Abstract
Background
Immune checkpoint blockade (ICB) and BRAFV600-targeted therapy have demonstrated substantial clinical efficacy for patients with stage 4 melanoma in clinical trials; however, their impact on survival and barriers to treatment in the “real-life” setting remains unknown.
Methods
Patients who presented with cutaneous melanoma during 2004–2015 using the National Cancer Database, which comprises > 70% of all newly diagnosed cancers in the U.S., were evaluated for predictors of presenting with stage 4 disease and receiving ICB, and for their associated unadjusted and risk-adjusted overall survival (OS).
Results
17,975 patients presented with stage 4 metastatic cutaneous melanoma. Overall, patients who presented after the FDA’s initial approvals (starting in 2011) for ICB and BRAFV600-targeted therapy demonstrated a 31% relative improvement in 4-year OS (p < 0.001), compared to pre-2011. Following the initial approvals in 2011, improved OS was associated in risk-adjusted analyses with ICB (HR 0.57, 95CI 0.52–0.63). ICB demonstrated improved median and 4-year OS of 16.9 months (95CI 15.6–19.3; vs. 7.7 months, 95CI 7.2–8.4) and 32.4% (95CI 29.5–35.3; vs. 21.0%, 95CI 19.6–22.2, all p < 0.001), respectively; improved OS was persistent in unadjusted and risk-adjusted landmark survival analyses. Uninsured patients and management in the community setting were less likely to receive ICB in multivariable analyses.
Conclusions
In a national “real-life” treatment population, we show that the wide availability of the novel treatment modalities ICB and BRAFV600-targeted therapy has significantly improved the survival of patients with stage 4 melanoma. Our findings additionally suggest that there are opportunities for expanding coverage and access to these novel immunotherapies in community practice.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2241-x) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Metastasis, Immune checkpoint blockade, Targeted therapy, Immunotherapy
Introduction
Melanoma incidence rates continue to rise faster than any other solid tumor, with the current lifetime risk for a person developing melanoma in the U.S. estimated to be 1 in 54 [1–3]. Although a majority of cases are diagnosed at an early stage where surgical excision is often curative, treatment of stage 4 melanoma has historically been limited given the low anti-tumor activity of conventional chemotherapies and cytokine therapy, resulting in a median OS time of less than 1 year [4].
However, the approvals of vemurafenib, a BRAFV600 inhibitor, and ipilimumab, a monoclonal antibody directed against the inhibitory receptor CTLA-4, by the U.S. Food and Drug Administration (FDA) in 2011 heralded two new classes of drug treatments—oncogene-targeted therapy and ICB—that have drastically changed the systemic therapy of advanced melanoma. Current approved checkpoint immunotherapies include monoclonal antibody inhibitors of PD-1 (nivolumab and pembrolizumab) and CTLA-4 (ipilimumab) as well as the combination of nivolumab and ipilimumab. Approved targeted therapies for MAPK pathway dysregulation by BRAF mutation, which is implicated approximately half of melanomas, include BRAF inhibitors (vemurafenib, dabrafenib, and encorafenib) and MEK inhibitors (trametinib, cobimetinib, and binimetinib) [5–7].
By blocking the CTLA-4 receptor’s inhibitory interactions with B7 ligands expressed on antigen-presenting cells, anti-CTLA-4 monoclonal antibodies (e.g., ipilimumab) permit the binding of the costimulatory B7 ligands with T cells’ CD28 receptors, thus supplying the secondary activation signals needed for persistent T-cell activation [8]. Separately, expression of PD-1 surface receptors leads to T-cell exhaustion and terminal differentiation; anti-PD-1 monoclonal antibodies (e.g., nivolumab and pembrolizumab) can counteract this T-cell inhibitory mechanism. Thus, anti-CTLA-4 and anti-PD1 immunotherapies enable a robust expansion of tumor-specific T cells that mediate clinical efficacy in advanced melanoma patients. The initial randomized controlled trials (RCTs) of ipilimumab in stage 3 unresectable and stage 4 melanoma patients revealed improved response rates and OS in both previously treated and untreated patients. A substantial proportion of patients developed immune-related adverse events of varying severity on these trials [9–11]. The KEYNOTE-006 RCT comparing pembrolizumab to ipilimumab demonstrated significantly improved progression-free survival and OS, durable objective response rates, and reduced serious adverse effects associated with anti-PD-1 immunotherapy [12, 13]. Combined PD-1 and CTLA-4 blockade in the CheckMate 067 and 069 RCTs demonstrated improved PFS and OS compared to ipilimumab [14, 15]. Targeted agents including small molecules that specifically inhibit downstream effectors of the MAPK pathway, such as mutated BRAF (e.g., vemurafenib, dabrafenib, and encorafenib) and MEK (e.g., trametinib, cobimetinib, and binimetinib) have demonstrated remarkable clinical efficacy in BRAFV600 mutant melanoma [3, 5–7, 16–20].
The current National Cancer Comprehensive Network (NCCN, v2.2018) guidelines for the initial treatment of stage 4 metastatic melanoma recommend systemic therapy with ICB and BRAFV600/MEK-targeted therapy for patients with BRAF-mutant melanoma and ICB for BRAF-wildtype melanoma [21]. Specifically, first-line immunotherapy treatment includes anti-PD-1 monotherapy with nivolumab or pembrolizumab or combination anti-CTLA-4/PD-1 therapy with nivolumab/ipilimumab. For patients with BRAF-mutant melanoma, first-line-targeted therapy includes combination BRAF/MEK inhibitor therapy with either dabrafenib/trametinib or vemurafenib/cobimetinib, or BRAF inhibitor monotherapy with vemurafenib or dabrafenib. These recommendations have been shaped by the efficacy and safety results from a number of phase 2 and 3 randomized clinical trials [5, 13, 22–27]. With the success of these new therapeutic classes, conventional cytotoxic chemotherapy (e.g., dacarbazine, temozolomide, carboplatin/paclitaxel, and/or fotemustine) and biochemotherapy regimens (including high-dose IL-2 and interferon alfa-2b) no longer have a role in the first-line treatment of stage 4 metastatic cutaneous melanoma.
The introduction of these new therapeutic classes has been exciting for both melanoma patients and providers, given their initial successes in multiple RCTs and retrospective analyses [28]. Although multiple RCTs have rigorously examined the safety and efficacy of novel checkpoint immunotherapies and oncogene-targeted therapies in advanced melanoma, there has yet to be a wide-scale evaluation of OS in stage 4 melanoma following the approval of checkpoint immunotherapies and oncogene-targeted therapies in 2011. In this study, we examine the survival and management of melanoma patients who initially presented with stage 4 disease in the contemporary era of ICB and oncogene-targeted therapies from the National Cancer Database (NCDB), one of the largest cancer databases that include data from the initial presentation for more than 70% of U.S. cancer patients [29].
Materials and methods
Data source and study design
The NCDB, a hospital-based nationwide cancer registry developed as a joint initiative between the American College of Surgeons and American Cancer Society and comprising more than 70% of newly diagnosed cancers in the United States, was queried for all patients newly diagnosed with cutaneous melanoma from 2004 to 2015 [29]. Cutaneous melanoma was identified by World Health Organization ICD-O3 morphological codes for malignant melanoma (i.e., 8720–8723, 8726, 8730, 8740–8746, 8750, 8760–8761, 8770–8774, and 8780, with behavior codes 2 and 3) and skin topographical codes (i.e., C44.0–44.9); as previously described [30, 31]. Patients were excluded if they were younger than 20 years, previously diagnosed with other cancers (i.e., a sequence of case greater than 1), lacked data on metastases, or only diagnosed at their index institution but were entirely treated elsewhere.
Variable design
Cases were classified as stage 4 (i.e., disseminated metastases) based on American Joint Committee on Cancer (AJCC, 7th ed.) M staging, using NCDB’s AJCC variables and metastasis collaborative stage site-specific factors for cutaneous melanoma [30]. Clinicopathologic characteristics at presentation, including age, sex, race, insurance status, Charlson–Deyo comorbidity index (CDI), geographic region and type of treating hospital, year of diagnosis, AJCC pT and pN classification, LDH level, and the primary lesion’s characteristics (i.e., site, histologic subtype, ulceration status, and mitotic proliferation index) were summarized and compared. Management characteristics included surgery for the primary lesion (i.e., no surgery, local excision, gross excision, or wide excision), resection of a metastatic lesion, radiotherapy, chemotherapy (including targeted therapy), and immunotherapy. Because the NCDB only encodes the initial first-line therapies for a patient and the NCCN guidelines have relegated both cytotoxic chemotherapeutics and biochemotherapeutics (i.e., interferon alfa-2b and high-dose IL-2) to second-line therapy for stage 4 patients who fail initial checkpoint immunotherapy, the overwhelming majority of immunotherapies and chemotherapies encoded in NCDB following FDA approvals in 2011 for melanoma patients should represent checkpoint immunotherapy and oncogene-targeted therapies, respectively.
Statistical analyses
Clinicopathologic characteristics were compared by χ2 test and t test between melanoma patients who presented with disseminated metastasis and those who did not, and among the patients with disseminated disease, between those that were treated with immunotherapy vs. those that were not. Data elements missing ≥ 10% of data were excluded from multivariable analyses. If patients were missing any data elements, they were excluded from any analyses of those data elements. Risk-adjusted predictors of presenting with disseminated disease or of receiving immunotherapy were assessed by multivariable logistic regression. For survival analysis in stage 4 melanoma patients, OS was evaluated from the date of diagnosis, with unadjusted OS differences compared by Kaplan–Meier plots and log-rank tests, as previously described [31]. The endpoint was date of death, with patients censored at the date of last follow-up. Due to the limited follow-up, the NCDB does not include survival information for patients diagnosed in the most recent year of the dataset, which for this release was 2015. Estimated OS was compared for stage 4 melanoma diagnosed before and after the start of FDA approvals of checkpoint immunotherapy and oncogene-targeted therapy (i.e., 2011). For stage 4 patients diagnosed after initial FDA approval (i.e., 2011+), OS was compared between those who received immunotherapy vs. those who did not; with additional survival landmark analyses to account for any immortal time bias. Landmark timepoints were selected for the median and mean time from diagnosis to receipt of immunotherapy. Predictors of OS were additionally assessed by multivariable Cox proportional hazards, adjusted for all clinicopathologic variables missing < 10% of data; and repeated for patients that reached the landmark timepoint. A two-sided p value < 0.05 was considered significant. Statistical analyses were performed with STATA (v14.2, StataCorp).
Results
Characteristics of patients presenting with stage 4 melanoma
A total of 407,386 patients diagnosed with cutaneous melanoma from 2004 to 2015 met inclusion criteria, of whom 4.4% (n = 17,795) initially presented with distant metastases (i.e., AJCC stage 4 or M1). The presenting characteristics of those with and without distant metastases are shown in Supplemental Table 1, along with multivariable logistic regression results for predictors of presenting with stage 4 disease at the time of first diagnosis. In multivariable logistic regression adjusted for variables with < 10% missing data, male sex [odds ratio (OR) 1.23, 95% confidence interval (95CI) 1.18–1.30], higher number of co-morbidities (CDI 1 vs. 0: OR 1.36, 95CI 1.27–1.45), black or Hispanic race (vs. white: OR 1.61, 95CI 1.35–1.93 and OR 1.46, 95CI 1.28–1.68; respectively), location of primary lesion, uninsured status (vs. privately insured: OR 2.36, 95CI 2.13–2.61), pT0 (i.e., no evidence of primary tumor) or pT4 (vs. pT1: OR 16.89, 95CI 15.32–18.64 and OR 19.83, 95CI 18.17–21.64; respectively), and nodal metastases (pN1 vs. pN0: OR 1.84, 95CI 1.70–1.98) remained significant independent predictors of presentation with stage 4 disease (p < 0.001 in each case). Without treatment, stage 4 patients had a median OS of 6.1 mos (95CI 5.4–6.6).
Improved overall survival of stage 4 melanoma patients following FDA approval of ICB and BRAFV600-targeted therapies
Of the stage 4 melanoma patients, 47.1% (n = 8389) presented following FDA approval of the checkpoint immunotherapy ipilimumab and BRAF inhibitor vemurafenib in 2011 (i.e., 2011–2015, including the subsequent approvals of PD-1, MEK, and BRAF inhibitors). The median time to death/censorship was 8.4 months pre-approval (interquartile range [IQR] 3.2–24.1, with 84.9% reaching endpoint) and 9.4 months post-approval (IQR 3.3–24.7), with 70.9% reaching the endpoint. Following the FDA approvals, median OS among stage 4 melanoma patients increased to 10.2 months (95CI 9.6–10.7; p < 0.001) from 8.6 mos (95CI 8.3–8.9) pre-approval and 4-year OS improved to 23.5% (95CI 22.3–24.8) from 18.0% (95CI 17.2–18.8). Patients presenting with AJCC M1b (i.e., with metastatic lung involvement; 27.5%, 95CI 24.1–31.0; vs. 20.1%, 95CI 17.7–22.6; p = 0.007), M1c (i.e., any non-lung visceral metastasis or any distant metastasis with elevated LDH; 18.4%, 95CI 16.6–20.3; vs. 8.3%, 95CI 7.4–9.2; p < 0.001) demonstrated improved 4-year OS following FDA approval, as compared to pre-approval; whereas patients with M1a disease did not (45.7%, 95CI 41.9–49.5; vs. 42.6%, 95CI 39.9–45.3; p = 0.16).
Characteristics associated with receipt of ICB in stage 4 melanoma patients
The proportion of patients who received ICB rose from 16.1% in 2011 to 37.4% in 2015. The characteristics of post-approval stage 4 melanoma patients who were treated with and without ICB are shown in Table 1. Only 3.1% (n = 194) of those who did not receive ICB had additional information encoded about why ICB was not administered; of which, ICB was contraindicated in 25.3%, ICB was refused in 44.9%, and patients died prior to receiving ICB in 21.7%. In multivariable logistic regression adjusted for clinicopathologic variables with < 10% missing data, younger age (50–59 vs. 60–69 years: OR 1.22, 95CI 1.02–1.47), lower number of co-morbidities (CDI 1 vs. 0: OR 0.68, 95CI 0.57–0.82), being insured privately (vs. uninsured: OR 2.62, 95CI 1.89–3.65) or through Medicare (OR 2.14, 95CI 1.50–3.04), more recently diagnosed (2015 vs. 2011: OR 3.04, 95CI 2.47–3.73) at an academic/research hospital (vs. community cancer program: OR 2.07, 95CI 1.56–2.74), receiving radiotherapy (OR 1.59, 95CI 1.38–1.83), not receiving targeted therapy (vs. received targeted therapy: OR 4.86, 95CI 4.05–5.84), and not having brain metastases (vs. only subcutaneous metastasis: OR 0.72, 95CI 0.57–0.90) remained significant predictors of receiving ICB (Table 1). Patient sex, race, and site of the primary lesion, and excision of the primary lesion were not significantly associated with ICB treatment.
Table 1.
Characteristics of post-approval stage 4 patients treated with immune checkpoint blockade
| % of stage 4 pts that received ICB | |||||
|---|---|---|---|---|---|
| Total (n) | % received ICB | Univariate χ2 p value | |||
| Multivariable logistic regression | |||||
| OR | 95CI | p value | |||
| Sex | < 0.001 | ||||
| Male | 5644 | 24.8 | Reference | ||
| Female | 2664 | 25.8 | 0.99 | 0.87–1.14 | 0.92 |
| Age (year) | < 0.001 | ||||
| 20–29 | 161 | 36.7 | No observations in multivar | ||
| 30–39 | 403 | 34.7 | No observations in multivar | ||
| 40–49 | 897 | 30.2 | 1.61 | 1.29–2.01 | < 0.001 |
| 50–59 | 1873 | 28.6 | 1.22 | 1.02–1.47 | 0.03 |
| 60–69 | 2147 | 26.6 | Reference | ||
| 70–79 | 1647 | 22.7 | 0.81 | 0.67–0.98 | 0.03 |
| 80–89 | 999 | 12.3 | 0.35 | 0.27–0.45 | < 0.001 |
| 90+ | 154 | 4.6 | 0.11 | 0.05–0.27 | < 0.001 |
| Year of diagnosis | < 0.001 | ||||
| 2011 | 1558 | 16.1 | Reference | ||
| 2012 | 1525 | 16.7 | 0.99 | 0.78–1.25 | 0.93 |
| 2013 | 1703 | 24.2 | 1.69 | 1.36–2.10 | < 0.001 |
| 2014 | 1701 | 28.8 | 2.12 | 1.72–2.63 | < 0.001 |
| 2015 | 1821 | 37.4 | 3.04 | 2.47–3.73 | < 0.001 |
| Comorbidity Index | < 0.001 | ||||
| 0 | 6336 | 27.5 | Reference | ||
| 1 | 1388 | 19.0 | 0.68 | 0.57–0.82 | < 0.001 |
| 2 | 380 | 17.6 | 0.64 | 0.47–0.89 | 0.007 |
| 3 | 204 | 9.3 | 0.37 | 0.22–0.61 | < 0.001 |
| Race/ethnicity | 0.28 | ||||
| White | 7788 | 25.1 | Reference | ||
| Black | 128 | 23.4 | 0.91 | 0.54–1.55 | 0.73 |
| Asian/pacific islander | 51 | 23.5 | 0.96 | 0.41–2.28 | 0.93 |
| Hispanic | 235 | 23.4 | 0.85 | 0.56–1.28 | 0.42 |
| Other/unknown | 106 | 34.0 | 1.08 | 0.62–1.88 | 0.78 |
| Primary payer | < 0.001 | ||||
| Not insured | 490 | 17.6 | Reference | ||
| Private insurance | 3226 | 31.6 | 2.62 | 1.89–3.65 | < 0.001 |
| Medicaid | 748 | 22.5 | 1.30 | 0.88–1.93 | 0.18 |
| Medicare | 3537 | 20.9 | 2.14 | 1.50–3.04 | < 0.001 |
| Other government | 135 | 25.9 | 2.10 | 1.15–3.86 | 0.02 |
| Unknown status | 172 | 23.8 | 1.63 | 0.88–3.02 | 0.12 |
| AJCC pTa | < 0.001 | ||||
| Is | 40 | 7.5 | |||
| 0 | 2415 | 26.6 | |||
| 1 | 350 | 18.9 | |||
| 2 | 274 | 25.9 | |||
| 3 | 389 | 26.5 | |||
| 4 | 1315 | 29.7 | |||
| X | 1994 | 21.9 | |||
| AJCC pNa | < 0.001 | ||||
| 0 | 879 | 20.0 | |||
| 1 | 702 | 27.5 | |||
| 2 | 338 | 29.9 | |||
| 3 | 468 | 31.6 | |||
| X | 3289 | 24.6 | |||
| Primary lesion site | 0.01 | ||||
| Upper limb/shoulder | 567 | 27.0 | Reference | ||
| Lower limb/hip | 604 | 27.0 | 1.19 | 0.83–1.70 | 0.35 |
| Trunk | 1223 | 25.2 | 0.89 | 0.65–1.22 | 0.48 |
| Face | 219 | 22.4 | 0.81 | 0.49–1.35 | 0.43 |
| Scalp/neck | 465 | 31.8 | 1.31 | 0.90–1.91 | 0.15 |
| Ear | 77 | 24.7 | 1.04 | 0.47–2.31 | 0.92 |
| Lip | 6 | 33.3 | 8.31 | 0.66–104.05 | 0.10 |
| Eyelid | 8 | 37.5 | 3.23 | 0.43–24.52 | 0.26 |
| Overlapping sites | 16 | 0 | Omitted due to 0 events | ||
| NOS | 5123 | 24.3 | 0.81 | 0.60–1.09 | 0.16 |
| Melanoma subtypea | 0.57 | ||||
| Superficial spreading | 258 | 29.5 | |||
| Nodular | 808 | 31.7 | |||
| Acral lentiginous | 51 | 23.5 | |||
| Lentigo maligna | 50 | 34.0 | |||
| Histology ulcerationa | 0.42 | ||||
| No | 1786 | 26.1 | |||
| Yes | 1499 | 27.4 | |||
| LDH levela | < 0.001 | ||||
| Within normal limits | 681 | 40.5 | |||
| < 1.5× normal | 666 | 39.2 | |||
| 1.5–10× normal | 268 | 27.6 | |||
| > 10× normal | 99 | 23.2 | |||
| Facility type | < 0.001 | ||||
| Community program | 610 | 15.3 | Reference | ||
| Cancer center | 2979 | 19.6 | 1.24 | 0.93–1.65 | 0.14 |
| Academic/research | 3300 | 30.6 | 2.07 | 1.56–2.74 | < 0.001 |
| Integrated network | 855 | 23.7 | 1.64 | 1.18–2.28 | 0.003 |
| Facility Location | < 0.001 | ||||
| New England | 396 | 27.8 | Reference | ||
| Middle Atlantic | 1107 | 29.0 | 1.06 | 0.78–1.43 | 0.72 |
| South Atlantic | 1785 | 22.7 | 0.84 | 0.62–1.13 | 0.24 |
| East North Central | 1229 | 24.2 | 0.87 | 0.64–1.18 | 0.38 |
| East South Central | 550 | 23.3 | 0.81 | 0.57–1.16 | 0.25 |
| West North Central | 605 | 24.3 | 0.93 | 0.66–1.32 | 0.70 |
| West South Central | 564 | 17.6 | 0.60 | 0.41–0.88 | 0.009 |
| Mountain | 469 | 31.8 | 1.24 | 0.87–1.77 | 0.24 |
| Pacific | 1039 | 22.4 | 0.91 | 0.66–1.25 | 0.54 |
| Metastatic sites | < 0.001 | ||||
| M1a (distant skin/LN) | 1151 | 24.0 | Reference | ||
| M1b (lung) | 1091 | 26.0 | 1.38 | 1.09–1.74 | 0.008 |
| M1c (other sites) | 3220 | 29.9 | 1.48 | 1.22–1.81 | < 0.001 |
| Brain involvement | 2229 | 20.5 | 0.71 | 0.56–0.89 | 0.003 |
| Excision of primary lesion | 0.62 | ||||
| Gross excision | 467 | 23.1 | Reference | ||
| None | 5797 | 24.7 | 1.31 | 0.96–1.78 | 0.09 |
| Local excision | 174 | 26.4 | 1.48 | 0.90–2.43 | 0.12 |
| Wide excision | 1105 | 26.0 | 1.20 | 0.88–1.64 | 0.25 |
| Resection of metastatic lesion | 0.03 | ||||
| None | 5842 | 24.3 | Reference | ||
| Resected | 2388 | 26.6 | 0.98 | 0.86–1.13 | 0.83 |
| Targeted therapy | < 0.001 | ||||
| None | 5961 | 30.2 | Reference | ||
| Treated | 2101 | 12.0 | 0.21 | 0.17–0.25 | < 0.001 |
| Radiotherapy | < 0.001 | ||||
| None | 5027 | 23.6 | Reference | ||
| Irradiated | 3240 | 27.7 | 1.59 | 1.38–1.83 | < 0.001 |
Italicized p values refer to the significance of that variable in univariate X2 analysis
Bold p values indicate statistical significance in multivariable regression
aVariables missing ≥ 10% of data were excluded from multivariable regression
Immune checkpoint blockade was associated with improved risk-adjusted overall survival
Following FDA approval in 2011, 25.1% (n = 2088) of stage 4 patients received ICB and had significantly improved OS in risk-adjusted analyses (HR 0.57, 95CI 0.52–0.63, p < 0.001). ICB was associated with improved median OS (16.9 mos, 95CI 15.6–19.3; vs. 7.7 mos, 95CI 7.2–8.4, p < 0.001) and 4-year OS (32.4%, 95CI 29.5–35.3; vs. 21.0%, 95CI 19.6–22.2; Fig. 1). In these patients, the median and mean times to ICB receipt were 2.0 months and 2.5 months, respectively; ICB demonstrated improved median and 4-year OS in all survival landmark analyses using these timepoints as landmarks: in patients who survived at least 2.0 months, ICB resulted in improved median OS (19.3 mos, 95CI 17.1–21.0; vs. 13.0 mos, 95CI 12.2–13.8, p < 0.001) and 4-year OS (32.9%, 95CI 29.9–35.9; vs. 25.9%, 95CI 24.4–27.6; Fig. 2a); in patients who survived at least 2.5 months, ICB resulted in improved median OS (19.6 mos, 95CI 17.9–22.0; vs. 14.1 mos, 95CI 13.3–15.1, p < 0.001) and 4-year OS (33.6%, 95CI 31.5–37.7; vs. 27.1%, 95CI 25.5–28.8; Fig. 2b).
Fig. 1.
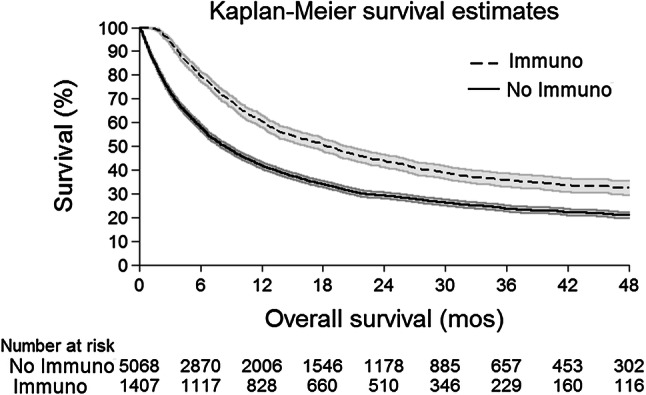
Kaplan–Meier OS Curves for ICB Treatment in post-approval stage 4 melanoma patients. Survival curve of patients treated with ICB (dashed line, n = 1407) demonstrated significantly improved OS compared to no ICB (solid line, n = 5068; p < 0.001), displayed with the associated number at risk table. 95% confidence interval: gray shading
Fig. 2.
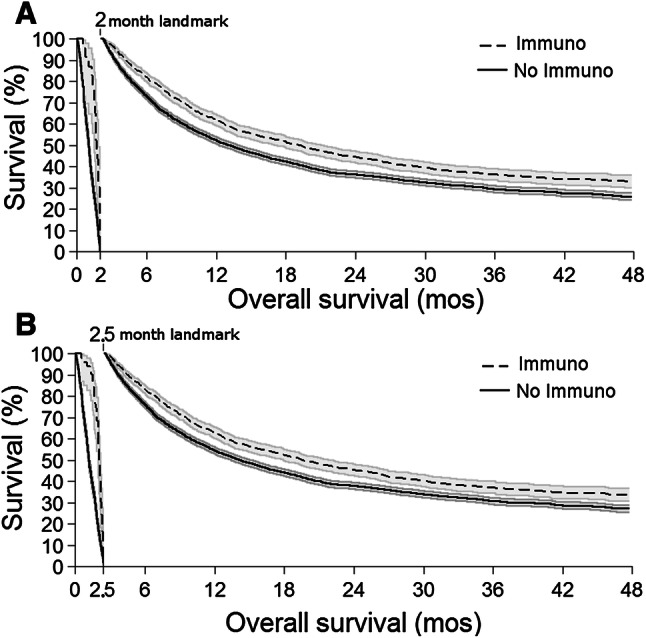
Kaplan–Meier landmark OS curves for ICB treatment in post-approval stage 4 melanoma patients. Using landmark timepoints of a 2.0 and b 2.5 months, all survival curves of patients treated with ICB (dashed lines) demonstrated significantly improved OS compared to no ICB patients (solid lines, all p < 0.001). a There were 23 ICB (1.6%) and 1040 no ICB (20.5%) patients who reached endpoint/censorship before the 2.0 month landmark; and b 51 ICB (3.6%) and 1224 no ICB (24.1%) patients who reached endpoint/censorship before the 2.5 month landmark. 95% confidence interval: gray shading
Overall survival for stage 4 melanoma patients diagnosed from 2011 to 2015 was risk-adjusted for variables with less than 10% of data missing (i.e., sex, age, year of diagnosis, CDI, race, insurance status, site of primary lesion, facility type, facility location, M stage, and treatment by primary excision, resection of metastatic lesion, radiotherapy, targeted therapy, and ICB), for which 4867 patients had complete data and 72.7% (n = 3538) reached the endpoint (Table 2 left panel). Clinicopathologically, patients who were female (hazard ratio [HR] 0.89, 95CI 0.83–0.96, p = 0.002), younger (≤ 49 vs. 60–69 years: HR 0.88, 95CI 0.77–1.00, p = 0.04), without comorbidities (CDI of 1 vs. 0: HR 1.29, 95CI 1.18–1.40, p < 0.001), insured privately (HR 0.67 vs. uninsured, 95CI 0.58–0.77, p < 0.001), insured through Medicare (HR 0.77 vs. uninsured, 95CI 0.66–0.90, p = 0.001), or had M1a disease (reference M1b: HR 0.59, 95CI 0.51–0.68, p < 0.001) had significantly improved OS; whereas race had no association with OS. With regards to treatment, resection of a metastatic lesion (HR 0.48, 95CI 0.44–0.52), targeted therapy (HR 0.73, 95CI 0.68–0.79), and ICB (HR 0.57, 95CI 0.52–0.63) were significantly associated with improved OS (p < 0.001 in each case). The significant OS improvement associated with ICB persisted in risk-adjusted Cox proportional hazards for patients who reached the landmark timepoints of 2.0 months (HR 0.79, 95CI 0.72–0.88, p < 0.001; Table 2 middle panel) and 2.5 months (HR 0.85, 95CI 0.77–0.94, p = 0.001; Table 2 right panel).
Table 2.
Multivariable proportional hazards for OS in post-approval stage 4 melanoma patients, stratified by landmark timepoints
| Multivariable Cox regression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Landmark = 2.0 mos | Landmark = 2.5 mos | ||||||||||
| HR | 95CI | p value | HR | 95CI | p value | HR | 95CI | p value | ||||
| Sex | ||||||||||||
| Female (ref male) | 0.89 | 0.83–0.96 | 0.002 | 0.87 | 0.81–0.95 | 0.001 | 0.86 | 0.79–0.94 | 0.001 | |||
| Age (year) | ||||||||||||
| ≤ 49 | 0.88 | 0.77–1.00 | 0.04 | 0.81 | 0.70–0.93 | 0.003 | 0.81 | 0.70–0.93 | 0.004 | |||
| 50–59 | 0.97 | 0.88–1.07 | 0.57 | 0.90 | 0.81–1.01 | 0.08 | 0.91 | 0.81–1.03 | 0.13 | |||
| 60–69 | Reference | Reference | Reference | |||||||||
| 70–79 | 1.11 | 1.01–1.23 | 0.04 | 1.09 | 0.97–1.23 | 0.14 | 1.09 | 0.97–1.23 | 0.16 | |||
| 80+ | 1.35 | 1.21–1.52 | < 0.001 | 1.42 | 1.24–1.62 | < 0.001 | 1.45 | 1.26–1.67 | < 0.001 | |||
| Year of diagnosis | ||||||||||||
| Per year | 0.96 | 0.93–0.99 | 0.01 | 0.92 | 0.89–0.96 | < 0.001 | 0.92 | 0.88–0.95 | < 0.001 | |||
| Comorbidity Index | ||||||||||||
| 0 | Reference | Reference | Reference | |||||||||
| 1 | 1.29 | 1.18–1.40 | < 0.001 | 1.15 | 1.04–1.27 | 0.009 | 1.16 | 1.04–1.29 | 0.006 | |||
| 2 | 1.54 | 1.33–1.78 | < 0.001 | 1.52 | 1.27–1.81 | < 0.001 | 1.46 | 1.21–1.76 | < 0.001 | |||
| 3 | 1.85 | 1.54–2.23 | < 0.001 | 1.72 | 1.36–2.18 | < 0.001 | 1.64 | 1.27–2.12 | < 0.001 | |||
| Race/ethnicity | ||||||||||||
| White | Reference | Reference | Reference | |||||||||
| Black | 1.12 | 0.85–1.49 | 0.42 | 1.07 | 0.76–1.52 | 0.68 | 1.09 | 0.77–1.56 | 0.62 | |||
| Asian/pacific islander | 0.91 | 0.59–1.41 | 0.68 | 0.96 | 0.59–1.56 | 0.88 | 0.96 | 0.58–1.58 | 0.87 | |||
| Hispanic | 0.87 | 0.70–1.08 | 0.21 | 0.98 | 0.77–1.26 | 0.89 | 1.02 | 0.79–1.31 | 0.90 | |||
| Other/unknown | 0.95 | 0.71–1.27 | 0.72 | 1.07 | 0.77–1.47 | 0.70 | 1.01 | 0.71–1.42 | 0.97 | |||
| Primary payer | Reference | Reference | Reference | |||||||||
| Not insured | ||||||||||||
| Private insurance | 0.67 | 0.58–0.77 | < 0.001 | 0.76 | 0.63–0.90 | 0.002 | 0.73 | 0.61–0.87 | 0.001 | |||
| Medicaid | 0.93 | 0.78–1.11 | 0.43 | 1.02 | 0.83–1.26 | 0.86 | 0.99 | 0.80–1.23 | 0.92 | |||
| Medicare | 0.77 | 0.66–0.90 | 0.001 | 0.84 | 0.69–1.01 | 0.07 | 0.80 | 0.65–0.97 | 0.02 | |||
| Other government | 0.65 | 0.48–0.89 | 0.006 | 0.81 | 0.58–1.14 | 0.22 | 0.79 | 0.55–1.12 | 0.18 | |||
| Unknown status | 0.84 | 0.62–1.13 | 0.26 | 0.89 | 0.62–1.26 | 0.51 | 0.87 | 0.60–1.25 | 0.44 | |||
| Primary lesion site | ||||||||||||
| Upper limb/shoulder | Reference | Reference | Reference | |||||||||
| Lower limb/hip | 1.05 | 0.86–1.29 | 0.64 | 1.11 | 0.89–1.38 | 0.36 | 1.15 | 0.92–1.45 | 0.23 | |||
| Trunk | 1.05 | 0.88–1.26 | 0.59 | 1.02 | 0.84–1.24 | 0.87 | 1.07 | 0.87–1.32 | 0.50 | |||
| Face | 0.75 | 0.57–0.98 | 0.04 | 0.77 | 0.58–1.04 | 0.09 | 0.84 | 0.62–1.14 | 0.26 | |||
| Scalp/neck | 0.68 | 0.54–0.86 | 0.001 | 0.72 | 0.57–0.92 | 0.01 | 0.77 | 0.60–0.99 | 0.04 | |||
| Ear | 1.41 | 0.92–2.15 | 0.11 | 1.41 | 0.89–2.24 | 0.14 | 1.58 | 0.99–2.52 | 0.05 | |||
| Lip | 0.59 | 0.08–4.24 | 0.60 | 0.68 | 0.09–4.87 | 0.70 | 0.74 | 0.10–5.33 | 0.77 | |||
| Eyelid | 1.74 | 0.64–4.72 | 0.28 | 2.10 | 0.66–6.65 | 0.21 | 2.58 | 0.81–8.21 | 0.11 | |||
| Overlapping sites | 1.43 | 0.67–3.06 | 0.36 | 1.29 | 0.47–3.50 | 0.62 | 1.46 | 0.54–3.98 | 0.46 | |||
| NOS | 0.85 | 0.71–1.01 | 0.06 | 0.86 | 0.71–1.05 | 0.14 | 0.92 | 0.75–1.13 | 0.42 | |||
| Facility type | ||||||||||||
| Community program | Reference | Reference | Reference | |||||||||
| Cancer center | 1.03 | 0.91–1.17 | 0.64 | 0.97 | 0.83–1.12 | 0.65 | 0.96 | 0.82–1.12 | 0.61 | |||
| Academic/research | 0.81 | 0.71–0.92 | 0.002 | 0.80 | 0.69–0.93 | 0.004 | 0.79 | 0.68–0.92 | 0.002 | |||
| Integrated network | 1.02 | 0.88–1.20 | 0.76 | 0.98 | 0.81–1.17 | 0.79 | 0.94 | 0.78–1.14 | 0.54 | |||
| Facility location | ||||||||||||
| New England | Reference | Reference | Reference | |||||||||
| Middle Atlantic | 0.92 | 0.78–1.10 | 0.36 | 1.06 | 0.87–1.30 | 0.58 | 1.09 | 0.89–1.35 | 0.41 | |||
| South Atlantic | 0.94 | 0.80–1.11 | 0.49 | 1.06 | 0.87–1.28 | 0.59 | 1.09 | 0.89–1.33 | 0.42 | |||
| East North Central | 1.14 | 0.96–1.35 | 0.13 | 1.27 | 1.04–1.55 | 0.02 | 1.31 | 1.06–1.61 | 0.01 | |||
| East South Central | 0.97 | 0.80–1.17 | 0.72 | 1.08 | 0.86–1.35 | 0.52 | 1.13 | 0.89–1.43 | 0.31 | |||
| West North Central | 1.14 | 0.94–1.37 | 0.18 | 1.32 | 1.06–1.63 | 0.01 | 1.33 | 1.06–1.67 | 0.01 | |||
| West South Central | 0.98 | 0.81–1.19 | 0.85 | 1.14 | 0.91–1.44 | 0.25 | 1.18 | 0.93–1.50 | 0.17 | |||
| Mountain | 0.93 | 0.76–1.13 | 0.47 | 1.08 | 0.86–1.35 | 0.54 | 1.10 | 0.87–1.39 | 0.44 | |||
| Pacific | 0.81 | 0.68–0.97 | 0.02 | 0.96 | 0.78–1.18 | 0.69 | 0.97 | 0.78–1.20 | 0.78 | |||
| Metastatic sites | ||||||||||||
| M1a (distant skin/LN) | Reference | Reference | Reference | |||||||||
| M1b (lung) | 1.69 | 1.47–1.95 | < 0.001 | 1.63 | 1.41–1.90 | < 0.001 | 1.63 | 1.40–1.91 | < 0.001 | |||
| M1c (other sites) | 2.66 | 2.36–3.00 | < 0.001 | 2.26 | 1.98–2.57 | < 0.001 | 2.20 | 1.93–2.51 | < 0.001 | |||
| Brain involvement | 3.17 | 2.77–3.62 | < 0.001 | 2.57 | 2.22–2.97 | < 0.001 | 2.50 | 2.16–2.91 | < 0.001 | |||
| Excision of primary lesion | ||||||||||||
| Gross excision | Reference | Reference | Reference | |||||||||
| None | 1.53 | 1.29–1.81 | < 0.001 | 1.21 | 1.01–1.46 | 0.04 | 1.19 | 0.98–1.44 | 0.08 | |||
| Local excision | 1.10 | 0.84–1.44 | 0.47 | 1.02 | 0.76–1.37 | 0.88 | 1.01 | 0.75–1.37 | 0.93 | |||
| Wide excision | 0.84 | 0.70–1.00 | 0.05 | 0.88 | 0.73–1.06 | 0.17 | 0.91 | 0.75–1.10 | 0.31 | |||
| Resection of metastatic lesion | ||||||||||||
| Yes (ref no.) | 0.48 | 0.44–0.52 | < 0.001 | 0.58 | 0.53–0.63 | < 0.001 | 0.60 | 0.55–0.66 | < 0.001 | |||
| Targeted therapy | ||||||||||||
| Yes (ref no.) | 0.73 | 0.68–0.79 | < 0.001 | 1.04 | 0.95–1.14 | 0.36 | 1.13 | 1.03–1.23 | 0.008 | |||
| Radiotherapy | ||||||||||||
| Yes (ref no.) | 1.06 | 0.99–1.15 | 0.11 | 1.37 | 1.25–1.49 | < 0.001 | 1.35 | 1.23–1.48 | < 0.001 | |||
| ICB | ||||||||||||
| Yes (ref no.) | 0.57 | 0.52–0.63 | < 0.001 | 0.79 | 0.72–0.88 | < 0.001 | 0.85 | 0.77–0.94 | 0.001 | |||
Bold p values indicate statistical significance in multivariable regression
Discussion
Over the past few decades, melanoma care has been advanced by improved screening, standardized surgical protocols, and increased use of sentinel lymph node biopsy [32]. Stage 4 melanoma has historically been challenging to treat and portended a poor prognosis. In the absence of treatment, median OS for stage 4 melanoma at initial presentation is approximately 6 months in our analyses. Preventative measures such as regular skin exams are increasingly being used to detect melanoma at an earlier stage, yet certain subpopulations continue to be at high risk of presenting with advanced melanoma. Specifically, our multivariable logistic analyses showed that patients who presented with stage 4 melanoma were significantly more likely to be male, non-white, uninsured, afflicted by more co-morbidities, diagnosed at community facilities, with AJCC pT0 (i.e., no evidence of primary tumor) or pT4 disease, and positive ulceration status—as compared to those who present at an earlier stage. Less visible or accessible sites of primary melanoma were also associated with significantly higher rates of presenting with stage 4 disease, reinforcing the need for comprehensive skin exams during screening. With the series of FDA approvals for ICB and BRAF-targeted therapies beginning in 2011, new, more effective agents are now available for the first-line treatment of metastatic melanoma.
Our findings are in line with NCCN management recommendations for stage 4 melanoma
The NCDB, by containing more than 70% of all cancer patients newly diagnosed in the U.S., enables the robust examination of melanoma patients’ management and survival in the “real-world” setting beyond clinical trials. Our findings are in line with the NCCN guidelines (version 2.2018) for first-line therapy for stage 4 melanoma, in which a multidisciplinary approach including ICB, BRAF-targeted therapy, and resection of metastatic disease is recommended based on improved OS of these approaches in clinical trials. Following FDA approval of ipilimumab and vemurafenib in 2011, and including subsequent approvals of the BRAF inhibitor dabrafenib (2013), the MEK inhibitor trametinib (2013), and the anti-PD-1 monoclonal antibodies pembrolizumab (2014) and nivolumab (2014), we detected a 31% relative increase in 4-year OS of patients with stage 4, as compared to patients diagnosed prior to 2011. Treatment with ICB was associated with improved median and 4-year OS in our unadjusted and risk-adjusted landmark survival analyses.
In our analyses, stage 4 patients treated with ICB were younger, more recently diagnosed, privately insured, treated at academic facilities, had fewer co-morbidities, and were treated with radiotherapy, and less likely to receive targeted therapy, as compared to patients who did not receive immunotherapy. Uninsured patients were significantly less likely to receive immunotherapy than patients insured privately or through Medicare, which translated into worse OS outcomes in risk-adjusted analyses, suggesting that improved coverage and access to these treatments is critical for stage 4 melanoma patients. Additionally, in this “real-world” treatment cohort we observed that stage 4 melanoma patients were significantly more likely to receive immunotherapy at academic centers, even though melanoma care is primarily delivered in the community setting in the U.S. Because ICB provides the best chance for durable disease control, and potentially even a cure, for advanced melanoma patients, our findings suggest that community practice paradigms may trail behind NCCN guidelines.
Clinicopathologic characteristics and overall survival in stage 4 melanoma
In evaluating additional therapeutic modalities for stage 4 patients, we demonstrate that resection of metastatic lesions also conferred a survival advantage. The NCCN recommends resection of non-primary lesions for patients with limited or symptomatic metastases [21]. The surgical debulking of metastatic lesions reduces the overall tumor burden and is hypothesized to synergistically decrease tumor-induced immune suppression [33]. We additionally demonstrated that wide excision of the primary melanoma lesion was associated with improved OS. Radiation therapy is recommended with palliative intent, particularly for brain metastases to reduce tumor size and to ameliorate neurologic symptoms. Despite reports of synergy between checkpoint immunotherapy and radiation therapy via an abscopal effect, a survival advantage was not associated with radiotherapy in our risk-adjusted analyses [34]. Additionally, female sex has long been associated with a favorable prognosis in melanoma patients, particularly in patients with in-transit and lymph node metastases [35]. We found that female sex was also an independent predictor of improved OS in stage 4 patients.
Limitations
Although the NCDB represents one of the largest cancer registries worldwide, the NCDB only captures data from a patient’s initial presentation. Therefore, our findings specifically pertain to patients diagnosed with stage 4 disease at the time of initial diagnosis and do not include the (larger) proportion of patients diagnosed at an earlier stage who later develop disseminated metastasis [29]. Additionally, the NCDB only includes OS data and does not allow for the evaluation of recurrence or progression. The NCDB lacks detailed data about symptomatology and resectability of metastases; as well as granular details about systemic therapy agents, doses, combinations, toxicities, and subsequent courses; and does not rigorously collect data on reasons why patients did not receive immunotherapy. Additionally, in the era of precision oncology where various cancer types are increasingly characterized and categorized by molecular alterations, a key limitation of the NCDB is its lack of molecular data—in particular, BRAF mutational status in melanoma patients. Receipt of targeted therapy was incorporated into multivariable analysis in part as a proxy for BRAF mutational status.
Conclusions
Using a large-scale database analysis of the “real-world” treatment population of melanoma patients, we demonstrate substantial improvement in OS for patients presenting with stage 4 cutaneous melanoma following the 2011 FDA approvals of checkpoint immunotherapy and BRAF-targeted therapies. Our findings are in line with the compelling clinical efficacy found in phase 3 clinical trials, leading to the FDA approvals of the new agents and substantiate the OS benefit that has led to the recommendations delineated in the current NCCN guidelines for first-line management with ICB and BRAF-targeted therapy in stage 4 melanoma. Our results portray the dramatic successes in recent years in the management of metastatic melanoma and suggest that there are opportunities for expanding coverage and access to these novel agents in community practice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CDI
Charlson–Deyo comorbidity index
- FDA
Food and Drug Administration
- HR
Hazard ratio
- ICB
Immune checkpoint blockade
- IQR
Interquartile range
- NCCN
National Cancer Comprehensive Network
- NCDB
National Cancer Database
- OR
Odds ratio
- RCT
Randomized controlled trial
- 95CI
95% confidence interval
Author contributions
ASD and CKZ contributed to the data analysis and interpretation, and manuscript writing. FSH, TRS, and PAO contributed to the experimental design, interpretation, and manuscript writing. JBI designed and supervised the study, conducted data analysis and interpretation, and wrote the manuscript.
Funding
J. Bryan Iorgulescu is supported by an NIH 5T32-HL7627-34 award. Cheryl K. Zogg is supported by the NIH Medical Scientist Training Program Training Grant T32GM007205.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was conducted in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. This study was deemed exempt from Partners Healthcare’s IRB review (2018P001413) and for this type of study formal consent is not required. There were no human participants, procedures, animal experiments, or cell line experiments.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Glazer AM, Winkelmann RR, Farberg AS, Rigel DS. Analysis of trends in US melanoma incidence and mortality. JAMA Dermatol. 2017;153:225–226. doi: 10.1001/jamadermatol.2016.4512. [DOI] [PubMed] [Google Scholar]
- 3.Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383:816–827. doi: 10.1016/S0140-6736(13)60802-8. [DOI] [PubMed] [Google Scholar]
- 4.Force J, Salama AK. First-line treatment of metastatic melanoma: role of nivolumab. ImmunoTargets Ther. 2017;6:1–10. doi: 10.2147/ITT.S110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 6.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 8.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 11.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611–622. doi: 10.1016/S1470-2045(17)30231-0. [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 13.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 18.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 19.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 20.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network (2018) NCCN clinical practice guidelines in oncology: Melanoma version 2.2018. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#melanoma. Accessed 1 Mar 2018 [DOI] [PubMed]
- 22.Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1:433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 23.Atkins MB, Larkin J. Immunotherapy combined or sequenced with targeted therapy in the treatment of solid tumors: current perspectives. J Natl Cancer Inst. 2016;108:414. doi: 10.1093/jnci/djv414. [DOI] [PubMed] [Google Scholar]
- 24.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 26.Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016;17:943–955. doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasquali S, Chiarion-Sileni V, Rossi CR, Mocellin S. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: a network meta-analysis. Cancer Treat Rev. 2017;54:34–42. doi: 10.1016/j.ctrv.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3:1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
- 30.LeBoit PE, Burg G, Weedon D, Sarasain A. Pathology and genetics of skin tumours. 3. Lyon: IARC Press; 2006. [Google Scholar]
- 31.Iorgulescu JB, Harary M, Zogg CK, et al. Improved risk-adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: results from a national cohort. Cancer Immunol Res. 2018;6:1–7. doi: 10.1158/2326-6066.CIR-18-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaikh WR, Dusza SW, Weinstock MA, et al. Melanoma thickness and survival trends in the United States, 1989–2009. J Natl Cancer Inst. 2016;108:1. doi: 10.1093/jnci/djv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) Ann Surg Oncol. 2012;19:2547–2555. doi: 10.1245/s10434-012-2398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88:986–997. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joosse A, de Vries E, Eckel R, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Investig Dermatol. 2011;131:719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


