Abstract
The methionine sulfoxide reductase (Msr) system is known for its function in reducing protein-methionine sulfoxide to methionine. Recently, we showed that one member of the Msr system, MsrA, is involved in the ubiquitination-like process in Archaea. Here, the mammalian MsrA is demonstrated to mediate the ubiquitination of the 14–3-3 zeta protein and to promote the binding of 14–3-3 proteins to alpha synuclein in brain. MsrA was also found to enhance the ubiquitination and phosphorylation of Ser129 of alpha synuclein in brain. Furthermore, we demonstrate that, similarly to the archaeal MsrA, the mammalian MsrA can compete for capturing ubiquitin using the same active site it contains for methionine sulfoxide binding. Based on our previous observations showing that MsrA knockout mice have elevated expression levels of dopamine and 14–3-3 zeta and our current data, we propose that MsrA-dependent 14–3-3 zeta ubiquitination affects the regulation of alpha synuclein degradation and dopamine synthesis in the brain.
Keywords: Ubiquitin, 14–3-3 proteins, alpha synuclein, brain
Graphical Abstract
Introduction
Accumulation of aggregated forms of α-synuclein (αSyn) and phosphorylated tau are associated with the development of the incurable neurodegenerative diseases, Parkinson’s disease (PD) and Alzheimer’s disease (AD). One family of proteins that regulates the stability and function of these forms of αSyn and tau are the 14–3-3 proteins that serve in signaling and interact with various protein partners including modifying their function as adaptors that stimulate protein-protein interactions. For example, the isotype 14–3-3ζ induces oligomerization of its target proteins (e.g., phosphorylated tau) [1,2]. Proteins bound to 14–3-3ζ are relatively resistant to protein phosphatases [1–3]. Thus, high levels of 14–3-3ζ are proposed to increase the levels of phosphorylated tau that is a marker associated with AD [1]. Furthermore, binding of 14–3-3 proteins to phosphorylated tyrosine hydroxylase (TH) enhances dopamine (DA) production that can cause the generation of toxic levels of cytosolic DA, quinones, and reactive oxygen species (ROS) [4]. The αSyn protein binds to 14–3-3 proteins as well as the dephosphorylated form of TH, the latter resulting in reduced TH activity [4]. Thus, the sequestration and regulation of 14–3-3 proteins by αSyn play an important role in the pathogenesis of neurodegenerative diseases.
MsrA is a stereospecific methionine sulfoxide (MetO) reductase, conserved across all domains of life, and provides antioxidant defense by catalyzing the reduction of S-MetO to methionine [5]. MsrA knockout (KO) mice exhibit high levels of DA and 14– 3-3ζ compared with the corresponding wild-type (WT) strain [6]. Recently, we found the archaeal MsrA can direct ubiquitin-like (Ubl) protein modification by a mechanism that is distinct from its MetO reduction/oxidation activity [7]. These observations prompted us to investigate the interaction between the MsrA, 14–3-3ζ, αSyn, and the ubiquitin (Ub) system and how these systems may relate to PD in brain.
Specifically, using mouse brain extracts and immunoprecipitation experiments we described positive effect of MsrA on the ubiquitination of 14–3-3ζ and the MsrA- dependent interaction between 14–3-3 and αSyn. We also determined that MsrA is able to bind Ub and that the level of its MetO reductase activity is upregulated in the heterozygote and knockout 14–3-3ζ mouse brain, compared to the control WT strain.
Material and Methods
Key resources
Key resources used in this study, including mouse strains, antibodies, proteins, and reagents, are listed in Supplemental Table S1.
The WT and MsrA KO strains were generated as previously described [8]. The 14–3-3ζ heterozygotes and homozygotes as well as the corresponding parent WT strain were also generated, as previously described [9]. These strains crossbred for more than 20 generations.
Identification of ubiquitinated 14–3-3ζ in mouse brain
Whole brains of MsrA KO and WT mice were extracted in phosphate buffered saline (PBS) supplemented with a protease inhibitor cocktail and MG132 proteasome inhibitor using a Teflon homogenizer. Equal amounts of the brain extracts [500 μg of protein, determined by Bradford reagent (Bio-Rad, Hercules, CA)] were incubated with 10 μg of His-tagged Ub (His-Ub) in the presence or absence of 10 mM ATP and 10 mM MgCl2 (Mg-ATP) for 30 min at 37°C. At the end of the incubation time, the reaction mixtures were added to 50 μl of pre-washed His-tag Purification Nickel Resin. After 1 h incubation at room temperature, the mixtures were centrifuged (500 × g, room temperature), and precipitated protein-bound resin mixtures were washed with PBS. Proteins were eluted from the resin using 1X SDS-PAGE sample buffer supplemented with 0.1 M imidazole and 0.1 M EDTA. The eluates were subjected to Western blot analysis using anti-Ub as the primary antibody. A parallel gel was stained by Coomassie blue R-250 and the ubiquitinated protein bands, observed in the Western blot analysis, were excised from the gel. In-gel tryptic digestion of the acquired protein band was performed, followed by tandem mass-spectrometry (MS/MS) analysis of the resulting peptides for protein and sequence identification of the target protein, as we previously described [10]. Briefly, samples were analyzed by LC-MS/MS using a NanoAcquity chromatographic system (Waters Corp., Milford, MA) coupled to an LTQ-FT mass spectrometer (ThermoFinnigan, Bremen, Germany). Peptides were separated on a reverse phase C18 column. MS/MS spectra were analyzed using Mascot (Matrix Science, London, UK; version 2.3), Sequest (Thermo Fisher Scientific, San Jose, CA; version 27, rev. 12) and X! Tandem (The GPM, thegpm.org; version 2007.01.01.1). X! Tandem was set up to search a subset of the SwissProt_2012 database. Scaffold software (version 3.6, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identification.
Immunoprecipitation experiments
Immunoprecipitation was conducted using extracts (0.5 mg protein) of whole brain from MsrA KO and WT mice, prepared as described above. The brain extracts were incubated with the His-Ub (5 μg) in the presence or absence of 10 mM Mg-ATP. After 20 min incubation at 37°C, the rabbit anti-14–3-3 antibody was added to each reaction mixture and incubated for an additional 1 h at room temperature. After incubation, each sample was added to pre-washed protein G Sepharose and rotated overnight at 4°C. The protein G beads were washed with PBS (5 × 1 mL) to remove unbound proteins. After washing, the proteins were eluted from the protein G Sepharose using 1X SDS-PAGE sample buffer, containing β-mercaptoethanol, and boiled. The eluates were subjected to Western blot analyses using mouse anti-αSyn antibody or mouse anti-Ub antibody. Immunoprecipitation was also conducted following the same procedure, while alternatively using a mouse anti-Ub antibody and probing the Western blot membrane with either rabbit anti-αSyn antibody or rabbit anti-14–3-3 antibody. In all cases, Western blots were repeated at least three times with essentially the same outcomes. Representative blots are shown.
Phosphorylation of α-synuclein in mouse brain extract. Alpha synuclein (αSyn) of MsrA
KO and WT brain extracts was immunoprecipiated using rabbit anti-αSyn antibody. Following the immunoprecipitation, the precipitates were subjected to Western blot analyses using mouse anti-αSyn antibody or mouse anti- αSyn phospho-Ser129 antibody.
In vitro Parkin and E2 auto-ubiquitination assays
Full length recombinant parkin protein was prepared as described previously [11], except that the solubility factor .GB1 was incorporated into the N-terminus in place of GST. This parkin construct used has an N-terminal GB1 tag giving the construct a molecular weight of 51.7 KDA. This N-terminally tagged Parkin is known to be in an uninhibited form (i.e. activated) form [12].
In addition, parkin has been shown capable of E2-independent ubiquitination but only under conditions where there is a high concentration of the E1 ligase and at pH greater than 8.5 [13]. Those conditions don’t apply here. We have also determined that the observed activity is E2-dependent (Ubch7) (results not shown). Accordingly, ecombinant proteins were added to a reaction mixture as follows: 5 μM of MsrA, 10-μM His-Ub, and 22 nM E1. For the autoubiquitination assay of the E3 ligase parkin, parkin (0.9 mg/mL) and its relevant 1.4 μM E2 (UbcH7) were added to the reaction mixture. For the E2 autoubiquitination assay, 1.4 μM E2 (UbcH5a) replaced E3 parkin and E2 UbcH7 in the reaction. The reaction buffer contained 50 mM Tris-HCl (pH 8.0) and 1 mM DTT supplemented with and without 10 mM Mg-ATP. The reaction mixtures were incubated for 30 min at 37°C. At the end of the incubation time, the extracts were diluted to 1X SDS-PAGE sample buffer and subjected to Western blot analysis using anti-Ub monoclonal antibody.
Msr activity assay
Msr activity was determined as previously described [8]. Briefly, the reaction mixture (100 μl) contained dabsyl-MetO as a substrate (200 μM), 25 mM Tris-HCl (pH 7.5), 20 mM DTT, and 100 μg of brain protein extract and 10 μg for recombinant MsrA. The reaction mixture was incubated for 30 min at 37°C and stopped by the addition of 100 μl acetonitrile. Thereafter, the sample was subjected to HPLC-based C-18 chromatography to identify the product, dabsyl-methionine. Reaction mixtures containing recombinant MsrA in conjunction with the Ub system (in the presence or absence of E1+ UbcH5a ± Mg-ATP) were pre-incubated for 15 min at 37°C. The samples were incubated for an additional 15 min, in the presence of the substrate (dabsyl-MetO) prior to detection of Msr reaction product (dabsyl-methionine) by HPLC. Msr activity was also determined in the whole brain extracts of 14–3-3ζ heterozygotes and homozygotes and their corresponding wild-type mice, which were homogenized in PBS in the presence of a protease inhibitor cocktail and MG132 proteasome inhibitor.
Results
14–3-3ζ identified as a high-level ubiquitinated protein in wild type relative to MsrA knockout mice
To examine the impact of MsrA on ubiquitination in vitro, equal proteins brain extracts of wild type (WT) and MsrA knockout (KO) mice were incubated with His-tagged Ub (His- Ub) in the presence or absence of Mg-ATP. The advantages of using this type of His-Ub pull procedure are the protection against deubiquitinating enzymes, strong avidity, and the low unspecific binding. Proteins covalently modified by the His-Ub were enriched by Ni2+ -affinity purification and subjected to anti-Ub Western blot analysis. Protein bands with ubiquitination levels that showed both MsrA and ATP dependency were observed. In particular, an ubiquitinated protein band of ~36 kDa was detected in the WT mouse brain with Mg-ATP that was not detected in the MsrA KO or in the absence of Mg-ATP (Figure 1). A parallel gel stained with Coomassie blue R-250 was used to identify the protein bands of interest by tandem mass spectrometry (MS/MS) analysis (data not shown). By this approach, the 36-kDa ubiquitinated protein was identified as 14–3-3ζ (Figure 2). Moreover, diglycine footprints were found on the lysine of the tryptic peptides (Figure 2), that indicated 14–3-3ζ was ubiquitinated at lysine residues K138 and K139. The increased molecular mass of 14–3-3ζ from ~28 kDa to ~36 kDa suggested that one Ub molecule was covalently bound to this protein, as the mass of Ub is ~8 kDa (although it can run at a lower mass in SDS-gel-electrophoresis). Thus, our data (Figures 1 & 2) indicated that 14–3-3ζ ubiquitination was significantly enhanced in the presence of MsrA.
Fig 1. Detection of ubiquitinated proteins in extracts of wild type (W) and MsrA knockout (M) mouse 14–3-3ζ brains.
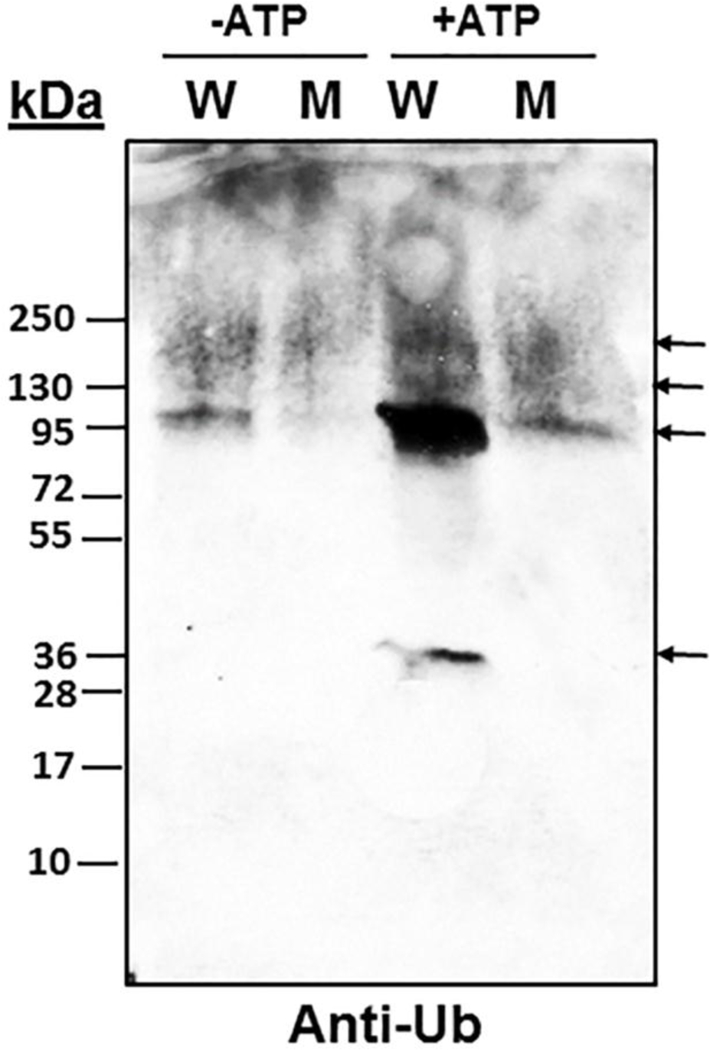
Brain extracts were prepared in PBS in the presence of protease and proteasome inhibitors. Equal amounts of protein extracts (see loading control gel) were incubated with His-Ub with and without Mg-ATP (10 mM of each) for a period of 30 min at 37°C. The proteins were enriched by Ni2+ -affinity purification prior to adding SDS-gel-electrophoresis sample buffer, followed by Western blot analysis using anti-Ub antibody. Both Anti-Ub Western blot (WB) and Coomassie blue staining showed the same protein pattern and intensity (the protein-stained gel was subjected to MS/MS analysis and thus is not shown). kDa, molecular mass markers for both the loading control protein stained gel (Commassie blue staining) and the WB. Arrows indicate the position of major ubiquitinated proteins.
Fig 2. Identification 14–3-3ζ as the MsrA- and ATP-dependent ubiquitinated protein by mass spectrometry analyses.

The identified 36-kDa ubiquitinated protein band (see Figure 1) was subjected to trypsin digestion followed by mass-spectrometry analyses for protein identification (see “Experimental procedures”). The “ylaevaagddkk” sequence indicated in bold was a tryptic peptide detected by MS/MS analysis. The underlined KK residues are the site of ubiquitination detected on the peptide by the presence of a diglycine (-Gly-Gly) remnant of the C-terminal residues of ubiquitin. Either of these lysines was found to be modified and these two lysines could not be distinguished due to their close proximity.
MsrA influences the interaction between αSyn and 14–3-3 proteins and their levels of ubiquitination
The interrelation between 14–3-3 proteins and αSyn has been already investigated in details demonstrating the following observations: a) Specific 14–3-3 isoforms co-localize with αSyn in a transgenic mouse model of PD [14]; b) 14–3-3 and αSyn from rat brain and the substantia nigra of PD patients co-immunoprecipitated [15, 16]; c) most importantly, large soluble complexes of αSyn and 14–3-3 were observed in human neuronal cells overexpressing αSyn [17]; d) Likewise, the 14–3-3ƞ isotype was shown to strongly interact with only oligomeric αSyn aggregates but not monomeric αSyn [18]; suggesting that14–3-3ƞ may reduce αSyn-mediated cellular toxicity [18]. Consequently, we hypothesize that the binding process of 14–3-3 proteins to αSyn through the production of large complexes is both dependent on 14–3-3 modification (e.g. ubiquitination) and the presence of MsrA.
Accordingly, we first investigated how MsrA affecting not only the ubiquitination levels of 14–3-3ζ, but how it may also impact the binding of 14–3-3ζ to αSyn in brain. The 14–3-3 antibody, which recognizes 14–3-3ζ and other members of the 14–3-3 protein family, was used for this analysis. In addition, an antibody specific for αSyn and an anti- Ub antibody that recognizes the multiple forms of Ub (mono- and poly-Ub protein conjugates, free poly-Ub chains, and free Ub) were used. First, we immunoprecipitated proteins from the brain extracts of MsrA WT and KO mice using mouse anti-Ub antibody and then probed these fractions for 14–3-3 proteins or αSyn by Western blot analysis. As shown in Figure 3A, MsrA KO brain showed somewhat lower levels of the ubiquitinated products of both αSyn and 14–3-3 proteins compared with WT brain. The reduction in the molecular mass of the mono-ubiquitinated form of 14–3-3 to the observed less than 36kDA molecular mass protein products (Figure 3A), and together with the observed multiple banding patterns of both proteins, indicates that they are partially degraded. A similar pattern was observed with respect to the ubiquitinated αSyn forms, in which their molecular masses depend on the level of ubiquitination and SDS-resistant oligomers of αSyn (Figure 3A). These results suggest that both αSyn and 14–3-3 proteins are ubiquitinated and degraded more efficiently in the presence of MsrA in vivo (note that protease and proteasome inhibitors were present during the brains extraction). The efficiency of using only Western blot analyses for the detection of ubiquitinated 14–3-3 forms is limited. Accordingly, immunoprecipitation experiments were performed to enrich the detection of the ubiquitinated 14–3-3 forms. Immunoprecipitation with anti-14–3-3 antibody suggests that either 14–3-3 proteins, or their protein-binding partners, or both are indeed more ubiquitinated and Ub-dependent degraded in the WT strain (as reflected by the presence of mid to low size molecular mass bands) (Figure 3B). This later result is in agreement with the ability of 14–3-3 and αSyn to produce large soluble complexes [17, 18]. Furthermore, it seems like the profile of the 14–3-3 immunprecipiates is nearly the same for both anti- αSyn and anti-Ub Western blots, suggesting that the vast majority of the detected protein bands in Figure 3B (lanes 1 and 3) are either ubiquitinated 14–3-3, or ubiquitinated αSyn, or a mixture of both that interact with each other in an MsrA- dependent manner. Next, to show the dependency on ATP in this process, a comparative experiment in which the brain extracts were incubated in the presence or absence of Mg-ATP for 30 min at 37°C was performed, prior to the immunoprecipitation by 14–3-3 antibody. The WT brain showed an enhanced degradation and ubiquitination of αSyn in the presence of Mg-ATP. However, in the brain of the MsrA KO strain, the levels of the monomer and the ~36kDa form of αSyn were increased by the addition of Mg-ATP, while no mid-to-low molecular mass ubiquitinated proteins were observed (Figure 3C). The level of protein phosphorylation may retard the mobility of any protein in an SDS-gel electrophoresis due to increased polarity. Thus, it is possible that the observed ~36kDa αSyn represents one of the phosphorylated forms of αSyn. Furthermore, the enhanced non-ubiquitinated αSyn band shown in the presence of Mg- ATP in the MsrA KO mouse brain extract points to the possibility of an enhanced αSyn stability in the MsrA KO versus the WT strain (Figure 3C). This observation correlates with a previous suggestion that 14–3-3 proteins protect the phosphorylated form of αSyn from being dephosphorylated at the presence of high DA levels (like in the case of MsrA KO brain) [6]. This function of 14–3-3 is predicated to be an Ub and MsrA-independent process, as suggested by the protection of αSyn from degradation in the absence of MsrA (i.e. in MsrA KO brain and in yeast msrA KO overexpressing MsrA [19]). Western blot analyses showed equal levels of the loading controls (αSyn, 14–3-3, and β-actin) in each brain extract used for the immunoprecipitation experiments (Figure 3D).
Fig 3. Binding affinity interactions between Ub, α-synuclein (αSyn), and 14–3-3 proteins in extracts of wild type (W) and MsrA knockout (M) mouse brains.
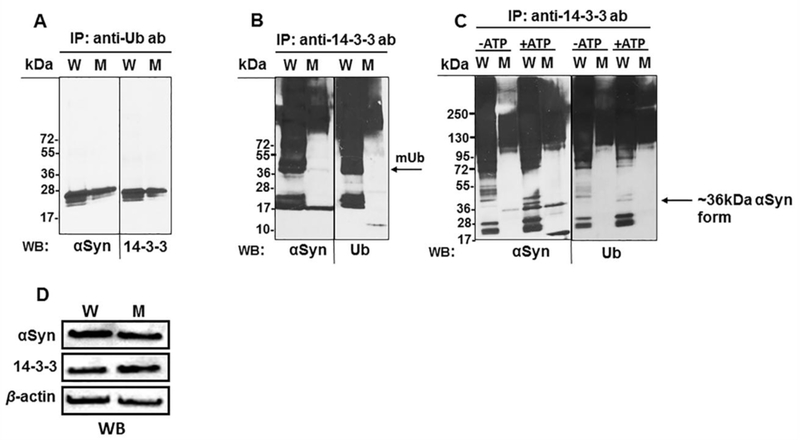
Immunoprecipitation (IP) experiments (A-C) were performed on brain extracts, followed by Western blot (WB) analysis using the protein antibodies (mouse anti-αSyn, mouse anti-Ub, and rabbit anti-14–3-3 antibodies) as indicated. The exception was in panel A, where the anti-αSyn antibody was from rabbit (since the precipitating anti-Ub antibody was from mouse). In panel C, the brain extracts were incubated for 20 min at 37°C with the His-Ub (5 μg) in the presence or absence of 10 mM Mg-ATP, prior to performing the IP procedure (D). Western blot analyses for loading controls of the W and M extracts prior to the immunoprecipitation (mouse anti-β actin antibody, mouse anti-αSyn, and rabbit anti-14–3-3 antibodies were used). kDa, molecular mass markers; mUb arrow, mono ubiquitination band of 14–3-3.
To determine whether αSyn in the MsrA KO brain is indeed hyper-phosphorylated compared to the WT strain, we have immunoprecipitated αSyn in each brain extract and probed the precipitates in Western blot analyses using mouse anti-αSyn or rabbit anti- αSyn phospho-Ser129 (the major phosphorylation site that is important for αSyn regulation). As demonstrated in Figure 4B, the relative level of phospho-Ser129 of a ~24 kDa form of αSyn was higher in the MsrA KO strain than the WT strain (while both strains had similar expression levels of non-phosphorylated αSyn, as indicated by probing with the anti-αSyn antibody) (Figure 4A). Noteworthy, the extracts used in Figure 4 were the same extracts used in Figure 3 and thus their loading controls are showed in Figure 3D. The level of protein phosphorylation may retard the mobility of any protein in an SDS-gel electrophoresis due to increased polarity. Thus, it is possible that the observed ~24kDa αSyn represents one of the phosphorylated forms of αSyn.
Fig 4. Phosphorylation of α-synuclein (αSyn) in wild type (W) and MsrA KO brains (M).
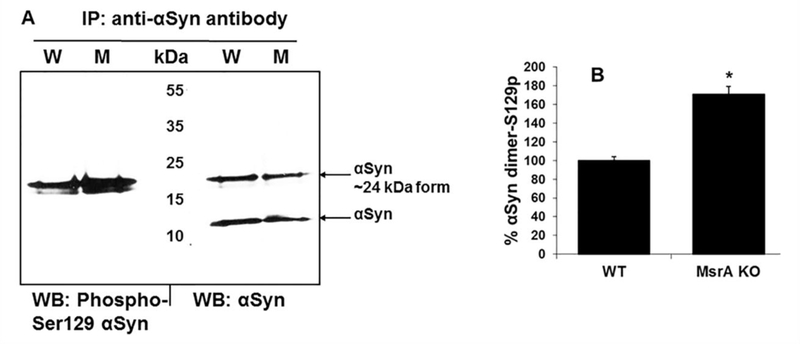
A. Alpha synuclein of each brain extract was immunoprecipiated using rabbit anti- αSyn antibody. Following the immunoprecipitation (IP), the precipitates were subjected to Western blot (WB) analyses using mouse anti-αSyn antibody or rabbit anti-αSyn phospho-Ser129 antibody. B. Ratio of the densitometry analyses (using NIH Image-J program) of the phosphorylated αSyn dimer over the total non-phosphorylated αSyn homodimer, presented as percent of wild type (WT) ratio. *, indicates significant statistical differences between the MsrA KO and WT group of mice (P< 0.01 using t-test program). Note, the loading controls for the brain extracts used in this figure are shown in Figure 3D.
Competition between MsrA and E2 and E3 enzymes on Ub binding
The data presented above suggest that ubiquitination of specific proteins is mediated by MsrA. To determine and evaluate the ability of MsrA to bind Ub, the effect of MsrA on the in vitro autoubiquitination of parkin was assessed. Parkin catalyzes E3 Ub ligase activity and is thought to directly or indirectly mediate proteasomal degradation of aggregation- prone proteins, including αSyn. Moreover, mutations in the Parkin gene are the most common cause of hereditary parkinsonism [20]. In agreement with previous findings [20], parkin showed a strong autoubiquitination in the presence of E1 and E2 (UbcH7) in the expected 55 kDa region (Figure 5A Lane 3). Surprisingly, this auto ubiquitination disappeared when MsrA was introduced to the system (Figure 5A, Lane 5). Autoubiquitination of MsrA was not observed in this in-vitro system (Figure 5A). This result suggests that MsrA inhibits the autoubiquitination of parkin, possibly through competing for binding Ub and/or its intermediates in the process. Similarly, the known auto-ubiquitination of another E2 (UbcH5a) was inhibited upon the addition of MsrA to the reaction mixture, especially in the reaction mixtures containing ATP (compare Lanes 2 and 4 in Figure 5B). UbcH5a is a major E2 protein that is active in a wide-ranging number of in vitro ubiquitination reactions and binds Ub non-covalently on a surface that is distinct from its SH-containing active site [21]. Thus, MsrA is found to inhibit not only the ubiquitin-dependent degradation of 14–3-3 and α-Syn (in vivo) but also the autoubiquitination of parkin and E2 (UbcH5a) via a direct mechanism (as monitored in vitro). It is important to note that no autoubiquitination of the E1 used in this system was detected (data not shown).
Fig 5. Effect of MsrA on Parkin and E2 (UbcH5a) auto-ubiquitination.
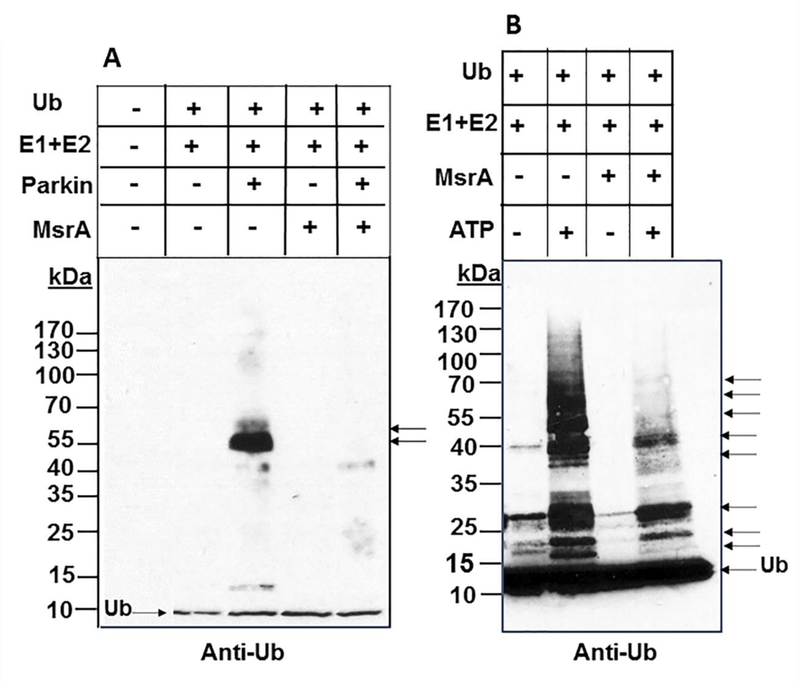
Recombinant proteins were added to reaction mixtures as shown above the images. The reaction mixtures were incubated for 30 min at 37°C. The reactions were stopped by adding 1X SDS-PAGE sample buffer, followed by Western blot analysis using anti-Ub antibody. Panel A: detection of parkin auto-ubiquitination as indicated by arrowheads. Panel B: detection of UbcH5a ubiquitinations as indicated by arrowheads. No E1 autoubiquitination was detected when the reaction contained only E1, Ub, and ATP (data not shown). kDa, molecular mass markers; Ub, ubiquitin.
Next, we correlated the MetO reductase activity of MsrA with the Ub system. MsrA MetO reductase activity was measured in the presence or absence of the Ub system (E1, E2, and Ub) and ATP. As shown in Figure 6, the MetO reductase activity of MsrA was strongly inhibited when the Ub system was activated by Mg-ATP (vs. absence of Mg-ATP). The complexity of the reaction mixture makes it difficult to determine the linearity of the events that result in either MetO reduction or Ub binding by the MsrA enzyme. However, the presented data clearly demonstrate that the Mg-ATP -dependent processes, yielding activated Ub, can compete with dabsyl-MetO binding by the MsrA’s active site (Figure 5A). It is important to note that MsrA was not ubiquitinated under these conditions (data not shown). Therefore, the MsrA activity was not affected by Ub modification to MsrA. Taken together, our data imply that the active site of the mammalian MsrA for MetO reduction can bind either MetO or the intermediates and/or products of ubiquitination, as it has been shown to be the case for archaeal MsrA [7].
Fig 6. MsrA activity in inhibited in the presence of E1, E2, Ub, and ATP.
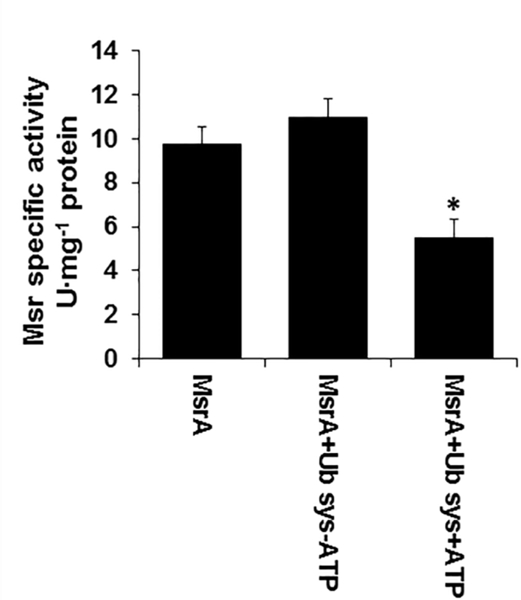
Recombinant human MsrA was incubated at 37°C for 30 min in the presence or absence of E1+E2+Ub and ± ATP followed by Msr activity determination (according to the related procedure described in “Materials and Methods”). *, indicates significant statistical differences between the MsrA+Ub system (Sys) + ATP group and the other two groups of mice (P< 0.01 using one-way Anova program).
Effect of 14–3-3ζ ablation on Msr activity in mouse brain
MsrA inhibits the autoubiquitinylation of parkin (Figure 5A) and E2 (UbcH7) (Figure 5B) and fosters 14–3-3ζ ubiquitination (Figure 1) and stabilization (Figure 3A). Accordingly, if 14–3-3ζ is a major substrate for MsrA-dependent ubiquitination, its ablation will cause the active site of MsrA to be less occupied with autoubiquitinylation intermediates and/or products and, therefore, will be more available for MetO reduction activity. Supporting evidence for this hypothesis is shown in Figure 7A, in which the MetO reductase activity of Msr was significantly elevated in brain of the 14–3-3ζ−/− and 14–3-3ζ−/+ mice compared with the 14–3-3ζ+/+ mouse. Although the MetO reductase activity was the highest in brain of the 14–3-3ζ−/− mouse, it was comparable to the 14–3-3ζ−/+ strain (Figure 7A). The latter observation suggests that a 50 % reduction of 14–3-3ζ expression is sufficient to elevate MsrA activity by enhancing its MetO reduction activity. The levels of MsrA were also found elevated in the 14–3-3ζ−/+ and 14–3-3ζ−/− strains compared to 14–3-3ζ+/+ (Figure 7B). Thus, the levels of MsrA protein and MetO reductase activity were increased in parallel by progressive KO of 14–3-3ζ
Fig 7. MsrA expression is upregulated in the absence of 14–3-3 ζ in mouse brain.
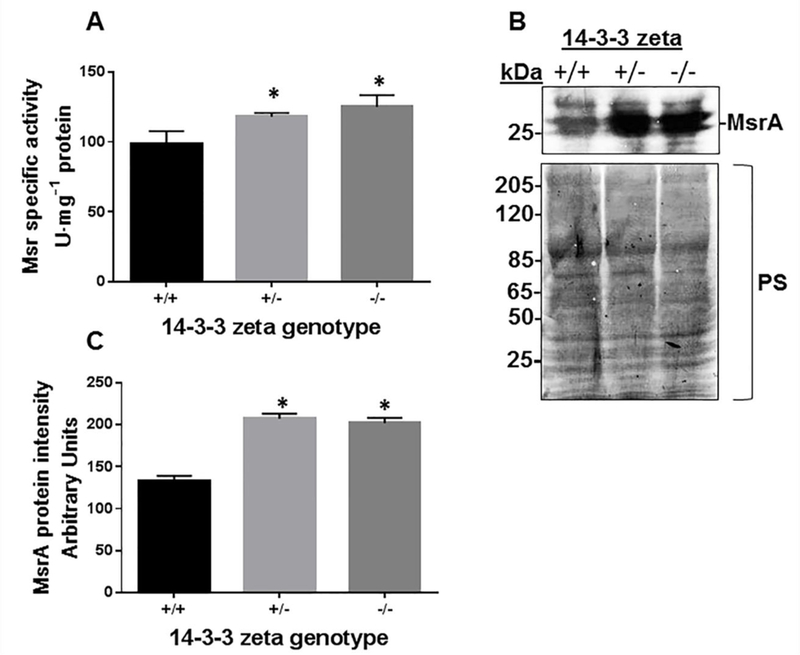
Protein extracts from mouse brains of three strains (14–3-3 ζ +/+, 14–3-3 ζ +/− and 14–3-3 ζ −/− ) were analyzed for Msr activity (A), MsrA expression (through Western blot analysis using anti-MsrA antibody) (B) that was also quantified using NIH Image-J program (C). kDa, molecular mass markers; PS, Ponceau-S staining used for total protein staining; *, indicates significant statistical differences between the 14–3-3 ζ +/+ group and each of the other group of mice (P< 0.01 using one-way Anova program).
Discussion
In agreement with the role of 14–3-3 proteins described in the introduction section, we have provided supportive evidence demonstrating that lack of MsrA protein inhibits the degradation rate of αSyn in a yeast ex vivo system [19], while increasing DA ,14–3-3ζ, and tau-phosphorylation levels in an MsrA knockout (MsrA KO) mouse [6,22]. 14–3-3 proteins and αSyn are abundantly expressed in the brain (~1% of total proteins) and are degraded by the Ub system. 14–3-3 proteins interact with many binding partners, including αSyn, affecting cell cycle and transcriptional control, signal transduction, intracellular trafficking, and regulation of ion channels [23]. Furthermore, studies performed on animal models and on post mortem brains of patients with neurodegenerative diseases, like PD and AD, point to the involvement of 14–3-3 proteins in disease-associated processes [23]. In PD, deposition of misfolded and aggregated forms of αSyn in Lewy bodies in the substantia nigra region of brain is associated with the development of this disease. Both 14–3-3 and αSyn can regulate DA levels in the striatum; thus, dysregulation and abnormal structure of either protein may affect DA synthesis (through TH activation). Accordingly, the cellular mechanisms that are responsible to control the levels of each protein (synthesis vs degradation pathways) play a significant role in maintaining normal function of the dopaminergic cell. As with other neurodegenerative diseases, the sporadic form of PD is an age and oxidative stress associated disease. As such, oxidative modification of αSyn and other proteins may lead to posttranslational modifications that are either “repaired” by specific enzymes or degraded via the Ub or autophagy systems. One established oxidative damage repair mechanism is mediated by the Msr system [5]. We and others have shown that lack of MsrA exacerbates the accumulation of oxidized proteins in the cell, reduces cell/organism survival under oxidative stress conditions, and alters the function of specific proteins (e.g., DA D2-receptor) [5, 24]. Given the dual function of MsrA in the current studies, we propose that MsrA enhances 14–3-3 ubiquitination (Figures 1 & 2), which in term may facilitate the degradation of 14–3-3 through the Ub-proteasome system. MsrA is an ideal candidate for the selection process aiming to either “repair” or “destroy” a protein, as MsrA can “repair” proteins by reducing MetO residues (e.g., of αSyn) or make a targeted protein (e.g., 14–3-3) available for destruction by the Ub system. The increased affinity of the interaction of αSyn with 14–3-3 proteins, due to the presence of MsrA and their increased ubiquitination levels (Figure 3), suggests that MsrA plays an important role in modulating the ubiquitination of both proteins; either directly (e.g., by functioning as an adaptor for the ubiquitination system) or indirectly (through a yet to be discovered protein factor). The 14–3-3 proteins can bind αSyn in the presence of high levels of DA and protect 14–3-3 from being degraded via the Ub system, through binding to a specific phosphorylated Ser129 of αSyn [25]. Thus, it is suggested that the presence of MsrA enhances αSyn degradation by eliminating this protective effect of 14– 3-3ζ through ubiquitination and degradation (Figure 8). Ub and ATP-dependent competition experiments suggest that MsrA can bind either auto-Ub system intermediates/products or MetO residues via its active site (Figures 5 and 6); a phenomenon that agrees with our previous observation in Archaea [7]. Lack of 14–3-3ζ protein in the 14–3-3ζ KO brain caused an upregulation of MsrA expression and associated activity (Figure 7). The protein 14–3-3ζ is required for Klotho’s anti-oxidant activity via the ASK1/p38 MAPK pathway, while interacting with thioredoxin [26].
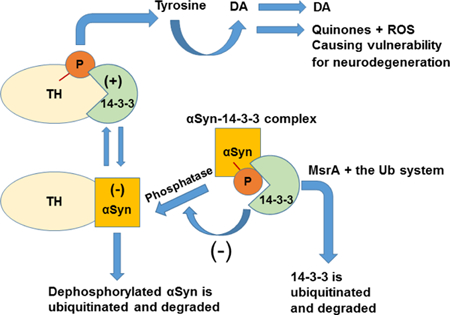
Fig 8. A summary figure describing the Involvement of MsrA in the regulation of 14–3-3 proteins and its effect on synuclein (αSyn) levels and function.
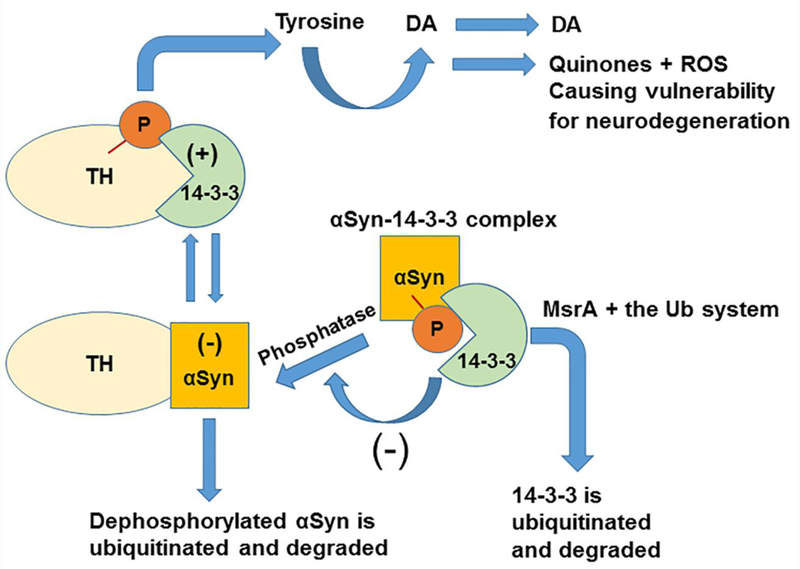
14–3-3 proteins (green) that bind to phosphorylated tyrosine hydroxylase (TH) (yellow), and αSyn (orange) that binds to unphosphorylated TH, differentially regulate TH activity to maintain optimal dopamine levels. In the case of reduced levels of MsrA (e.g. in MsrA KO mice), 14–3-3 (e.g. 14–3-3 ζ) is not efficiently degraded by the ubiquitin system and thus preferentially binds to phosphorylated αSyn (P in red, a phosphorus group). This situation provides protection of αSyn from dephosphorylation events, which promote its ubiquitination and degradation. We have provided supportive evidence demonstrating that lack of MsrA protein inhibits the degradation rate of αSyn in a yeast ex vivo system [18] and increases the stability of 14–3-3ζ in mouse brain [6]. It is hypothesized that upon lower levels of MsrA (e.g. the MsrA KO brain), 14–3-3ζ bind strongly to non-aggregated phosphorylated forms of αSyn. This phenomenon leads to a higher stability of αSyn and an enhanced activation of TH, causing an increase in dopamine (DA) formation and release [6]. However, increased levels of ubiquitinated forms of 14–3-3ζ in WT brain (a phenomenon supported by this paper) seem to bind more strongly to non-monomeric αSyn forms, causing the formation of high molecular mass aggregates of 14– 3-3-αSyn complexes. (+), positive regulatory effect of 14–3-3 on TH activity. (−), negative regulatory effect of αSyn on TH activity and negative regulatory effect of 14–3-3 on αSyn dephosphorylation. ROS, reactive oxygen species.
Consequently, enhanced oxidative stress in the 14–3-3ζ KO brain is hypothesized to cause an upregulation of MsrA expression as a compensatory mechanism. If so, the MsrA may protect against the resulting ROS-mediated damage to proteins by either its MetO reduction activity or by its Ub-modifier activity, or both. A summary of the proposed role of MsrA in DA regulation, with respect to 14–3-3ζ and αSyn functions is illustrated in Figure 8. Future studies will focus on screening for other Ub related factors and conditions that regulate and mediate the interactions between MsrA and its substrates.
Conclusions
Posttranslational modification to proteins such as oxidation of methionine residues ubiquitination may alter their function and/ or make them more prone to degradation, respectively. Here we show that the enzyme methionine sulfoxide reductase type A (MsrA) is able to function not only as a “repair” mechanism to “oxidative damaged” proteins but also as mediator for the ubiquitination of 14–3-3 proteins and specifically 14– 3-3ζ. Furthermore, the presence of MsrA enhances the phosphorylation levels of αSyn through the ubiquitination and degradation of 14–3-3ζ. The dual involvement of MsrA in both protein repair and degradation pathways strengthen its important role as a modulator of cellular function under oxidative stress conditions.
Supplementary Material
Highlights.
MsrA mediates 14–3-3 protein ubiquitination
MsrA enhances the interaction between specific forms of 14–3-3 and alpha synuclein
Phosphorylation levels of alpha synuclein are affected by MsrA
Acknowledgments
This research was supported by the Hedwig Miller Fund for Aging Research and by KU ADC P30-AG-035982 and the Landon Center on Aging provided to J. Moskovitz. Also, this research was supported by the NIH R01 GM57498 grant to J.A. Maupin-Furlow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Agarwal-Mawal A, Qureshi HY, Cafferty HY, Yuan PWZ, Han D, Lin R, Paudel HK, 14–3-3 connects glycogen synthase kinase-3β to tau within a brain microtubuleassociated tau phosphorylation complex. J Biol Chem 278 (2003) 12722–12728. [DOI] [PubMed] [Google Scholar]
- [2].Luo Z Z, Tzivion G G, Belshaw PJ, Vavvas D, Marshall M, Avruch J, Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature 383 (1996)181–185. [DOI] [PubMed] [Google Scholar]
- [3].Dent P, Jelinek T,, Morrison DK, Weber MJ, Sturgill TW, Reversal of Raf-1 activation by purified and membrane-associated protein phosphatases. Science 268 (1995) 1902–1906. [DOI] [PubMed] [Google Scholar]
- [4].Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ, A role for alpha- synuclein in the regulation of dopamine biosynthesis. J Neurosci. 22 (2002) 3090–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oien DB, Moskovitz J, Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr Top Dev Bio 80 (2008) 93–133. [DOI] [PubMed] [Google Scholar]
- [6].Oien DB, Osterhaus GL, Latif SA, Pinkston JW, Fulks J, Johnson M, Fowler SC, Moskovitz J, MsrA knockout mouse exhibits abnormal behavior and brain dopamine levels. Free Radic Biol Med 45 (2008) 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fu X, Adams Z, Liu R, Hepowit NL, Wu Y, Bowmann CF, Moskovitz J, Maupin-Furlow JA JA, Methionine Sulfoxide Reductase A (MsrA) and Its Function in Ubiquitin-Like Protein Modification in Archaea. MBio 8 Pii (2017) e01169–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER, Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 98 (2001) 12920–12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheah PS, Ramshaw HS, Thomas PQ, Toyo-Oka K, Xu X, Martin S, Coyle P, Guthridge MA, Stomski F, van den Buuse M, Wynshaw-Boris A, Lopez AF, Schwarz QP, Neurodevelopmental and neuropsychiatric behavior defects arise from 14– 3-3zeta deficiency. Mol Psychiatry 17 (2012) 451–466. [DOI] [PubMed] [Google Scholar]
- [10].Moskovitz J, Detection and localization of methionine sulfoxide residues of specific proteins in brain tissue. Protein Pept Lett 21 (2014) 52–55. [DOI] [PubMed] [Google Scholar]
- [11].Rankin CA, Joazeiro CA, Floor E, Hunter T, E3 ubiquitin-protein ligase activity of parkin is dependent on cooperative interaction of RING finger (TRIAD) elements. J Biomed Sci 8 (2001) 421–429. [DOI] [PubMed] [Google Scholar]
- [12].Chaugule VK, Burchell L, Barber KR, Sidhu A, Leslie SJ, Shaw GS, Walden H, Autoregulation os Parkin activity through its ubiquitin-like domain. The EMBO J 30 (2011) 2853–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chew KC, Matsuda N, Saisho K, Lim GG, Chai C, Tan HM, Tanaka K, Lim KL, Parkin mediates aApparent E2-independent monoubiquitination in vitro and contains an Intrinsic activity that catalyzes polyubiquitination. Plos One 6 (2011) e19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shirakashi Y, Kawamoto Y, Tomimoto H, Takahashi R, Ihara M, Alpha-synuclein is colocalized with 14–3-3 and synphilin-1 in A53T transgenic mice. Acta Neuropathol 112 (2006) 681–689. [DOI] [PubMed] [Google Scholar]
- [15].Ostrerova N, Petrucelli L,M, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B, Alpha-synuclein shares physical and functional homology with 14–3-3 proteins. J Neurosci 19 (1999) 5782–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sato S, Chiba T, Sakata E, Kato K, Mizuno Y, Hattori N, Tanaka K, 14–3-3eta is a novel regulator of parkin ubiquitin ligase. EMBOJ 25 (2006) 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu J, Kao SK, Lee FJ, Song W, Jin LW, Yankner BA, Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8 (2002) 600–606. [DOI] [PubMed] [Google Scholar]
- [18].Plotegher N, Kumar D, Tessari I, Brucale M, Munari F, Tosatto L, Belluzzi E, Greggio E, Bisaglia M, Capaldi S, Aioanei D, Mammi S, Monaco HL, Samo B, Bubacco L, The chaperone-like protein 14–3-3ƞ interacts with human α-synuclein aggregation intermediates rerouting the amyloidogenic pathway and reducing α- synuclein cellular toxicity. Hum Mol Genet 23(21) (2014) 5615–29. [DOI] [PubMed] [Google Scholar]
- [19].Oien DB, Shinogle HE, Moore DS, Moskovitz J, Clearance and phosphorylation of alpha-synuclein are inhibited in methionine sulfoxide reductase A null yeast cells. J Mol Neurosci. 39 (2009) 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rankin CL, Roy A, Zhang Y, Richter M, Parkin A Top Level Manager in the Cell’s Sanitation Department. Open Biochem J 5 (2011) 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brzovic PS, Klevit RE, Ubiquitin transfer from the E2 perspective: why is UbcH5 so promiscuous? Cell Cycle 5 (2006) 2867–2873. [DOI] [PubMed] [Google Scholar]
- [22].Pal R, Oien DB, Ersen FY, Moskovitz J, Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res 180 (2007) 765–774. [DOI] [PubMed] [Google Scholar]
- [23].Cheah PS, Ramshaw HS, Thomas PQ, Toyo-Oka K, Xu X, Martin S, Coyle P, Guthridge MA, Stomski F, van den Buuse M, Wynshaw-Boris A, Lopez AF, Schwarz QP, Neurodevelopmental and neuropsychiatric behavior defects arise from 14– 3-3zeta deficiency. Mol Psychiatry 17 (2012) 451–466. [DOI] [PubMed] [Google Scholar]
- [24].Oien DB, Ortiz AN, Rittel AG, Dobrowsky RT, Johnson MA, Levant B B, Fowler SC, Moskovitz J, Dopamine D(2) receptor function is compromised in the brain of the methionine sulfoxide reductase A knockout mouse. J Neurochem 114 (2010) 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Berg D, Holzmann C, Riess O, 14–3-3 proteins in the nervous system. Nat Rev Neurosci 4 (2003) 752–762. [DOI] [PubMed] [Google Scholar]
- [26].Brobey RK, Dheghani M, Foster PP, Kuro-O M, Rosenblatt KP, Klotho Regulates 14–3-3ζ Monomerization and Binding to the ASK1 Signaling Complex in Response to Oxidative Stress. PLoS One 10(10) (2015) e0141968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


