Supplemental Digital Content is available in the text.
Key Words: Alzheimer disease center, National Alzheimer’s Coordinating Center, Alzheimer disease, Lewy body disease, frontotemporal degeneration, MCI
Abstract
Introduction:
In 2015, the US Alzheimer’s Disease Centers (ADC) implemented Version 3 of the Uniform Data Set (UDS). This paper describes the history of Version 3 development and the UDS data that are freely available to researchers.
Methods:
UDS Version 3 was developed after years of coordination between the National Institute on Aging-appointed Clinical Task Force (CTF), clinicians from ∼30 ADCs, and the National Alzheimer’s Coordinating Center (NACC). The CTF recognized the need for updates to align with the state of the science in dementia research, while being flexible to the diverse needs and diseases studied at the ADCs. Version 3 also developed a nonproprietary neuropsychological battery.
Results:
This paper focuses on the substantial Version 3 changes to the UDS forms related to clinical diagnosis and characterization of clinical symptoms to match updated consensus-based diagnostic criteria. Between March 2015 and March 2018, 4820 participants were enrolled using UDS Version 3. Longitudinal data were available for 25,337 of the 37,568 total participants using all UDS versions.
Discussion:
The results from utilization of the UDS highlight the possibility for numerous research institutions to successfully collaborate, produce, and use standardized data collection instruments for over a decade.
In 1984, the US National Institute on Aging (NIA) of the National Institutes of Health inaugurated the Alzheimer’s Disease Centers (ADC) program, aimed at providing a comprehensive approach to research on Alzheimer disease (AD) and related disorders. Through their research, ADCs are expected to aid in early detection, diagnosis, treatment, and prevention of neurodegenerative disease. As part of the ADC program, Centers’ data must be collected in a standardized manner, but also in a way that allows individual Centers to maintain their unique research foci and strategies. To date, the NIA has funded 39 past and present ADCs that are located at medical institutions across the United States, and the ADC program has continually evolved to adapt to the state of the science.
At the start, ADCs collected data primarily retrospectively (eg, chart review) as part of the Minimum Data Set (MDS), with data collection coordinated by the National Alzheimer’s Coordinating Center (NACC) starting in 1999. The MDS was a cross-sectional data set consisting of limited data on demographics, clinical diagnoses, and neuropathologic findings (when available). It largely served as a library/cataloging service.
In 2005, ADCs began collecting longitudinal demographic, clinical, neuropsychological, and diagnostic data on participants using Version 1 of the Uniform Data Set (UDS). Details on the data collected and the process of developing UDS Version 1 can be found elsewhere.1 UDS Version 2 was implemented in 2008, resulting in a mostly minor update to the original data collection instruments such as adding a few new questions to the forms, restructuring of form logic (eg, skip patterns), and adding a few neuropsychological test elements [eg, Mini Mental State Exam (MMSE)2 pentagon item score].
In contrast, numerous important changes were made with the implementation of UDS Version 3, including the addition of the Form C2 neuropsychological test battery.3 The aims of this paper are to describe the history of development and implementation of UDS Version 3, the UDS data that are currently available, and the types of studies that can be conducted using this important resource. Previous published papers focus on the UDS Version 3 neuropsychological test battery only.3,4 This paper describes the overall development of the UDS Version 3, which consists of a number of instruments beyond the neuropsychological test battery; the particular clinical data it collects; and the supplementary data that are available.
METHODS
The original impetus for the development and adoption of UDS Version 3 was the desire for an updated neuropsychological test battery, details of which have been thoroughly described elsewhere.3 Some of the benefits of the updated test battery are that it is nonproprietary and that it includes a new episodic memory test to reduce practice effects observed in repeatedly using the prior version. A primary goal in the development of the UDS Version 3 neuropsychological battery was a set of nonproprietary tests that would allow sharing and comparison across various studies. Thus, some of the chosen tests were initially missing norms with which to reference until sufficient sample size allowed UDS Version 3 norms to be posted on the NACC website and to be published in 2018.3 In addition, the new test battery now captures visuospatial and nonverbal memory function, domains not previously tested in UDS Versions 1 and 2. The careful review, evaluation, and implementation of the new battery took a number of years and was overseen by the Neuropsychology Work Group, a subcommittee of the ADCs’ Clinical Task Force (CTF). Before implementation, the ADCs pilot tested the new battery (ie, the Crosswalk Study4), which allowed the Neuropsychology Work Group to assess if new and old tests capturing the same domains could be equated and used in longitudinal analyses. Results from the crosswalk study indicated that Version 3 neuropsychological tests were well correlated with Version 2 tests (ρ=0.68 to 0.78).4 The crosswalk study also suggested methods to convert scores on the Version 3 tests to comparable scores from the Version 2 battery. These conversion factors allow for comparison of all available initial visit test scores among individuals, whether they received UDS Version 2 or 3 at baseline, and for longitudinal comparison of test scores for participants who received both Version 2 and subsequently Version 3 tests during follow-up.
Along with the neuropsychological test changes, a number of other substantive edits were made to the forms in Version 3. These changes were instituted by the CTF in order to streamline the forms and adapt to the latest diagnostic criteria, including biomarker measures. Moreover, new questions were added to assess clinical characteristics of related neurodegenerative disorders [eg, frontotemporal lobar degeneration (FTLD) mutations, repeated traumatic brain injuries (TBIs)], reflecting the ADC program’s expanding and diversifying research priorities. Decisions were made by the entire CTF, wherein consensus if not unanimity almost always was achieved whenever possible. The CTF Chair was tasked with resolving any impasses that arose.
With the goal of streamlining the UDS, the CTF worked with the NACC to review UDS Version 2, form by form, identifying questions that were redundant across forms and opportunities to increase the clarity of the questions and/or instructions. The decision to eliminate entire forms or large numbers of items was made by the CTF if items were both infrequently used by researchers and deemed no longer necessary to the basic clinical work-up.
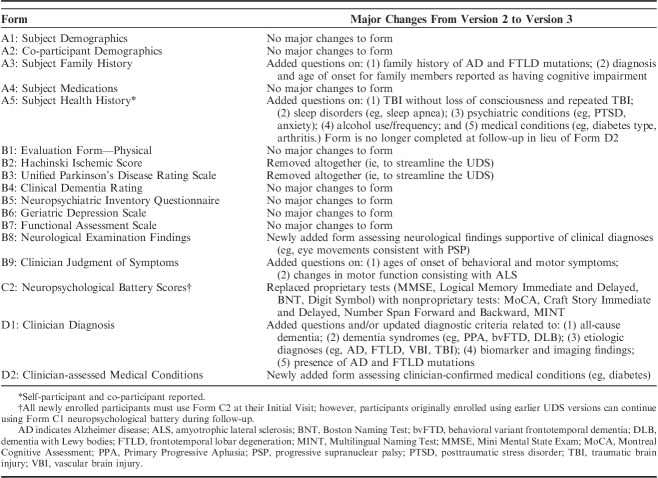
After a comprehensive review of the forms, few data elements from Version 2 were eliminated due to redundancy, and a few wording and coding changes were suggested to clarify data elements. In the end, the major changes (Table 1) included modifications to the clinical diagnosis form (Form D1), the removal of the Hachinski Ischemic Score (Form B2) [given the increased use of magnetic resonance imaging (MRI) to document ischemic burden] and the Unified Parkinson’s Disease Rating Scale (UPDRS; Form B3), and the addition of summary neurological examination findings (Form B8) and clinician-assessed medical conditions (Form D2) forms. Of note, the UPDRS was incorporated into a module that assesses Lewy body disorders (LBD module), which ADCs implemented in August, 2017.
TABLE 1.
Major Changes Implemented With Version 3 of the Uniform Data Set Initial Visit Packet
The Clinical Diagnosis Form D1 was updated to not only adapt to the 2011 National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria for AD that incorporated biomarkers [preclinical AD,5 mild cognitive impairment due to AD (MCI-AD),6 and AD dementia7], but also to the latest diagnostic criteria for conditions such as frontotemporal degeneration (eg, behavioral variant frontotemporal degeneration8 and primary progressive aphasia9) and vascular brain injury. In addition, Version 3 of Form D1 incorporated new specific questions on AD and FTLD mutations and on conditions including posterior cortical atrophy (PCA), multiple system atrophy, human immunodeficiency virus–associated neurocognitive disorder (HAND), and psychiatric conditions including bipolar disorder and posttraumatic stress disorder. The Neurological Exam Findings Form B8 was a new addition for Version 3, aimed at capturing signs from the neurological exam consistent with specific neurodegenerative conditions such as Parkinson disease (PD), progressive supranuclear palsy, amyotrophic lateral sclerosis, and cerebrovascular disease. Finally, Form D2 was added to gather data on medical conditions that may confer risk for AD or dementia, such as cardiovascular disease and diabetes, with the goal of improving reliability and validity compared with the participant/co-participant self-reported medical conditions collected on Subject Health History Form A5.
In the months preceding implementation of UDS Version 3, NACC staff developed the documentation necessary for ADCs to begin using the forms. This included developing data element dictionaries10 (coding guides for all variables), guidebooks11 (to guide clinicians on form completion), neuropsychological battery instructions12 for clinicians, and data quality checks (to stop or flag potentially erroneous data entry), among other documentation. The data element dictionaries and accompanying documentation were provided to ADCs in advance so that they could program their databases to submit the data to NACC on the implementation date. In addition, NACC created electronic forms for ease of data entry and modified the UDS Oracle database and UDS data submission system to allow for entry and storage of the Version 3 data. Before implementation, ADC clinical leaders participated in a webinar to learn about Version 3 changes and how to complete the forms. UDS Version 3 was implemented by all ADCs on March 15, 2015.
In October 2017, the Spanish translation of UDS Version 3 became available (Initial and Follow-up Visit Packet; Neuropsychological Battery). Spanish translation involved preliminary translation and content review by expert clinicians, and pilot testing at 3 US and 4 Latin American sites (see the Acknowledgments section). Pilot testing revealed minimal issues with the initial translation for the Montreal Cognitive Assessment (MoCA) (original Spanish version), Benson Complex Figure (Copy and Delayed Recall), Number Span Forward and Backward, Category Fluency (Animals and Vegetables List Generation), and Trail Making Test Parts A & B. However, adaption was required for the Craft Story (Immediate and Delayed Recall), Verbal Fluency: Phonemic Test (English: words beginning with F and L; Spanish: words beginning with P and M), and the Multilingual Naming Test (MINT). Once the Spanish translation became available to ADCs, the Centers were asked to submit a Linguistic History form to ascertain levels of acculturation for all UDS participants who identify as Hispanic or Latino. In addition to the Spanish translation, UDS Version 3 has been translated into Chinese, with plans for use in Alzheimer’s research centers in China.
RESULTS
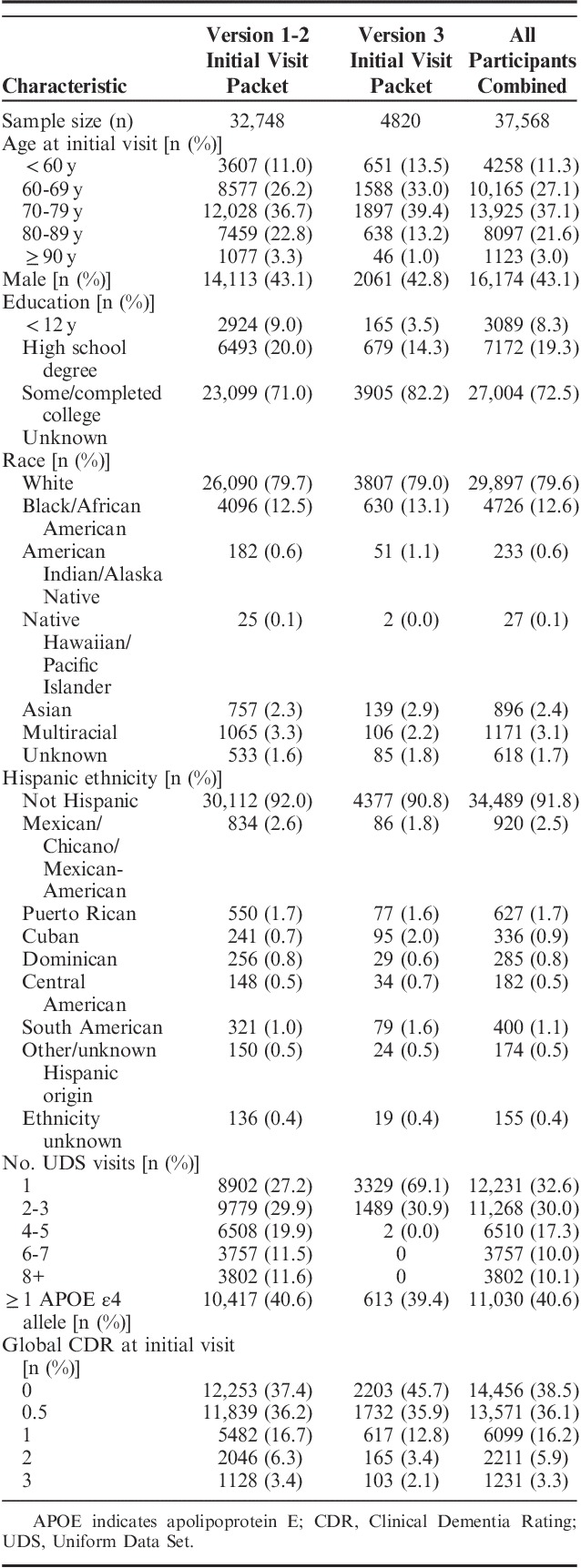
Table 1 outlines the major changes to the UDS with Version 3 implementation. As of March 1, 2018, the UDS (all versions) contains data on 37,568 participants followed at 39 past and present ADCs. Longitudinal data (≥2 visits) are available for 25,337 of the participants, with some participants having up to 13 UDS visits to date. Version 3 initial visit packets have been submitted on 4820 individuals. Results reported below are based on data collected from September 2005 through March 2018 using all 3 UDS versions.
Approximately 4000 participants were younger than 60 years of age and ∼1000 were 90 years or older at their initial visit (Table 2). Reflecting the shift in AD research toward earlier detection, the age demographic shifted to younger ages when comparing those enrolled using UDS Version 3 to those enrolled using UDS Version 1 to 2 forms. In addition, the number of individuals who were asymptomatic (global Clinical Dementia Rating=0) increased from 37% among those enrolled using UDS Version 1 to 2 to 46% among those enrolled using Version 3. Forty-one percent had at least one apolipoprotein E (APOE) ε4 allele. Although the large majority of the participants are white (80%) and non-Hispanic (92%), 4726 participants are African American, 896 are Asian, and 3079 are Hispanic.
TABLE 2.
Demographics and Clinical Characteristics of Uniform Data Set Participants
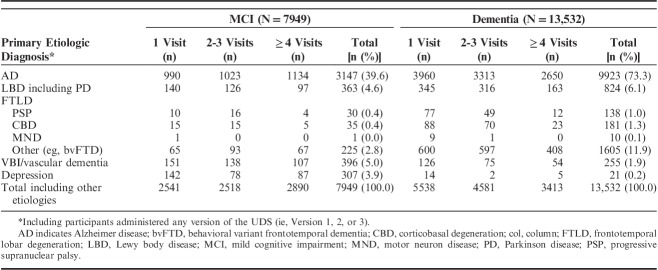
MCI and dementia were clinically diagnosed in 7949 and 13,532 individuals at their initial visit, respectively (Table 3). ADC clinicians are required to indicate the primary and contributing etiologic diagnosis for MCI or dementia. AD was the primary etiologic diagnosis for 40% of those with MCI and 73% of those with dementia. Among the MCI participants, primary etiologic diagnoses other than AD were not as common (eg, 5% LBD, 5% vascular brain injury/dementia, 4% depression), and in the remainder of dementia participants, most had a diagnosis of LBD including PD (6%) or FTLD with behavioral variant frontotemporal degeneration or primary progressive aphasia syndromes (12%). Table 3 additionally provides the number of participants who have at least 2 UDS visits (ie, longitudinal data) by their primary etiologic diagnosis at their initial visit. For example, among MCI participants with a primary etiologic diagnosis of LBD at their initial visit, 126 completed 2 or 3 UDS visits and 97 completed at least 4 UDS visits.
TABLE 3.
Primary Etiologic Diagnoses for Mild Cognitive Impairment or Dementia at Initial Visit, by Number of Visits
A number of major additions and edits were made to Clinician Diagnosis Form D1, including newly collected information whether the subject had an AD (n=14) or FTLD (n=27) mutation. Table 4 highlights the new data elements collected with the implementation of UDS Version 3. Twenty-seven individuals reported having family members with a known AD mutation (PS1, PS2, or APP), and 51 reported family with an FTLD mutation (MAPT, PGRN, C9ORF72, FUS).
TABLE 4.
Highlights of Data Added to Assessment for Version 3, By Form
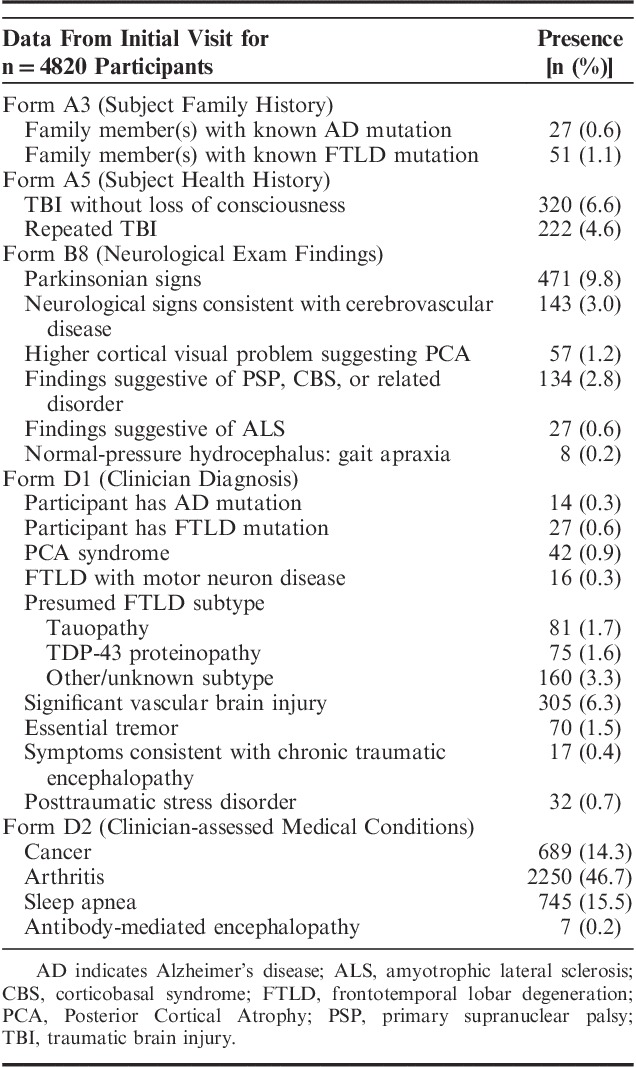
With Version 3, additional details are collected on TBI, including individuals with TBI without loss of consciousness (n=320) and repeated TBI (n=222) (Table 4). Detailed neurological examination findings on the new Form B8 included parkinsonian signs (n=471 participants) and higher cortical visual problem suggesting PCA (n=143). A number of diagnoses not previously collected were also added to Form D1, including PCA syndrome (n=42 participants), FTLD with motor neuron disease (n=16), essential tremor (n=70), symptoms consistent with chronic traumatic encephalopathy (n=17), and posttraumatic stress disorder (n=32). Finally, the new Clinician-assessed Medical Conditions Form D2 records data on a variety of clinician-endorsed conditions, including cancer (n=689 participants), arthritis (n=2250), and sleep apnea (n=745).
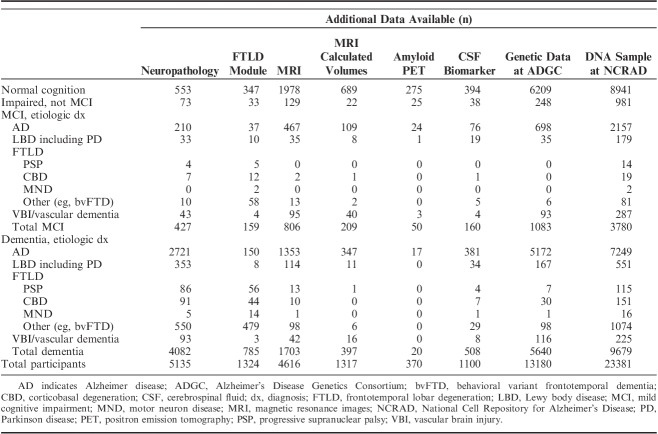
A substantial number of UDS participants have additional data sets that are also available at NACC. Neuropathology (NP) data13 are available for 5135 UDS participants who have died and consented to autopsy (Table 5). Data from the FTLD module (symptoms, diagnoses, imaging evidence, and neuropsychological test findings specific to FTLD) are available for 1324 UDS participants. MRI are available to download for 4616 participants. Among individuals with MRI, 1317 have measures of regional brain volumes (eg, hippocampal volume). Amyloid positron emission tomography (PET) is available for download for 370 participants and cerebrospinal fluid (CSF) biomarker data for 1100 participants. In addition, genetic data are available for 13,180 UDS participants by request from the Alzheimer’s Disease Genetics Consortium (ADGC), and deoxyribonucleic acid (DNA) samples are available for 23,381 by request from the National Cell Repository for Alzheimer’s Disease (NCRAD).
TABLE 5.
Additional Data Available on Uniform Data Set Participants by Primary Etiologic Diagnosis at Most Recent Visit
Table 5 provides finer grained details on the number of participants who have these additional data by their primary etiologic diagnosis. For instance, the large majority of individuals (81%) with amyloid PETs available for download had a clinical diagnosis of normal cognition or impaired not MCI (some impairment that does not meet MCI criteria) at their most recent visit, and 166 MCI participants and 540 dementia participants with a diagnosis of LBD (including PD) have DNA samples stored at NCRAD.
DISCUSSION
After considerable deliberation, compromise, and collaboration, the CTF, the ADCs, and NACC implemented UDS Version 3 in March 2015. There was a relatively lengthy period of evaluation, discussion, and negotiation amongst the expert ADC clinicians who made up the regular CTF and those who were part of the associated ad hoc subcommittees as well as other ADC clinicians. The CTF considered how to incorporate new developments in the field, while preserving longitudinal continuity with the >10 years of data collected in the prior UDS versions. Deletions, updates, and additions to the UDS needed to balance the needs of ∼30 Centers that each have their distinct research priorities and different participant characteristics (eg, age, primary diagnosis). Clinician opinion differs regarding the most important forms and questions to collect in the UDS within and across multiple ADCs. The CTF weighed the need to assess key clinical characteristics for the increasing number of neurodegenerative diseases studied within the ADC program against the need to keep the UDS as parsimonious as possible, being mindful of the clinicians’, research staffs’, and participants’ time. The resulting UDS Version 3, as in the prior UDS versions, demonstrates the ability of researchers from numerous institutions to collaborate and implement a standardized data collection instrument for over a decade.
An important contribution of the UDS is that it provides standardized instruments that can be supplemented with additional modules such as the FTLD and LBD modules, which were implemented by ADCs in January 2012 and August 2017, respectively. ADCs voluntarily submit the modules for individuals suspected to have FTLD or LBD, but additionally for individuals with AD or controls, which aids in determining the discriminative capabilities of the module instruments. To date, 1324 participants have completed the FTLD module, 464 of which have longitudinal FTLD module visits. As of March 2018, the LBD module data have not accumulated in sufficient numbers to provide to researchers, but the initial visit forms and documentation for the LBD and FTLD modules are viewable on the NACC website (www.alz.washington.edu/WEB/dataforms_main.html). In addition, researchers outside of the ADCs may request permission to use the UDS or associated modules. In the future, additional modules can be added to the UDS to collect more detailed data on a specific subpopulation or neurodegenerative disease (eg, preclinical disease).
The UDS serves as a foundation to integrate not only standardized clinical assessments of participants with NACC’s NP data set, but also to incorporate novel MRI, PET, and CSF biomarker data with these important clinical characteristics. The NACC database is adaptable and thus capable of receiving supplemental data such as the imaging and fluid biomarker data that complement the UDS and also reflect the trending research priorities of the ADCs. Along with the data that are either readily available at NACC or that can be linked to the UDS via the ADGC (eg, genome-wide association study) or NCRAD (eg, DNA samples), researchers can supplement the UDS data through a special request to one or more ADCs to obtain additional data not available at NACC (requests for UDS data, supplemental data and modules, and special requests for external data/specimens can be initiated at http://www.alz.washington.edu). This process of requesting external data from ADCs is initially coordinated by NACC. As an example, one such study gained approval from 15 ADCs to survey participants of 4 different racial/ethnic groups about their willingness to commit to post mortem brain donation.14 Researchers also work with NACC to determine samples of brain tissue that may be available for sharing from one or more ADCs. An example is a published study that combined existing NP data at NACC with supplemental data on number of microinfarcts through abstraction of University of Washington and Oregon Health and Science University ADC records to examine the associations between mixed pathologies and domain-specific cognitive decline.15
The growing depth and breadth of the UDS and of the additional data that can be linked to the UDS has resulted in an ever increasing number of published manuscripts using the NACC data (n=481 as of March 16, 2018) (viewable under “Publications and Productivity” link at www.alz.washington.edu). Early papers using the UDS were limited in terms of sample size and tended to focus on descriptions of the UDS sample or various methodological issues. In contrast, papers published using the UDS data in the first quarter of 201816–32 investigated a wide range of topics, such as inappropriate medication use among those with incident dementia,27 TBI history and early age of onset in autopsy-confirmed AD,28 big data approaches to preclinical trial enrichment,24 and predicting sex differences in MCI and AD using data on white matter hyperintensities and hippocampal volume.17 Thus, the demonstrated increase in publications using UDS data suggests that the UDS revisions described in this article have tailored the standardized data collection to measures that are more useful for researchers.
The volume of the published articles and abstracts using the UDS and the continually increasing diversity of topics are evidence of the value of the data to junior and seasoned researchers alike. The strengths of the UDS include not only the types of data that can be linked to it, but its longitudinal nature and the availability of sufficient sample sizes to study rarer conditions or special populations not otherwise feasible. For instance, UDS data provide opportunities for finer grain analyses focused on Hispanics/Latinos of specific origins. In addition, the ability to merge clinical data with NP data is critical, as NP remains the gold standard for neurodegenerative diseases such as AD.
Although the UDS exhibits a number of strengths desirable to researchers, anyone using the data must acknowledge its weaknesses when conducting a study or interpreting findings. Recruitment methods for the UDS sample have varied between ADCs and within each ADC over time, and are often based on convenience samples such as recruitment through clinics or participant referrals. The benefit of this approach is that ADCs are able to enrich their samples with individuals who have or are more likely to develop neurodegenerative disease. In addition, as mentioned above, ADCs have distinct research priorities and sample characteristics. The differences in recruitment and sample characteristics across the ADCs provide a heterogenous and diverse group with which to study, but this also means that the sample is not population-based and thus may not truly reflect local ADC communities or the larger US population. Another limitation is that the UDS Version 3 neuropsychological battery is missing a verbal list learning task such as the Rey Auditory Verbal Learning Test. The Neuropsychology Work Group decided against adding a verbal list learning task because the options were mostly proprietary (a major goal in developing the UDS 3 battery was to be nonproprietary), and because of the wide range of verbal list instruments used across ADCs, which would have created burden on the ADCs that have long established cohorts using an instrument not chosen for UDS Version 3. Also, the UDS instruments have changed over time, which can present a challenge to investigators wishing to use the longitudinal data (ie, neuropsychological test battery changed from UDS Version 2 to 3) or wishing to use the largest possible samples sizes (definition of TBI history changed from UDS Version 2 to 3). NACC developed the researchers data dictionary (RDD-UDS33) to harmonize data across the UDS versions, whenever possible, to try to minimize this issue and ensure researchers are aware of important UDS version changes. In addition, NACC research staff are available to consult with researchers who have questions about version changes, or other details about the UDS data.
UDS Version 3 demonstrates a number of advantages and improvements over the prior versions, collecting new and pertinent data in line with the evolving research interests at the ADCs. UDS Version 3 was developed in a manner that allows it to be combined with the previous versions, so that the longitudinal nature of the UDS and the legacy data remain accessible to researchers. A distinct advantage of UDS Version 3 is that it is nonproprietary. With permission, Version 3 can be used in other studies, allowing for a common method of assessing cognition and clinical characteristics among those with normal cognition and various neurological diseases and also providing the ability to compare findings across various studies and cohorts. Examples of research studies that are using UDS forms include the Dominantly Inherited Alzheimer Network (DIAN), the Advancing Research and Treatment for Frontotemporal Lobar Degeneration Consortium (ARTFL) and the Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS). In addition, the UDS data can be combined with valuable data internal and external to NACC, including imaging, CSF biomarker, and genetic data. Since its inception in 2005, the UDS has highlighted the possibility for numerous research institutions with sometimes disparate priorities to collaborate with each other to produce and use standardized data collection instruments. Version 3 was no exception, and with its implementation, it provides researchers a unique, valuable resource to facilitate acceleration of novel AD and related disorders research.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.alzheimerjournal.com.
ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH Grant U01 AG016976. Additional acknowledgements to the Alzheimer’s Disease Centers are provided in Supplemental Digital Content 1 (http://links.lww.com/WAD/A207). The authors thankfully acknowledge all of the faculty and staff at the ADCs, UDS participants and co-participants, as well as the following sites for participating in the pilot study of the Spanish translation of UDS Version 3: University of California, San Diego; University of Southern California; Mount Sinai School of Medicine; Memory Clinic at the Institute on Aging, Pontificia Universidad Javeriana School of Medicine, Bogota, Colombia; Fundación para la Lucha contra las Enfermedades Neurológicas de la Infancia, Buenos Aires, Argentina; Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suárez,” Mexico City, Mexico; Peru Young-Onset Dementia Network: Universidad Peruana Cayetano Heredia and Clínica Internacional. Lima, Peru.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Supported by NIA/NIH Grant U01 AG016976 (W.K.). Supported by the NIH (P50 AG16574) (D.S.K.). Supported by NIA grant AG05131(D.G.). Supported by NIH/NIA P30 AG028383 (G.J.). Research supported from the National Institutes of Health (P50AG033514, R01AG027161, R01AG011099, U19AG010483, R01AG049872, UF1AG051216, R01AG021155, R56AG037639, U2CAG057441, R01AG052324), the Department of Veterans Affairs (CSR&D I01 CX001261), the RECALL Foundation, the Alzheimer’s Association and Foundation for NIH, Janssen, and Lilly (C.C.). Receives research support from the National Institutes of Health (R01AG058557, R01AG053312, R01AG034614, R01AG03367, R01AG043962, P30AG035982, U10NS077356, UL1TR000001); received research support in the last 2 years for clinical trials from Lilly, Avid Radiopharmaceuticals, Toyama Chemical Company, Merck, Biogen, AbbVie, vTv Therapeutics, and Janssen (J.B.). Supported by NIA AG08017 (J.Q.). Supported by NIA AG 046499 and AG 017586 (K.R.). Receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants # P50AG005681; P01AG003991; P01AG026276; and UF01AG032438 (J.C.M.).
D.S.K.: serves on a Data Safety Monitoring Board for the DIAN study; is an investigator in clinical trials sponsored by, Lilly Pharmaceuticals, Biogen and the Alzheimer’s Treatment and Research Institute at the University of Southern California. D.G.: serves on Data Safety Monitoring Boards for Cognition Therapeutics and Proclara Biosciences and is an Editor of Alzheimer’s Research & Therapy. J.B.: serves as a consultant for Stage 2 Innovations; and received honoraria and travel support for speaking from Astra-Zeneca. C.P.: serves on 2 Data Safety Monitoring Boards for Biogen. J.C.M.: currently participating in clinical trials of antidementia drugs from Eli Lilly and Company and Biogen. The remaining authors declare no conflicts of interest.
Contributor Information
Collaborators: the Neuropsychology Work Group, Directors, and Clinical Core leaders of the National Institute on Aging-funded US Alzheimer’s Disease Centers
REFERENCES
- 1.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 2.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monsell SE, Dodge HH, Zhou XH, et al. Results from the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord. 2016;30:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Alzheimer’s Coordinating Center. NACC Uniform Data Set Data Element Dictionary For Initial Visit Packet. 2015. Available at: www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER3/ivp_ded.pdf. Accessed April 15, 2018.
- 11.National Alzheimer’s Coordinating Center. NACC Uniform Data Set Coding Guidebook For Initial Visit Packet. 2015. Available at: www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER3/UDS3_ivp_guidebook.pdf. Accessed April 14, 2018.
- 12.National Alzheimer’s Coordinating Center. NACC Uniform Data Set Instructions for the Neuropsychological Battery (Form C2). 2015. Available at: www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER3/UDS3_npsych_instructions_C2.pdf. Accessed April 14, 2018.
- 13.Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer’s Coordinating Center’s Neuropathology Form—available data and new analyses. J Neuropathol Exp Neurol. 2018;77:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boise L, Hinton L, Rosen HJ, et al. Willingness to be a brain donor: a survey of research volunteers from 4 racial/ethnic groups. Alzheimer Dis Assoc Disord. 2017;31:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and associations with domain-specific cognitive decline. Neurology. 2017;89:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agogo GRC, Gnjidic D, Moga D, et al. Longitudinal associations between different dementia diagnoses and medication. Int Psychogeriatr. 2018;18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke SL, Hu T, Fava NM, et al. Sex differences in the development of mild cognitive impairment and probable Alzheimer’s disease as predicted by hippocampal volume or white matter hyperintensities. J Women Aging. 2018:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke SL, Maramaldi P, Cadet T, et al. Decreasing hazards of Alzheimer’s disease with the use of antidepressants: mitigating the risk of depression and apolipoprotein E. Int J Geriatr Psychiatry. 2018;33:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleary EG, Cifuentes M, Grinstein G, et al. Association of low-level ozone with cognitive decline in older adults. J Alzheimers Dis. 2018;61:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis M, O’Connell T, Johnson S, et al. Estimating Alzheimer’s disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. 2018;15:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Leon MJ, Pirraglia E, Osorio RS, et al. The nonlinear relationship between cerebrospinal fluid Abeta42 and tau in preclinical Alzheimer’s disease. PLoS One. 2018;13:e0191240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur A, Edland SD, Peavy GM. The MoCA-Memory Index Score: an efficient alternative to paragraph recall for the detection of amnestic mild cognitive impairment. Alzheimer Dis Assoc Disord. 2018;32:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirson NY, Scott Andrews J, Desai U, et al. Patient characteristics and outcomes associated with receiving an earlier versus later diagnosis of probable Alzheimer’s disease. J Alzheimers Dis. 2018;61:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin M, Gong P, Yang T, et al. Big data analytical approaches to the NACC Dataset: aiding preclinical trial enrichment. Alzheimer Dis Assoc Disord. 2018;32:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell JA, Cadet T, Burke S, et al. The paradoxical impact of companionship on the mental health of older African American men. J Gerontol B Psychol Sci Soc Sci. 2018;73:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian W, Fischer CE, Schweizer TA, et al. Association between psychosis phenotype and APOE genotype on the clinical profiles of Alzheimer’s disease. Curr Alzheimer Res. 2018;15:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey CM, Gnjidic D, Agogo GO, et al. Longitudinal patterns of potentially inappropriate medication use following incident dementia diagnosis. Alzheimers Dement (N Y). 2018;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaffert J, LoBue C, White CL, et al. Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer’s disease. Neuropsychology. 2018;32:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stipho F, Jackson R, Sabbagh MN. Pathologically confirmed Alzheimer’s disease in APOE varepsilon2 homozygotes is rare but does occur. J Alzheimers Dis. 2018;62:1527–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting SKS, Foo H, Chia PS, et al. Dyslexic characteristics of Chinese-speaking semantic variant of primary progressive aphasia. J Neuropsychiatry Clin Neurosci. 2018;30:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tse KH, Cheng A, Ma F, et al. DNA damage-associated oligodendrocyte degeneration precedes amyloid pathology and contributes to Alzheimer’s disease and dementia. Alzheimers Dement. 2018;14:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintraub S, Besser L, Dodge H, et al. Version 3 of the Alzheimer’s Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Alzheimer’s Coordinating Center. NACC Uniform Data Set Researchers Data Dictionary. 2015. Available at: www.alz.washington.edu/WEB/rdd_uds.pdf. Accessed April 15, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.alzheimerjournal.com.