Abstract
Background:
Despite drastic improvement in overall survival for pediatric patients with cancer, those with osteosarcoma have stable rates of survival since the 1980’s. This project evaluates the effect of several variables on survival after first recurrence in patients with osteosarcoma.
Methods:
Data from three prospective North American cooperative group trials for newly diagnosed osteosarcoma are included: INT-0133, POG-9754, and AOST0121. The analytic population for this study is all enrolled patients with first event free survival (EFS) event of relapse. The primary outcome measure for this retrospective analysis was survival after recurrence (SAR).
Results:
The analytic population consisted of N=431 patients. SAR was statistically significantly associated with age at enrollment (< 10 years, p = 0.027), presence of metastatic disease at diagnosis (localized, p <0.0001), site of relapse (combination lung + bone, unfavorable, p=0.005) and time to first relapse (2+ years, favorable, p<0.0001) in multivariate analysis. Ethnicity, primary site of tumor, race, and sex were not significantly related to SAR.
Conclusions:
Prolonged SAR in patients with relapsed osteosarcoma is associated with age, extent of disease at diagnosis, site of and time to relapse. Adolescent and young adult patients with osteosarcoma have shorter SAR than younger patients, consistent with studies showing decreased overall survival in this group. Although patients with primary metastatic disease have shorter SAR, there is a subset of patients who relapse greater than 2 years from initial diagnosis that will become survivors. Histological response was significantly associated with time to relapse, but was not predictive of SAR.
Keywords: Recurrent osteosarcoma, prognosis, survival after recurrence, adolescent/young adult (AYA), histological response
INTRODUCTION
Osteosarcoma is the most common malignant bone tumor in children, adolescents, and young adults1. Following the inclusion of adjuvant chemotherapy in the frontline treatment of osteosarcoma in the 1970’s, the 6-year event free survival (EFS) of patients with non-metastatic disease improved from 11% to 61%.2 However, progress over the last 30 years has stalled with no significant improvement in event free survival, despite multiple randomized studies and international collaborations3. In addition, the outlook for children with metastatic disease remains poor, with an estimated EFS of <30% at 5 years.4,5 For those patients who experience disease relapse, the outcome is very poor with approximately 20% or less surviving, though limited data has been reported for this group.6,7,8,9
The identification of novel therapeutic agents that can be utilized for patients with newly diagnosed osteosarcoma is of critical importance to decrease relapse.10 The Children’s Oncology Group (COG) has initiated a series of phase II clinical trials aimed at identifying novel agents that have activity against osteosarcoma.11 However, the design of these studies is limited by the historical data for survival of relapsed patients treated on prior clinical trials. To expand our baseline understanding of patients with relapsed osteosarcoma and determine potential prognostic factors, we have reviewed the prior three COG studies for patients with newly diagnosed osteosarcoma and analyzed data from those who have relapsed.
METHODS
Patients
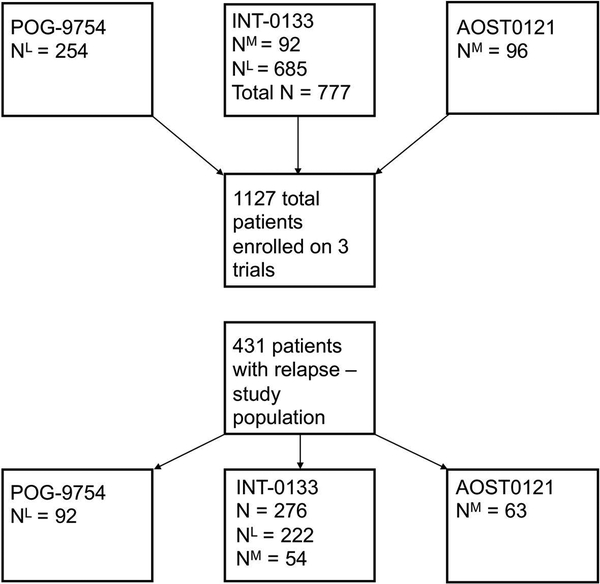
Data from the three most recent prospective, COG trials for newly diagnosed osteosarcoma were included in this analysis; INT-0133, POG-9754, and AOST0121 (Figure 1). Inclusion criteria for these trials have been previously published and eligibility criteria for each trial differed slightly.7,12,13 INT-0133 enrolled both patients with metastatic and non-metastatic disease regardless of resectability at institutions of the Children’s Cancer Group (CCG), but only patients with non-metastatic, resectable disease were included at the Pediatric Oncology Group (POG) sites. POG-9754 enrolled patients with non-metastatic disease, and AOST0121 enrolled those with metastatic disease, respectively. Patients with unresectable disease were enrolled only in the studies and/or treatment arms that included those with metastatic disease (Figure 1). The analytic population includes all patients enrolled on one of these three therapeutic trials with a first event defined as relapse. Diagnosis was defined as date of initial enrollment on therapeutic study, and relapse was defined as known recurrence of disease as reported to the COG. Therefore, our analysis included a variety of patients with newly diagnosed metastatic and non-metastatic disease, inclusive of any primary site. All three studies were approved by the institutional review boards of the participating institutions prior to enrollment. In addition, the patient and/or legal guardians were apprised of the nature of the research and consented to treatment.
Figure 1:
Patients enrolled on POG9754, INT0133, and AOST0121 were eligible for the current analysis if they experienced relapse as a first event.
Treatment
All patients enrolled received a standard chemotherapy backbone consisting of high dose methotrexate, doxorubicin, and cisplatin (MAP). Patients enrolled on INT-0133 received MAP with or without muramyl tripeptide-phosphatidyl ethanolamine (MTP-PE) and/or ifosfamide as previously described7,12. POG-9754 contained three arms that attempted to dose intensify chemotherapy using different combinations of MAP + IE based on initial tumor response12. Finally, all patients enrolled on AOST0121 received MAP with the addition of Ifosfamide and Etoposide (MAPIE); patients with tumors positive for the ERBB2 marker also received trastuzumab (Supplemental Table S1).
Recurrence of Disease
Site of disease at first recurrence was described as two variables: local recurrence (yes/no) and site of recurrence (bone only, lung only, bone and lung, other). On INT-0133, site of recurrence was recorded as a series of nine yes/no questions (recurrent disease of primary site, recurrent disease at previous metastatic site, new pulmonary metastases, new pleural metastases, new mediastinal disease, new regional metastases, new metastases to bone, new lymph node metastases, and other sites of relapse). “Recurrent disease at primary site” translated directly to local recurrence. If “recurrent disease at previous metastatic site” was indicated, the recorded metastatic site from enrollment was examined to determine the correct classification. Otherwise, sites were categorized as bone recurrence only, lung recurrence only, both bone and lung recurrence, or other (for lymph node, mediastinal or other sites). On POG-9754 and AOST0121, site of recurrence involved a classification as “Progression” or “Relapse” plus one or more ICD-O codes and one or more free text responses. Two of the authors (HSP, KAJ) reviewed the primary data and classified the recurrence sites accordingly.
Statistical Analysis
The primary outcome measure for this retrospective analysis was survival after recurrence (SAR). SAR was the time from the first recurrence of disease to death or last patient contact, whichever occurred first. Patients were considered as censored at last contact, if death had not occurred. The SAR of patient groups was estimated by the method of Kaplan and Meier14. The effect of parameters including demographic variables, tumor histologic response, site of disease at recurrence, and time to recurrence were analyzed for effect on SAR.
The outcome in terms of SAR was compared between patient groups in the analytic population. The relative risk for death was compared across the groups using the log-rank test14. The conventional alpha level of 0.05 was used to designate a comparison statistically significant. Demographic and covariates of interest included gender, age, race, ethnicity, site of primary tumor at diagnosis, metastasis at diagnosis, histologic response, site of disease at recurrence, and time to recurrence. Age category at enrollment, histologic response, site of relapse, local recurrence, time to first relapse, sex, metastatic status at diagnosis, ethnicity, race, and primary tumor site were used in a proportional hazards model and a stepwise model selection procedure was used to develop a multivariate analysis12. All analyses and graphs were done in SAS 9.3.
Age at enrollment was used as a categorical variable (< 10, 10–17, ≥ 18 years) and as a continuous variable using the t-test or a one-way analysis of variance (ANOVA), as appropriate. The age grouping above has been used in past Ewing sarcoma trials which we felt was also a reasonable age breakdown for this analysis. Each pair of measured demographic characteristics were checked for association within the analytic population by the exact conditional test of proportions15. When ANOVA was done, the patient groups were assessed for significant differences using Tukey 95% confidence intervals on the differences16.
Histologic response was converted to a categorical variable coded as “Good” (> 95% necrosis) and “Poor” (≤ 95% necrosis) as this was collected differently on the three studies. For INT-0133 there were 5 categories used to describe necrosis (“no effect,” “more than 50% viable tumor,” “5–50% tumor viable,” “less than 5% tumor viable,” “no viable tumor noted”), while this data was collected as integer percentages on both POG-9754 and AOST-0121. Site of disease recurrence was described as two variables – local recurrence (yes/no) and site of recurrence (bone only, lung only, bone and lung, other).
Time to recurrence, measured from the date of enrollment on therapeutic study (diagnosis) to the date of first relapse, was considered as a categorical variable defined by 0–1 years, (≤ 365 days) 1–2 years, (366–730 days), and 2+ years (≥ 731 days), respectively.
INT-0133 was opened May 1993 and closed November 1997. Data current to September 2005 were used for analysis. POG-9754 was opened September 1999 and closed February 2002. Data current to July 2008 were used for analysis. AOST0121 was opened July 2001 and closed November 2005. Data current to July 2008 were used for analysis.
RESULTS
Patient Characteristics
INT-0133 enrolled 777 eligible patients (685 non-metastatic and 92 metastatic), POG-9754 enrolled 254 eligible non-metastatic patients, and AOST0121 enrolled 96 eligible metastatic patients for a total of 1127 enrolled patients (Figure 1). The analytic population, consisting of patients with first event free survival (EFS) event of relapse or progression, consisted of 431 relapsed patients: 276 patients were enrolled on INT-0133 (64%), 92 patients were enrolled on POG-9754 (21%), and 63 were enrolled on AOST0121 (15%). Median follow up for the analytic population was 3.5 years (maximum 9.7 years).
Over half of the patients in the analytic population (60%) were male. Most patients were white (66%) and non-Hispanic (77%) and were 10–17 years of age at time of enrollment on initial therapy (70%). Most patients had an extremity tumor at diagnosis (94%), while 4% of patients had a pelvic tumor, and 2% had “other” tumors (non-extremity, non-pelvic). Localized disease was present in 73% of the population initially and over half (54%) had a “poor” response to neoadjuvant chemotherapy (22% “good”, 24% “unknown”) (Table 1). There were no statistically significant differences between patients by study of enrollment aside from extent of initial disease due to the inclusion criteria differences among the studies (data not shown).
Table 1:
Demographics of Patient Cohort with Relapsed Osteosarcoma
| N = 431 | N (%) | |
|---|---|---|
| Sex | Male | 258 (60) |
| Female | 173 (40) | |
| Race | Black | 64 (15) |
| Other | 25 (6) | |
| White | 286 (66) | |
| Unknown | 56 (13) | |
| Ethnicity | Non-Hispanic | 331 (77) |
| Hispanic | 56 (13) | |
| Unknown | 44 (10) | |
| Age at enrollment | < 10 years | 63 (15) |
| 10–17 years | 303 (70) | |
| ≥ 18 years | 65 (15) | |
| Site of Initial Tumor | Extremity | 405 (94) |
| Pelvis | 17 (4) | |
| Other1 | 9 (2) | |
| Disease at Diagnosis | Non-metastatic | 314 (73) |
| Metastatic | 117 (27) | |
| Histologic Response | Good | 95 (22) |
| Poor | 231 (54) | |
| Unknown | 105 (24) |
The “other” primary sites included jaw, rib, skull, and vertebral primary disease
Survival after First Recurrence
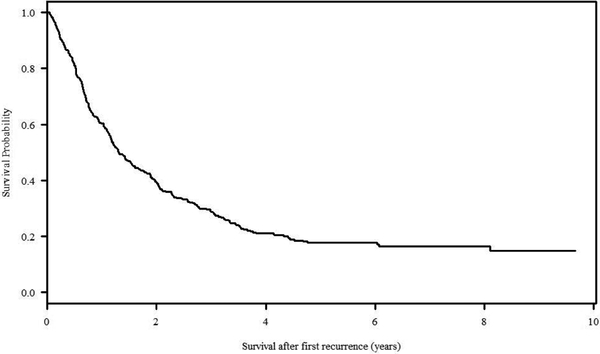
One hundred eight (108/431, 25.1%) total relapsed patients were alive at last contact with a median follow up of 3.53 years (follow up 0– 9.66 years). The median time from enrollment on a front-line trial to first relapse was 1.31 years. The overall SAR at 5 years was 17.7% (Figure 2). Several of the variables of interest were significantly related to SAR in univariate analysis when stratified by disease extent at diagnosis (non-metastatic versus metastatic patients) including age at study enrollment (p=0.027), site of relapse (p=0.005; only significant in the non-metastatic cohort), and time to relapse (p<0.00001) (Table 2).
Figure 2:
Overall survival of all patients enrolled on one of the three included studies initially and went on to have a relapse event (entire study population).
Table 2:
Association of Covariates of Interest with 5 Year Survival after Recurrence
| Total N | Hazard Ratio | 95% CI | p value | ||
|---|---|---|---|---|---|
| Extent of Disease | |||||
| Non-metastatic | 314 | 1 (ref) | |||
| Metastatic | 117 | 1.4793 | 1.1319 – 1.9333 | 0.0041 | |
| Site of Relapse | |||||
| Lung only | 215 | 1 (ref) | |||
| Bone only | 95 | 1.0988 | 0.8071 – 1.4960 | 0.5494 | |
| Lung & bone | 38 | 1.8854 | 1.2841 – 2.7682 | 0.0012 | |
| Other sites | 50 | 1.5690 | 1.0902 – 2.2582 | 0.0153 | |
| Time to First Relapse | |||||
| 0–1 year | 155 | 1.7830 | 1.3399 – 2.3726 | <0.0001 | |
| 1–2 years | 164 | 1 (ref) | |||
| 2+ years | 112 | 0.5698 | 0.4138 – 0.7845 | 0.0006 | |
| Age at Enrollment | |||||
| < 10 years | 63 | 0.6543 | 0.4545 – 0.9419 | 0.0225 | |
| 10–17 years | 303 | 1 (ref) | |||
| ≥ 18 years | 65 | 2.2709 | 1.4377 – 3.5870 | 0.0004 |
Age and Disease Extent at Diagnosis
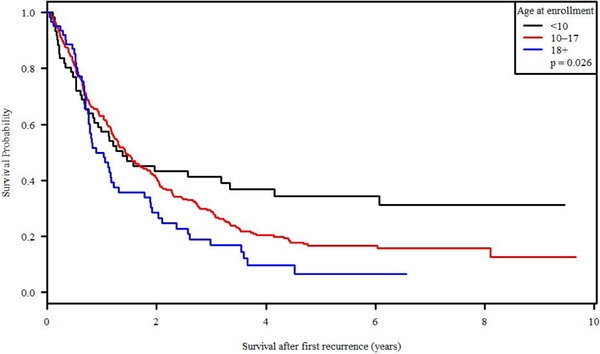
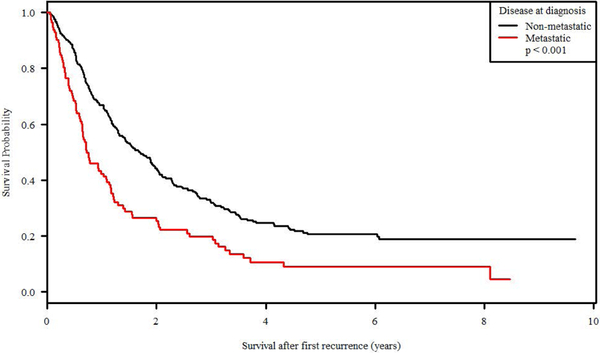
Survival after recurrence (SAR) was significantly associated with age at enrollment at initial diagnosis, with a 34.3% 5-year SAR for patients <10 years old versus 6.4% for patients >18 years old (p = 0.0265; Figure 3). Disease extent at diagnosis was also statistically significant with metastatic versus non-metastatic patients demonstrating a 5-year SAR of 9% v 20.6% (p<0.0001; Figure 4). Ethnicity, primary site of tumor at diagnosis, race, and sex were not significantly related to survival after recurrence. Therapeutic trial of enrollment was significantly associated with SAR (p <0.0001), but the effect was entirely due to the metastatic status eligible for enrollment on each study; POG-9754 and INT-0133 (non-metastatic patients) had similar SAR (p = 0.2214) as did AOST0121 and INT-0133 (metastatic patients; p = 0.9806).
Figure 3:
Survival after recurrence of disease by age at initial diagnosis of osteosarcoma.
Figure 4:
Survival after recurrence of disease by disease extent at initial diagnosis (metastatic versus non-metastatic).
Site of disease at recurrence
This category was defined by two variables - local recurrence (yes/no) and site of recurrence (bone, lung, bone & lung, other). For those participants who relapsed 0–1 years from completion of therapy, 62.9% had a local recurrence and 28.1% had a distant recurrence (P<0.00001), although if recurrent disease occurs in metastatic sites, biopsy of the initial tumor site may not be performed. This, and the difficulty with clear imaging due to prior surgery with hardware placement in many cases, may lead to an underestimation of the rate of local or combined recurrence. From the database data, it is unknown how many of these were true recurrence versus progression of disease at the primary site. The presence of a local recurrence (yes/no) was not significantly associated with SAR. However, the site of recurrence was related to SAR, primarily in those initially diagnosed with localized disease initially (p=0.0182). In this group, having pulmonary or bone relapse were approximately equivalent in terms of SAR (HR lung only (ref) 1, HR bone only 1.10; p=0.54, survival estimate 25.5% v 22.0% at 5 years; Table 2), yet a combination lung and bone relapse were at increased risk for post-recurrence death (HR of combined lung and bone 1.89, p=0.001; survival estimate 10.4% at 5 years; Table 2).
Time to relapse
Time to relapse is measured from date of diagnosis to first relapse. Over half of patients with metastatic disease who developed relapse do so within the first year of diagnosis (53.0% v 29.6% of non-metastatic patients, p<0.0001). The group of patients that relapsed in < 1 year from diagnosis includes those with very early relapse after completion of therapy, local progression on therapy, or new sites of disease during initial therapy. Time to relapse was associated with SAR at 5 years in both metastatic (p=0.008) and non-metastatic cohorts (p<0.0001). Patients who relapse at greater than or equal to 2 years from initial diagnosis are more likely to be alive 5 years after recurrence than those who relapse in 0–1 or 1–2 years. Those with initially metastatic disease who had a recurrence with a time to relapse of 0–1 year had a 5-year SAR of 2.2%, while those who relapsed after 1–2 years and 2+ years, respectively, had a SAR of 16.1% and 26.3% at 5 years. Similarly, those with localized disease at diagnosis who relapsed 0–1 years, 1–2 years, and 2+ years from diagnosis had a 5-year SAR of 10.6%, 15.6%, and 39.3%, respectively (p<0.0001).
Histological response
Histological response in the primary tumor specimen was significantly associated with time to relapse, but was not predictive of SAR (p=0.20). Of the 431 relapsed patients in this study, 231 (53.6%) initially had “poor” histological response (≤ 95% necrosis) and were more likely to relapse early (74.9% of relapses occur at <2 years). Only 95 (22.0%) patients initially had “good” histological response, and these patients were more likely to relapse late (46.3% of relapses occur at 2+ years; p <0.0001). Histological response was unknown for 105 patients.
Multivariate Analysis
The covariates that remained significant in multivariate analysis were age at enrollment on study, extent of disease at diagnosis, site of relapse, and time to relapse (Table 2). Patients between 10 and 17 years at enrollment had a decreased likelihood of survival after relapse than those less than 10 years old (HR = 1.53 (1.06, 2.20), p = 0.0225). Likewise, patients who were 18 years or older at enrollment are less likely than both younger age groups to survive after relapse (HR = 1.49 (1.06, 2.07), p = 0.0199 compared to 10–17 year; Table 2).
Patients with relapsed disease who initially had metastatic disease at diagnosis are less likely to survive after recurrence than relapsed patients with initially localized disease (HR = 1.48 (1.13, 1.93), p = 0.004; Table 2).
Pulmonary relapse remains most common. Patients with isolated pulmonary compared to isolated bone relapses were essentially equivalent in terms of SAR (lung only ref HR=1; bone only HR=1.10 (0.81, 1.50), p = 0.55) while a combined bone and lung relapse negatively impacted SAR (HR=1.89, (1.28, 2.77), p = 0.0012). Relapses in “other” sites (such as pleural, mediastinal, regional or lymph node sites) demonstrated intermediate SAR (HR 1.57, (1.09, 2.26), p = 0.0153, Table 2).
Finally, compared to patients who relapse between one and two years from initial diagnosis, those who relapse prior to one year have an increased risk of death (HR = 1.78 (1.34, 2.37), p <0.0001) and those who relapse greater than two years from study enrollment are more like to survive after recurrence (HR = 0.5698 (0.41, 0.78), p = 0.0006, Table 2).
DISCUSSION
Treatment for patients with recurrent osteosarcoma remains a critical challenge with limited data providing prognostic information to guide therapy. A variety of approaches can be undertaken including surgery, chemotherapy, and in select cases, radiation therapy. To better understand the historical outcome for patients with recurrent osteosarcoma and the factors that have the greatest effect on survival outcome, we have undertaken an assessment of the largest cohort of patients with newly diagnosed osteosarcoma treated on multi-institutional cooperative group studies in North America. In this study, we have reviewed the outcome of 431 patients with relapsed osteosarcoma and found that 20.6% of localized and 9.0% of metastatic patients survived beyond 5 years after first relapse. The overall SAR at 5 years is 17.7% which is consistent with other reports showing 10-year survival estimates of 11.8% to 18%.17,8,9
Previous retrospective analyses have identified several factors that may influence outcome in the setting of relapse. Those with pulmonary relapse who have unilateral disease or a solitary nodule have been identified to be in the best prognostic group with up to 50% surviving.9,18,19 Additional factors previously described include a longer disease-free interval from initial diagnosis to relapse, good histologic response to neoadjuvant chemotherapy, absence of pleural involvement, and the ability to achieve a second complete surgical remission.2,4,20,17,21,22,9
In our analysis, we have found similar results as prior studies for age, extent of disease, and time to relapse, though we did not find histological response to initial therapy to correlate with SAR (p=0.20). Despite the utility of histologic response for prognostication, translating this to improved survival has been difficult. Several therapeutic trials were able to show improved histologic response with additional neoadjuvant therapy, yet this did not lead into gains in overall survival.23,24 Data from the EURAMOS trial showed that although histological response following initial therapy with MAP is prognostic, the addition of Ifosfamide and Etoposide (IE) to poor responders did not result in better EFS or overall survival.25 Data from the European Osteosarcoma Intergroup (EOI) does suggested that patients with initial good histologic response were more likely to have improved post relapse survival (HR 0.7, p=0.015) in 1,067 patients, yet all of these patients had initially localized, resectable, extremity disease.22 Perhaps, the diversity of our cohort of patients in terms of site and extent of disease influenced our findings relating histologic response to SAR.
We have demonstrated that patients who were diagnosed at 18 years of age or older had a significant decrease in the likelihood of surviving after disease recurrence than both the less than 10 year and 10–17-year age groups. Bacci et al showed that patients with relapsed disease who were 14 or younger were more likely to attain a second complete remission and therefore long-term survival (71% vs 56.4%, p=0.006).21 In both data sets, as patients approach young adulthood, their likelihood for survival after first relapse diminishes. There are several hypotheses about why older patients may have poorer survival in osteosarcoma including biology of the tumor, delays in diagnosis, decreased participation in clinical trials, and decreased adherence or increased toxicity leading to limitations or delays in therapy.12 From other studies, the poorer prognosis seen in 18–30 year old patients is not explained by histological response, tumor location, or the presence of metastatic disease at diagnosis.12
Extent of disease at diagnosis was significantly associated with survival after recurrence. Patients with metastatic disease at diagnosis were more likely to die of disease after relapse. This association intuitively may speak to the biologic differences in metastatic and localized tumors.9 Patients with localized tumors at diagnosis and subsequent local recurrence have overall SAR up to 30%.26 However, patients with localized disease at diagnosis with non-lung, distant or combination relapses tend to have very poor survival after recurrence.22 Data from the Rizzoli Institute has shown patients with pulmonary recurrence with two or fewer nodules are most likely to survive after relapse.20 Our data set did not include the number of pulmonary nodules at recurrence, so we are unable to make direct comparisons regarding the probability of survival with a single or multiple nodules at recurrence, but surgical resection of recurrent lesions has been associated with improved survival in many studies.27,20,21,28,17,9 These data will likely be collected prospectively on future COG osteosarcoma clinical trials.
More time from the initial diagnosis to the first relapse was found to be favorably associated with survival after recurrence. Other studies have shown this as well, yet with varying time point cutoffs for significance, such as relapse occurring at 18 months or greater from initial diagnosis.20,9 Given the nature of surgical management of osteosarcoma, it is not always possible to differentiate between recurrence and progression in a known site of disease while on therapy. We chose to include patients receiving upfront therapy for whom it was not possible to determine whether complete remission was achieved which may have had the effect of lowering the outcome in the group with early recurrence (0–1 years after diagnosis).
The ability to achieve a second complete surgical resection of disease has been associated with improved long term survival after recurrence.2,4,20,17,21,22,9 However, how the use of chemotherapy in the setting of relapsed disease impacts outcome is debatable.20,17 Due to significant limitations in the available data we were unable to assess the impact of post-relapse surgical management and/or chemotherapy on outcome. The maximal survival period reported here is 10 years where relapse is uncommon, yet possible. Longer term data collection for this cohort will identify patients who are essentially cured of their disease. Finally, this data includes patients with first relapse of disease and does not follow their course through possible subsequent relapse events, yet prognostic factors after re-recurrence are thought to be similar to those seen at time of first relapse.29
In summary, patients with relapsed osteosarcoma were more likely to survive after first relapse if they were <18 years, had localized disease at diagnosis, relapsed 2 or more years post-diagnosis, and did not have a combination relapse (bone/lung). The 5-year SAR for this entire group of patients (N=431) was 17.7% although certain subgroups, as above, may have better outcomes. The Children’s Oncology Group is focused on novel, informative phase II trials for osteosarcoma to more rapidly identify active drugs that could be moved to front line therapy, all in the continued hopes of improving survival for these patients.11 Identifying therapies that will decrease the risk of recurrence remains of paramount importance as up-front remission continues to remain the best chance for overall cure.
Supplementary Material
Acknowledgments
Funding sources: Research was supported by the Chair’s Grant U10CA98543 and Human Specimen Banking Grant U2CA114766 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. Additional support was provided by both the NCTN Operations Center Grant U10CA180886 and NCTN Statistics & Data Center Grant U10CA180899 (current grants, as of 3/1/14). Additional support for research is provided by a grant from the WWWW (QuadW) Foundation, Inc. (www.QuadW.org) to the Children’s Oncology Group. MI receives funding from the Reid R. Sacco AYA Cancer Alliance. Finally, this research was also supported by the St. Baldrick’s Foundation.
Glossary
- EFS
Event free survival
- SAR
Survival after recurrence
- COG
Children’s Oncology Group
- CCG
Children’s Cancer Group
- POG
Pediatric Oncology Group
- MAP
Methotrexate/Doxorubicin/Cisplatin
- MTP-PE
Muramyl tripeptide-phosphatidyl ethanolamine
- MAPIE
Methotrexate/Doxorubicin/Cisplatin/Ifosfamide/Etoposide
- ANOVA
One-way analysis of variance
- HR
Hazard ratio
- OS
Overall survival
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
REFERENCES:
- 1.Youn P, Milano MT, Constine LS, Travis LB. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: A population-based study of 28,844 patients. Cancer. 2014;120(15):2334–2342. doi:10.1002/cncr.28733. [DOI] [PubMed] [Google Scholar]
- 2.Link MP, Goorin a M, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991:8–14. [PubMed] [Google Scholar]
- 3.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11(3):449–453. [DOI] [PubMed] [Google Scholar]
- 5.Petrilli AS, De Camargo B, Odone Filho V, et al. Results of the Brazilian osteosarcoma treatment group studies III and IV: Prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161–1168. doi:10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- 6.Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011–2018. doi:10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 7.Chou AJ, Merola PB, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: The memorial sloan-kettering cancer center experience. Cancer. 2005;104(10):2214–2221. doi:10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 8.Crompton BD, Goldsby RE, Weinberg VK, Feren R, O’Donnell RJ, Ablin AR. Survival after recurrence of osteosarcoma: A 20-year experience at a single institution. Pediatr Blood Cancer. 2006. doi:10.1002/pbc.20580. [DOI] [PubMed] [Google Scholar]
- 9.Leary SES, Wozniak AW, Billups CA, et al. Survival of pediatric patients after relapsed osteosarcoma: The St. Jude Children’s Research Hospital experience. Cancer. 2013. doi:10.1002/cncr.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison D, Geller D, Gill J, Lewis V, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39–50. [DOI] [PubMed] [Google Scholar]
- 11.Janeyway K, Gorlick R. The case for informative phase 2 trials in osteosarcoma. Lancet Oncol. 2016;17:1022–23. [DOI] [PubMed] [Google Scholar]
- 12.Janeway KA, Barkauskas DA, Krailo MD, et al. Outcome for adolescent and young adult patients with osteosarcoma: A report from the Children’s Oncology Group. Cancer. 2012;118(18):4597–4605. doi:10.1002/cncr.27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival - A report from the children’s oncology group. J Clin Oncol. 2008;26(4):633–638. doi:10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed New York: John Wiley and Sons; 2002. [Google Scholar]
- 15.Bishop S, feinberg SE, Holland YMM. Discrete Multivariate Analysis. Cambridge, MA: MIT Press; 1975. [Google Scholar]
- 16.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. 4th ed New York: McGraw Hill/Irwin; 2005. [Google Scholar]
- 17.Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23(3):559–568. doi:10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Arndt CAS, Koshkina NV., Inwards CY, et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: Effects on disease-free survival and immunomodulation. A report from the Children’s Oncology Group. Clin Cancer Res. 2010;16(15):4024–4030. doi:10.1158/1078-0432.CCR-10-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daw NC, Chou AJ, Jaffe N, et al. Recurrent osteosarcoma with a single pulmonary metastasis: a multi-institutional review. Br J Cancer. 2014;112. doi:10.1038/bjc.2014.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: Prognostic factors for long-term survival. J Clin Oncol. 2003;21(4):710–715. doi:10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 21.Briccoli A, Rocca M, Salone M, et al. Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer. 2005;104(8):1721–1725. doi:10.1002/cncr.21369. [DOI] [PubMed] [Google Scholar]
- 22.Gelderblom H, Jinks RC, Sydes M, et al. Survival after recurrent osteosarcoma: Data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur J Cancer. 2011;47(6):895–902. doi:10.1016/j.ejca.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: Results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16(7):2452–2458. [DOI] [PubMed] [Google Scholar]
- 24.Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the european osteosarcoma intergroup. J Natl Cancer Inst. 2007;99(2):112–128. doi:10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 25.Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. 2016. doi:10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi A, Lewis VO, Satcher RL, Moon BS, Lin PP. What are the factors that affect survival and relapse after local recurrence of osteosarcoma? Clin Orthop Relat Res. 2014;472(10):3188–3195. doi:10.1007/s11999-014-3759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari S, Briccoli A, Mercuri M, et al. Late Relapse in Osteosarcoma. J Pediatr Hematol Oncol. 2006;28:418–422. [DOI] [PubMed] [Google Scholar]
- 28.Bacci G, Briccoli A, Longhi A, et al. Treatment and outcome of recurrent osteosarcoma: Experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol (Madr). 2005. doi:10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- 29.Bielack SS, Kempf-Bielack B, Branscheid D, et al. Second and subsequent recurrences of osteosarcoma: Presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009;27(4):557–565. doi:10.1200/JCO.2008.16.2305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.