Abstract
Objective:
To empirically identify subgroups of patients with obesity and investigate their association with post-operative weight change.
Methods:
A longitudinal analysis of 2458 adults in the Longitudinal Study of Bariatric Surgery (LABS) study. Baseline data was used to identify subgroups. The outcome was three-year weight change after bariatric surgery.
Results:
We identified 4 classes (subtypes) of obesity which could be characterized as diabetes with low HDL (Class 1), disordered eating (Class 2), mixed (class 3), and extreme obesity with early onset (Class 4). Approximately 98% of participants in class 1 had diabetes vs. < 40% in the other classes. There were high rates of binge eating in class 2 and more than 92% of those in this class reported eating when not hungry. Class 4 was characterized by a higher BMI at baseline. Adults in Class 4 lost an average of 25.0% (males) – 30.3% (females) of their baseline weight over three years. In contrast to participants in Class 1, those in Classes 2 and 3 had significantly larger 3-year weight losses than their peers in Class 4.
Conclusions:
Obesity is a heterogeneous disease. Bariatric surgery may be most beneficial for adults with disordered eating.
Keywords: bariatric surgery, observational, prospective, weight change
INTRODUCTION
Relatively few people are able to lose weight and maintain weight loss over long periods of time.1–3 Despite being the most effective weight loss method, there is considerable variability in weight loss following bariatric surgery. Weight loss is greater with Roux-en Y gastric bypass than gastric banding, but even among patients undergoing the same type of procedure, there are different patterns of weight change.4,5 Within the Longitudinal Assessment of Bariatric Surgery (LABS) study, one group started regaining weight after 6 months and by 3 years weighed, on average, only 10% less than their baseline weight. In contrast, participants in two other groups continued to lose weight through year two.5 The results suggest that obesity is a heterogeneous disease and that research may be hampered by considering all people above a set BMI cut-off to have a homogeneous disease and expecting equal success from all in response to a given treatment. By better understanding subtypes of obesity, we might be able to move closer to a “precision medicine” model of obesity treatment.
One obesity subtype that has been suggested is binge eating disorder (BED).6 BED is characterized by at least weekly episodes of binge eating, involving consuming a very large amount of food, larger than most people would eat in a similar circumstance, and feeling a loss of control (LOC) over the episode. Unlike bulimia nervosa, people with BED do not engage in frequent attempts to counteract the high caloric intake of the binge by using vomiting, laxatives, or other unhealthy methods. Food addiction is a related and more controversial construct that also involves compulsive eating. Binge eating, food addiction, and night eating syndrome are all prevalent among patients seeking bariatric surgery,7 and if these behavioral traits remain active postoperatively, may negatively impact weight loss durability. LABS reported 17.7% of participants had night eating syndrome, 43.4% reported LOC eating, and 15.7% met criteria for BED.7 Those with BED were more likely to have impaired glucose levels, but less likely to have cardiovascular disease.8
We have suggested that there are likely at least five obesity subtypes,9 but to date there have not been large studies focused on identifying subtypes of obesity. Eating behaviors and social and environmental risk factors for obesity may cluster together and have strong associations with dietary intake and physical activity patterns.10,11 This suggests that examining groupings of obesity-related traits, behaviors, and risk factors, rather than considering them individually, could permit a better understanding of obesity pathogenesis.
Latent class analysis (LCA) is an analytic approach that classifies people into mutually-exclusive categories based on their patterns of response to the exposures or outcomes of interest and has been helpful for identifying some possible subtypes of obesity. Several latent class analyses considering appetitive behaviors, eating behavior, and/or physical activity have observed evidence of multiple subtypes of obesity.10,12,13 No large studies of severely obese patients have attempted to identify obesity subtypes using a broad range of psychological, behavioral, and biological parameters. The goal of this project was to use data on appetitive behaviors, disordered eating, family history of obesity, and markers of cardiovascular health and hormones related to glucose metabolism to identify subtypes of obesity among adults enrolled in the LABS study and to examine if these empirically derived subgroups of obesity differ in their three year weight change post bariatric surgery.
METHODS
Participants
The LABS Consortium was formed, in part, to acquire long-term data on the safety, efficacy, and durability of bariatric surgical procedures currently performed in the United States using standardized data collection practices. LABS participants were at least 18 years old and underwent first-time bariatric procedures by a surgeon participating in the LABS consortium at one of 10 hospitals at six geographically diverse clinical centers in the United States (New York, NY; Pittsburgh, PA; Seattle, WA; Fargo, ND; Greenville, NC; and Portland, OR) between March 2006 and April 2009. The institutional review boards at each center approved the protocol and consent forms and all study participants provided written consent. LABS is registered at ClinicalTrials.gov (NCT00465829). .
Of those consenting, 2,458 participants completed a baseline research visit within 30 days prior to surgery. After their surgery, follow-up occurred 30 days, 6 months, 1 year and annually thereafter up to 7 years. For this analysis, 2 people were excluded due to biologically implausible fasting insulin values (>971 mU/ml), thus records from 2456 participants remained for the analysis.
Assessments and Outcome Measures
Weight and weight change
Weight was measured by LABS-certified trained personnel within 30 days of surgery using a standard protocol on a study-purchased Tanita® Body Composition Analyzer (model TBF-310) scale. 14 If a protocol weight was not obtained, weight was measured by research or medical personnel on a non-study scale and is referred to as a “clinical weight”. If neither a protocol nor clinical weight was available, a patient self-reported weight was used. Differences between measured and self-reported weights in this cohort were small, and did not systematically differ by measured body mass index (BMI) or degree of postoperative weight change. The average degree of under-reporting by self-report was 0.7 kg for women and 1.0 kg for men.20 At baseline BMI was calculated from measured weight and height. BMI at age 18 was recalled by participants using the weight history questionnaire.15,16 Participants also used silhouettes to reported the weight status of their parents.17 Weight was measured annually using the procedure described above. Weight change was modeled as the difference between weight at baseline and three months after surgery.
Dream and disappointed weight
Participants were asked to report what they considered their “dream weight” (after weight loss surgery), as well as their “happy weight”, “acceptable weight” and “unhappy weight”. We converted these into dream and disappointed BMI.
Eating for reasons other than hunger
Participants were asked how often they ate when not hungry and how often they ate despite feeling full. The responses to each question were “rarely or never,” “occasionally,” “frequently,” and “nearly every day.” Considering small numbers in each category, for comparing the subtypes these variables were dichotomized into “frequently” or “nearly every day” vs. “rarely or never” or “occasionally”, however, in the main LCA analysis they were not collapsed.
Loss of control and binge eating
Two questions were used to assess grazing with loss of control (LOC): “During the past six months have you had periods of time where you ate continuously during the day or parts of the day without planning what and how much you would eat?” and “did you experience a loss of control: that is you felt like you could not control your eating?” . Participants had to endorse both questions to be classified as engaging in grazing with LOC. Binge eating disorder was assessed with the Questionnaire of Eating and Weight Patterns – Revised.18 Participants who reported weekly episodes in the last 6 months of eating what other would consider to be a very large amount of food in a short amount of time and feeling out of control during the episode were considered to be binge eaters. Participants who engaged in at least weekly binge eating and at least three of the following were considered to have binge eating disorder: eating much more rapidly than usual, eating until uncomfortably full, eating a large amount when not hungry, eating alone because they would be embarrassed by how much they were eating, and/or feeling disgusted with oneself or depressed or guilty after eating. Night eating syndrome is characterized by consuming at least 25% of daily caloric intake after dinner or after waking from sleep and morning insomnia. We classified participants as engaging in night eating if they reported consuming at least half of their daily food intake after suppertime or if they reported snacking on at least half of the occasions when they got up in the middle of the night. Questions on night eating came from a Night Eating Questionnaire.19
Drugs and alcohol
Smoking status and use of drugs were assessed at the pre-operative visit. In addition, alcohol use was assessed with the Alcohol Use Disorders Identification Test (AUDIT), which is a validated tool to identify unhealthy drinking behaviors and alcohol use disorders.
Biological measures
Markers of cardiovascular risk health (high density lipoprotein (HDL), low density lipoprotein (LDL), high sensitivity C reactive Protein (hsCRP), and triglyceride levels), glucose and hormones related to glucose metabolism (leptin, ghrelin, and insulin) were measured from a fasting blood sample taken at baseline.
Statistical Analysis
We conducted a latent class analysis (LCA) to identify subtypes of obesity. LCA starts with random split of participants into classes, reclassifies based on an improvement criterion (fit criterion) until the best classification is found. LCA accounts for clustering (repeated measurements), as well as incomplete (missing) data under missing at random (MAR) assumptions, with estimation conducted via the Expectation-Maximization (EM) algorithm.21
Model fit was evaluated using the Bayesian Information Criterion (BIC),22 the sample-size adjusted BIC (aBIC),23; the Consistent Akaike Information Criterion (cAIC),24 and minimum class sizes, and entropy were used to guide the optimal number of classes, with particular emphasis on minimizing cAIC while maintaining class sizes of at least 25.25 We characterize each of the classes obtained using LCA by features common among its members that distinguish them from other classes. Because the distributions were skewed, we presented medians and interquartile ranges rather than means and standard deviations. Diabetes and other possible comorbidities were not included in the LCA. Instead we examined the class-specific prevalence of comorbidities after conducting the LCA.
Mixed effects models (using Proc Mixed) were used to examine prospective associations between latent classes and percent weight change over three years. Models adjusted for surgical group (Roux-en-Y or gastric bypass), weight at baseline, age, and interactions between age and surgical group and baseline weight, and the interaction between baseline weight and surgical group. Analyses were stratified by gender because the interaction between latent class and gender was significant.
RESULTS
The median BMI of the 2456 adults in the analysis was 45.9 kg/m2. Participants ranged in age from 18 to 78 years. We identified four distinct classes within the LABS subjects. Although the model fit was slightly better with a 5-class model (AIC=239166.8, BIC=240188.7, and sample adjusted BIC=239630), one of the classes had only 20 people. Therefore, we selected the 4-class model (AIC=240277, BIC=241108, and sample adjusted BIC=240653). The classes ranged in size from 91 people (Class 1) to 1108 people (Class 3). The median age was oldest in Class 1 (49 years) and lowest in Class 4 (43 years). The smallest percentages of females were in Classes 1 (63.7%) and 4 (65.5%) (Table 1). The median baseline BMI in Class 4 was substantially higher (58.3 kg/m2) than in the other classes; however, the prevalence of diabetes was much lower in Class 4 (38.5%) than Class 1 (97.8%).
Table 1.
Baseline demographic factorsa within the 4 classes identified in the Longitudinal Assessment of Bariatric Surgery (LABS) Study
| Class 1 (Diabetes) (N=91) |
Class 2 (Disordered Eating) (N=892) |
Class 3 (Mixed) (N=1108) |
Class 4 (Early onset) (N=365) |
|
|---|---|---|---|---|
| Age (Years) | 49.0 (40.0–57.0) |
46.0 (37.0–54.0) |
47.0 (37.0–55.0) |
43.0 (35.0–52.0) |
| Gender (female) | 63.7% | 83.4% | 80.2% | 65.5% |
| BMI (kg/m2) | 45.5 (40.4–49.2) |
44.6 (41.1–48.7) |
44.7 (41.3–48.7) |
58.3 (54.1–63.3) |
| Type 2 diabetes | 97.8% | 27.1% | 31.2% | 38.5% |
Continuous variables are characterized by median (25th–75th percentile). Categorical variables are characterized by percentages
The four classes could be described as diabetes with low HDL (Class 1), disordered eating (Class 2), mixed (Class 3), and extreme obesity with an early onset16 (Class 4). As shown in Table 2, Class 2 was characterized by high percentages of participants having grazing with LOC (60.8%), BED (36.6%), night eating (26.0%), often eating when not hungry (92.4%), and often eating when full (73.9%). In contrast, smaller percentages of Class 3 participants had those behaviors and disorders. Less than 2% reported BED, 7.2% reported often eating when not hungry, and only 0.1% reported often eating when full.
Table 2.
Eating patterns a within classes in the LABS study
| Class 1 (Diabetes) (N=91) |
Class 2 (Disordered eating) (N=892) |
Class 3 (Mixed) (N=1108) |
Class 4 (Early onset) (N=365) |
|
|---|---|---|---|---|
| Grazing with LOC | 41.7% | 60.8% | 19.1% | 27.2% |
| Binge Eating Disorder | 19.8% | 36.6% | 1.9% | 10.2% |
| Night eating | 14.1% | 26.0% | 12.1% | 14.0% |
| Often eat when not hungry | 37.0% | 92.4% | 7.2% | 29.3% |
| Often eat when full | 31.7% | 73.9% | 0.1% | 18.8% |
| >4 meals and snacks per day | 32.9% | 52.9% | 41.2% | 41.8% |
Missing: loss of control n=221; binge eating disorder n=233, night eating syndrome n=672; often eat when not hungry n=214; often eat when full n=204; meals and snacks per day n=249.
Although the median BMI was much higher in Class 4 than the other classes, it was Class 1 that was characterized as having elevated glucose levels and triglycerides, but the lowest HDL levels of the four classes (Table 3). Class 4 had the lowest median ghrelin levels. The pattern was consistent with the higher weights of the participants in Class 4, who had a higher median baseline BMI (58.3 kg/2). They also had a higher BMI at age 18 and a dream BMI in the overweight range (Table 4).
Table 3.
Baselinea glucose, hormones related to glucose metabolism, and markers of cardiovascular health b across classes in the LABS study
| Class 1 (Diabetes) (N=91) |
Class 2 (Disordered eating) (N=892) |
Class 3 (Mixed) (N=1108) |
Class 4 (Early onset) (N=365) |
|
|---|---|---|---|---|
| Glucose (mg/dl) | 246.5 (217–309) |
97 (89–110) |
98 (89–112) |
97 (89–111) |
| Fasting insulin (mU/ml) |
26.1 (17.4–43.3) |
18.5 (12.1–28.7) |
18.5 (12.8–28.7) |
23.6 (16.4–33.9) |
| Ghrelin (pg/ml) | 677.8 (580.6–819.9) |
745.3 (633.1–916.4) |
732.0 (611.2–890.1) |
622.4 (551.0–736.2) |
| Leptin (ng/ml) | 39.0 (26.8–52.8) |
56.8 (43.2–71.4) |
54.2 (41.4–72.0) |
71.6 (54.6–89.0) |
| Triglycerides (mg/dl) |
249.0 (167.0–363.0) |
145.5 (104.0–197.0) |
133.0 (97.0–186.0) |
127.0 (92.0–170.0) |
| LDL (mg/dl) | 107.5 (73.0–132.0) |
114.0 (90.0–136.0) |
108.0 (85.0–131.0) |
100.5 (80.5–121.0) |
| HDL (mg/dl) | 37.0 (32.0–45.0) |
44.0 (38.0–53.0) |
43.0 (36.0–51.0) |
40.0 (35.0–47.0) |
| hsCRP (mg/dl) | 0.8 (0.5–1.4) |
0.7 (0.4–1.2) |
0.7 (0.3–1.2) |
0.9 (0.6–1.3) |
Median (25th–75th percentile).
Missing: glucose n=453; insulin n=444; ghrelin n=473; triglycerides n=442; LDL n=432; HDL n=101; CRP n=444
Table 4.
Distributiona of current, late adolescent, dream, and unacceptable BMI (kg/m2) and parental weight statusb within classes in the LABS study
| Class 1 (Diabetes) |
Class 2 (Disordered eating) |
Class 3 (Mixed) |
Class 4 (Early onset) |
|
|---|---|---|---|---|
| Pre-surgery BMI | 45.5 (40.4–49.2) |
44.6 (41.1–48.7) |
44.7 (41.3–48.7) |
58.3 (54.1–63.3) |
| BMI at age 18 | 25.8 (23.2–31.2) |
25.0 (21.9–29.1) |
25.6 (22.0–30.0) |
32.0 (28.1–39.5) |
| Dream BMI | 24.2 (22.4–25.8) |
22.8 (21.5–24.4) |
23.3 (21.6–25.0) |
26.5 (24.2–29.4) |
| Unacceptable BMI | 34.9 (31.3–37.4) |
32.3 (30.0–35.4) |
33.3 (30.4–35.9) |
43.9 (40.4–48.3) |
| Overweight Mother | 27.1% | 29.0% | 30.7% | 37.4% |
| Overweight Father | 35.0% | 37.8% | 32.2% | 40.5% |
Continuous variables are characterized by median (25th–75th percentile). Categorical variables are characterized by percentages.
Missing: BMI at age 18 n=921; dream BMI n=190; unacceptable BMI n=219; maternal weight status n=190; paternal weight status n=296
Obesity class was predictive of three-year weight change (Figure 1a–d). Adults in Class 4 weighed an average of 25.0% (males) – 30.3% (females) less than of their baseline weight at three years post-bariatric surgery. Participants in Classes 2 (Males: −3.41% 95% confidence interval (CI) −5.03- −1.79, Females: −3.00% 95%CI −4.01 – −1.99) and 3 (Males: −3.05% 95% CI −4.58- −1.51, Females: −1.97% 95%CI −2.96 – −0.97) had significantly larger 3-year weight losses than their peers in Class 4. Weight losses of adults in Class 1 did not significantly differ from Class 4 (Table 5).
Figure 1a.
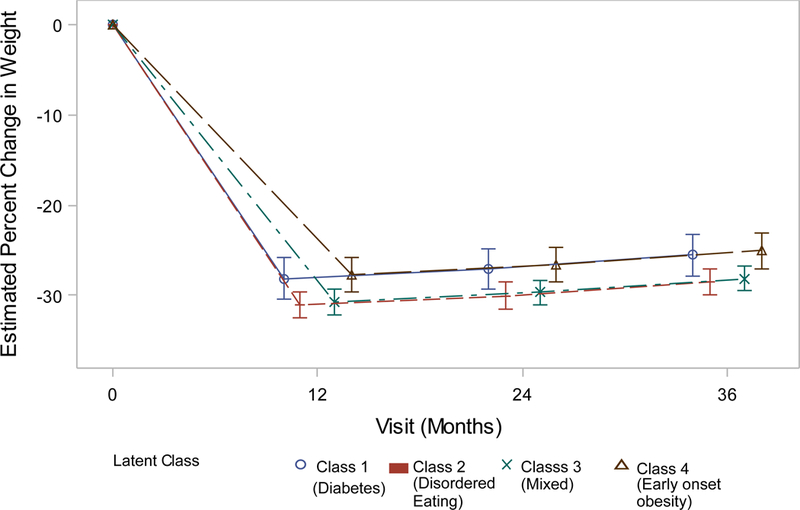
Weight change (%) patterns by obesity class among males in the LABS study who underwent Roux-en-Y Bypass surgery.
Table 5.
Prospective association between obesity class and 3-year weight change (%) after bariatric surgery among adults in the LABS study.
| Males | Females | |||
|---|---|---|---|---|
| β | 95% confidence interval |
β | 95 % confidence interval |
|
| Intercept | −25.05 | −26.99 – −23.12 | −30.28 | −31.24 – −29.32 |
| Age | 0.15 | −0.10 – 0.21 | 0.12 | 0.09 – 0.15 |
| Obesity class | ||||
| Class 1 (Diabetes) | −0.47 | −2.89 – 1.95 | 0.06 | −1.73 – 1.86 |
| Class 2 (Disordered eating) | −3.41 | −5.03 – −1.79 | −3.00 | −4.01 – −1.99 |
| Class 3 (Mixed) | −3.05 | −4.58 – −1.51 | −1.97 | −2.96 – −0.97 |
| Class 4 (Early onset) | Referent | Referent | ||
| Baseline weight | −0.10 | −0.14 – −0.07 | −0.05 | −0.07 – −0.02 |
| Surgical group | ||||
| Gastric band | − 14.29 | 12.27 – 16.31 | 15.57 | 14.46 – 16.67 |
| Roux-en-Y | Referent | Referent | ||
DISCUSSION
Among 2456 severely obese adults in the LABS study, four different groups were identified and group membership was related to weight change at 3 years.. The smallest class, which included 91 adults, was a metabolically unhealthy subgroup. This group was characterized as being at high cardiovascular disease risk due to highly elevated glucose and triglyceride levels and suboptimal HDL concentrations. Among the females, this group had the largest loss in weight three years after bariatric surgery. A much larger class of 892 people exhibited high levels of aberrant eating patterns, with almost all of the members reporting often eating when not hungry and when full. In addition, more than half of the participants reported episodes of LOC eating. In contrast, the largest class, which included 1108 adults, contained very few participants who reported aberrant eating behavior. Interestingly, no other factors distinguished this group from the other classes. Both of these groups lost more weight than their peers in Class 4.
Latent class analysis has been used to identify subtypes of eating disorders,26 as well as classify adolescents into homogenous subgroups based on behavioral patterns10. A similar technique, latent profile analysis, has been used to identify subgroups based on eating behavior patterns.13 Not only can these techniques identify homogeneous groups within a heterogeneous sample, but the groups they identify can, in some applications, be used to improve prediction of outcomes. For example, Savage and Birch used latent class analysis to identify subgroups within 176 adults based on weight control behaviors. They observed differences between the subgroups (classes) in terms of subsequent weight change.27 However, to date the classification of weight-related disorders have focused on a relatively narrow set of constructs, such as dietary intake, physical activity, and weight concerns.8 No studies have incorporated biological measures. Our results highlight the merits of including both behavioral and biological constructs in classification systems. Had we only included indicators of eating behavior, we would not have identified the sharp contrast in metabolic health of Class 1 compared to Classes 2–4 and may have only identified the group who may be at particularly high risk for poor outcomes due to the high rates of aberrant eating behaviors.
Our results suggest that there are distinct subgroups/subtypes of obesity and some benefit more than others from bariatric surgery. One subtype appears to be in need of close monitoring for cardiovascular risk and this may be the group which benefits most from the reductions in diabetes that have been consistently observed with bariatric surgery. In addition, numerous cross-sectional studies have found that rates of LOC eating and BED are elevated in obese populations. In the LABS cohort, although rates of grazing with LOC were high, there was substantial variability among the classes, from 19% to 61%. In one group almost all of the 892 adults were often eating when they were not hungry or despite being full; whereas, in another subgroup these behaviors were rare. The subgroup of obese patients who may be particularly sensitive to external cues to eat and/or have disrupted or dysregulated satiety signaling lost more weight than other subgroups. Research is needed to understand whether the greater weight loss in this subgroup reflects becoming less sensitive to external cues, changes in appetite, or other changes in appetitive behaviors..
There are many strengths to this study, including the large sample size and the inclusion of a wide range of behaviors, cognitions, and biological measures. It is important to note that the participants in this study were extremely obese and planning to have bariatric surgery within 30 days. As is typical of such cohorts, the LABS cohort was approximately 80% female. Therefore, it is unclear whether the same subtypes would be identified in a sample that was not seeking bariatric surgery. It is possible that if the same subtypes are present in less obese adults or those not seeking bariatric surgery, the relative distribution of the subtypes may vary from what we observed in LABS. Because the sample was predominantly non-Hispanic Caucasian, it is unknown whether the results are generalizable to obese adults of other groups. Replication of the approach should be undertaken using a more racially and ethnically diverse sample. Savage and Birch found that weight change varied by behavioral subtypes.27 Since this analysis was cross-sectional, we do not yet know whether outcomes vary across the subtypes we have identified. Nevertheless, the differences we observed between empirically-identified subgroups have important implications for understanding causes of and treatments for obesity.
In future research we will investigate whether longer term post-operative weight loss trajectories or change in comorbidity response vary across these obesity subtypes. Previous attempts to identify preoperative factors that predict weight loss and weight maintenance after bariatric surgery have been largely null;28,29 thus identifying subgroups of people who are undergoing bariatric surgery may help to identify those who would most benefit from bariatric surgery, as well as identify those whose in need of additional modalities to achieve an optimal postoperative weight change. The current study demonstrates that using preoperative information, one can discern obesity subtypes who have different weight change trajectories after surgery.
Figure 1b.
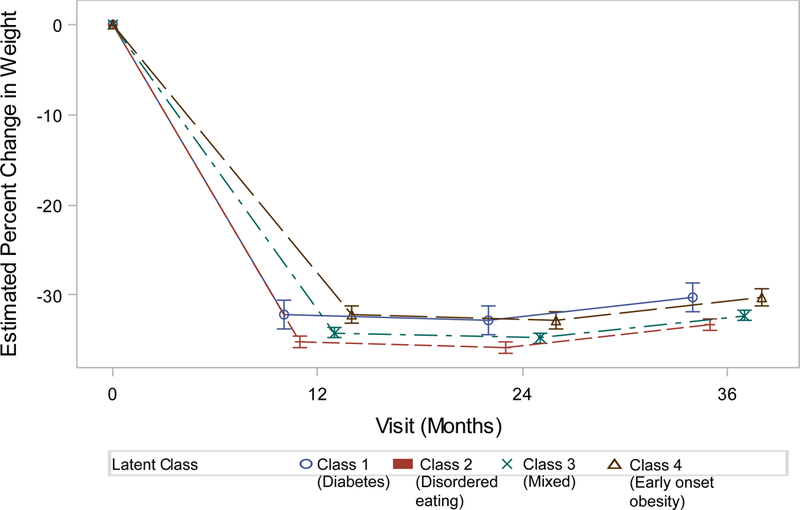
Weight change (%) patterns by obesity class among females in the LABS study who underwent Roux-en-Y Bypass surgery.
Figure 1c.
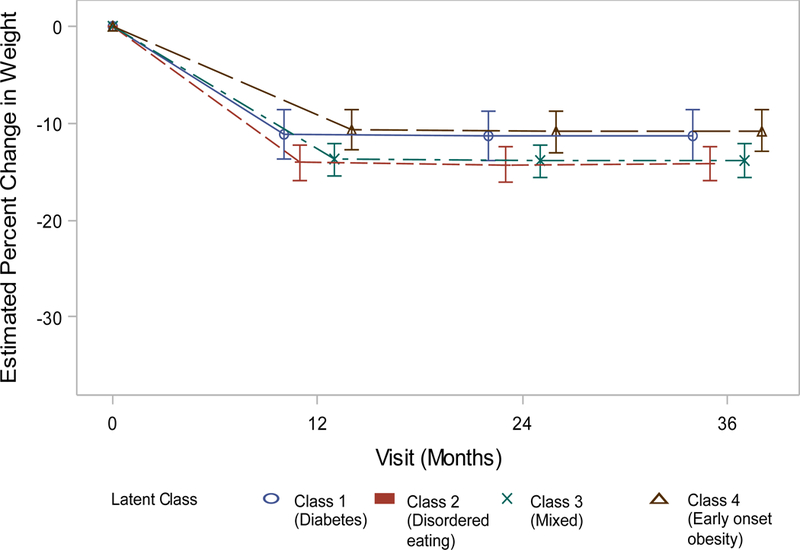
Weight change (%) patterns by obesity class among males in the LABS study who underwent gastric banding
Figure 1d.
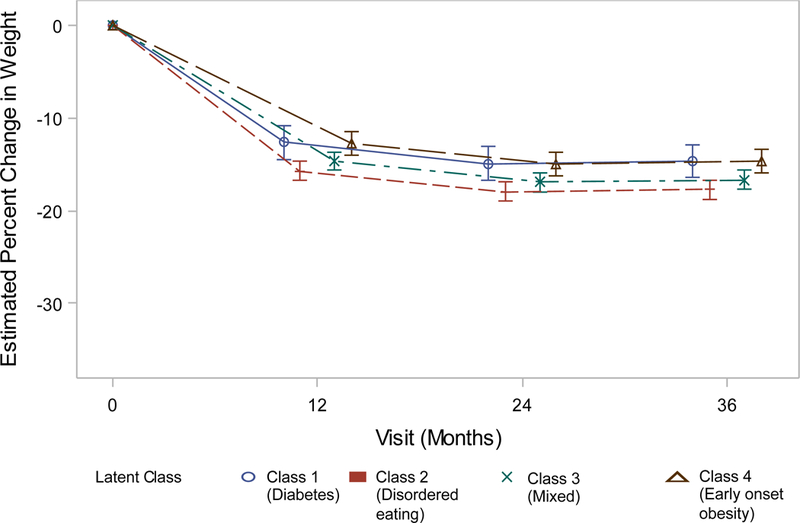
Weight change (%) patterns by obesity class among females in the LABS study who underwent gastric banding.
What is known:
There is heterogeneity in weight change after bariatric surgery.
What does this study add:
We empirically identified subgroups within adults with obesity:
We show how the subgroups differ from each other in terms of baseline characteristics and three-year weight change.
Acknowledgments
Funding/Support: LABS-2 was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: Data Coordinating Center – U01 DK066557; Columbia University Medical Center – U01-DK66667 (in collaboration with Cornell University Medical Center CTRC, Grant UL1- RR024996); University of Washington – U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute – U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555 ; and in collaboration with the Teen-LABS consortium - UM1DK072493.
Role of the Sponsor: The NIDDK scientists contributed to the design and conduct of the study, which included collection, and management of data. The project scientist from the NIDDK served as a member of the steering committee, along with the principal investigator from each clinical site and the data coordinating center. The data coordinating center housed all data during the study and performed data analyses according to a prespecified plan developed by the data coordinating center biostatistician and approved by the steering committee and independent data and safety monitoring board. The decision to publish was made by the Longitudinal Assessment of Bariatric Surgery-2 steering committee, with no restrictions imposed by the sponsor.
Thomas H. Inge has received research grant funding from Ethicon Endosurgery and has served as consultant for Sanofi Corporation.
Dr. Pories receives research grants from Johnson & Johnson, Nestle’s Corporation
Dr Pomp receives honoraria for speaking from Medtronic/Covidien, Ethicon and W. L Gore & Associates
Dr. Courcoulas has received research grants from Covidien and Ethicon J & J Healthcare.
Footnotes
Conflict of Interest Disclosures:
Drs. Belle, Dakin, Mitchell, Field, Wahed, and Mr. Johnson have no conflicts of interest to declare.
References
- 1.Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014;348:g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenoir L, Maillot M, Guilbot A, Ritz P. Primary care weight loss maintenance with behavioral nutrition: An observational study. Obesity (Silver Spring) 2015;23(9):1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherwood NE, Crain AL, Martinson BC, et al. Enhancing long-term weight loss maintenance: 2 year results from the Keep It Off randomized controlled trial. Prev Med 2013;56(3–4):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. Jama 2014;312(9):934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. Jama 2013;310(22):2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson JI, Lalonde JK, Berry JM, et al. Binge-eating disorder as a distinct familial phenotype in obese individuals. Arch Gen Psychiatry 2006;63(3):313–319. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell JE, King WC, Courcoulas A, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord 2015;48(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JE, King WC, Pories W, et al. Binge eating disorder and medical comorbidities in bariatric surgery candidates. Int J Eat Disord 2015;48(5):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field AE, Camargo CA Jr.,Ogino S The merits of subtyping obesity: one size does not fit all. JAMA 2013;310(20):2147–2148. [DOI] [PubMed] [Google Scholar]
- 10.Huh J, Riggs NR, Spruijt-Metz D, Chou CP, Huang Z, Pentz M. Identifying patterns of eating and physical activity in children: a latent class analysis of obesity risk. Obesity (Silver Spring) 2011;19(3):652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leech RM, McNaughton SA, Timperio A. The clustering of diet, physical activity and sedentary behavior in children and adolescents: a review. Int J Behav Nutr Phys Act 2014;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutelle KN, Peterson CB, Crosby RD, Rydell SA, Zucker N, Harnack L. Overeating phenotypes in overweight and obese children. Appetite 2014;76:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vannucci A, Tanofsky-Kraff M, Crosby RD, et al. Latent profile analysis to determine the typology of disinhibited eating behaviors in children and adolescents. J Consult Clin Psychol 2013;81(3):494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis 2007;3(2):116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics 2013;132(6):1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins TM, Buncher CR, Akers R, et al. Validation of a weight history questionnaire to identify adolescent obesity. Obes Surg 2013;23(9):1404–1412. [DOI] [PubMed] [Google Scholar]
- 17.Stunkard AJ, Sorensen T, Schulsinger F. Use of a Danish Adoption Register for the study of obesity and thinness. In: Kety SS, Rowland LP, Sidman RL, Matthysse SW, eds. The Genetics of Neurological and Psychiatric Disorders New York: Raven Press; 1983:115–120. [Google Scholar]
- 18.Spitzer RL, Yanovski SZ, Marcus MD. The Questionnaire of Eating and Weight Patterns-Revised (QEWP-R, 1993) 1993. [Google Scholar]
- 19.Allison KC, Lundgren JD, O’Reardon JP, et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the Night Eating Syndrome. Eat Behav 2008;9(1):62–72. [DOI] [PubMed] [Google Scholar]
- 20.Christian NJ, King WC, Yanovski SZ, Courcoulas AP, Belle SH. Validity of self-reported weights following bariatric surgery. JAMA 2013;310(22):2454–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempster AP, Laird NM, Rubin DB. Maximum Likelihood from Incomplete Data Via Em Algorithm. Journal of the Royal Statistical Society Series B-Methodological 1977;39(1):1–38. [Google Scholar]
- 22.Schwarz G Estimating the dimension of a model. Ann Stat 1978;6:461–464. [Google Scholar]
- 23.Sclove LS. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika 1978;52:333–343. [Google Scholar]
- 24.Bozdogan H Model selection and Aikaike’s information criterion (AIC): The general theory and its analytic extensions. Psychometrika 1987;52:345–370. [Google Scholar]
- 25.Swanson SA, Lindenberg K, Bauer S, Crosby RD. A Monte Carlo investigation of factors influencing latent class analysis: an application to eating disorder research. Int J Eat Disord 2012;45(5):677–684. [DOI] [PubMed] [Google Scholar]
- 26.Swanson SA, Horton NJ, Crosby RD, et al. A latent class analysis to empirically describe eating disorders through developmental stages. Int J Eat Disord 2014;47(7):762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savage JS, Birch LL. Patterns of weight control strategies predict differences in women’s 4-year weight gain. Obesity (Silver Spring) 2010;18(3):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courcoulas AP, Christian NJ, O’Rourke RW, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng J, Seip R, Stone A, Ruano G, Tishler D, Papasavas P. Ethnic variation in weight loss, but not co-morbidity remission, after laparoscopic gastric banding and Roux-en-Y gastric bypass. Surg Obes Relat Dis 2015;11(1):94–100. [DOI] [PubMed] [Google Scholar]


