Abstract
Objective.
To evaluate the validity and acceptability of at-home self-collection to test for high-risk human papillomavirus (HPV) and sexually transmitted infections(STIs) among women overdue for cervical cancer screening by national guidelines.
Methods.
Low-income, infrequently screened women were recruited from the general population in North Carolina to participate in an observational study. Participants provided two self-collected cervico-vaginal samples (one at home and one in the clinic), and a clinician-collected cervical sample. Samples were tested for high-risk HPV, Chlamydia trachomatis(C. trachomatis), Neisseria gonorrhoeae(N. gonorrhoeae), Trichomonas vaginalis(TV), and Mycoplasma genitalium(M. genitalium). Cervical samples were also tested by liquid-based cytology.
Results.
Overall, 193 women had conclusive high-risk HPV results for all three samples and cytology results. Prevalence of high-risk HPV within self-home samples (12.4%) was not different from that within clinician samples (11.4%; p = 0.79) and to that within self-clinic samples (15.5%; p = 0.21) Positivity for high-risk HPV in all sample types increased with increasing grades of cervical abnormality (p<0.001). Self-home samples detected high-risk HPV in all identified cases of HSIL and of CIN2+. Detection was comparable across sample types for TV(range: 10.2%−10.8%), M. genitalium (3.3%−5.5%), C. trachomatis(1.1%−2.1%), and N. gonorrhoeae (0%−0.5%). Kappa values between sample types ranged from 0.56–0.66 for high-risk HPV, 0.86–0.91 for TV, and 0.65–0.83 for M. genitalium. Most participants reported no difficulty understanding self-collection instructions(93.6%), and were willing to use self-collection in the future(96.3%).
Discussion:
Mail-based, at-home self-collection for high-risk HPV and STI detection was valid and well-accepted among infrequently screened women in our study. These findings support the future use of high-risk HPV self-collection to increase cervical cancer screening rates among higher-risk women in the United States.
INTRODUCTION
Cervical cancer is preventable with regular screening and treatment.(1) However, an estimated 13,240 women in the United States will develop and 4,170 will die from cervical cancer in 2018.(2) Almost 20% of eligible U.S. women report not having received a Pap test within the last 3 years,(3) the maximum interval recommended for Pap testing alone.(4)
High-risk human papillomavirus (HPV) is the primary cause of high-grade precancerous lesions and cervical cancer.(5) Testing for high-risk HPV is usually performed on cervical samples collected by a clinician. The development of new collection devices, and sensitive molecular diagnostic assays, have made it possible to conduct high-risk HPV testing on genital samples self-collected by women.(6) Studies in Europe and Canada found that offering at-home high-risk HPV self-collection by mail to women overdue for screening increases screening completion compared to invitation to in-clinic screening.(7,8) Self-collection for high-risk HPV testing with sensitive amplification tests has a similar sensitivity and specificity as clinician collection for high-grade cervical precancerous detection, though most studies have evaluated samples self-collected in clinical settings.(6,9,10)
Few studies have assessed validity of self-collection for high-risk HPV testing conducted entirely by mail(11,12). Assessing validity of mail-based self-collection for high-risk HPV and STI testing among women overdue for screening is key, as a self-collection intervention conducted by mail may be more scalable than in-person distribution of kits with face-to-face instruction, with potential to reach women not in regular clinical care.
MATERIALS AND METHODS
Our primary aim was to examine the clinical performance of high-risk HPV testing on self-collected cervico-vaginal samples for CIN2+ detection in a population of US women at elevated risk of cervical cancer due to underscreening. We also examined detection of C. trachomatis, N. gonorrhoeae, Trichomonas vaginalis (TV), and Mycoplasma genitalium (M. genitalium) in self-collected compared to clinician-collected samples.
Data presented here are from the second phase of the My Body, My Test observational study (MBMT-2). The first phase of the My Body My Test study found that using samples self-collected at home and returned by mail for high-risk HPV testing was feasible and well-accepted among underscreened low-income women in North Carolina, United States.(13,14)
Women were eligible to participate in the My Body, My Test −2 study if they were 30 to 64 years of age; reported no history of Pap testing in the past 4 years (overdue for screening by national guidelines at the start of the study); had a household income below 250% of the poverty level; were not pregnant; had not had a hysterectomy; and were uninsured, underinsured, or had Medicaid insurance. Eligibility measures were assessed by self-report. Income and insurance criteria were defined to ensure eligibility for free cervical cancer screening services through collaborating safety net clinics and programs.
From February 2012 to October 2014, participants were recruited from the general population in 5 counties in North Carolina: Alamance, Buncombe, Chatham, Durham, and Orange. Recruitment was conducted via direct outreach by study personnel and collaborators; referral from the United Way 2–1-1 social assistance hotline; word-of-mouth; and posters and flyers distributed in social service agencies, shelters, churches, supermarkets, and other locations likely to reach low-income women. Women were screened for eligibility by phone by a call center run by the American Sexual Health Association, or in person at time of recruitment.
Participants were asked to provide three types of genital samples: (i) a cervico-vaginal sample self-collected by brush at home and returned by mail (self-home sample); (ii) a cervico-vaginal sample self-collected by brush in a clinic and handed to a nurse (self-clinic sample), and (iii) a cervical sample collected by brush by a clinician during a pelvic examination (clinician sample). Upon determination of a woman’s eligibility, study personnel mailed her a packet containing a self-collection kit, an informed consent form, and Health Insurance Portability and Accountability Act authorization for the study to access participant medical records related to cervical cancer screening and treatment. At the time of enrollment, all participants were also scheduled for an appointment at a collaborating clinic for collection of the self-clinic and clinician samples, which were collected before self-home results were known. At study completion, participants completed a questionnaire eliciting feedback on their experiences of and attitudes towards self- and clinician-collection. Self-collection was referred to as the “self-test” on the questionnaire for participant comprehension. All participants were referred to in-clinic screening and were provided an incentive of $35 USD for returning the self-home sample and attending an appointment for collection of the self-clinic, and for clinician samples, and $10 for completing the questionnaire.
Self-collection of the cervico-vaginal samples was performed by using a Viba brush (Rovers Medical Devices B.V., The Netherlands). Participants were instructed to introduce the brush into the vagina as far as it could comfortably go and rotate 5 times, remove the brush head and place it into a collection tube containing 4.3 mL of Aptima sample transport media (Hologic, Inc., Marlborough, Mass.). The sample was then mailed in a prepaid, preaddressed envelope to study staff at the University of North Carolina (UNC). Illustrated instructions for completing self-collection instructions were pilot tested for comprehension by low-literacy populations before use. Instructions were slightly revised during project implementation to emphasize that vials should be closed tightly, which resolved an emergent issue of a number of samples leaking in transit. Home self-collected samples were returned an average of 15 days before the clinic appointment.
At the study clinic appointment, participants self-collected a second vaginal sample using the same brush, preservation solution, and instructions used for at-home self-collection. Participants then underwent a standard pelvic examination, during which a clinician collected a cervical sample using an endocervical brush (Cytobrush Plus GT) and spatula (Pap-Perfect), preserved in PreservCyt media (Hologic, Inc.) for high-risk HPV and cytology testing. The in-clinic self- and clinician-collected cervical samples were then mailed or hand-delivered to study staff.
Upon receipt, study staff de-identified the self-collected samples. The self-home and self-clinic samples were shipped at ambient temperature to Hologic laboratories in San Diego, Calif., for high-risk HPV and STI testing. Two 1-mL aliquots were taken from the clinician-collected sample and each placed into a vial containing 2.9 mL of Aptima sample transport media. One of these vials was sent at ambient temperature to Hologic laboratories for high-risk HPV and STI testing, and the other vial was stored at UNC in case of need for confirmatory testing by the Microbiology Core Laboratory of the Southeastern Sexually Transmitted Diseases Cooperative Research Center. The remainder of the clinician sample was delivered to the McLendon laboratory at UNC Hospitals for liquid-based cytology testing on a ThinPrep processor.
Testing for high-risk HPV testing was performed using the Aptima HPV assay (Hologic, Inc.), a molecular amplification assay that detects qualitatively E6/E7 mRNA of 14 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). Testing procedures were identical for self-collected samples and aliquots from clinician samples. Samples testing positive for the high-risk HPV panel according to manufacturer’s instructions(15) were then tested for types 16 and for 18/45 as part of standard of care using the FDA-approved Aptima 16 18/45 assay. Testing for STIs was performed using the Aptima Combo2 assay for C. trachomatis and N. gonorrhoeae, the Aptima Trichomonas vaginalis assay, and the Aptima analyte-specific reagent-based assay for M. genitalium (all from Hologic, Inc.). Testing was performed on the automated Panther system by a trained operator, according to the manufacturer’s instructions. Cytology samples were analyzed using the ThinPrep 2000 Processor and classified according to the 2001 Bethesda System. If any cytological cervical abnormality or high-risk HPV infection was identified by a clinician-collected cervical sample, the clinician referred the participant to follow-up diagnostics and treatment per standard guidelines.(16)
Participants attending in-clinic appointments received the results of their clinician-collected tests from clinic staff per standard protocols. Participants who did not attend an in-clinic appointment received at-home self-collection results from study staff by phone or letter, along with information to schedule a clinic appointment with a local clinic offering low-cost cervical cancer screening. The UNC Institutional Review Board approved the study protocol.
We used the McNemar’s test to assess differences in detection rates between sample collection methods, and in participants’ attitudes toward self-collection as compared to Pap testing. Wilson score intervals were calculated for high-risk HPV and STI detection by sample type. Agreement between sample collection methods was measured by pairwise calculation of the Kappa statistic (κ). A kappa value of 0.41–0.60 is considered a moderate agreement, 0.61–0.80 a good agreement, and 0.81–0.99 an excellent agreement.(17) Difference in high-risk HPV prevalence across cytology grades was assessed by Fisher’s exact test. Sensitivity and specificity of high-risk HPV testing for the detection of high-grade squamous intraepithelial lesions (HSIL) and of CIN2+ were computed for each sample type.
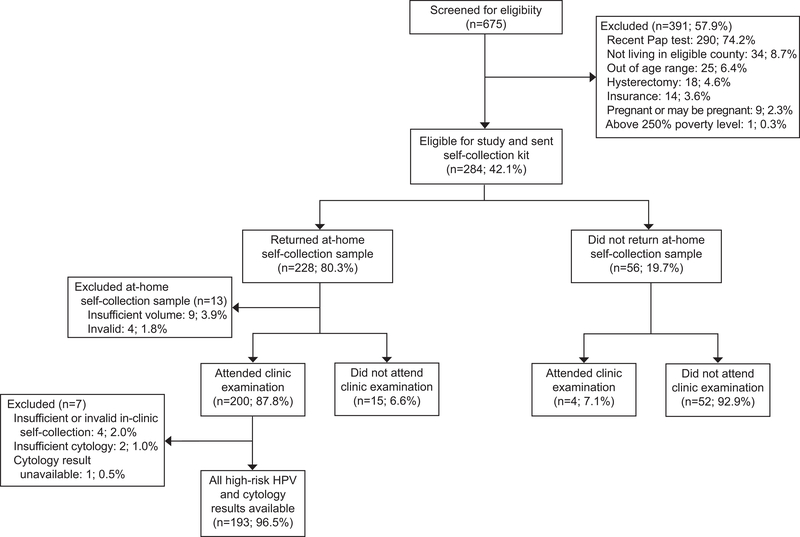
Of the 675 women screened, 42.1% (n = 284) were eligible for the study and were sent home self-collection kits by mail (Figure 1). Of these 284 women, 80.3% (n = 228) returned a self-home sample and 70.4% (n = 200) also attended a clinic appointment. Of the 228 self-home samples returned, 94.3% (n = 215) had valid high-risk HPV results, after excluding 13 samples: 9 (3.9%) with insufficient volume and 4 (1.8%) with inconclusive high-risk HPV results. Four women who tested high-risk HPV positive by self-collection and one woman who tested positive for trichomonas by self-collection were lost to follow-up. Of the 200 self-clinic samples collected, 98.1% (n = 196) had valid high-risk HPV results, after excluding 4 samples: 3 (1.5%) with insufficient volume and 1 (0.5%) with an inconclusive high-risk HPV result. All clinician samples resulted in valid high-risk HPV results. Two clinician samples (0.9%) contained insufficient cells for cytology diagnosis, and one cytology result (0.4%) was unavailable due to processing error. Thus, a final analytic sample of 193 women with conclusive high-risk HPV results for the three different sample types and corresponding cytology results were included in data analyses. No demographic differences were found between the included (n = 193) and excluded (n = 91) women (data not shown).
Figure 1.
Study flowchart of My Body My Test-2 participants.
Women were referred to colposcopy or repeat cytology based on the clinician sample results according to national consensus guidelines.(16) We tracked attendance of recommended follow-up among women with abnormal cytology until follow-up procedures had been completed or, in the case of participant non-attendance, until the patient was lost to follow-up. Of 11 women referred to colposcopy, 8 (72.7%) attended. The 3 colposcopy referrals lost to follow-up were excluded from histology analysis, leaving an analytic sample of 190 for CIN2+ calculations. Six women were referred to treatment for biopsy-confirmed CIN2+ by loop electrosurgical excision procedure or cold-knife conization, of whom 5 (83.3%) completed treatment and one was lost to follow-up.
RESULTS
The median age of the 193 participants was 45 years (range: 30–63 years, Table 1). The median time since last Pap test was 5 years (range: 4–20 years). About half of the participants (45%) were white, 26% were Black, 26% were Hispanic, and 4% reported another race. The majority had no post-high school education (61%). Most participants lived on income at or below 100% of the federal poverty level (74%) and were uninsured or under-insured (80%).
Table 1.
Socio-demographic characteristics and attitudes of 193 low-income, underscreened women
| Characteristics and attitudes | ||
|---|---|---|
| Median Age (range) in years | 45 (30–63) | |
| Median time since last Pap test (range) in years | 5 (4–20) | |
| N* | % | |
| Race White |
85 | 44.5 |
| Black | 49 | 25.7 |
| Hispanic | 49 | 25.7 |
| Other† | 8 | 4.2 |
| Education | ||
| High school diploma or less | 101 | 61.2 |
| Some college or more | 64 | 38.8 |
| Income | ||
| 100% of FPL‡ or more | 47 | 26.0 |
| Below 100% FPL | 134 | 74.0 |
| Current health insurance | ||
| No insurance or underinsured | 154 | 80.2 |
| Medicaid or Medicare | 38 | 19.8 |
| Overall thoughts about the self-test | ||
| Mostly positive | 127 | 67.6 |
| Neutral | 42 | 22.3 |
| Mostly negative | 18 | 9.6 |
| Don’t know | 1 | 0.5 |
| Willing to use the self-test again | ||
| Yes | 182 | 96.3 |
| No | 6 | 3.2 |
| Don’t know | 1 | 0.5 |
| Preference for receiving self-test or Pap results | ||
| Phone | 39 | 20.5 |
| 84 | 44.2 | |
| No preference | 67 | 35.3 |
| Trusts the self-test is safe | ||
| Completely | 116 | 62.0 |
| A moderate amount | 42 | 22.5 |
| A little | 17 | 9.1 |
| Not at all | 5 | 2.7 |
| Don’t know | 7 | 3.7 |
| Hard to understand the self-test instructions | ||
| No | 175 | 93.6 |
| Yes | 12 | 6.4 |
| Physical discomfort when using the self-test | ||
| None | 126 | 66.3 |
| A little | 59 | 31.1 |
| A lot | 5 | 2.6 |
| Pain when using the self-test | ||
| None | 157 | 82.2 |
| A little | 32 | 16.8 |
| A lot | 2 | 1.0 |
| Bleeding when using the self-test | ||
| None | 173 | 92.0 |
| A little | 15 | 8.0 |
Includes 193 participants with complete hrHPV and cytology results. Counts may not total 193 due to missing values: Race=2; Education=28, Income=10, Health insurance=1, Overall thoughts about the self-test=5, Willing to use the self-test again=4, Preference for receiving self-test or Pap results=3, Trusts the self-test is safe=6, Hard to understand the self-test instructions=6, Physical discomfort when using the self-test=3, Pain when using the self-test=2, Bleeding when using the self-test=5.
Other includes: Asian (N=1), American Indian or Alaska Native (N=1), “mixed” (N=3), and other not specified (N=3).
FPL=Federal Poverty Level.
Prevalence of high-risk HPV within self-home samples (12.4%) was not different from that within clinician samples (11.4%; p = 0.79) and to that within self-clinic samples (15.5%; p = 0.21) (Table 2). Detection of TV was nearly identical across sample types: 10.2% for self-home, 10.8% for self-clinic, and 10.2% for clinician samples. Detection of M. genitalium in self-home (5%) and self-clinic (5.5%) samples was not statistically different from clinician-samples (3.3%; p = 0.38 and p = 0.13, respectively). Detection of C. trachomatis and N. gonorrhoeae was less common, with 4 (2.1%) C. trachomatis infections and 1 (0.5%) N. gonorrhoeae infection detected by any sample type (Table 2).
Table 2.
Prevalence of high-risk human papillomavirus and other sexually transmitted infections, stratified by sample type
| Overall N* | Home Self-Collection | Clinic Self-Collection | Clinician Collection | ||||
|---|---|---|---|---|---|---|---|
| N positive | % | N positive | % | N positive | % | ||
| (95%CI) | (95%CI) | (95%CI) | |||||
| hrHPV† | 193 | 24 | 12.4 | 30 | 15.5 | 22 | 11.4 |
| (8.1–17.9) | (10.9–21.2) | (7.3–16.5) | |||||
| CT‡ | 189 | 2 | 1.1 | 4 | 2.1 | 3 | 1.6 |
| (0.1–3.8) | (0.6–5.3) | (0.3–4.6) | |||||
| NG§ | 189 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 |
| (0.0–1.9) | (0.0–2.9) | (0.0–1.9) | |||||
| TV || | 186 | 19 | 10.2 | 20 | 10.8 | 19 | 10.2 |
| (6.3–5.5) | (6.7–16.1) | (6.3–15.5) | |||||
| MG ¶ | 181 | 9 | 5.0 | 10 | 5.5 | 6 | 3.3 |
| (2.3–9.2) | (2.7–9.9) | (1.2–7.1) | |||||
Overall N defined for each infection by participants with conclusive results for all sample types. Missing data included N=4 each for CT and NG, N=7 for TV, and N=12 for MG.
hrHPV=high risk human papillomavirus;
CT=Chlamydia trachomatis;
NG= Neisseria gonorrhoeae;
TV= Trichomonas vaginalis;
MG= Mycoplasma genitalium;
For high-risk HPV detection, agreement was good between self-home and self-clinic (κ = 0.66) and between self-home and clinician samples (κ =0.66), while agreement was moderate between self-clinic and clinician samples (κ = 0.56) (Table 3). For TV detection, agreement between all sample types was excellent (range of κ = 0.86–0.91). For M. genitalium detection, agreement was excellent between self-home and self-clinic samples (κ = 0.83), and was good between self-home and clinician samples (κ = 0.65) and between self-clinic and clinician samples (κ = 0.74). C. trachomatis and N. gonorrhoeae prevalence was too low to assess agreement.
Table 3.
Kappa agreement of infection detection between sample types
| Result (Sample 1/Sample 2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Positive/ Positive | Positive/ Negative | Negative/ Positive | Negative/ Negative | Kappa | (95%CI) | |
| hrHPV* N=193 |
Self-home† | Self-clinic‡ | 19 | 5 | 11 | 158 | 0.66 | (0.49–0.80) |
| Self-home | Clinician§ | 16 | 8 | 6 | 163 | 0.66 | (0.46–0.80) | |
| Self-clinic | Clinician | 16 | 14 | 6 | 157 | 0.56 | (0.36–0.73) | |
| TV || N=186 |
Self-home | Self-clinic | 17 | 2 | 3 | 164 | 0.86 | (0.71–0.96) |
| Self-home | Clinician | 17 | 2 | 2 | 165 | 0.88 | (0.74–0.98) | |
| Self-clinic | Clinician | 18 | 2 | 1 | 165 | 0.91 | (0.80–1.00) | |
| MG¶ N=181 |
Self-home | Self-clinic | 8 | 1 | 2 | 170 | 0.83 | (0.59–1.00) |
| Self-home | Clinician | 5 | 4 | 1 | 171 | 0.65 | (0.27–0.92) | |
| Self-clinic | Clinician | 6 | 4 | 0 | 171 | 0.74 | (0.44–0.94) | |
hrHPV=high-risk human papillomavirus;
Self-home=cervico-vaginal sample self-collected at home;
Self-clinic=cervico-vaginal sample self-collected in clinic,
Clinician=cervical sample collected by clinician;
TV= Trichomonas vaginalis;
MG= Mycoplasma genitalium; 95%CI=95% confidence interval.
Analyses for each infection among women with available results for all three sample types.
Overall prevalence of ASC-US (atypical squamous cells of undetermined significance) or worse cytology in the study population was 7.8% (n = 15 of 193 participants), and that of HSIL was 1.6% (n = 3/193) (Table 4). Histologically-confirmed CIN2+ was detected in 6 women referred to colposcopy based on abnormal cytology, representing an overall CIN2+ prevalence of 3.2% in our population.
Table 4.
High-risk HPV prevalence stratified by cytology and histology results
| Total N | Home-based Self-Collection |
Clinic Self-Collection | Clinician Collection | ||||
|---|---|---|---|---|---|---|---|
| hrHPV%* | (95%CI) | hrHPV% | (95%CI) | hrHPV% | (95%CI) | ||
| Cytology (n=193)† | |||||||
| Normal | 178 | 8.4 | (5.2–13.4) | 12.4 | (8.3–18.0) | 7.3 | (4.3–12.1) |
| ASC-US‡ | 6 | 33.3 | (9.7–70.0) | 16.7 | (3.0–56.4) | 33.3 | (9.7–70.0) |
| LSIL§ | 3 | 66.7 | (20.8–93.9) | 66.7 | (20.8–93.9) | 66.7 | (20.8–93.9) |
| ASC-H || | 3 | 66.7 | (20.8–93.9) | 66.7 | (20.8–93.9) | 66.7 | (20.8–93.9) |
| HSIL+ ¶ | 3 | 100.0 | (43.9–100.0) | 100.0 | (43.9–100.0) | 100.0 | (43.9–100.0) |
| Histology (n=190)# | |||||||
| Normal** | 184 | 8.7 | (5.4–13.7) | 12.5 | (8.5–18.1) | 7.6 | (4.6–12.4) |
| CIN2+†† | 6 | 100.0 | (61.0–100.0) | 83.3 | (43.7–97.0) | 100.0 | (61.0–100.0) |
hrHPV=high-risk human papillomavirus;
Analyses limited to participants with conclusive cytology and hrHPV results on all three sample types;
ASC-US=atypical squamous cells of undetermined significance;
LSIL=low-grade squamous intraepithelial lesion;
ASC-H=atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion;
HSIL+=high-grade squamous intraepithelial lesion or worse;
Excludes 1 ASC-US hrHPV-positive, 1 ASC-H, and 1 LSIL referred to colposcopy but lost to follow-up. No CIN1 cases were detected.
Includes women with normal cytology for whom colposcopy was not recommended and thus histology not conducted.
CIN2+=cervical intraepithelial neoplasia 2 or more severe; Histology results: 3 cases CIN2/3, 2 cases CIN3, 1 case CIN3/CIS
Positivity for high-risk HPV in all sample types increased with increasing grades of cervical abnormality (p<0.001). Among the 165 women with normal cytology and high-risk HPV-negative clinician-collected samples, 4.8% (n= 8/165) of the self-home and 8.5% (n=14/165) of the self-clinic samples were high-risk HPV-positive. Six women with normal cytology and high-risk HPV-positive clinician results had high-risk HPV-negative self-home samples. Among the 24 self-home high-risk HPV-positive samples, prevalence of abnormal cytology was 37.5% (n = 9/24) and of HSIL was 12.5% (n = 3/24), compared to 3.6% (n = 6/169) and 0%, respectively, among self-home high-risk HPV-negative samples. Prevalence of CIN2+ was 0% among the 168 self-home high-risk HPV negative samples and 27.3% (n = 6/22) among self-home high-risk HPV positive samples with histological status.
All identified cases of HSIL and CIN2+ tested high-risk HPV-positive by self-home samples (Table 2). Self-home sampling sensitivity was 100% for HSIL and 100% for CIN2+, and specificity was 88.9% (95% CI: [83.6–93%]) for HSIL and 91.1% [86–94.8] for CIN2+. Self-clinic sensitivity was 100% for HSIL and 83.3% [35.9–99.6] for CIN2+, and specificity was 85.8% [80–90.4] for HSIL and 87.2% [81.5–91.7] for CIN2+. Clinician sample sensitivity was 100% for HSIL and 100% for CIN2+, and specificity was 90% [84.8–93.9] for HSIL and 92.2% [87.3–95.7] for CIN2+.
The prevalence of high-risk HPV type 16 was 2.6% (n = 5) in self-home, 2.1% (n = 4) in self-clinic, and 2.1% (n = 4) in clinician samples, respectively, and the prevalence of high-risk HPV types 18/45 was 1% (n = 2), 0.5% (n = 1), and 1.6% (n = 3), respectively. HrHPV type 16 was not detected in any HSIL case by any sample type, and was detected in 1 (16.7%) case of CIN2+ by all sample types. HrHPV types 18/45 were detected in 1 (33.3%) case of HSIL by all sample types, in 2 (33.3%) cases of CIN2+ by self-home and clinician samples, and in 1 (16.7%) case by a self-clinic sample.
Nearly all participants reported being willing to do the self-collection again (96.3%) and reported that it was not hard to understand the self-collection instructions (93.6%) (Table 1). The majority had mostly positive (67.6%) or neutral (22.3%) “overall thoughts” about the self-collection. Most participants reported no or little physical discomfort (97.4%) or pain (99%) during self-collection; however, 2 (1%) reported “a lot” of pain. Five participants reported that they “hurt or injured” themselves during self-collection, of whom 2 provided a response when asked what had happened: “I had some bleeding,” and “both the Pap and the self-test were uncomfortable.” A small proportion of participants reported “a lot of pain” from the Pap test (n = 5, 2.9%) or from the self-collection (n = 2, 1%) (p = 0.37). Most participants were willing to do the Pap test (97.9%) and the self-collection (96.3%) again (p = 0.72). Participants reported similar levels of overall positive thoughts about the Pap test (60.7%) and the self-collection (67.6%; p = 0.15). Five women (2.7%) reported lack of trust in the safety of self-collection. More participants expressed preference for receiving their self-collection results by mail (n = 84, 44.2%) than by phone (n = 39, 20.5%) (p = <0.001), and 67 (35.3%) expressed no preference.
DISCUSSION
HrHPV testing on samples self-collected at home and returned by mail detected high-risk HPV infection in all histologically-confirmed CIN2+ cases among ~200 infrequently screened women. The study population had a relatively high CIN2+ prevalence (3.2%). At-home self-collection was comparable to clinician-based collection for high-risk HPV, TV, and M. genitalium detection. Participants responded positively to conducting self-collection at home using simple illustrated instructions, and reported high willingness to do self-collection again.
These results provide evidence that high-risk HPV testing on samples self-collected by brush at home, placed in preservation solution, and returned by mail may be as accurate as testing on clinician-collected samples for high-grade lesion detection. Self-collected samples returned by mail and clinician-collected samples showed comparable sensitivity for CIN3+ detection among Swedish patients referred for cervical precancer treatment,(11) and identical sensitivity for CIN2+ detection in a Chinese population.(12) Other studies have found high concordance of high-risk HPV detection in mailed self-collected and clinician-collected samples, although study designs did not allow for assessment of sensitivity and specificity given that only self-collection high-risk HPV-positive women were referred to in-clinic screening.(18,19)
Home-based self-collection to test for sexually transmitted infections (STIs; e.g., C. trachomatis and N. gonorrhoeae) has been found highly feasible and well-accepted, with comparable sensitivity and specificity to clinician collected samples,(20–22) and could be a good option for those unable or unwilling to attend in-clinic screening. We found comparable TV detection rates between self- and clinician-collected samples, consistent with findings in other populations.(23–25) M. genitalium infection was detected more in self-collected cervico-vaginal samples than clinician-collected cervical samples – although this difference was relatively imprecise – consistent with findings from our recent study conducted in Kenya.(26) Higher M. genitalium detection in self-collected samples may be attributable to differences in the type and volume of media used for sample preservation, or to the relatively low M. genitalium load in cervical cells of infected women.(27) Further research is needed to determine whether sample type affects sensitivity for M. genitalium infection.
An important study strength is that all self-collection results were paired with standard-of-care high-risk HPV and cytology co-testing. Our focus on validation of self-collection among infrequently screened women is novel for U.S.-based studies. Estimated CIN2+ prevalence of 3.2% among our participants, compared to prevalence of <1% in the general U.S. screening population,(28) indicates that we successfully identified a population of women at elevated risk for high-grade lesions and cervical cancer. All identified CIN2+ cases were detected by high-risk HPV testing on home self-collected samples, indicating the safety of a home-based high-risk HPV self-collection approach with referral of self-collection high-risk HPV-positive women to in-clinic screening.
This study could have been strengthened by assessing self-collected sample adequacy (i.e., β-globin testing), although very high adequacy has been previously found in mailed samples self-collected by the Viba brush with liquid preservation solution (97.7%−99.7%).(18,29) A larger sample with more cases of CIN2+ would provide more robust assessment of sensitivity and specificity estimates and detection of smaller differences between sample types. Self-home and clinician-collected samples showed good agreement (Kappa=0.66) among all women tested, and complete agreement in CIN2+ cases, although power was limited for definitive assessment. Our population-based recruitment approach, essential to identifying medically underserved women who might fall outside regular care, made confirmation of eligibility through medical records review infeasible. As participants self-selected into the study, our sample may not be representative of the overall U.S. population of infrequently screened women. Financial incentives for screening completion and lack of comparator group, prevented assessment of behavioral outcomes such as effect on screening uptake or follow-up to further care.
Almost all women in our study were willing to perform self-collection again, and found the instructions not difficult to understand. These findings add to evidence of high acceptability of mailed self-collection among infrequently screened women worldwide(30) and diverse groups of U.S. women(30–33) Broad acceptability is critical if this practice is to be implemented in national screening programs, as has been done recently in the Netherlands and Denmark.(34,35)
In conclusion, we found at-home self-collection for high-risk HPV and STI detection to be valid and well-accepted among this population of infrequently screened women in North Carolina. While findings are promising for the future use of self-collection in U.S.S screening programs to improve access and coverage of cervical cancer screening in infrequently screened women in the United States, future implementation research on program efficacy and cost-effectiveness, including a comprehensive assessment of continuity from screening to treatment, is needed.
Supplementary Material
Precis.
At-home self-collection was comparable to clinician-based collection for detection of high-risk human papillomavirus, Trichomonas vaginalis, and Mycoplasma genitalium among approximately 200 infrequently screened women in North Carolina.
Acknowledgments:
The authors thank our collaborators at Alamance Regional Health Services, Marlene Warren and Barbara Toth at the Buncombe County Health Department, Dr. Elliot-Bynum and Virginia Mitchell at Caare Inc., Dr. Lorraine Cummings, and our collaborators at Western North Carolina Community Health Services for providing clinical services to our participants. We thank the leadership and call center agents at the United Way’s 2–1-1 of Western NC and the American Sexual Health Association. We thank Anna Pfaff, Lanya Shapiro, and Xian Brooks for contributing to study implementation; Florence Paillard for editing the manuscript; Dana Lapple for performing STI testing in Dr. Hobbs’s laboratory; and Anne Menkens for providing substantial feedback on the draft manuscript.
Supported by grants from the National Cancer Institute U54 CA156735 and NIH NCI R01 CA183891, and from the University Cancer Research Fund at the University of North Carolina at Chapel Hill. Confirmatory hrHPV and STI testing conducted by Dr. Hobbs’s laboratory was funded by U19-AI-031496. The China Scholarship Council sponsored Yuqian Zhao. The authors adhered to the Good Publication Practice (GPP3) guidelines with regards to all industry sponsored contributions received for this study.
Abbreviations used:
- ASC-H
atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion
- ASC-US
atypical squamous cells of undetermined significance
- CI
confidence interval
- C. trachomatis
Chlamydia trachomatis
- CIN2+
cervical intraepithelial neoplasia grade 2 or more severe
- CIN3+
cervical intraepithelial neoplasia grade 3 or more severe
- FPL
federal poverty leve
- HSIL
high-grade squamous intraepithelial lesion
- LBC
liquid-based cytology
- LSIL
low-grade squamous intraepithelial lesion
- MBMT
My Body, My Test
- M. genitalium
Mycoplasma genitalium
- N. gonorrhoeae
Neisseria gonorrhoeae
- STI
sexually transmitted infection
- STM
sample transport media
- TV
Trichomonas vaginalis
- UNC
University of North Carolina at Chapel Hill
- U.S.
United States
Footnotes
The other authors did not report any potential conflicts of interest.
Financial Disclosure
HrHPV and STI testing, sample preservation media, ThinPrep processor slides, assay reagents, and cervical samples collection brushes and spatulas were donated by Hologic. Self-collection brushes were donated by Rovers Medical Devices. Some conference travel expenses for Andrea C. Des Marais were paid by Hologic. Marcia M. Hobbs has consulted for Hologic. Jennifer S. Smith has received research grants, supply donations, and consultancies; served on paid advisory boards, and/or has been a paid speaker for Arbor Vita, BD Diagnostics, Hologic, Rovers Medical Devices, and Trovagene in the past 5 years. Neither Hologic nor Rovers had input into the research design, analysis, or interpretation of results.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.Smith JS, Brewer NT, Saslow D, Alexander K, Chernofsky MR, Crosby R, et al. Recommendations for a national agenda to substantially reduce cervical cancer. Cancer Causes Control. 2013;24(8):1583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noone A, Howlander N, Krapcho M, Miller D, Breast A, Yu M, et al. SEER Cancer Statistics Review, 1975–2015 [Internet]. National Cancer Institute. Bethesda, MD 2018. Available from: https://seer.cancer.gov/cr/1975_2015/
- 3.Gamble S, Mawokomatanda T, Xu F, Chowdhury P, Pierannunzi C, Flegel D, et al. Surveillance for Certain Health Behaviors and Conditions Among States and Selected Local Areas — Behavioral Risk Factor Surveillance System, United States, 2013. and 2014. Vol. 66, MMWR Surveillance Summaries. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyer VA. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–91. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high‐grade cervical lesions: a meta‐analysis update. Int J cancer. 2007;121(3):621–32. [DOI] [PubMed] [Google Scholar]
- 6.Arbyn M, Verdoodt F, Snijders PJF, Verhoef VMJ, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–83. [DOI] [PubMed] [Google Scholar]
- 7.Racey CS, Withrow DR, Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: a systematic review and meta-analysis. Can J Public Heal. 2013;104(2):159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Racey CS, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. Randomized intervention of self-collected sampling for human papillomavirus testing in under-screened rural women: uptake of screening and acceptability. J women’s Heal. 2016;25(5):489–97. [DOI] [PubMed] [Google Scholar]
- 9.Snijders PJF, Verhoef VMJ, Arbyn M, Ogilvie G, Minozzi S, Banzi R, et al. High‐risk HPV testing on self‐sampled versus clinician‐collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J cancer. 2013;132(10):2223–36. [DOI] [PubMed] [Google Scholar]
- 10.Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: Focused literature review. Can Fam Physician. 2017;63(8):597–601. [PMC free article] [PubMed] [Google Scholar]
- 11.Leinonen MK, Schee K, Jonassen CM, Lie AK, Nystrand CF, Rangberg A, et al. Safety and acceptability of human papillomavirus testing of self-collected specimens: A methodologic study of the impact of collection devices and HPV assays on sensitivity for cervical cancer and high-grade lesions. J Clin Virol. 2018;99:22–30. [DOI] [PubMed] [Google Scholar]
- 12.Chang CC, Tseng CJ, Liu WW, Jain S, Horng SG, Soong YK, et al. Clinical evaluation of a new model of self-obtained method for the assessment of genital human papilloma virus infection in an underserved population. Chang Gung Med J. 2002;25(China (Republic : 1949-) PT-Journal Article PT-Research Support, Non-U.S. Gov’t LG-English DC-20030109 OVID MEDLINE UP 20161215):664–71. [PubMed] [Google Scholar]
- 13.Smith JS, Des Marais AC, Deal AM, Richman AR, Perez-Heydrich C, Yen-Lieberman B, et al. Mailed Human Papillomavirus Self-Collection With Papanicolaou Test Referral for Infrequently Screened Women in the United States. Sex Transm Dis. 2018;45(1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson C, Breithaupt L, Des Marais A, Rastas C, Richman A, Barclay L, et al. Acceptability and ease of use of mailed HPV self-collection among infrequently screened women in North Carolina. Sex Transm Infect. 2018;94(2):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hologic. Aptima HPV Assay Package Insert [Internet]. 2017. [cited 2018 May 15]. Available from: https://www.hologic.com/sites/default/files/package-insert/AW-14517-001_003_01.pdf
- 16.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 Updated Consensus Guidelines for the Management of Cervical Cancer Screening Test and Cancer Precursors. Am Soc Colposc Cerv Pathol. 2013;17(5):S1–27. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. Biometrics [Internet]. 1977;33(1):159. Available from: http://www.jstor.org/stable/2529310?origin=crossref [PubMed]
- 18.Gök M, van Kemenade FJ, Heideman DAM, Berkhof J, Rozendaal L, Spruyt JWM, et al. Experience with high‐risk human papillomavirus testing on vaginal brush‐based self‐samples of non‐attendees of the cervical screening program. Int J Gynecol Cancer. 2012;130(5):1128–35. [DOI] [PubMed] [Google Scholar]
- 19.Wikström I, Lindell M, Sanner K, Wilander E. Self-sampling and HPV testing or ordinary Pap-smear in women not regularly attending screening: a randomised study. Br J Cancer. 2011;105(3):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9(2):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fajardo‐Bernal L, Aponte‐Gonzalez J, Vigil P, Angel‐Müller E, Rincon C, Gaitán HG, et al. Home‐based versus clinic‐based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections. Cochrane Libr. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunny C, Taylor D, Hoang L, Wong T, Gilbert M, Lester R, et al. Self-collected versus clinician-collected sampling for Chlamydia and Gonorrhea screening: a systemic review and meta-analysis. PLoS One. 2015;10(7):e0132776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chernesky M, Jang D, Gilchrist J, Randazzo J, Elit L, Lytwyn A, et al. Ease and Comfort of Cervical and Vaginal Sampling for Chlamydia trachomatis and Trichomonas vaginalis with a New Aptima Specimen Collection and Transportation Kit. J Clin Microbiol. 2014;52(2):668–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrow SC, Smith DW, Harnett GB. The diagnosis of chlamydia, gonorrhoea, and trichomonas infections by self obtained low vaginal swabs, in remote northern Australian clinical practice. Sex Transm Infect. 2002;78(4):278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knox J, Tabrizi SN, Miller P, Petoumenos K, Law M, Chen S, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29(11):647–54. [DOI] [PubMed] [Google Scholar]
- 26.Lockhart A, Psioda M, Ting J, Campbell S, Mugo N, Kwatampora J, et al. Prospective Evaluation Of Cervico-Vaginal Self And Cervical Physician-Collection For The Detection Of Chlamydia Trachomatis, Neisseria Gonorrhoeae, Trichomonas Vaginalis, And Mycoplasma Genitalium Infections. Sex Transm Dis. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross JDC, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect. 2006;82(4):269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting J, Kruzikas DT, Smith JS. A global review of age-specific and overall prevalence of cervical lesions. Int J Gynecol Cancer. 2010;20(7):1244–9. [DOI] [PubMed] [Google Scholar]
- 29.Bais AG, van Kemenade FJ, Berkhof J, Verheijen RHM, Snijders PJF, Voorhorst F, et al. Human papillomavirus testing on self‐sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J cancer. 2007;120(7):1505–10. [DOI] [PubMed] [Google Scholar]
- 30.Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Vol. 93, Sexually Transmitted Infections. 2017. p. 56–61. [DOI] [PubMed] [Google Scholar]
- 31.Ilangovan K, Kobetz E, Koru-Sengul T, Marcus EN, Rodriguez B, Alonzo Y, et al. Acceptability and feasibility of human papilloma virus self-sampling for cervical cancer screening. J Women’s Heal. 2016;25(9):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarinci IC, Litton AG, Garcés-Palacio IC, Partridge EE, Castle PE. Acceptability and usability of self-collected sampling for HPV testing among African-American women living in the Mississippi Delta. Women’s Heal Issues. 2013;23(2):e123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montealegre JR, Mullen PD, Jibaja-Weiss ML, Mendez MMV, Scheurer ME. Feasibility of cervical cancer screening utilizing self-sample human papillomavirus testing among Mexican immigrant women in Harris County, Texas: a pilot study. J Immigr Minor Heal. 2015;17(3):704–12. [DOI] [PubMed] [Google Scholar]
- 34.Bonde J HPV self-sampling to become new public cervical cancer screening alternative in the Capital Region of Denmark [Internet]. 2017. Available from: https://www.linkedin.com/pulse/hpv-self-sampling-become-new-public-cervical-cancer-screening-bonde
- 35.National Institute for Public Health and the Environment. New population screening for cervical cancer is more effective and introduces self-collection of samples. Annual Report RIVM 2016 [Internet]. 2017; Available from: https://magazines.rivm.nl/en/2017/06/annual-report-rivm-2016/new-population-screening-cervical-cancer-more-effective-and
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.