Abstract
Introduction
The development of de novo donor-specific antibodies (dnDSA) has been associated with rejection and graft loss in kidney transplantation, and DSA screening is now recommended in all kidney transplant recipients. However, the clinical significance of dnDSA detected by screening patients with a stable creatinine remains unclear.
Methods
103 patients <18 years old receiving a first, kidney alone transplant between 12/1/2007 and 12/31/2013 underwent DSA screening every 3 months for 2 years posttransplant, with additional testing as clinically indicated. No treatment was given for DSAs in the absence of biopsy-proven rejection.
Results
20 patients (19%) had dnDSA first detected on a screening test and 13 patients (13%) had dnDSA first detected on a for-cause test. Mean follow-up time posttransplant was 4.4 years. Screening-detected dnDSA was associated with an increased risk of rejection within 3 years, microvascular inflammation, and C4d staining on a 2 year protocol biopsy. In a Cox proportional hazards regression, screening-detected dnDSA was not associated with time to 30% decline in eGFR (aHR 0.88, 95%CI 0.30–2.00 p=0.598) or graft loss. dnDSA first detected on for-cause testing was associated with a 2.8 times increased risk of decline in graft function (95% CI 1.08–7.27 p=0.034) and a 7.34 times increased risk of graft loss (95%CI 1.37–39.23 p=0.020) compared to those who did not develop dnDSA.
Conclusion
The clinical setting in which dnDSA is first detected impacts the association between dnDSA and graft function. Further research is needed to clarify the role of dnDSA screening in pediatric kidney transplantation.
Introduction
In 2014 17,814 individuals in the United States received a kidney transplant; 712 of them were children.1 Over the past 30 years there has been substantial increase in kidney allograft survival, but most of this has been due to improvements in short-term rather than long-term survival.2 Chronic allograft nephropathy (including interstitial fibrosis with tubular atrophy (IFTA) and transplant glomerulitis) remains the leading cause of graft loss,3 and human leukocyte antigen (HLA) antibodies are thought to play a key role in its development.4
Donor specific antibodies (DSAs) are antibodies developed by the transplant recipient against HLA antigens present on the donor kidney. Numerous studies have linked the development of de novo DSAs (dnDSA) after kidney transplantation to poor graft outcomes in both adults and children.1,5–12 This has resulted in recommendations that patients undergo routine screening for the development of dnDSA posttransplant.13 However, many of these original studies combined screening with testing done in the setting of graft dysfunction1,6,10,14,15 or screened stored serum without regard to the patient’s clinical status.7–9,12 This raises concern that the association between dnDSA and graft outcome seen in prior studies may not be representative of a population with stable kidney function undergoing screening.
Multiple studies have shown that a large subset (34–48%) of patients who develop dnDSAs develop neither rejection nor have a decline in graft function.1,7,9,14,15 In a subgroup analysis of their study of 244 adult patients, Cooper et al reported that the 2 year graft survival among those with dnDSA detected on a protocol test was 93% compared to 97.8% among those without dnDSA, a difference that was not statistically significant.14 In this study we aim to examine whether or not patients <18 years old at the time of transplant with de novo DSAs first detected in the setting of stable kidney graft function have worse outcomes than those with no dnDSA.
Methods
We performed a retrospective cohort study of all pediatric patients receiving a kidney transplant at Seattle Children’s Hospital between 12/1/2007 and 12/31/2013. Inclusion criteria were age less than 18 years at the time of transplant, receipt of a primary, kidney-alone transplant, and at least 2 years of DSA monitoring. Exclusion criteria included a history of prior kidney transplant, previous or concurrent other solid organ transplant, and previous hematopoietic stem cell transplant. All patients had a negative crossmatch and no DSA prior to transplant.
Induction immunosuppression was with methylprednisolone and either thymoglobulin or an IL-2 receptor antagonist (basiliximab or daclizumab). Maintenance immunosuppression was primarily with tacrolimus and mycophenolate mofetil. Maintenance tacrolimus level goals were 10–12 ng/dl from 3–59 days posttransplant, 7–10 ng/ml 60–84 days posttransplant, 5–7 ng/ml 85–365 days posttransplant, and 3–5 ng/ml >365 days posttransplant. Mycophenolate mofetil was dosed at 600 mg/m2/dose (maximum 1000mg/dose) IV every 12 hours, beginning in the operating room, and transitioned to 450mg/m2/dose (maximum 750mg/dose) orally every 12 hours once the tacrolimus level was at goal. Mycophenolate mofetil dosing was decreased to 300 mg/m2/dose (maximum 500mg/dose) orally every 12 hours beginning 14 days posttransplant. Maintenance steroids were reserved for patients on a sirolimus protocol or who required steroids for other underlying diseases. All patients received pneumocystis jirovecii pneumonia prophylaxis with trimethoprim-sulfamethoxazole or pentamidine for 12 months posttransplant, antifungal prophylaxis with nystatin or clotrimazole for 1 month posttransplant, and CMV antiviral prophylaxis with valganciclovir for 6 months posttransplant.
As a surrogate for medication adherence, we calculated the coefficient of variation (CV), equal to the standard deviation divided by the mean, multiplied by 100, of tacrolimus trough levels16. CVs were calculated separately for the time periods of 85–365 days posttransplant and 366–730 days posttransplant; a weighted average of the 2 coefficients was then calculated for each patient. Tacrolimus whole blood concentration was measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) using Waters Quattro micro or Waters TQ-S micro with a variability of 4.1 – 6.5%. CV was not calculated for patients on sirolimus.
Patients underwent surveillance kidney biopsies between 3 and 6 months posttransplant, between 6 and 12 months posttransplant, and at approximately 24 months posttransplant. All rejection episodes were biopsy-proven; no rejection treatment was given in the absence of a Banff-criteria diagnosis of rejection. Acute cellular rejection episodes were treated with 3 days of methylprednisolone (30mg/kg/day IV, maximum 1000mg/day) followed by a prednisone taper. Antibody-mediated rejection was treated with plasmapheresis daily for 3 days followed by intravenous immunoglobulin and rituximab.
Since January 2008 pediatric kidney transplant patients at Seattle Children’s Hospital have undergone DSA screening every 3 months as part of routine clinical care. BloodWorks Northwest (Seattle, WA), a United Network for Organ Sharing-accredited laboratory that specializes in testing for organ transplantation, performed all DSA tests and provided the results; all DSA results within the first 2 years posttransplant were included in analysis. DSA screening was performed by Luminex technology (either the Luminex 200 or FLEXMAP 3D platform) using single-antigen beads (OneLambda, CA). At our institution, a median fluorescence intensity (MFI) of <2500 is considered ‘weak’, a MFI of 2500 to <8000 is considered ‘moderate’, and a MFI >8000 is considered ‘strong’. All other data were collected from the electronic medical record.
Patients were divided into 3 categories for analysis: patients who developed dnDSA first detected on a screening test, patients who developed dnDSA first detected on a for-cause test, and those who never developed a dnDSA. For all patients with a positive DSA test, the creatinine at the time of the positive test was compared to a baseline in a window 3–6 months prior, defined as the lowest creatinine measured on 2 occasions at least 1 week apart. The screening-detected group was defined as those whose creatinine at the time of first positive dnDSA test was no more than 0.1 mg/dl higher than the baseline in a window 3–6 months prior. The for-cause group included those patients whose creatinine at the time of the first positive dnDSA test was at least 0.2mg/dl higher that it had been in the previous 3 to 6 months. Once patients were grouped based on their first positive DSA test, group assignment did not change, regardless of future changes in DSA results or creatinine. No threshold was used to define a positive DSA result; the lowest reported MFI in this study was 300. No immunosuppression was adjusted, nor was treatment was given, for dnDSA in the absence of biopsy-proven acute rejection.
Estimated glomerular filtration rate (eGFR) was calculated using the Modified Schwartz equation17 for patients less than 18 years old and the CKD-EPI equation18 once patients were 18 years or older. Baseline eGFR posttransplant was calculated using the lowest serum creatinine obtained on 2 occasions at least 1 week apart within the first 60 days after transplant.
The primary outcome of this study was a 30% decline in eGFR, defined as the number of days after transplant when the eGFR fell below 30% of the posttransplant baseline and did not recover within 30 days. A 30% decline in eGFR has been endorsed as a surrogate end point for end stage renal disease that can allow studies to be conducted over shorter time periods and with a smaller number of patients.19,20 Secondary outcomes included graft loss, eGFR at 3 years posttransplant, the number of biopsy-proven rejection episodes within 3 years after transplant, and the incidence of microvascular inflammation, C4d staining, and IFTA on the 2-year protocol biopsy. All biopsies for this study were examined by our renal pathologist using Banff 2007 criteria. Microvascular inflammation was defined as a glomerulitis or peritubular capillaritis score greater than 1. C4d staining was assessed on formalin fixed paraffin embedded sections stained by immunohistochemistry; cases with staining in greater than 10% of tissue were considered positive.
Descriptive statistics used means and standard deviations for normally distributed data and medians and interquartile ranges for skewed data. Using Cox proportional hazards regression, transplant recipients with and without dnDSA were compared for the time to a 30% decline in eGFR and time to graft loss. We employed linear regression to examine the relation between developing dnDSA and eGFR at 3 years posttransplant and logistic regression to examine the relative incidence of interstitial fibrosis and tubular atrophy. The hazard of rejection was compared using a Cox proportional hazards model with dnDSA as a time-varying covariate and allowing for multiple rejection episodes per transplant recipient. Once recipients developed dnDSA they were considered to be DSA positive for the remainder of the follow-up time, even if DSA later resolved. In cases where patients had multiple rejection episodes, time to rejection was calculated from the previous episode. The previous number of episodes was adjusted for in the model by stratification, and robust standard error estimates were used to account for correlation caused by multiple episodes in the same patient. All outcomes were adjusted for confounding by age, type of transplant donor, delayed graft function, cold ischemia time, and baseline eGFR posttransplant, determined on an a priori basis.
Two sensitivity analyses for the primary outcome were performed, 1 using varying MFI thresholds for defining a positive DSA test and 1 dichotomizing the highest MFI on the first positive DSA test into weak (MFI <2500) or moderate/strong (MFI≥2500). Dichotomized MFI groups were also used to look at pathology outcomes using Fisher Exact testing. Analysis was conducted using STATA 12.
This study was approved by the Seattle Children’s Hospital Institutional Review Board, study #13646.
Results
Study population
138 kidney transplants were performed at Seattle Children’s Hospital between 12/1/2007 and 12/31/2013. Among the transplant recipients, 15 were excluded because they were over 18 years old, 12 had a previous solid organ transplant, 1 underwent a multi-organ transplant, and 7 had less than 2 years of DSA screening due to either graft failure prior to 1 year (n=2) or transfer of care to another institution (n=5) (Figure 1). In total 103 patients were included in the study. 81% of patients had 6 or more DSA tests during the first 2 years after transplant. Mean follow up time was 4.4 years posttransplant.
Figure 1.
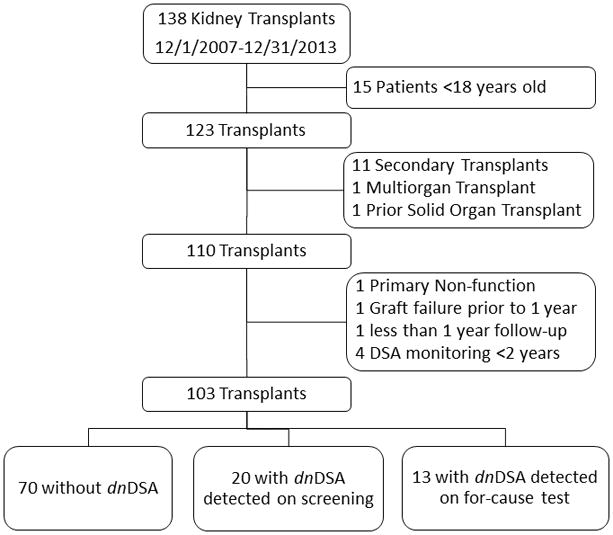
Study Flow Diagram
Thirty-three patients (32%) developed dnDSA within 2 years posttransplant; 20 patients (19%) had dnDSA first detected by screening while 13 patients (13%) were detected on for-cause testing. Patients with screening-detected dnDSA tended to be younger than those without dnDSA, while those with for-cause detected dnDSA tended to be older. Those with screening-detected dnDSA were more likely to have had delayed graft function, a longer cold ischemia time, and EBV or CMV mismatch compared to those without dnDSA. Patients with for-cause detected dnDSA were more likely to have received a deceased donor transplant, have a longer cold ischemia time, and undergo induction with an IL-2 inhibitor. Patients with dnDSA detected in any setting had a median of 4 renal biopsies, 1 more than was generally seen among those without dnDSA. Patients in the 3 study groups were otherwise similar (Table 1).
Table 1.
Baseline characteristics, immunosuppression management, and transplant characteristics of 103 pediatric renal transplants performed 12/1/2008–12/31/2013, subdivided by subsequent diagnosis of dnDSA and clinical stability at time of dnDSA detection.
| No dnDSA n=70 |
dnDSA detected on
screening n=20 |
dnDSA detected on for-cause
testing n=13 |
|
|---|---|---|---|
| Age (years) (median (IQR)) | 12.2 (7.4–14.8) | 6.8 (3.8–16.3) | 16 (6.5–16.3) |
| Male (n(%)) | 44 (63%) | 15 (75%) | 8 (62%) |
| Caucasian (n(%)) | 46 (66%) | 11 (55%) | 6 (46%) |
| ESRD cause (n(%)) | |||
| CAKUT | 36 (54%) | 9 (45%) | 8 (65%) |
| Other | 34 (46%) | 11 (55%) | 5 (35%) |
| Deceased donor transplant (n(%)) | 31 (44%) | 10 (50%) | 11 (85%) |
| Induction(n(%)) | |||
| Thymoglobulin | 56 (80%) | 17 (85%) | 8 (62%) |
| IL-2 blockade | 14 (20%) | 3 (15%) | 5 (38%) |
| # of thymoglobulin doses (mean(sd)) | 4.4 (1.2) | 4.6 (1.1) | 4.8 (1.6) |
| Steroids at induction (n(%)) | 61 (87%) | 19 (95%) | 12 (92%) |
| Maintenance MMF (n(%)) | 70 (100%) | 20 (100%) | 13 (100%) |
| Maintenance Immunosuppression (n(%)) | |||
| Tacrolimus | 68 (97%) | 19 (95%) | 13 (100%) |
| Sirolimus | 2 (3%) | 1 (5%) | 0 (0%) |
| Maintenance steroids (n(%)) | 4 (6%) | 1 (10%) | 0 (0%) |
| Warm ischemia time (min) (median (IQR)) | 56 (44–67) | 52 (45–59) | 51 (47–60) |
| Cold ischemia time (min) (median (IQR)) | 178 (126–714) | 449 (139–691) | 698 (494–996) |
| Delayed graft function (n(%)) | 4 (6%) | 3 (15%) | 0 (0%) |
| cPRA (median(IQR)) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| # Class I HLA mismatches (mean(sd)) | 4.2 (1.6) | 4.8 (1.2) | 4.9 (1.1) |
| # DQ mismatches (mean(sd)) | 1.2 (0.73) | 1.2 (0.75) | 1.2 (0.69) |
| # DR mismatches (mean(sd)) | 0.8 (0.73) | 1 (0.73) | 0.9 (0.86) |
| EBV D+/R− (n(%)) | 21 (30%) | 9 (45%) | 2 (20%) |
| CMV D+/R− (n(%)) | 20 (29%) | 9 (45%) | 4 (33%) |
| Baseline eGFR (ml/min/1.73m2) (mean(sd)) | 85.7 (21.2) | 93.9 (36.6) | 89.0 (20.1) |
| Renal biopsies in 2 years (median(IQR)) | 3 (2–4) | 4 (2.5–4.5) | 4 (3–5) |
| Time in Study (years) (mean(sd)) | 4.5 (1.6) | 4.5 (1.7) | 3.2 (1.8) |
ESRD: End-stage renal disease; MMF: mycophenolate mofetil; cPRA: calculated panel reactive antibodies; D+/R-: Donor positive/recipient negative
dnDSA characteristics
Patients with screening-detected dnDSA tended to develop dnDSA earlier, and the dnDSA persisted longer, than those with dnDSA detected on a for-cause test. Those with dnDSA on a for-cause test were more likely to have antibodies against Class I HLA (15%) or both Class I and Class II HLA (31%) compared to those with screening dnDSA (10% and 10%, respectively). Conversely, those with screening-detected dnDSA were more likely to have isolated Class II HLA antibodies (80%). The 2 groups had similar peak MFIs and rates of C1q positivity. Approximately 30% of detected dnDSAs resolved prior to the end of 2 years of monitoring in both groups (Table 2). There was no difference in our surrogate marker for adherence, mean CV of tacrolimus trough level, between those with screening-detected dnDSA and those with dnDSA detected on for-cause testing (p=0.924, Table 2).
Table 2.
Characteristics of de novo donor specific antibodies detected in pediatric patients after kidney transplant, by clinical stability at time of dnDSA detection.
| dnDSA detected on
screening n=20 |
dnDSA detected on for-cause
testing n=13 |
|
|---|---|---|
| ≥6 DSA tests in 2 years of monitoring | 17 (85%) | 10 (77%) |
| Time to DSA (days) (median(IQR)) | 332 (87–738) | 543 (394–630) |
| Class of Initial DSA (n(%)) | ||
| Class I | 2 (10%) | 2 (15%) |
| Class II | 16 (80%) | 7 (54%) |
| Class I & Class II | 2 (10%) | 4 (31%) |
| Initial DSA MFI (median(IQR)) | 2500 (1050–4350) | 2400 (1300–3400) |
| Class of DSA overall (n(%)) | ||
| Class I | 2 (10%) | 2 (15.4%) |
| Class II | 13 (65%) | 7 (15%) |
| Class I & Class II | 5 (25%) | 4 (31%) |
| Peak MFI overall (median(IQR)) | 6150 (2750–11600) | 4100 (2200–8900) |
| C1q positive (n(%)) | 6 (43%) | 2 (40%) |
| Biopsy within 3 months of positive DSA | 10 (50%) | 10 (77%) |
| Antibody-mediated rejection | 1 (10%) | 1 (10%) |
| Acute cellular rejection | 3 (30%) | 6 (60%) |
| Duration of DSA (days) (median(IQR)) | 378 (85–596) | 172 (85–241) |
| DSA resolved before 2 years (n(%)) | 6 (30%) | 4 (31%) |
| CV for tacrolimus trough level (mean(sd)) | 34.9% (12.5) | 35.3% (12.4) |
CV: coefficient of variation
Outcomes of patients with dnDSA
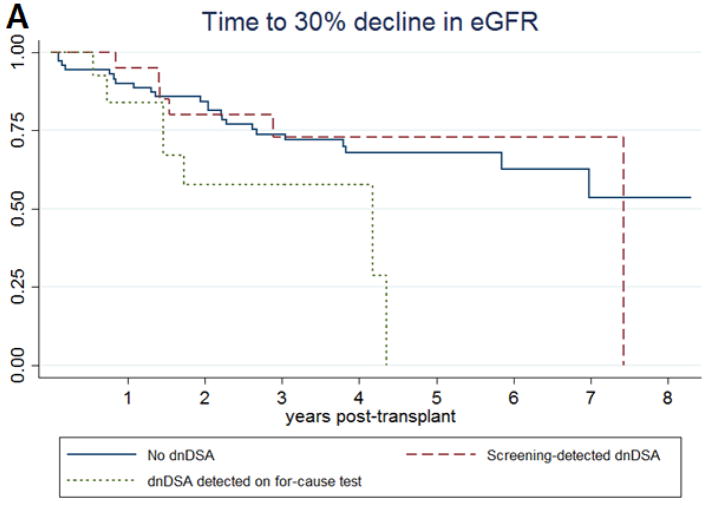
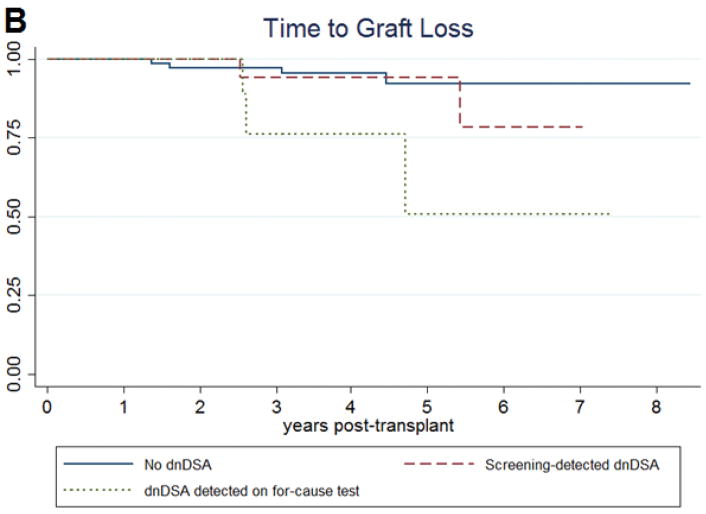
In a Cox proportional hazards regression controlling for age at transplant, type of transplant donor, incidence of delayed graft function, cold ischemia time, and baseline posttransplant eGFR, there was no significant difference in the occurrence of a 30% decline in eGFR between those with screening-detected dnDSA and those who never developed DSA (aHR 0.88 95%CI 0.30–2.00 p=0.598) (Table 3a; Figure 2a). There was an increased hazard ratio for graft loss, but the result was not statistically significant (aHR 1.75 95%CI 0.28–10.73 p=0.546). There was also no difference the eGFR at 3 years posttransplant (difference -1.08ml/min/1.73m2 p=0.616).
Table 3a.
Clinical outcomes among pediatric kidney transplant patients subdivided by presence of dnDSA and clinical stability at the time of dnDSA detection. Results are adjusted for age at transplant, type of transplant donor, incidence of delayed graft function, cold ischemia time, and baseline estimated glomerular filtration rate.
| Outcome | No
dnDSA n=70 |
dnDSA detected on
screening n=20 |
dnDSA detected on for-cause
testing n=13 |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| aHR | aHR (95%CI) | p | aHR (95%CI) | p | ||||
| eGFR 30% decline | Ref | 0.88 (0.30–2.00) | 0.598 | 2.80 (1.08–7.27) | 0.034 | |||
| Graft loss | Ref | 1.75 (0.28–10.73) | 0.546 | 7.34 (1.37–39.23) | 0.020 | |||
| Mean | SD | Mean | SD | p | Mean | SD | p | |
| eGFR at 3 years (ml/min/1.73m2) | 75.34 | 23.65 | 74.26 | 36.20 | 0.632 | 60.84 | 30.31 | 0.054 |
| n=244.7 person-years | n=41.8 person-years | n=22.5 person-years | ||||||
|
|
|
|
||||||
| IR | RR | IR | aHR (95% CI) | p | IR | aHR (95% CI) | p | |
| Rejection (at 3 years) | 0.15 | Ref | 0.36 | 2.41 (1.27–4.54) | 0.007 | 0.71 | 2.43 (1.00–5.90) | 0.050 |
Figure 2.
Kaplan-Meier survival curves for (A) time to a 30% decline in estimated glomerular filtration rate and (B) time to graft loss among pediatric kidney transplant patients, subdivided by presence of dnDSA and clinical setting of dnDSA detection.
Patients with dnDSA detected on for-cause testing did have an increased risk of decline in graft function (aHR 2.80 95%CI 1.08–7.27 p=0.034), an increased risk of graft loss (aHR 7.34 95%CI 1.37–39.23 p=0.020), and a lower 3-year eGFR (difference -14.5ml/min/1.73m2 p=0.054) compared to those without dnDSA after adjustment for age at transplant, type of transplant donor, incidence of delayed graft function, cold ischemia time, and baseline eGFR (Table 3a; Figure 2b).
The development of dnDSA was associated with an increased risk of an episode of any type of rejection within 3 years posttransplant regardless of circumstances of dnDSA detection. The incidence of rejection in patients without dnDSA was 15 per 100 person-years, compared to 36 per 100 person-years in those with screening-detected dnDSA and 71 per 100 person-years in those with dnDSA done for-cause (Table 3a). In a Cox proportional hazards analysis with dnDSA status as a time varying exposure, allowing for multiple rejection episodes per patient, and adjusted for age at transplant, type of transplant donor, incidence of delayed graft function, cold ischemia time, and baseline-posttransplant eGFR, patients with screening-detected dnDSA and those with dnDSA on for-cause testing had a similar increased hazard for subsequent rejection episodes (dnDSA on screening: aHR 2.41 95%CI 1.27–4.54 p=0.007; dnDSA on for-cause testing: aHR 2.43 95%CI 1.00–5.90 p=0.050).
Patients who developed dnDSA were more likely to have microvascular inflammation and C4d staining on their protocol biopsy 2 years after transplant compared to those without dnDSA (Table 3b). The incidence of microvascular inflammation was higher among those with dnDSA on for-cause testing (42%) compared to those with screening-detected dnDSA (24%), while the incidence of C4d staining was similar between the 2 groups. There was no association between dnDSA and IFTA in this study.
Table 3b.
Biopsy findings among pediatric kidney transplant patients subdivided by presence of dnDSA and clinical stability at the time of dnDSA detection. Results are adjusted for age at transplant, type of transplant donor, incidence of delayed graft function, cold ischemia time, and baseline estimated glomerular filtration rate.
| No dnDSAn=65 |
dnDSA detected on
screening n=17 |
dnDSA detected on for-cause
test n=12 |
||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | aOR1 (95% CI) | p | n (%) | aOR (95% CI) | p | ||
| Microvascular Inflammation1 | Ref | 11.46 (1.69–77.58) | 0.012 | 31.02 (3.76–256.0) | 0.001 | |||
| Absent | 63 (97%) | 13 (76%) | 7 (58%) | |||||
| Present | 2 (3%) | 4 (24%) | 5 (42%) | |||||
| C4d2 | Ref | 11.49 (2.34–56.36) | 0.003 | 7.73 (1.26–47.44) | 0.027 | |||
| 0 | 61 (95%) | 11 (65%) | 8 (67%) | |||||
| 1, 2, or 3 | 3 (5%) | 6 (35%) | 4 (33%) | |||||
| IFTA | Ref | 2.41 (0.71–8.14) | 0.158 | 0.36 (0.04–3.30) | 0.363 | |||
| I | 53 (82%) | 11 (65%) | 11 (92%) | |||||
| II or III | 12 (18%) | 6 (35%) | 1 (8%) | |||||
Microvascular inflammation defined as a glomerulitis or peritubular capillaritis score >0;
Delayed graft function omitted from regression model; only 3 out of 7 patients with DGF had a 2 year protocol biopsy, and none of these 3 had c4d on biopsy
In a sensitivity analysis that used different MFI thresholds to define a positive DSA test, there was no change in time to a 30% decline in eGFR when using MFI thresholds of 1000, 1500, 2500, or 8000 (Table 4). In a separate analysis, we dichotomized patients with dnDSA by their highest MFI on their initial positive DSA test into <2500 and ≥2500. In this analysis we found similar results for the primary outcome of a 30% decline in eGFR. We also found a similar incidence of antibody-mediated rejection, graft failure, microvascular inflammation, c4d positivity, and IFTA between the 2 groups (Table 5).
Table 4.
Risk of 30% decline in estimated glomerular filtration rate among pediatric kidney transplant patients, comparing patients with dnDSA detected by screening to patients who did not develop dnDSA within 2 years of kidney transplant, using different MFI thresholds to adjudicate DSA positivity. Results are adjusted for age at transplant, type of transplant donor, delayed graft function, cold ischemia time, and baseline estimated glomerular filtration rate.
| No dnDSA |
dnDSA detected on screening | 30% decline in renal function: No dnDSA vs dnDSA detected on screening | ||
|---|---|---|---|---|
|
|
|
|
||
| n1 | n1 | aHR (95%CI) | p | |
| Any MFI | 70 | 20 | 0.88 (0.30–2.00) | 0.598 |
| MFI ≥1000 | 72 | 17 | 1.01 (0.38–2.69) | 0.976 |
| MFI ≥1500 | 75 | 17 | 0.97 (0.33–2.85) | 0.955 |
| MFI ≥2500 | 79 | 17 | 1.04 (0.39–2.76) | 0.938 |
| MFI ≥8000 | 90 | 9 | 0.99 (0.23–4.29) | 0.994 |
Total number of patients does not consistently equal 90 due to some patients being reclassified as ‘dnDSA on for-cause testing’ at each threshhold
Table 5.
Sensitivity analysis subdividing pediatric kidney transplant recipients with dnDSA by clinical setting of DSA detection and the highest MFI on their first positive DSA test. Primary outcome of 30% decline in eGFR evaluated by Cox proportional hazards regression adjusted for age at transplant, type of transplant donor, delayed graft function, cold ischemia time, and baseline estimated glomerular filtration rate. All other outcomes evaluated using Fisher Exact testing without adjustment.
| Initial MFI | 30% decline in eGFR | Antibody-mediated rejection | Graft failure | |||
|---|---|---|---|---|---|---|
| n (%) | aHR (95% CI) | p value | n (%) | incidence rate (p-y) | n (%) | |
|
|
|
|||||
| Screening dnDSA | ||||||
| <2500 | 9 (45%) | 0.86 (0.26–2.77) | p=0.796 | 2 (22%) | 0.11 | 1 (11%) |
| ≥2500 | 11 (55%) | 0.63 (0.14–2.70) | p=0.539 | 3 (27%) | 0.13 | 1 (9%) |
| p=1.00 | p=1.00 | |||||
| For-cause dnDSA | ||||||
| <2500 | 8 (62%) | 3.50 (1.14–10.68) | p=0.028 | 1 (13%) | 0.07 | 3 (38%) |
| ≥2500 | 5 (38%) | 1.90 (0.40–3.09) | p=0.421 | 1 (20%) | 0.11 | 0 (0%) |
| p=1.00 | p=0.231 | |||||
| Microvascular inflammation2 | c4d score 1,2, or 3 | IFTA II or III | ||||
| n (%) | n (%) | n (%) | n (%) | |||
|
|
|
|||||
| Screening dnDSA | ||||||
| <2500 | 8 (47%) | 2 (25%) | 3 (37%) | 3 (38%) | ||
| ≥2500 | 9 (53%) | 2 (22%) | 3 (33%) | 3 (33%) | ||
| p=1.00 | p=1.00 | p=1.00 | ||||
| For-cause dnDSA | ||||||
| <2500 | 8 (66%) | 4 (50%) | 2 (25%) | 0 (0%) | ||
| ≥2500 | 4 (33%) | 1 (25%) | 2 (50%) | 1 (25%) | ||
| p=0.576 | p=0.547 | p=0.333 | ||||
Each episode of antibody mediated rejection occurred in 1 individual patient;
Microvascular inflammation defined as a glomerulitis or peritubular capillaritis score >0; p-y: person-years
Discussion
In this study we show that clinically stable patients who are first diagnosed with dnDSAs on a screening test have an increased risk of acute rejection, microvascular inflammation, and C4d deposition compared to those patients who never develop dnDSA. However, we could not show an increased risk of decline in graft function or graft loss. Consistent with previous studies, we show that patients who develop dnDSA detected on for-cause testing do have an increased risk for decline in graft function and graft loss. To the best of our knowledge, this is the largest study to examine the outcomes of screening dnDSA in pediatric kidney transplant patients.
Much of the previous literature on dnDSA and graft outcomes has combined data on screening in clinically asymptomatic patients with data from patients with graft dysfunction.1,5,10,14 While this was logical given the goals of those studies, it may not be appropriate to apply those results to a clinically stable population undergoing DSA screening. Our data and data from Wiebe et al15 and Cooper et al14 suggest that patients who are clinically stable and those with graft dysfunction represent distinct groups with different risks of poor graft outcome. This means that the results of those prior studies could overstate the risk of dnDSA in clinically stable patients while understating the risk in those with graft dysfunction. This is perhaps best illustrated in the study by Cooper et al, who reported an overall hazard ratio for 2 year graft loss of 7.7 among those with dnDSA. However, our review of their reported outcomes shows that, when their patient population was divided into those with dnDSA on protocol testing vs nonprotocol testing, the relative risk of 2 year graft loss was 3.2 for the protocol group and 28.6 for the nonprotocol group - a 9-fold difference.14
There is already evidence that not all dnDSA have equal propensity to damage the kidney. Studies have shown an increased risk of graft loss among patients with anti-HLA class II antibodies,21 anti-DQ antibodies,22 and antibodies with a higher MFI.8 DSAs are thought to damage the renal microvasculature via complement fixation, and several research groups have found an increased risk of graft loss caused by DSAs that bind complement proteins C1q23,24 or C3d.25 However, the strong association between C1q or C3d binding ability and DSA MFI has raised concerns about the validity of these assays.26,27 It is possible that the types of dnDSA that develop in patients who are otherwise clinically stable may differ from the dnDSA in patients with graft dysfunction, which could explain the difference in outcome seen in our study. A higher percentage of patients with dnDSA detected on for-cause testing had both class I and class II antibodies; however this study was not designed or powered to detect differences in antibody characteristics. Further research with larger patient populations is needed to better clarify the types of antibodies that are most harmful to the renal allograft.
Both acute rejection2 and microvascular inflammation28–30 have been associated with decreased graft survival, while the impact of C4d staining on graft survival is more questionable.28,30–32 However, in our study screening-detected dnDSA was not associated with a faster decline in graft function despite being associated with both acute rejection and microvascular inflammation. One explanation for this may be that acute rejection and microvascular inflammation represent the first steps leading to a later decline in graft function that was not captured in our follow-up. However, those transplant recipients with dnDSA detected on for-cause testing were shown to have a higher rate of decline in graft function and graft failure within the study follow up time, despite generally developing dnDSA later (median 543 days) than those patients with screening-detected dnDSA (median 332 days). Alternatively, the association between acute rejection and graft function may be more nuanced than previously thought. In their original study of the association between acute rejection and graft loss, Meier Kriesche et al noted that graft survival among patients whose renal function returned to baseline after a rejection episode was no different than patients without rejection episodes.2 It is possible that the biopsy findings associated with screening-detected dnDSA represent a different, more indolent, process than in patients with dnDSA detected on for-cause testing.
The study utilized a rich clinical data source that included DSA screening results and biopsy data on patients during times of both clinical stability and graft dysfunction. This allows fuller assessment of both dnDSA incidence and pathologic changes throughout the posttransplant course. It also took advantage of a large pediatric cohort managed at a single institution under a single standardized set of transplant protocols, decreasing the variation in outcome that could be introduced by variations in immunosuppression management. The use of survival techniques allowed us to take full advantage of the long follow-up times available on many of the study’s patients.
The chief limitation of this study is that the follow-up period may not have been long enough to fully monitor the association between the presence of dnDSA and long-term outcome. Antibody-mediated damage is hypothesized to be an indolent process.15 Terasaki et al published data showing a mean time from detection of dnDSA to graft failure of approximately 2.9 years.33 While the mean posttransplant follow-up time in our study was 4.5 years, 25% of patients in the dnDSA-stable creatinine group had less than 2 years of follow-up after their first positive DSA test. However, our primary outcome was a 30% decline in renal function, not graft loss. A 30% decline in eGFR is a surrogate endpoint that allows for shorter trial durations while maintaining a chance of type I error of less than 5%.20 Therefore, the shorter duration of follow-up in our study may be somewhat mitigated. A second limitation is that we found an increased number of biopsies in the group with screening-detected dnDSA, suggesting that the detection of dnDSA, while not directly prompting treatment, may have led to increased surveillance and, therefore, detection of rejection.
In conclusion, we demonstrated that the clinical setting in which dnDSA is first detected impacts the association between dnDSA and graft function. Screening-detected dnDSA was associated with an increased risk of rejection episodes, microvascular inflammation, and C4d deposition but not associated with a decline in graft function, suggesting that there may be ongoing subclinical renal damage. Further study is needed to establish the role of dnDSA screening in the pediatric kidney transplant population, to clarify the types of antibodies that are most associated with harm to the graft, and to guide interventions.
Acknowledgments
Funding:
Dr. Engen was supported by National Institute of Health grant 5 T32 DK007662. Dr. Schumacher was supported by National Institute of Health grant NIEHS T32ES015459.
Abbreviations
- aHR
adjusted hazard ratio
- CI
confidence interval
- CMV
cytomegalovirus
- CV
coefficient of variation
- dnDSA
de novo donor-specific antibody
- DSA
donor-specific antibody
- EBV
Epstein-Barr Virus
- eGFR
estimated glomerular filtration rate
- HLA
human leukocyte antigen
- IFTA
interstitial fibrosis and tubular atrophy
- IL-2
interleukin-2
- IQR
interquartile range
- MFI
median fluorescence intensity
Footnotes
Authorship:
Rachel M Engen participated in the research design, performance of the research, data analysis, and writing of the paper.
Giulia E Park participated in the performance of the research.
Cooper Schumacher participated in data analysis.
Idoia Gimferrer participated in the performance of the research and the writing of the paper.
Paul Warner participated in the performance of the research.
Laura S Finn participated in performance of the research.
Noel S Weiss participated in the research design and writing of the paper.
Jodi M Smith participated in the research design and writing of the paper.
Disclosures:
The authors declare no conflicts of interest.
Contributor Information
Rachel M Engen, Department of Pediatrics, University of Washington Seattle, WA.
Giulia E Park, Division of Nephrology, Seattle Children’s Hospital Seattle, WA.
Cooper S Schumacher, Department of Biostatistics, University of Washington Seattle, WA.
Idoia Gimferrer, Bloodworks Northwest, Seattle, WA.
Paul Warner, Bloodworks Northwest, Seattle, WA.
Laura S Finn, Department of Pathology, University of Washington Seattle, WA.
Noel S Weiss, Department of Epidemiology, University of Washington Seattle, WA.
Jodi M Smith, Department of Pediatrics, University of Washington Seattle, WA.
References
- 1.Kim JJ, Balasubramanian R, Michaelides G, et al. The clinical spectrum of de novo donor-specific antibodies in pediatric renal transplant recipients. Am J Transplant. 2014;14(10):2350–2358. doi: 10.1111/ajt.12859. [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche H-U, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–535. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 4.Terasaki PI. A personal perspective: 100-year history of the humoral theory of transplantation. Transplantation. 2012;93(8):751–756. doi: 10.1097/TP.0b013e3182483713. [DOI] [PubMed] [Google Scholar]
- 5.Christiaans MH, Overhof-de Roos R, Nieman F, van Hooff JP, van den Berg-Loonen EM. Donor-specific antibodies after transplantation by flow cytometry: relative change in fluorescence ratio most sensitive risk factor for graft survival. Transplantation. 1998;65(3):427–433. doi: 10.1097/00007890-199802150-00024. [DOI] [PubMed] [Google Scholar]
- 6.Mihaylova A, Baltadjieva D, Boneva P, et al. Clinical relevance of anti-HLA antibodies detected by flow-cytometry bead-based assays--single-center experience. Hum Immunol. 2006;67(10):787–794. doi: 10.1016/j.humimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Lachmann N, Terasaki PI, Budde K, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87(10):1505–1513. doi: 10.1097/TP.0b013e3181a44206. [DOI] [PubMed] [Google Scholar]
- 8.Freitas MCS, Rebellato LM, Ozawa M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95(9):1113–1119. doi: 10.1097/TP.0b013e3182888db6. [DOI] [PubMed] [Google Scholar]
- 9.Banasik M, Boratyńska M, Kościelska-Kasprzak K, et al. The impact of de novo donor-specific anti-human leukocyte antigen antibodies on 5-year renal transplant outcome. Transplant Proc. 2013;45(4):1449–1452. doi: 10.1016/j.transproceed.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Dieplinger G, Ditt V, Arns W, et al. Impact of de novo donor-specific HLA antibodies detected by Luminex solid-phase assay after transplantation in a group of 88 consecutive living-donor renal transplantations. Transpl Int. 2014;27(1):60–68. doi: 10.1111/tri.12207. [DOI] [PubMed] [Google Scholar]
- 11.Ntokou I-SA, Iniotaki AG, Kontou EN, et al. Long-term follow up for anti-HLA donor specific antibodies postrenal transplantation: high immunogenicity of HLA class II graft molecules. Transpl Int. 2011;24(11):1084–1093. doi: 10.1111/j.1432-2277.2011.01312.x. [DOI] [PubMed] [Google Scholar]
- 12.Ginevri F, Nocera A, Comoli P, et al. Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12(12):3355–3362. doi: 10.1111/j.1600-6143.2012.04251.x. [DOI] [PubMed] [Google Scholar]
- 13.Tait BD, Süsal C, Gebel HM, et al. Consensus Guidelines on the Testing and Clinical Management Issues Associated With HLA and Non-HLA Antibodies in Transplantation. Transplant J. 2013;95(1):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91(10):1103–1109. doi: 10.1097/TP.0b013e3182139da1. [DOI] [PubMed] [Google Scholar]
- 15.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12(5):1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigo E, San Segundo D, Fernández-Fresnedo G, et al. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation. 2016;100(11):2479–2485. doi: 10.1097/TP.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, et al. New Equations to Estimate GFR in Children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson A, Lawrence J, Stockbridge N. GFR Decline as an End Point in Trials of CKD: A Viewpoint From the FDA. Am J Kidney Dis. 2014;64(6):836–837. doi: 10.1053/j.ajkd.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Greene T, Teng C-C, Inker LA, et al. Utility and Validity of Estimated GFR–Based Surrogate Time-to-Event End Points in CKD: A Simulation Study. Am J Kidney Dis. 2014;64(6):867–879. doi: 10.1053/j.ajkd.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Campos EF, Tedesco-Silva H, Machado PG, Franco M, Medina-Pestana JO, Gerbase-DeLima M. Post-Transplant Anti-HLA Class II Antibodies as Risk Factor for Late Kidney Allograft Failure. Am J Transplant. 2006;6(10):2316–2320. doi: 10.1111/j.1600-6143.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 22.Willicombe M, Brookes P, Sergeant R, et al. De Novo DQ Donor-Specific Antibodies Are Associated With a Significant Risk of Antibody-Mediated Rejection and Transplant Glomerulopathy. Transplant J. 2012;94(2):172–177. doi: 10.1097/TP.0b013e3182543950. [DOI] [PubMed] [Google Scholar]
- 23.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-Binding Anti-HLA Antibodies and Kidney-Allograft Survival. N Engl J Med. 2013;369(13):1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16(1):12–7. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 25.Comoli P, Cioni M, Tagliamacco A, et al. Acquisition of C3d-Binding Activity by De Novo Donor-Specific HLA Antibodies Correlates With Graft Loss in Nonsensitized Pediatric Kidney Recipients. Am J Transplant. 2016;16(7):2106–16. doi: 10.1111/ajt.13700. [DOI] [PubMed] [Google Scholar]
- 26.Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM. C1q Binding Activity of De Novo Donor-specific HLA Antibodies in Renal Transplant Recipients With and Without Antibody-mediated Rejection. Transplantation. 2015;99(6):1151–1155. doi: 10.1097/TP.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 27.Messina M, Ariaudo C, Praticò Barbato L, et al. Relationship among C1q-fixing de novo donor specific antibodies, C4d deposition and renal outcome in transplant glomerulopathy. Transpl Immunol. 2015;33(1):7–12. doi: 10.1016/j.trim.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Einecke G, Sis B, Reeve J, et al. Antibody-Mediated Microcirculation Injury Is the Major Cause of Late Kidney Transplant Failure. Am J Transplant. 2009;9(11):2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 29.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4d Banff Scores in Early Protocol Biopsies of Kidney Transplant Recipients with Preformed Donor-Specific Antibodies (DSA) Am J Transplant. 2011;11(1):56–65. doi: 10.1111/j.1600-6143.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 30.Verghese P, Dunn T, Najafian B, Kim Y, Matas A. The impact of C4d and microvascular inflammation before we knew them. Clin Transplant. 2013;27(3):388–396. doi: 10.1111/ctr.12111. [DOI] [PubMed] [Google Scholar]
- 31.John R, Konvalinka A, Tobar A, Kim SJ, Reich HN, Herzenberg AM. Determinants of Long-Term Graft Outcome in Transplant Glomerulopathy. Transplantation. 2010;90(7):757–764. doi: 10.1097/TP.0b013e3181efcffd. [DOI] [PubMed] [Google Scholar]
- 32.Sharif A, Kraus ES, Zachary AA, et al. Histologic Phenotype on 1-Year Posttransplantation Biopsy and Allograft Survival in HLA-Incompatible Kidney Transplants. Transplantation. 2014;97(5):541–547. doi: 10.1097/01.TP.0000442513.27641.7e. [DOI] [PubMed] [Google Scholar]
- 33.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]