4 and NWTS-5. NWTS-4 patients were reclassified using NWTS-5 staging criteria. Following reclassification, the stage distribution included 17 stage I (25%) and 25 stage II (37%) NWTS4 patients. When stage I and II patients were combined for NWTS-4 and NWTS-5, there was a greater percentage of stage I/II patients enrolled on NWTS-4 (62%), compared to NWTS-5 (52%).
The outcome for patients with stage I/II disease (all classified using NWTS-5 criteria) appears to have improved with NWTS-5 treatment (Regimen I) (P=0.016) with five-year EFS of 91% (95% CI: 82%−99%), compared with 74% (95% CI: 61%−87%) for patient treated on NWTS-4 (Regimens DD, DD-4A). The overall survival for stage I/II patients also improved when treated on NWTS-5 (P=0.016) with five-year OS of 98% (95% CI: 94%−100%), compared to 88% (95% CI: 78%−98%) for patients treated on NWTS-4. No difference in outcome for patients with stage III/IV disease was seen between treatment studies. (See Table 1) The five-year EFS and OS for NWTS-4 was 72% (95% CI: 61%−83%) and 87% (95% CI: 78%−95%) in comparison to the five-year EFS and OS for NWTS-5 of 79% (95% CI: 71%−88%) and 90% (95% CI: 84%−96%). Figure 5 shows the survival for CCSK on NWTS 1–5.
Table 1.
| Stage | Study | ||
|---|---|---|---|
| NWTS-5 | NWTS-4 | ||
| NWTS-5 definition | NWTS-4 definition | NWTS-5 definition | |
| I | 12 (11%) | 25 (37%) | 17 (25%) |
| II | 44 (41%) | 17 (25%) | 25 (37%) |
| III | 45 (42%) | 24 (35%) | 24 (35%) |
| IV | 7 (6%) | 2 (3%) | 2 (3%) |
| Total | 108 | 68 | 68 |
| Five-year EFS | 79% (71%−88%) | 72% (61%−83%) | |
| Five-year OS | 90% (84%−96%) | 87% (78%−95%) | |
| Five-year EFS and 95% CI by stage |
|||
| I | 100%(100%−100%) | 76% (60%−93%) | 82% (64%−100%) |
| II | 88% (77%−99%) | 71% (49%−92%) | 68% (50%−86%) |
| I/II | 91% (82%−99%) | 74% (61%−87%) | 74% (61%−87%) |
| III | 73% (59%−87%) | 71% (51%−90%) | 71% (51%−90%) |
| IV | 29% (0%−76%) | 50% (0%−100%) | 50% (0%−100%) |
| III/IV | 67% (53%−81%) | 69% (50%−88%) | 69% (50%−89%) |
| Five-year OS and 95% CI by stage |
|||
| I | 100% (100%−100%) | 92% (81%−100%) | 100%(100%−100%) |
| II | 98% (92%−100%) | 82% (64%−100%) | 80% (64%−96%) |
| I/II | 98% (94%−100%) | 88% (78%−98%) | 88% (78%−98%) |
| III | 89% (79%−99%) | 87% (73%−100%) | 87% (73%−100%) |
| IV | 36% (0%−75%) | 50% (0%−100%) | 50% (0%−100%) |
| III/IV | 82% (71%−93%) | 84% (70%−99%) | 84% (70%−99%) |
EFS – Event-free survival; OS – Overall survival
Figure 5.
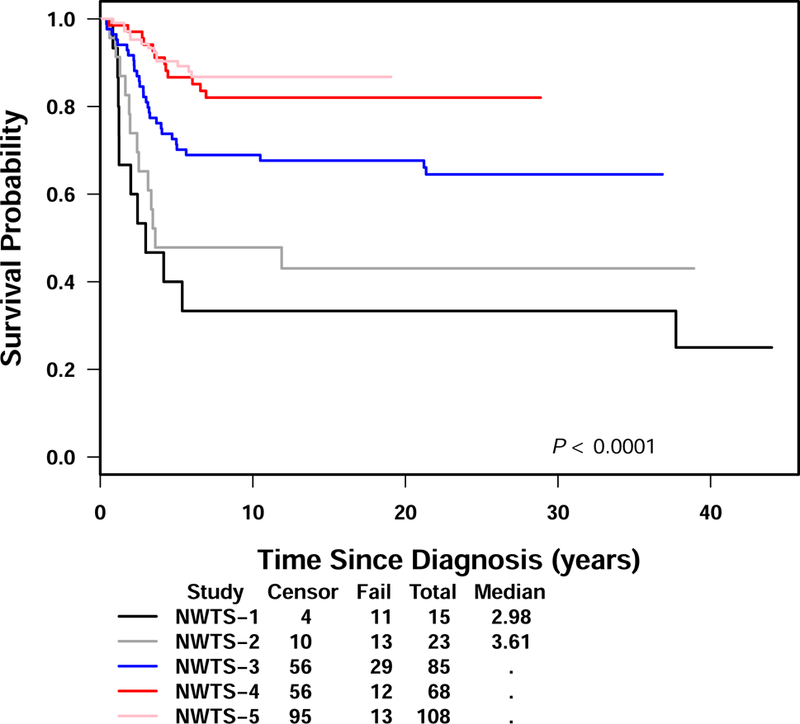
Overall survival for CCSK patients in NWTS-1 to NWTS-5
Discussion
The current analysis was undertaken to examine the impact of a new regimen that included cyclophosphamide and etoposide on the EFS and OS of patients with CCSK. The five-year EFS was 79% (95% CI: 71%−88%) and OS was 90% (95% CI: 84%−96%) for patients treated with regimen I. This was similar to the outcome of patients treated on NWTS-4.
On previous NWTS studies, CCSK patients were treated with therapy similar to that for higher stage, favorable histology Wilms tumor patients. On NWTS-5, in an attempt to improve the outcome of CCSK patients, a new regimen was developed that included vincristine and doxorubicin, added cyclophosphamide and etoposide, and omitted dactinomycin. On NWTS-5 treatment lasted 24 weeks whereas treatment on the arm of NWTS-4 with the better RFS lasted for 15 months.5 The cumulative anthracycline dose on regimen I was 225 mg/m2, compared to 300 mg/m2 on regimen DD4A. Radiation therapy was delivered to all sites of disease regardless of the completeness of surgical resection.
Patients with lower stage disease seemed to benefit from regimen I. Patients with stage I and stage II disease showed an improvement in five-year EFS and OS compared to patients with the same stages of disease on NWTS-4. To make sure this was not related to the change in staging criteria between NWTS-4 and NWTS-5, we combined the patients with stage I and II disease treated on NWTS-5 and NWTS-4 and compared their outcome. The five-year EFS and OS for stage I and II patients treated on NWTS-5 were 91% and 98%, respectively as compared to 74% and 88% for stage I/II patients treated on NWTS-4. Since all CCSK patients on NWTS-4 and NWTS-5 receive radiation to the tumor bed, the only difference is the chemotherapy regimen (DD-4A vs regimen I). Because of the excellent outcome of stage I patients on NWTS-5, Children’s Oncology Group (COG) renal tumor protocol, AREN0321, eliminated radiation to the tumor bed as long as the patient had undergone lymph node sampling that was negative for disease.
The relapse pattern of CCSK appears to be changing as has been observed with other pediatric tumors such as neuroblastoma.8 On NWTS-5, the first site of relapse was the brain in 12 out of 23 patients. This is different from what was observed on NWTS-4 on which the lungs and bones were the most common sites of recurrent disease. Prior to NWTS-4 the incidence of brain metastases was approximately 11%. This observation of the increase in relapses in the brain is not unique to this study since the International Society of Pediatric Oncology (SIOP) also has observed an increase in the frequency of recurrence in the brain.9,10 Although the results from NWTS-4 may not be representative of the behavior of CSSK, the incidence of brain metastases is higher on NWTS-5. This change could be a result of the chemotherapy regimen although cyclophosphamide used in this regimen does penetrate the central nervous system (CNS) and provides better CNS coverage than the three chemotherapeutic agents used in NWTS-4. In a report of 37 relapsed CCSK patients from Europe, 13 relapses occurred only in the brain and 1 occurred in combination with other sites. Of those patients with metastases to the brain, all except for two patients received carboplatin and ifosfamide or cyclophosphamide as part of their initial treatment. Three of these patients were stage 1 at initial diagnosis and did not receive radiation as part of their treatment.10 Another possibility to explain the difference in recurrence pattern is that the chemotherapy on this protocol kills the residual cells located in the common sites of recurrence such as lungs and bones whereas the previous chemotherapy did not provide adequate control of these sites. Therefore, patients developed recurrent disease in these sites initially and succumbed to their disease before they had time to develop brain recurrences.
Another aspect of CCSK that appears to be changing is the timing of the recurrences. In the past, CCSK was characterized by late relapses. In NWTS-4 30% of the recurrences occurred between 2 years and 37 months; however, none was reported after 37 months which was different from past studies.5 In the present study, most of the events occurred within three years (20/23). However, the latest relapse reported was 13.5 years from diagnosis and occurred in the remaining kidney. Tissue was not available to investigate whether this was a recurrence or a new primary. Recurrences occurred as late as four years after diagnosis in the SIOP study.9
The results observed on NWTS-5 are similar to those reported by SIOP. In the SIOP93–01/2001 protocols, 191 CCSK patients had a five-year EFS 78% and OS of 86% with a median follow-up of 6.2 years after diagnosis. The SIOP93–01/2001 trials chemotherapy included dactinomycin, vincristine, ifosfamide (or cyclophosphamide), etoposide, carboplatin and doxorubicin (or epirubicin). Stage 1 patients were not irradiated. Similar to our results, they found there was no difference in outcome for patients less than 24 months of age as compared to patients older than 24 months of age. Children less than 12 months of age (n=25) at diagnosis had a poorer five-year EFS of 49% and OS of 61% as compared to patients older than 12 months of age who had a five-year EFS of 84% and OS of 89%, similar to the outcome of patients older than 24 months of age. It is unknown whether the 50% reduction in chemotherapy doses for children < 12 months of age is a contributing factor in the inferior outcome observed. Advanced stage disease was the only independent unfavorable prognostic factor for survival. 9 It is important to remember that all patients treated on SIOP trials receive preoperative chemotherapy whereas in North America the practice is to have surgery first, if the tumor is resectable, followed by chemotherapy.
We considered whether a change in the diagnostic criteria for renal tumors could influence the CCSK treatment outcomes reported here and as shown in Figure 5. Although all tumors from patients enrolled on NWTS 1–5 have undergone central pathology review and in real time for NWTS-5, the ability to distinguish between CCSK and other renal tumors (including rhabdoid tumors) increased throughout the history of NWTS trials. BAF47 (INI-1) immunohistochemistry did not become available until later in NWTS-5. However, the experience during central review is that such cases are quite rare, and are unlikely to impact on the outcome of the current study. Mandatory staging requirements for CCSK patients evolved during NWTS 1–3 in that a chest xray was the only required study on NWTS-1. However during this time skeletal surveys, bone scans and brain MRIs were recommended and then skeletal surveys and brain MRI (although CT was acceptable) were required in NWTS-4. As noted for NWTS-5, CT scans of the primary tumor and lungs, bone scan, skeletal survey, brain MRI as well as bone marrow aspirates were required as part of the work up. Despite this, the breakdown in stage is fairly similar between NWTS 1–5 with a decrease in the stage I patients in NWTS 4 and 5 as compared to NWTS 1–3.1,5 The NWTS-5 results suggest that patients with stages I and II CCSK treated with regimen I have better disease control than NWTS-4 patients who were treated with three drugs delivered over 15 months. For higher stage disease, the benefit of regimen I is not apparent as compared to the three drugs utilized in NWTS-4. Patients with stage IV disease made up less than 10% of the study population but continue to have a poor prognosis and require new approaches. The addition of cyclophosphamide (14 g/m2 total dose) and etoposide is not without risk in terms of late effects. Cyclophosphamide is associated with sterility and both cyclophosphamide and etoposide are associated with subsequent malignancies.11–14 Regimen I may be more beneficial if it is feasible to eliminate radiation from stage I patients. However, these results from the COG study (AREN0321) are not yet available. Longer follow-up is necessary to fully evaluate the change in frequency of late effects from the addition of these agents for treatment in this group of patients and to justify the exposure.
Although the outcome is reasonable for patients with clear cell sarcoma of the kidney, much work remains to be done. Improvements are needed particularly for patients with stage 4 disease and patients who relapse. The role of radiation therapy in this cancer needs to be further elucidated. The identification of internal tandem duplications (ITD) in the BCOR gene in approximately 85% of CCSK, YWHAE (encoding 14–3-3ɛ)-NUTM2 fusions in 12% of cases and EGFR-ITD in a case of CCSK may provide additional insight into tumorigenesis in CCSK, as well as diagnosis and potentially therapeutic targets.15–18 Randomized clinical trials are difficult to do based on the incidence but, with international collaboration, some of the outstanding issues noted above may be addressed.
Supplementary Material
Acknowledgements
We thank the patients and families for their participation in this trial as well as investigators of the Children’s Oncology Group and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children entered on the National Wilms Tumor Studies. We also want to acknowledge the contributions of Norman E. Breslow to this study. He died in December, 2015 and he is deeply missed.
Supported by grants CA-42326, U10CA098543, U10CA180886, and U10CA180899
Abbreviations
- NWTS
National Wilms Tumor Study
- CCSK
Clear Cell Sarcoma of the Kidney
- EFS
Event-Free Survival
- OS
Overall Survival
- RFS
Relapse-Free Survival
- IRB
Institutional Review Board
- INI-1
Integrase interactor −1
- SIOP
International Society of Pediatric Oncology
- CNS
Central Nervous System
- ITD
Internal Tandem Duplications
- Kg
Kilogram
- IV
Intravenously
Footnotes
Conflict of Interest: Dr. Anderson reports other from Merck, outside the submitted work; none of the other authors reports a conflict.
References
- 1.Green DM, Breslow NE, Beckwith JB, et al. Treatment of children with clear-cell sarcoma of the kidney: a report from the National Wilms’ Tumor Study Group. J Clin Oncol 1994; 12:2132–2137. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, Kidd JM. Undifferentiated sarcoma of the kidney: a tumor of childhood with histopathologic and clinical characteristics distinct from Wilms tumor. Cancer 1978; 42:1916–1921. [DOI] [PubMed] [Google Scholar]
- 3.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumor: results of the first National Wilms Tumor Study. Cancer 1978; 41:1937–1948. [DOI] [PubMed] [Google Scholar]
- 4.Marsden HB, Lawler W. Bone-metastasizing renal tumor of childhood. Br J Cancer 1978; 38:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seibel NL, Li S, Breslow NE, et al. Effect of duration of treatment on treatment outcome for patients with clear cell sarcoma of the kidney: a report from the National Wilms’ Tumor Study Group. J Clin Oncol 2004; 22:468–473. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 33:457–481. [Google Scholar]
- 7.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc A 1972; 135:185–206. [Google Scholar]
- 8.Kramer K, Kushner B, Heller G, et al. Neuroblastoma metastatic to the central nervous system. The Memorial Sloan-Kettering Cancer Center experience and a literature review. Cancer 2001; 91:1510–1519. [PubMed] [Google Scholar]
- 9.Furtwangler R, Gooskens SL, van Tinteren H, et al. Clear cell sarcomas of the kidney registered on International Society of Pediatric Oncology (SIOP)93–01 and SIOP 2001 protocols: A report of the SIOP Renal Tumour Study Group. European J Cancer 2013; 49: 3497–3506. [DOI] [PubMed] [Google Scholar]
- 10.Gooskens SL, Furtwangler R, Spreafico F et al. Treatment and outcome of patients with relapsed clear cell sarcoma of the kidney: a combined SIOP and AIEOP study. Br J Cancer 2014;111: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenney LB, Laufer MR, Grant FD, et al. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer 2001; 91: 613–21. [DOI] [PubMed] [Google Scholar]
- 12.Smith MA, Rubinstein L, Anderson JR, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol 1999; 17:569–577. [DOI] [PubMed] [Google Scholar]
- 13.Davies SM. Therapy-related leukemia associated with alkylating agents. Med Pediatri Oncol 2001;36: 525–535. [DOI] [PubMed] [Google Scholar]
- 14.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014; 15:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno-Yokohata H, Okita H, Nakasato K, et al. Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet 2015; 47:861–3. [DOI] [PubMed] [Google Scholar]
- 16.Fehr A, Hansson MC, Kindblom LG, et al. YWHAE-FAM22 gene fusion in clear cell sarcoma of the kidney. J Pathol 2012; 227:35–37. [DOI] [PubMed] [Google Scholar]
- 17.O’Meara E, Stack D, Lee CH, et al. Characterization of the chromosomal translocation t(10;17) (q22;p13) in clear cell sarcoma of kidney. J Pathol 2012; 227:72–80. [DOI] [PubMed] [Google Scholar]
- 18.Santiago T, Clay MR, Azzato E, et al. Clear cell sarcoma of kidney involving a horseshoe kidney and harboring EGFR internal tandem duplication. Pediatr Blood Cancer 2017;64:e26602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


