Abstract
Providing evidence-based supportive care for children with cancer has the potential to optimize treatment outcomes and improve quality of life. The Children’s Oncology Group (COG) Supportive Care Guidelines sub-Committee conducted a systematic review to identify current supportive care clinical practice guidelines (CPGs) relevant to childhood cancer or pediatric hematopoietic stem cell transplant. Only 22 papers met the 2011 Institute of Medicine criteria to be considered a CPG. The results highlight the paucity of CPGs available to pediatric oncology healthcare professionals and the pressing need to create CPGs using current methodological standards.
Keywords: pediatric cancer, practice guidelines, supportive care, systematic review
Background
Collaborative research efforts and randomized clinical trials have resulted in substantial improvements in childhood cancer outcomes over the past three decades.[1,2] Despite large improvements in survival outcomes for children with cancer, patients experience a high burden of expected treatment-related and disease-related symptoms.[3–5] Across diverse clinical domains, delivering care consistent with clinical practice guideline (CPG) recommendations improves outcomes.[6–10] As such, provision of evidence-based supportive care to children with cancer has the potential to optimize treatment outcomes and improve quality of life.
In 2011, the Institute of Medicine (IOM) released a report entitled “Clinical Practice Guidelines We Can Trust” as a call for the development of methodologically sound evidence-based guidelines.[11] As part of this report, the IOM updated their long-standing definition of a CPG (Table I). The 2011 definition added a methodological requirement. That is, to be considered a CPG, recommendations must be “informed by a systematic review of evidence.”[11] Prior to 2011, the need for a systematic review was not explicitly stated as a necessary foundation upon which CPG recommendations are formulated.[12] In making this change, the IOM emphasized the importance of distinguishing CPGs from other forms of guidance, such as expert opinion and consensus statements.
TABLE I.
Change in IOM definition of a CPG
| IOM | Year | CPG definition |
|---|---|---|
| New definition[11] | 2011 | Clinical practice guidelines are statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options. |
| Original definition[12] | 1990 | Clinical practice guidelines are systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances |
The IOM defines a systematic review as using “explicit, prespecified scientific methods to identify, select, assess, and summarize the findings of similar but separate studies.”[13] The requirement of a systematic review as the basis for CPG recommendations helps to avoid bias in the selection and weighting of the evidence. As of June 2014, the National Guideline Clearinghouse (NGC) adopted the new IOM definition and only CPGs that are based on a systematic review are included in the NGC.[14] Additionally, the NGC requires that the systematic reviews supporting the CPGs must be updated at a minimum interval of every 5 years to be considered current.[14]
The Children’s Oncology Group (COG) recognizes the value of rigorously developed pediatric oncology supportive care CPGs. The COG Supportive Care Guidelines sub-Committee was created with the goal of identifying and evaluating supportive care CPGs for endorsement to help standardize supportive care recommendations in treatment protocols. The sub-Committee does not develop CPGs. The endorsement review process incorporates evaluation by committee members and COG stakeholders.[15] Links to endorsed supportive care CPGs are provided in COG protocols. In addition, information regarding COG supportive care endorsed CPGs, including COG-developed summaries, are available on an open-access website (https://childrensoncologygroup.org/index.php/cog-supportive-care-endorsed-guidelines). To identify current CPGs related to any supportive care topic relevant to pediatric oncology or hematopoietic stem cell transplant (HSCT) recipients undergoing active treatment or follow-up (i.e. excluding survivorship care), the committee conducted an initial systematic review which is now updated monthly. The purpose of this report is to present the results of this systematic review.
Methods
Searches of MEDLINE, MEDLINE in-Process, Embase and PubMed databases were performed with the assistance of a library scientist for articles indexed between January 2012 (since older CPGs would now be considered out-dated, as per NGC criteria) and January 2018. The complete search strategies and dates are provided in Supplemental Appendix 1. The eligibility criteria were defined a priori. Papers were included if they: (1) were fully published in English in 2012 or more recently (i.e. would be considered current according to NGC criteria); (2) were identified as a guideline by the authors of the paper; (3) addressed a supportive care topic; (4) were relevant to pediatric cancer or pediatric HSCT; (5) were intended for patients undergoing active therapy or follow-up care (i.e. excluded those specific to survivorship care); (6) were intended for use in economically developed countries (as indicated by the UN economic country classification 2012)[16]; and (7) met the NGC criteria for being based on a systematic review as outlined in Table II.
TABLE II.
NGC systematic review criteria [14]
| Required elements |
|---|
| 1. An explicit statement that the clinical practice guideline was based on a systematic review |
| 2. A description of the search strategy that includes a listing of database(s) searched |
| 3. A summary of search terms used |
| 4. The specific time period covered by the literature search including the beginning date and end date (month and year) |
| 5. A summary of inclusion and exclusion criteria |
| 6. The number of studies identified |
| 7. The number of studies included |
| 8. A synthesis of evidence from the selected studies, e.g., a detailed description or evidence tables |
| 9. A summary of the evidence synthesis (see 8. above) included in the guideline that relates the evidence to the recommendations, e.g., a descriptive summary or summary tables |
Two authors (JS, PDR) independently screened the citations identified by the electronic database searches. Any citation identified by either author as potentially relevant was retrieved for full text evaluation. For full-text screening, the eligibility criteria were applied using a two-step process. First, all papers that met inclusion criteria 1–6 above were identified. Next, these papers were further assessed to determine whether they met each element of the NGC systematic review criteria (Table II). This process was again conducted independently by two authors (JS, PR). A third author (LD) reviewed any discrepancies for final consensus. An NGC element did not need to be present in the original publication, but it needed to be indicated as available in a supplement or as available upon request. The patient population for whom the CPG was developed (i.e. pediatric-specific or both adult and pediatric) was also assessed.
Agreement of inclusion between reviewers (JS, PR) was assessed using the kappa statistic. Agreement was defined as (0.00 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), substantial (0.61 to 0.80) or almost perfect (0.81 to 1.00).[17]
Results
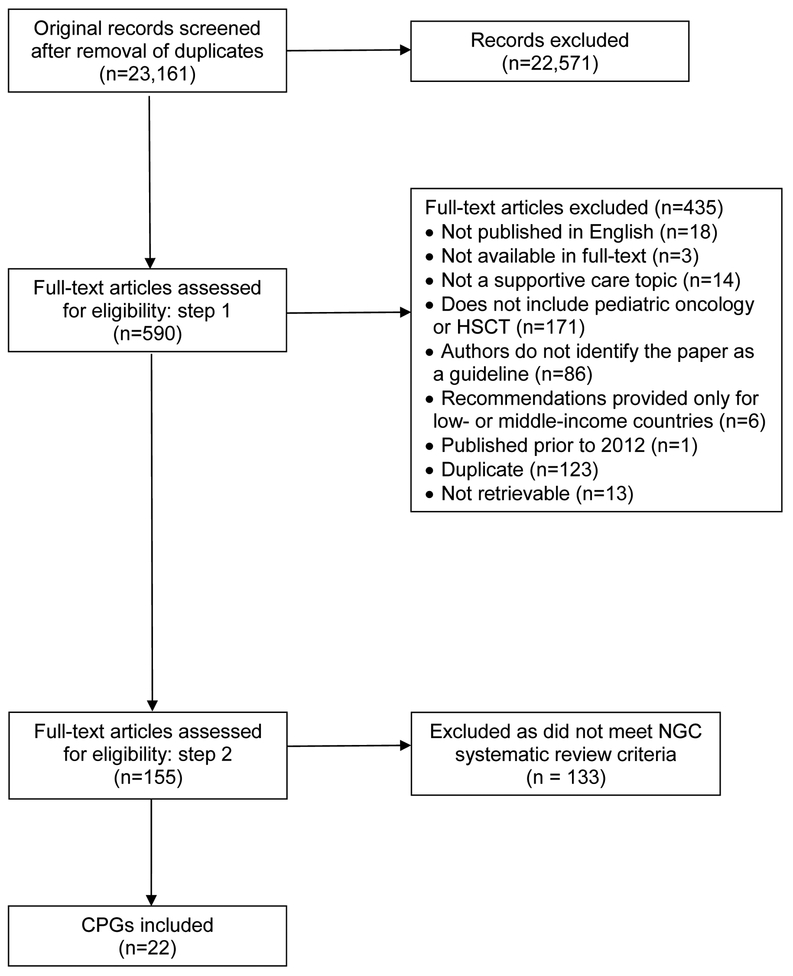
The search strategy identified 23,161 unique citations, of which 590 were retrieved for full-text evaluation. Of these, 155 provided current supportive care focused recommendations relevant to pediatric oncology or HSCT. However, after applying the NGC systematic review eligibility criteria (Table II), only 22/155 (14.2%) met the full criteria for inclusion (Figure 1). Inter-rater agreement was almost perfect (kappa=0.98, CI 0.93–1.00).
Figure 1.
Flowchart of paper identification and selection
Table III illustrates the number of elements from the NGC systematic review criteria that were missing in the 133 papers excluded for this reason. Of these, 7/133 (5.3%) were missing only a single element. However, 75.2% (100/133) were missing five or more of the nine NGC elements (Table III). Table IV lists the frequency with which specific NGC criteria were missing and 73.7% (98/133) did not make a statement indicating that a systematic review had been conducted. The most commonly omitted element was a summary of the eligibility criteria which was either missing or was not reported in sufficient detail to be evaluated or reproduced in 88.7% (118/133).
TABLE III.
Number of NGC elements missing from papers excluded for not meeting NGC systematic review criteria
| Number of NGC systematic review elements missing | Number of papers excluded N=133 |
|---|---|
| 1 | 7 (5.3%) |
| 2 | 6 (4.5%) |
| 3 | 6 (4.5%) |
| 4 | 14 (10.5%) |
| 5 | 11 (8.3%) |
| 6 | 16 (12.0%) |
| 7 | 25 (18.8%) |
| 8 | 17 (12.8%) |
| 9 | 31 (23.3%) |
TABLE IV.
Frequency of specific elements missing from papers excluded for not meeting NGC systematic review criteria (n=133)
| Specific NGC systematic review element | Number of excluded papers missing the element (N=133) |
|---|---|
| States based on systematic review | 98 (73.7%) |
| Databases searched | 53 (39.8%) |
| Search terms | 103 (77.4%) |
| Specific time period | 84 (63.2%) |
| Number of studies screened | 107 (80.5%) |
| Number studies included | 102 (76.7%) |
| Eligibility criteria | 118 (88.7%) |
| Evidence tables | 107 (80.5%) |
| Recommendations linked to evidence synthesis | 62 (46.6%) |
Of the 22 papers which met eligibility criteria, 13 were specific to the pediatric population.[18–29] The remaining nine CPGs included recommendations for both adult and pediatric patients (Table V). Almost one-third (7/22) of the included CPGs were focused on various aspects of chemotherapy-induced nausea and vomiting (CINV), of which five made exclusively pediatric recommendations.[18–22] Three pediatric-specific CPGs focused on different psychosocial aspects of care.[23–25] There were also two CPGs which addressed fever and neutropenia exclusively in the pediatric oncology and HSCT population.[26,27] These were published by the same guideline group first as an original guideline in 2012 and then as an update in 2017. The other pediatric-specific CPGs addressed: antifungal prophylaxis,[28] prevention of mucositis[30] and prevention of Pneumocytis jirovecii pneumonia in children with solid tumours.[29]
Table V.
Characteristics of included supportive care CPGs (n=22)
| Supportive Care Topic | Sub-Topic Focus | Number of CPGs | |
|---|---|---|---|
| Pediatric-Specific (year published) | Combined Adult/Pediatric(year published) | ||
| Chemotherapy-induced nausea and vomiting | Anticipatory[18,35] | 1 (2014) | 1 (2016) |
| Acute[19,21,22,36] | 3 (2013, 2017, 2017) | 1 (2017) | |
| Breakthrough/Refractory[20] | 1 (2016) | ||
| Hematology | Myeloid growth factor[37] | 1 (2015) | |
| Platelet transfusion[38] | 1 (2015) | ||
| Fungal infection | Prophylaxis[28] | 1 (2014) | |
| Mucositis | Prophylaxis[30] | 1 (2017) | |
| Psychosocial care | Parent support[24] | 1 (2015) | |
| Medication adherence[25] | 1 (2015) | ||
| Financial burden[24] | 1 (2015) | ||
| Fever and neutropenia[26,27] | 2 (2012, 2017) | ||
| Fertility preservation[39] | 1 (2013) | ||
| Central venous catheter care[40,41] | 2 (2013, 2018) | ||
| Graft-versus-host disease treatment | Photopheresis[42] | 1 (2014) | |
| Genetic testing | Cardiotoxictiy[43] | 1 (2016) | |
| Pneumocytis pneumonia | Prevention[29] | 1 (2017) | |
Discussion
The results of this systematic review highlight both the paucity of current supportive care CPGs available to healthcare professionals caring for children with cancer or pediatric HSCT recipients and the pressing need for the development of CPGs that meet current methodological standards. Given that 133 papers were identified that provided current supportive care focused recommendations for clinical care relevant to pediatric oncology or HSCT but did not meet NGC criteria, it appears many guideline developers have not yet updated their development processes to reflect the key requirement of conducting a systematic review as outlined by the IOM seven years ago.
While we have endeavoured to ensure that all current supportive care CPGs relevant to pediatric oncology or HSCT have been identified, we recognize that there are limitations to our approach. In particular, we chose to focus only on papers published in peer-reviewed journals and we did not search grey literature sources such as medical specialty association websites. In addition, while a very broad search literature strategy was used, it was cancer-focused, which raises the possibility of missing potentially relevant supportive care CPGs that are not cancer-specific, if they do not have indexing or text words related to cancer. Further, we did not contact the authors of the 133 papers excluded for not meeting NGC criteria. It is possible that one or more of these papers may not have fully documented their methods in the main text or supplements although none stated that additional information regarding their methods was available upon request. It is possible that one or more of the excluded papers may have met NGC criteria. Meeting the IOM definition of a CPG, as operationalized by NGC, is the first of several important characteristics of CPGs that should be considered prior to endorsing or using a CPG in practice. Tools that can facilitate the evaluation of CPGs are available to help highlight strengths, weaknesses and areas of potential concern. A commonly used tool is the Appraisal of Guidelines for Research and Evaluation (AGREE II) which evaluates CPGs on the basis of 6 domains, including: (1) Scope and Purpose; (2) Stakeholder Involvement; (3) Rigour of Development; (4) Clarity of Presentation; (5) Applicability; and (6) Editorial Independence.[31] Guideline developers who create CPGs with these domains in mind can greatly improve the quality of their CPG and the likelihood that its recommendations are implemented – the ultimate goal of every CPG effort.
Similarly, since CPG development is a time-consuming and resource-intensive process, it is important that guideline developers work collaboratively to avoid duplication of efforts in an already underserved field. Guideline development groups must be aware of which topic areas of supportive care in pediatric oncology/HSCT are deemed important by stakeholders, which topics have already been well addressed and which areas are lacking in CPGs. In 2015, Loeffen et al. used a Delphi survey to identify and prioritize relevant childhood cancer supportive care topics in the Netherlands from the perspective of health care professionals.[32] The ten topics of highest priority for CPG development were: infection, sepsis, fever and neutropenia, pain, nausea/vomiting, restrictions in daily life and activities, palliative care, procedural sedation, terminal care and oral mucositis.[32] Currently, there are up-to-date pediatric CPGs in only four of these ten topics areas. Information regarding COG-endorsed CPGs, including COG-developed summaries, links to the CPGs and implementation tools, are available on the open-access COG Supportive Care Endorsed Guidelines website (https://childrensoncologygroup.org/index.php/cog-supportive-care-endorsed-guidelines).
With open communication between collaborative groups and an awareness of which supportive care topics have been addressed, duplication of efforts may be prevented. An example of such a collaborative group is the international Pediatric Oncology Guidelines in Supportive Care (iPOG)[33] Network which was established by representatives of several pediatric oncology organizations with an interest in pediatric supportive care CPG identification, development and dissemination. The iPOG Network does not develop CPGs, rather, iPOG Network members collaborate, identify research gaps, and provide guidance and updates about ongoing CPGs being developed by their respective organizations in North America and Europe.[33,34] These efforts result in collaboration around the CPG development process, international planning of high priority topics and collegial support among guideline developers.
In summary, the results of this systematic review highlight a substantial need for pediatric-focused supportive care CPGs in oncology and HSCT that meet the current methodologic standards for guideline development. Given the potential for supportive care CPGs to improve patient outcomes, it is imperative to prioritize the development and implementation of supportive care CPGs that are created using robust and transparent methods.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institutes of Health to the Children’s Oncology Group (U10CA098543) and The National Clinical Trials Network (NCTN) Operations Center Grant (U10CA180886) and by the NCI Community Oncology Research Program (NCORP) Research Base (UG1CA189955). This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations
- AGREE II
Appraisal of Guidelines for Research and Evaluation
- CINV
Chemotherapy-induced Nausea and Vomiting
- COG
Children’s Oncology Group
- CPG
Clinical Practice Guideline
- HSCT
Hematopoietic Stem Cell Transplantation
- IOM
Institute of Medicine
- iPOG
international Pediatric Oncology Guidelines in Supportive Care Network
- NGC
National Guideline Clearinghouse
Footnotes
Conflict of Interest Statement
LS, JLR, JT, LLD and PDR are authors on one or more guideline identified by this systematic review. Individuals with conflicts of interest do not participate in the COG guideline endorsement review process for the guideline under review.
References
- 1.Hudson MM, Link MP, Simone JV. Milestones in the curability of pediatric cancers. J Clin Oncol 2014:32(23):2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 2012:30(14):1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggott C, Dodd M, Kennedy C, et al. Multiple symptoms in pediatric oncology patients: a systematic review. J Pediatr Oncol Nurs 2009:26(6): 325–339. [DOI] [PubMed] [Google Scholar]
- 4.Johnston DL, Hyslop S, Tomlinson D, et al. Describing symptoms using the Symptom Screening in Pediatrics Tool in hospitalized children with cancer and hematopoietic stem cell transplant recipients. Cancer Med 2018:7(5):1750–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupuis LL, Johnston DL, Baggott C, et al. Validation of the Symptom Screening in Pediatrics Tool in Children Receiving Cancer Treatments. J Natl Cancer Inst 2018:110(6):661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 2012:23(8):1986–1992. [DOI] [PubMed] [Google Scholar]
- 7.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010:38(2):367–374. [DOI] [PubMed] [Google Scholar]
- 8.Dexheimer JW, Borycki EM, Chiu KW, et al. A systematic review of the implementation and impact of asthma protocols. BMC Med Inform Decis Mak 2014:14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inwald EC, Ortmann O, Zeman F, et al. Guideline concordant therapy prolongs survival in HER2-positive breast cancer patients: results from a large population-based cohort of a cancer registry. BioMed research international 2014:2014:137304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA Intern Med 2013:173(7):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IOM (Institute of Medicine). Clinical Practice Guidelines We Can Trust. Washington, D.C.: The National Academies Press; 2011. [Google Scholar]
- 12.Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines In: Field MJ, Lohr KN, editors. Clinical Practice Guidelines: Directions for a New Program. Washington DC: National Academy of Sciences.; 1990. [PubMed] [Google Scholar]
- 13.IOM (Institute of Medicine). Finding What Works in Health Care: Standards for Systematic Reviews. Washington DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 14.National Guideline Clearinghouse. 2018. July 18 <https://www.ahrq.gov/gam/summaries/inclusion-criteria/index.html>. Accessed 2018 July 18.
- 15.Children’s Oncology Group. 2018. February 21 COG Supportive Care Endorsed Guidelines <https://www.childrensoncologygroup.org/index.php/cog-supportive-care-endorsed-guidelines>. Accessed 2018 Feb 21.
- 16.United Nations. 2012. February 21 World Economic Situation and Prospects 2012. United Nations; <http://www.un.org/en/development/desa/policy/wesp/wesp_archive/2012wesp.pdf>. Accessed 2018 Feb 21. [Google Scholar]
- 17.Koch GG, Landis JR, Freeman JL, et al. A general methodology for the analysis of experiments with repeated measurement of categorical data. Biometrics 1977:33(1):133–158. [PubMed] [Google Scholar]
- 18.Dupuis LL, Robinson PD, Boodhan S, et al. Guideline for the prevention and treatment of anticipatory nausea and vomiting due to chemotherapy in pediatric cancer patients. Pediatr Blood Cancer 2014:61(8):1506–1512. [DOI] [PubMed] [Google Scholar]
- 19.Dupuis LL, Boodhan S, Holdsworth M, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer 2013:60(7):1073–1082. [DOI] [PubMed] [Google Scholar]
- 20.Flank J, Robinson PD, Holdsworth M, et al. Guideline for the Treatment of Breakthrough and the Prevention of Refractory Chemotherapy-Induced Nausea and Vomiting in Children With Cancer . Pediatr Blood Cancer 2016:63(7):1144–1151. [DOI] [PubMed] [Google Scholar]
- 21.Dupuis LL, Sung L, Molassiotis A, et al. 2016 updated MASCC/ESMO consensus recommendations: Prevention of acute chemotherapy-induced nausea and vomiting in children. Support Care Cancer 2017:25(1):323–331. [DOI] [PubMed] [Google Scholar]
- 22.Patel P, Robinson PD, Thackray J, et al. Guideline for the prevention of acute chemotherapy-induced nausea and vomiting in pediatric cancer patients: A focused update. Pediatr Blood Cancer 2017. [DOI] [PubMed] [Google Scholar]
- 23.Kearney JA, Salley CG, Muriel AC. Standards of Psychosocial Care for Parents of Children With Cancer. Pediatr Blood Cancer 2015:62 Suppl 5:S632–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelletier W, Bona K. Assessment of Financial Burden as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer 2015:62 Suppl 5:S619–631. [DOI] [PubMed] [Google Scholar]
- 25.Pai ALH, McGrady ME. Assessing Medication Adherence as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer 2015:62:S696–S706. [DOI] [PubMed] [Google Scholar]
- 26.Lehrnbecher T, Phillips R, Alexander S, et al. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol 2012:30(35):4427–4438. [DOI] [PubMed] [Google Scholar]
- 27.Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J Clin Oncol 2017:35(18):2082–2094. [DOI] [PubMed] [Google Scholar]
- 28.Science M, Robinson PD, MacDonald T, et al. Guideline for primary antifungal prophylaxis for pediatric patients with cancer or hematopoietic stem cell transplant recipients. Pediatr Blood Cancer 2014:61(3):393–400. [DOI] [PubMed] [Google Scholar]
- 29.Proudfoot R, Phillips B, Wilne S. Guidelines for the Prophylaxis of Pneumocystis jirovecii Pneumonia (PJP) in Children with Solid Tumors. Journal of Pediatric Hematology/Oncology 2017:39(3):194–202. [DOI] [PubMed] [Google Scholar]
- 30.Sung L, Robinson P, Treister N, et al. Guideline for the prevention of oral and oropharyngeal mucositis in children receiving treatment for cancer or undergoing haematopoietic stem cell transplantation. BMJ support 2017:7(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol 2010:63(12):1308–1311. [DOI] [PubMed] [Google Scholar]
- 32.Loeffen EA, Mulder RL, Kremer LC, et al. Development of clinical practice guidelines for supportive care in childhood cancer--prioritization of topics using a Delphi approach. Support Care Cancer 2015:23(7):1987–1995. [DOI] [PubMed] [Google Scholar]
- 33.International Pediatric Oncology Guidelines in Supportive Care Network (iPOG Network). February 21 <http://www.sickkids.ca/Research/iPOG/index.html>. Accessed 2018 Feb 21.
- 34.Loeffen EA, Kremer LC, Mulder RL, et al. The importance of evidence-based supportive care practice guidelines in childhood cancer-a plea for their development and implementation. Support Care Cancer 2017:25(4):1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupuis LL, Roscoe JA, Olver I, et al. 2016 updated MASCC/ESMO consensus recommendations: Anticipatory nausea and vomiting in children and adults receiving chemotherapy. Support Care Cancer 2017:25(1):317–321. [DOI] [PubMed] [Google Scholar]
- 36.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update . J Clin Oncol 2017:35(28):3240–3261. [DOI] [PubMed] [Google Scholar]
- 37.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update . J Clin Oncol 2015:33(28):3199–3212. [DOI] [PubMed] [Google Scholar]
- 38.Nahirniak S, Slichter SJ, Tanael S, et al. Guidance on Platelet Transfusion for Patients With Hypoproliferative Thrombocytopenia. Transfusion Medicine Reviews 2015:29(1):3–13. [DOI] [PubMed] [Google Scholar]
- 39.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update . J Clin Oncol 2013:31(19):2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiffer CA, Bohlke K, Delaney M, et al. Platelet Transfusion for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update . J Clin Oncol 2018:36(3):283–299. [DOI] [PubMed] [Google Scholar]
- 41.Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline . J Clin Oncol 2013:31(10):1357–1370. [DOI] [PubMed] [Google Scholar]
- 42.Bredeson C, Rumble RB, Varela NP, et al. Extracorporeal photopheresis in the management of graftversus-host disease. Current Oncology 2014:21(2):e310–e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aminkeng F, Ross CJ, Rassekh SR, et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol 2016:82(3):683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.