Abstract
Background:
VSL#3 is a patented probiotic for which several clinical trials suggest benefits on motor function, bloating and symptoms of irritable bowel syndrome (IBS).
Objectives:
To quantify effects of VSL#3 on abdominal pain, stool consistency, overall response, abdominal bloating, and quality of life (QOL) in IBS through meta-analysis.
Methods:
MEDLINE (OvidSP and PubMed), EMBASE, Web of Science, and Scopus were searched up to May 2017. Using a fixed effects model, we pooled data from intention-to-treat analyses of randomized trials (RCTs) comparing VSL#3 to placebo in IBS. Data were reported as relative risk (RR), overall mean difference (MD) or standardized MD (SMD) with 95% confidence intervals (CI). Quality of evidence was rated using the GRADE approach.
Key Results:
Among 236 citations, five RCTs (243 patients) were included. No significant differences were observed for abdominal pain (SMD = −0.03; 95% CI −0.29–0.22), bloating (SMD = −0.15; 95% CI −0.40–0.11), proportion of bowel movements with normal consistency (overall MD = 0; 95% CI −0.09–0.08), or IBS-QOL (SMD = 0.08; 95% CI −0.22–0.39). VSL#3 was associated with a nearly statistically significant increase in overall response (RR=1.39; 95% CI 0.99–1.98).
Conclusions & Inferences:
In this systematic review and meta-analysis, there was a trend towards improvement in overall response with VSL#3, but no clear evidence effectiveness for IBS. However, the number and sample sizes of the trials are small and the overall quality of evidence for three of the five outcomes was low. Larger trials evaluating validated endpoints in well-defined IBS patients are warranted.
Keywords: irritable bowel syndrome, probiotic, systematic review, VSL#3
Graphical abstract
VSL#3 is a patented probiotic for which several clinical trials suggest benefits on symptoms of irritable bowel syndrome (IBS). In the current systematic review and meta-analysis, we were unable to demonstrate clear evidence for benefit with VSL#3 in IBS, although a strong trend towards improvement in overall response was observed. Further study of VSL#3 in well-defined IBS patients using validated clinical endpoints is required.
Irritable bowel syndrome (IBS) is a common functional bowel disorder with estimated regional prevalence rates ranging from 5.8 to 17.5%.1 The complex pathophysiology of IBS remains incompletely understood, but may involve both central and peripheral mechanisms including alterations in the brain-gut axis, gastrointestinal (GI) motility, intestinal permeability, local immune responses, low-grade inflammation, visceral hypersensitivity and intestinal microbiota.2 With increasing evidence linking the intestinal microbiota with numerous human syndromes and diseases including IBS,3 there has been growing interest in modulation of the microbiota as a therapeutic strategy for IBS. The intestinal microbiota may serve as a key factor in IBS pathophysiology through direct effects on the aforementioned central and peripheral mechanisms, and through their production of microbial metabolic products.4, 5 Composition of the human intestinal microbiota is shaped by various host-specific and environmental factors.5, 6 Thus, antibiotic and probiotic therapies have been considered as potential treatments to target the intestinal dysbiosis or microbial imbalance that may exist in IBS.
Probiotics are live microorganisms that when consumed by the host in adequate amounts, may confer beneficial health effects.7 Probiotics have long been used in the treatment of many GI symptoms. They are easily available and have been extensively studied. Unfortunately, most studies have been of suboptimal quality with significant heterogeneity in study endpoints and methodological rigor. A prior systematic review of probiotics in IBS reported evidence for efficacy on persistent symptoms, global IBS and abdominal pain scores. However, evidence for benefit with individual strains or specific combinations was not shown.8 Preclinical studies have demonstrated strain-specific effects of probiotics, which should be taken into consideration when prescribing probiotics in the clinical setting.9
Several strains and combinations of strains are currently available for IBS therapy, with Lactobacillus sp. and Bifidobacterium sp.8, 9 most frequently studied. A specific probiotic combination, VSL#3, is a patented probiotic preparation consisting of eight different strains of bacteria (Bifidobacterium longum, B. infantis, B. breve, Lactobacillus acidophilus, L. casei, L. delbrueckii ssp.bulgaricus, L. plantarum and Streptococcus salivarus ssp. thermophilus) that has demonstrated efficacy in IBS patients with reduction of bloating and abdominal symptoms.10–15 However, studies have generally been small and of limited duration. Furthermore, only a few have employed rigorous methodological standards utilizing FDA responder endpoints. The objectives of this systematic review are to examine clinical efficacy of the patented probiotic VSL#3 on outcomes of abdominal pain, stool consistency, overall response, bloating and quality of life (QOL) in patients with IBS.
METHODS
Search strategy and study selection:
The study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.16 A search of the medical literature was conducted in May 2017 using MEDLINE (OvidSP and PubMed interfaces), EMBASE, Web of Science, and Scopus by an experienced medical librarian (JH) with input from study investigators. Studies were identified using a search strategy that included relevant keywords and Medical Subject Headings (MESH) to retrieve randomized controlled trials of VSL#3 for patients with IBS. A focus on treatment outcome and impact on abdominal pain and bowel function was also included. The search strategy contained a combination of MESH terms and keywords that included terms related to “Irritable Bowel Syndrome”; “Probiotics” OR “VSL #3.mp”; “Treatment outcome” OR “Abdominal Pain”.) The search strategy is shown in the Appendix. Randomized trials (RCTs) comparing VSL#3 with placebo among patients with IBS were eligible for inclusion. No date limitations or language restrictions were applied. All trials were eligible for inclusion regardless of publication-type. First period data from cross-over studies were eligible for inclusion. Abstracts and titles identified from the search strategy were assessed by two independent investigators (AS and MC) for eligibility. All potentially relevant studies were subsequently obtained for a full-text review. Bibliographies of eligible studies, available reviews, and clinicaltrials.gov were reviewed. Conference abstracts and clinicaltrials.gov were manually searched to identify potentially eligible studies. Conflicting decisions were resolved by discussion.
Eligibility criteria:
Eligible for inclusion were randomized placebo-controlled trials examining effects of VSL#3 on outcomes of abdominal pain and stool consistency in patients with IBS. Any definition of IBS, including physician diagnosis or symptom-based diagnostic criteria, was satisfactory for inclusion. Studies investigating patients with other organic gastrointestinal conditions including inflammatory bowel disease, microscopic colitis, celiac disease, or infectious colitis were excluded. No minimum duration of therapy was applied. Studies had to report data on at least one of the primary outcomes: abdominal pain or stool consistency.
Study Outcomes:
The primary outcomes were effects of VSL#3 on mean improvements in abdominal pain and stool consistency. Secondary outcomes were effects of treatment on overall response, abdominal bloating, and quality of life (QOL). We also assessed the frequency and types of adverse events. Outcomes were selected based on previous recommendations established by the FDA for evaluating treatment benefit in clinical trials of IBS.
Data abstraction:
All data were abstracted independently by two investigators (AS and MC) for the primary and secondary outcomes on to a Microsoft Excel spreadsheet. Authors of included studies were contacted for missing data; their provided responses were included in the analyses. Data were recorded as continuous for: (a) mean improvement in abdominal pain scores from baseline, (b) proportion of stools with normal consistency while on treatment, (c) mean improvement in abdominal bloating scores from baseline, (d) mean improvement in QOL scores from baseline. Data were recorded as dichotomous outcomes for overall response, defined as proportion of patients achieving a pre-specified endpoint (e.g. satisfactory relief in bloating or IBS symptoms) in weekly response for 50% of treatment weeks. Total number of adverse events were recorded. Data were abstracted from each trial to assess study characteristics: location, number of centers, year published, intervention type, control type, dose and duration of therapy, and duration of follow-up. Patient characteristics including study population, total number of participants, mean age, and proportion of female patient were recorded. Data were also abstracted by two investigators (AS and MC) to evaluate individual study risk of bias according to the Cochrane Risk of Bias Assessment Tool17 with disagreements resolved by discussion. A third investigator (TI) was available to manage any disagreements if they could not be resolved by discussion.
Quality of Evidence:
We used the GRADE approach to evaluate strength of the body of evidence. In this approach, level of evidence is rated as high, moderate, low, or very low based on the following factors: (1) risk of bias, (2) indirectness, (3) unexplained heterogeneity or inconsistency, (4) imprecision, and (5) probability of publication bias. All factors were considered for each outcome, and were classified as “serious” or “very serious” if a reason could be found for downgrading the evidence.
Data synthesis and statistical analysis:
We used an intention-to-treat analysis for all dichotomous outcomes with dropouts categorized as non-responders. Continuous outcomes were recorded using all available data. Data were pooled using fixed effects models. Pooled estimates of treatment effect for continuous variables were reported as standardized mean difference (SMD) or mean difference (MD) with 95% confidence intervals (CI). Proportion of overall responders was reported as risk ratio (RR) with 95% CI using the Mantel-Haenszel method. For continuous outcomes, we analyzed the mean difference in change from baseline. If this variable was not reported and could not be calculated from other data, we used the mean difference at follow-up. We assessed statistical heterogeneity using the I2 statistic. The I2 statistic represents the proportion of variation across studies that is due to differences between studies beyond chance. I2 > 50% indicates significant heterogeneity among studies.18
Meta-analyses were performed using the “meta” package19 in R Statistical Software (version 3.4.2) and the significance level set to an alpha of 0.05. When no standard deviation (SD) was reported (Wong et al 2015), it was imputed from other studies using the same scale. When a specified endpoint of interest (e.g. proportion of patients achieving a pre-specified endpoint in weekly response for 50% of treatment weeks) was not provided (Staudacher et al 2017), but data on a similar endpoint were reported, these data were recorded as “surrogate” outcomes and pooled for the meta-analysis. We performed additional pre-specified sensitivity analyses to assess the effect of VSL#3 by excluding studies with SD imputation or a surrogate outcome.
RESULTS
Study Characteristics:
The search strategy used (Appendix) identified 236 citations, of which five RCTs including 243 patients (124 randomized to VSL#3 and 119 randomized to placebo) were eligible for inclusion. A flow diagram of studies identified in the search is shown in Figure 1. Characteristics of included studies are summarized in Table 1. Mean age among all study participants was 38.3 years (SD=16.2); 69% were women. The trial populations included patients with IBS using Rome II or III criteria. Two studies enrolled only patients with diarrhea-predominant IBS.11, 13 Patients were screened for prior antibiotic-use which was disallowed during the 2 weeks,12, 13 1 month10, 15 or 3 months11 preceding initiation of study procedures. Adverse events were recorded in all studies. All five studies were double-blinded, randomized, placebo controlled trials of VSL#3 doses of 450 billion lyophilized bacteria/day10 or 900 billion bacteria per day11–13, 15. Treatment duration ranged from 4 weeks15, to 8 weeks11–13. Three studies included a 2-week run-in phase.10, 12, 13 One study required an amendment to the protocol after 17 participants completed 8 treatment weeks to allow for a 2-week run-in phase followed by a 4-week treatment phase due to difficulties with patient recruitment.12 One study randomized patients to a co-intervention of sham diet vs. low FODMAP diet.15
Figure 1:
Study selection flow diagram
Table 1: Characteristics of included randomized placebo-controlled trials.
| Study, Year | Study Design | Location | Intervention (dose; N) | Control (N) | Treatment duration | Outcomes reported |
|---|---|---|---|---|---|---|
| Wong et al., 2015 | RCT | Singapore, Singapore | VSL#3 (450 × 109 LB daily; 20) |
PCBO (22) | 6 weeks | AP, SC@, AB, QOL |
| Kim et al., 2005 | RCT | Rochester, MN | VSL#3 (450×109 LB twice daily; 24) |
PCBO (24) | 8 weeks & 4 weeks$ | AP, SC, OR, AB |
| Kim et al., 2003 | RCT | Rochester, MN | VSL#3 (450 × 109 LB twice daily; 12) |
PCBO (13) | 8 weeks | AP, SC, OR, AB |
| Michail et al., 2011 | RCT | Dayton, OH | VSL#3 (900 × 109 LB daily; 15) |
PCBO (9) | 8 weeks | AP, AB, QOL |
| Staudacher et al., 2016 | RCT& | Multi-center* | VSL#3 (450 × 109 LB twice daily; 53) |
PCBO (51) |
4 weeks | AP, SC, OR, AB, QOL, |
Abbreviations: RCT = randomized controlled trial, PCBO = placebo, LB = lyophilized bacteria, AP = abdominal pain, SC = stool consistency, OR = overall response, AB = abdominal bloating, QOL = quality of life
*2 hospitals in London, UK;
@Scale used for stool consistency not provided;
&Study design including four arms (sham diet +PCBO, sham diet + VSL#3, low FODMAP diet + PCBO, low FODMAP diet + VSL#3),
$8 weeks treatment duration for 17 subjects and 4 weeks treatment duration for 31 subjects
Quality of Evidence:
Quality of evidence is summarized in Table 2 using the GRADE approach as described in the methods. Two studies were considered to be at moderate 10 or high risk of bias 11 due to concerns for selection and reporting bias, and evidence was rated down for outcomes in which these studies were included. Overall, there was high certainty suggesting a lack of benefit on stool consistency and moderate certainty suggesting a trend towards benefit in overall response with VSL#3. For all other outcomes, there was low certainty suggesting lack of benefit of VSL#3.
Table 2: Quality of evidence summarized using the GRADE Approach.
| Outcome studied | Risk of bias | Indirectness | Inconsistency* | Imprecision | Publication bias | Overall Quality |
|---|---|---|---|---|---|---|
| Abdominal pain | Serious1 | - | - | Serious2 | - | Low |
| Stool consistency | - | - | - | - | - | High |
| Overall response | - | - | - | - | Serious3 | Moderate |
| Abdominal bloating | Serious1 | - | - | Serious2 | - | Low |
| Quality of life | Serious1 | - | - | Serious | - | Low |
*Inconsistency could not be assess due to small number of trials
1High to moderate risk of bias for outcomes in two trials
2Wide 95% CI
3Overall response measured in one study, but results not reported
Primary Outcomes
Abdominal pain:
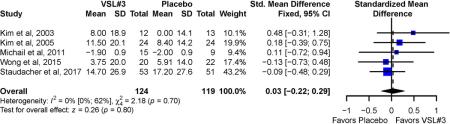
Four trials10, 12, 13, 15 appraised pain symptoms based on a 100 mm visual analogue scale. One trial11 used the Gastrointestinal Symptom Rating Scale (GSRS), a clinical rating scale for IBS.20. Overall, no significant difference was observed for mean improvement in abdominal pain between VSL#3 (SMD = 0.03; 95% CI −0.22 to 0.29) and placebo (Figure 2). No significant statistical heterogeneity was observed across studies (I2 = 0%, P-value for Cochrane’s Q = 0.7). Sensitivity analysis excluding one study that required the use of imputed data for the SD of the abdominal pain intensity score10 showed similar findings (SMD = 0.07; 95% CI −0.21 to 0.34). There was no observed heterogeneity among studies (I2 = 0%, P=0.6).
Figure 2:
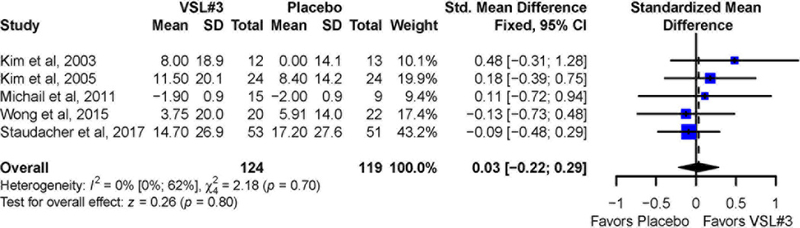
Pooled effects of VSL#3 vs. placebo on abdominal pain reported as standardized mean difference
Stool consistency:
Data for change in percentage of bowel movements with normal stool consistency before and after treatment was available for 177 patients in three studies.12, 13, 15 There was no significant difference in the change of the proportion of bowel movements with normal consistency (Figure 3) for VSL#3 vs. placebo (overall MD = 0; 95% CI= −0.09 to 0.08). There was no observed heterogeneity across studies (I2 = 0%, P= 0.71).
Figure 3:
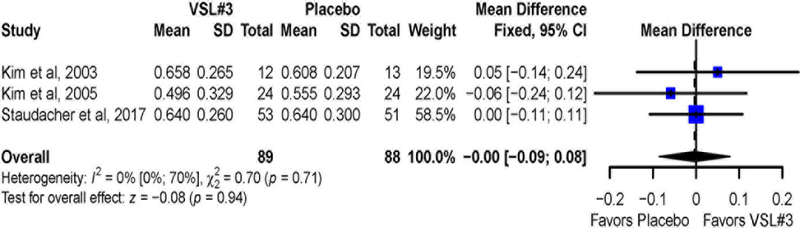
Pooled effects of VSL#3 vs. placebo on stool consistency reported as mean difference
Secondary Outcomes
Overall response:
Data for overall response and proportion of stools with normal consistency were available for 177 patients in three studies.12, 13, 15 Two studies defined a response as achieving a pre-specified endpoint (e.g. satisfactory relief in bloating or IBS symptoms) during at least 50% of the treatment weeks.12, 13 A third study defined overall response as adequate relief of symptoms over the past 7 days at the end of the follow-up period.15 Relative to the placebo group (32 of 88 [36%] responders), 45/89 (50%) of patients treated with VSL#3 reported an overall response (RR=1.39; 95% CI 0.98 to 1.96) (Figure 4). There was no significant heterogeneity across studies (I2 = 0%, p = 0.63). Sensitivity analysis excluding the trial with overall response defined over the past 7 days demonstrated no significant difference in overall response (RR=1.18; 95% CI 0.66 to 2.13) with no significant heterogeneity across studies (I2 = 0%, p = 0.48).
Figure 4:
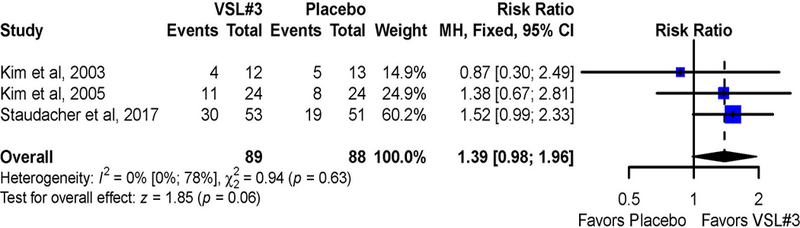
Pooled effects of VSL#3 vs. placebo on overall response reported as risk ratio
Abdominal bloating:
Among the five studies, no significant difference was observed for mean improvement in abdominal bloating (Figure 5) with VSL#3 vs. placebo (SMD = 0.15; 95% CI −0.11 to 0.4). There was no significant heterogeneity across studies (I2 = 5%, p = 0.38). Sensitivity analysis excluding one trial10 for which data imputation was required showed similar results (SMD= 0.16; 95% CI −0.12 to 0.44) with no significant heterogeneity across studies (I2 = 28%, p = 0.25).
Figure 5:
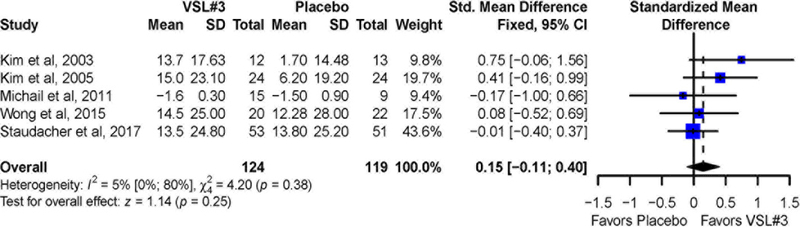
Pooled effects of VSL#3 vs. placebo on abdominal bloating reported as standardized mean difference
Quality of Life:
Data for QOL were available from three studies.10, 11, 15 No significant differences (Figure 6) between VSL#3 and placebo treated groups were observed for mean improvement in QOL (SMD = −0.08; 95% CI −0.39 to 0.22). There was no significant heterogeneity across studies (I2 = 0%, P = 0.65). Sensitivity analysis excluding trial10 with imputed data showed similar results (SMD = −0.14; 95% CI −0.49 to 0.21) and heterogeneity remained non-significant (I2 = 0%, P = 0.48).
Figure 6:
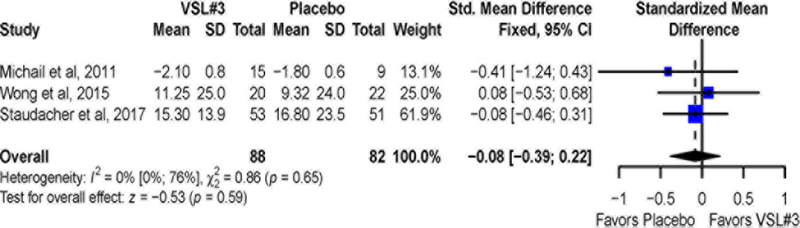
Pooled effects of VSL# vs. placebo on quality of life scores reported as standardized mean difference
Adverse Events:
Only one study15 reported adverse events, which included worsened GI symptoms, in 7.8% of placebo patients and 3.8% of VSL#3 patients.
DISCUSSION.
Although individual trials have shown evidence for benefit with VSL#3 vs. placebo for abdominal bloating and flatulence, there are no clear data demonstrating improved bowel function, pain reduction, or overall response. Few trials have incorporated at least 8 weeks of study treatment and study endpoints of interest. In recent years, the FDA has put forth recommendations21 to guide trial design and trial endpoints in IBS clinical trials in attempts to enhance reliability in assessment of treatment effects. Recommendations for evaluating treatment response have focused on “overall response” defined as a pre-specified improvement in weekly or daily response for at least 50% of treatment weeks. Additionally, both abdominal pain and stool frequency have been evaluated for response. Consequently, guidance for clinicians and patients regarding effective use of probiotics such as VSL#3 in treatment of IBS has been vague. Thus, we attempted to assess pooled effects of VSL#3 on abdominal pain, stool consistency, and overall response. In this systematic review and meta-analysis of randomized controlled trials, we were unable to demonstrate clear evidence for efficacy of VSL#3 compared to placebo among patients with IBS for any of the outcomes examined, with the exception of a nearly statistically significant difference in overall response between treatment groups. This lack of effect is generally consistent with findings reported in individual studies as well as a prior systematic review of probiotics in IBS, in which subgroup analysis of three trials using VSL#3 in IBS showed no significant benefit compared to placebo on global or abdominal pain scores.8 At the same time, few adverse events were reported, and VSL#3 appears to be safe and well tolerated in IBS.
The precise mechanisms by which VSL#3 exerts potentially beneficial effects are unknown. In vivo and in vitro studies have suggested that VSL#3 may modulate host-immune response, intestinal microbiota, anti-inflammatory pathways, visceral pain responses and epithelial barrier function.22–26 Mechanistic studies exploring potential beneficial effects of VSL#3 in patients with IBS have been limited, among which are the trials included in this analysis. In both studies by Kim et al,12, 13 investigators incorporated assessment of colonic transit to demonstrate retardation of colonic transit in patients treated with VSL#3 in the second, but not the first trial. Data on effects of VSL#3 on the intestinal microbiota of IBS patients has also been conflicting. Michail et al.11 could not detect any significant changes in total or relative composition of gut microbiota after treatment with VSL#3 and placebo, while a recent study examined effects of VSL#3 on intestinal microbiota from rectal biopsies of IBS patients to demonstrate significant reductions in Bacteroides compared to controls.27. Others have examined VSL#3 effects on rectal pain thresholds in IBS and association with salivary melatonin to demonstrate significant increases in rectal distension pressure thresholds with VSL#3, but not after placebo and significant increases in morning melatonin in males treated with VSL#3 but not females.10 Hence, the mechanistic rationale promoting use of VSL#3 remains an area of ongoing investigation. Further study to clarify the effect of VSL#3 on regulation of pathophysiologic pathways of IBS may be necessary to identify specific biomarkers that may predict therapeutic response or guide patient selection in future clinical trials, particularly given the heterogeneous nature of IBS. In a recent systematic review,28 VSL#3 was associated with a benefit over placebo in inducing remission in active ulcerative colitis, implying that probiotics and specific probiotic combinations may be effective in this particular patient subset. Enrichment of future IBS cohorts with clearly defined patient phenotypes may be helpful in defining the role of VSL#3 or other specific probiotics combinations in the treatment of this condition.
Although the results of our systematic review and meta-analysis could not demonstrate a clear benefit of VSL#3 compared to placebo, our ability to detect significant differences may have been affected by several factors. Limitations of this study are: 1) inclusion of multiple IBS subtypes in individual trials for which we were unable to perform subgroup analyses due to the small number of total patients and lack of access to individual patient data (which was obtained for only two trials)12,13; 2) availability of only five published trials with a small number of total participants; 3) variable treatment duration across studies, and; 4) inconsistent reporting of outcomes. Due to the small number of studies, non-significant results for assessment of heterogeneity cannot be interpreted as evidence for study homogeneity.
The inclusion of multiple IBS subtypes may have obscured the ability to detect the benefit of VSL#3, which may have differential effects on IBS subtypes due diverse mechanisms. It is hypothesized that probiotics may affect gastrointestinal symptoms through modulation of the gut microbiota and there have now been several studies to suggest that specific microbial perturbations are associated with bowel pattern phenotype. For example, increased gut microbial richness has been associated with longer colon transit29 while decreased species richness has been associated with looser stool30. In the study by Kim et al. 200512, VSL#3 slowed colonic transit relative to placebo in patients with IBS. Based on these observations, VSL#3 might be effective in patients with diarrhea-predominant IBS. In general; however, non-targeted investigation of heterogeneous patient populations would tend to diminish the ability to identify treatment efficacy. Currently approved therapies for IBS are based on trials that are anchored on bowel function phenotype;31 hence, it remains important for future trials of VSL#3 in IBS to incorporate phenotypic homogeneity.
In summary, probiotics have generated considerable interest as potential therapeutic agents in IBS. However, the biological effects of probiotics may be highly strain-specific and careful review of the existing evidence base for specific probiotics strains or combinations of strains is important. In our study, VSL#3 was not associated with a clear benefit in abdominal pain, stool consistency, abdominal bloating, or quality of life in patients with IBS. However, a trend towards improvement in overall response was observed. Notably, despite the numerous studies published on VSL#3, studies addressing meaningful outcomes of adequate duration of treatment in patients with IBS are limited and the overall quality of evidence for three of the five outcomes examined was low. Additional studies of VSL#3 are warranted before recommendations regarding its potential efficacy in IBS can be made. Such trials should be adequately powered for rigorous clinical endpoints, take into account potential biological actions of the specific microbial species included, and have longer follow-up in carefully selected patient populations.
KEY POINTS.
Studies have indicated a potential benefit of probiotics in IBS, although efficacy of individual strains or specific combinations is unknown.
While pooled analyses in the current study show no clear benefit with VSL#3 in IBS on abdominal pain or stool consistency, there is a strong trend towards improvement in overall response.
Further study of VSL#3 in well-defined IBS patients using validated clinical endpoints is required.
ACKNOWLEDGEMENTS
Not applicable
Funding Source: AS is supported, in part, by grants KL2TR001106, and UL1TR001108 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
APPENDIX. Detailed Search Strategy
MEDLINE was searched using the OvidSP interface on May 24, 2017
exp Colonic Diseases, Functional/ or exp Irritable Bowel Syndrome/
exp Probiotics/
vsl 3.mp.
vsl #3.mp.
2 or 3 or 4
exp Stomach/
exp Pain/
6 and 7
exp Abdominal Pain/
exp Disease Management/ or exp Disease Progression/
exp Gastrointestinal Motility/
exp Gastric Emptying/
exp Remission Induction/ colo
exp “Quality of Life”/
exp Digestive System/
exp Digestive System Physiological Phenomena/
exp Diarrhea/
exp Treatment Outcome/
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
1 and 5 and 19
limit 20 to (english language and humans)
limit 21 to randomized controlled trial
MEDLINE was searched using the PubMed interface on May 24, 2017
(((((((((((((“Treatment Outcome”[Mesh]) OR “Diarrhea”[Mesh]) OR “Digestive System Physiological Phenomena”[Mesh]) OR “Digestive System”[Mesh]) OR “Quality of Life”[Mesh]) OR “Remission Induction”[Mesh]) OR “Gastric Emptying”[Mesh]) OR “Gastrointestinal Motility”[Mesh]) OR “Disease Progression”[Mesh]) OR “Disease Management”[Mesh]) OR “Abdominal Pain”[Mesh]) OR ((“Pain”[Mesh]) AND “Stomach”[Mesh]))) AND (((“Colonic Diseases, Functional”[Mesh]) OR “Irritable Bowel Syndrome”[Mesh])) AND ((“Probiotics”[Mesh]) OR “vsl 3”) OR “vsl #3”
Web of Science was searched on May 25, 2017
# 22 144
#20 AND #19
Refined by: LANGUAGES: ( ENGLISH )
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 21 152
#20 AND #19
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 20 176,640
TOPIC: (“randomized controlled trial”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 19 527
#18 AND #17 AND #16
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 18 556,498
#15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 17 16,132
#4 OR #3
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 16 18,097
#2 OR #1
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 15 24,302
TOPIC: (“treatment outcome”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 14 62,326
TOPIC: (“diarrhea”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All year
# 13 4,405
TOPIC: (“digestive system”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 12 279,871
TOPIC: (“quality of life”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 11 83,178
TOPIC: (“remission” OR “remission induction”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 10 10,740
TOPIC: (“gastric emptying”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 9 4,669
TOPIC: (“gastrointestinal motility”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 8 60,962
TOPIC: (“disease progression”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 7 14,723
TOPIC: (“disease management”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 6 32,905
TOPIC: (“abdominal pain”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 5 2,727
TOPIC: (“stomach”) AND TOPIC: (“pain”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 4 307
TOPIC: (“vsl#3”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 3 16,007
TOPIC: (“probiotics”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 2 18,096
TOPIC: (“irritable bowel syndrome”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
# 1 3
TOPIC: (“functional colonic disease”)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC Timespan=All years
Scopus was searched on May 25, 2017
( ( ( ”abdominal pain” ) OR ( ”stomach” AND ”pain” ) ) AND ( ( ”abdominal pain” OR ”gastric emptying” OR ”gastrointestinal motility” OR ”remission induction” OR ”treatment outcome” OR ”disease progression” OR ”disease management” OR ”quality of life” OR ”diarrhea” OR ”digestive system” ) ) ) AND ( ( ”irritable bowel syndrome” OR ”colonic diseases, functional” ) AND ( ”probiotics” OR ”VSL#3” ) ) AND ( LIMIT-TO ( LANGUAGE , ”English” ) ) AND ( EXCLUDE ( DOCTYPE , ”re” ) OR EXCLUDE ( DOCTYPE , ”ch” ) OR EXCLUDE ( DOCTYPE , ”ed” ) OR EXCLUDE ( DOCTYPE , ”sh” ) OR EXCLUDE ( DOCTYPE , ”bk” ) OR EXCLUDE ( DOCTYPE , ”no” ) OR EXCLUDE ( DOCTYPE , ”le” ) ) AND ( LIMIT-TO ( EXACTKEYWORD , ”Humans” ) OR LIMIT-TO ( EXACTKEYWORD , ”Randomized Controlled Trial (topic)” ) ) AND ( LIMIT-TO ( EXACTKEYWORD , ”Randomized Controlled Trial” ) )
EMBASE was searching on May 26, 2017
#8#4 AND #5 AND #6 AND #7 109
#7’randomized controlled trial’/exp 445466
#6’irritable bowel syndrome’ 16461
#5’human’/exp 18195192
#4#1 AND #2 AND #3 1250
#3’probiotic agent’/exp OR ‘vsl3’/exp 24570
#2’irritable colon’/exp 21041
#1’abdominal pain’/exp OR ‘stomach emptying’/exp OR ‘gastrointestinal motility’/exp OR ‘remission’/exp OR ‘treatment outcome’/exp OR ‘diarrhea’/exp OR ‘disease management’/exp OR ‘disease course’/exp OR ‘quality of life’/exp OR ‘digestive system’/exp 6002848
Google Scholar was searched on May 26, 2017.
The following strategy was used:
allintitle: irritable bowel syndrome AND “VSL #3”
Footnotes
Author contributions: MC: data abstraction and writing of the manuscript; AS: study conceptualization, writing of the protocol and manuscript; TJS: study development, writing of the manuscript; HX: statistical analysis and writing of the manuscript; TFI: study development and writing of the manuscript; JH: literature search and writing of the manuscript.
Disclosures: NONE
REFERENCES
- 1.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut 2017;66:1075–1082. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 3.Riviere A, Selak M, Lantin D, et al. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkes GC, Brostoff J, Whelan K, et al. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol 2008;103:1557–67. [DOI] [PubMed] [Google Scholar]
- 5.Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol 2017;312:G52-G62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarner F, Requena T, Marcos A. Consensus statements from the Workshop “Probiotics and Health: Scientific evidence”. Nutr Hosp 2010;25:700–4. [PubMed] [Google Scholar]
- 8.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1547–61; quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- 9.Moraes-Filho JP, Quigley EM. The Intestinal Microbiota and the Role of Probiotics in Irritable Bowel Syndrome:: a review. Arq Gastroenterol 2015;52:331–8. [DOI] [PubMed] [Google Scholar]
- 10.Wong RK, Yang C, Song GH, et al. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci 2015;60:186–94. [DOI] [PubMed] [Google Scholar]
- 11.Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double-blind, Placebo-controlled Trial of VSL#3 in Diarrhea-predominant Irritable Bowel Syndrome. Probiotics Antimicrob Proteins 2011;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 2005;17:687–96. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2003;17:895–904. [DOI] [PubMed] [Google Scholar]
- 14.Guandalini S, Magazzu G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010;51:24–30. [DOI] [PubMed] [Google Scholar]
- 15.Staudacher HM, Lomer MCE, Farquharson FM, et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017;153:936–947. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarzer G meta: An R package for meta-analysis. R News 2007;7:40–45. [Google Scholar]
- 20.Wiklund IK, Fullerton S, Hawkey CJ, et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol 2003;38:947–54. [DOI] [PubMed] [Google Scholar]
- 21.(CDER) USDoHaHSFaDACfDeaR. Guidance for Industry: Irritable Bowel Syndrome - Clinical Evaluation of Drugs for Treatment, 2012. [Google Scholar]
- 22.Dai C, Zheng CQ, Meng FJ, et al. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-kappaB pathway in rat model of DSS-induced colitis. Mol Cell Biochem 2013;374:1–11. [DOI] [PubMed] [Google Scholar]
- 23.Distrutti E, Cipriani S, Mencarelli A, et al. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One 2013;8:e63893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do EJ, Hwang SW, Kim SY, et al. Suppression of colitis-associated carcinogenesis through modulation of IL-6/STAT3 pathway by balsalazide and VSL#3. J Gastroenterol Hepatol 2016;31:1453–61. [DOI] [PubMed] [Google Scholar]
- 25.Dolpady J, Sorini C, Di Pietro C, et al. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res 2016;2016:7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121:580–91. [DOI] [PubMed] [Google Scholar]
- 27.Ng SC, Lam EF, Lam TT, et al. Effect of probiotic bacteria on the intestinal microbiota in irritable bowel syndrome. J Gastroenterol Hepatol 2013;28:1624–31. [DOI] [PubMed] [Google Scholar]
- 28.Derwa Y, Gracie DJ, Hamlin PJ, et al. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 2017;46:389–400. [DOI] [PubMed] [Google Scholar]
- 29.Roager HM, Hansen LB, Bahl MI, et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol 2016;1:16093. [DOI] [PubMed] [Google Scholar]
- 30.Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016;65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147:1149–72 e2. [DOI] [PubMed] [Google Scholar]