Abstract
Purpose
To examine in persons with varying degrees of cognitive impairment the relationship between self-reports of cognitive complaints and quality of life (QOL).
Method
Older adults (n=259) with normal cognition, Mild Cognitive Impairment (MCI), and mild stage Alzheimer’s disease (AD) dementia completed tests of cognition and self-report questionnaires about QOL and three kinds of cognitive complaints: cognitive difficulties, distress from cognitive difficulties, and believing you had more memory problems than most people. Bivariate, multivariable, and multivariate regression analyses assessed relationships between domains of QOL and each cognitive complaint.
Results
Bivariate and multivariable analyses controlling for severity of cognitive and functional impairment found that cognitive complaints were related to relatively lower quality of daily life (QOL-AD, DEM-QOL), greater depression (GDS), more anxiety (BAI), higher perceived stress (PSS), and lower general mental wellbeing (SF-12 MCS).
Discussion
Cognitive complaints have robust associations with QOL. These findings have implications for AD prevention trials and management of clinical populations.
Keywords: self-rated health, Alzheimer’s disease, self-reported symptoms, mild cognitive impairment, quality of life
Introduction
In the United States (U.S.), over 5 million older Americans have Alzheimer’s disease (AD) dementia, and that number is expected to nearly triple in the next two decades.1 To address this leading cause of disability that has no therapies to stop or slow its progression, the U.S. national Alzheimer’s plan seeks to discover an effective therapy by 2025.1 To achieve this goal, older adults are being identified early in the disease process, either with Mild Cognitive Impairment (MCI) or with no cognitive impairment but with one or more biological risk factors. The premise is that to prevent or slow cognitive decline through both novel targeted pharmaceutical treatments and lifestyle changes, we need to detect the disease early.2
A plan to diagnose and treat AD early means patient-reported outcomes are becoming of even more important.3–7 Traditionally, patient self-report data have had limited use in AD research and clinical practice because the disease often causes problems in insight that can limit accurate self-report of disability.6,8,9 This is arguably less likely early in the disease process.9,10 Currently, much of the information about the patient’s health status and symptoms comes from the patient’s informant. However, with earlier diagnosis, patients may expect to provide reports of their health status and symptoms.
Cognitive complaints are self-report data about the experience of cognitive symptoms. These self-report data can reflect one’s beliefs about functioning, which in turn, can affect willingness to seek diagnosis and care.11–13 They can also convey information about subjective difficulties and distress that can guide treatment planning and affect treatment adherence.14–16 Although it is unclear whether cognitive complaints correspond to objective measures of cognitive impairment and pathology,10,17–21 patient-reported data about beliefs, perceived difficulities, and distress related to cognitive symptoms can offer useful information about the subjective experience and quality of life (QOL) of a patient.
QOL is a multidimensional construct. It is typically evaluated across both personal and situational domains to encompass a person’s overall functioning.22 For individuals with dementia, the conceptual framework for QOL often integrates cognitive functioning, physical functioning, social interactions, mental wellbeing, and mood.22 Prior QOL studies in persons with AD dementia have tended to evaluate multiple domains. However, when assessing cognitive symptoms these studies have focused almost exclusively on neuropsychiatric symptoms.7,23–27 These are symptoms that often occur at severe stages of disease and are uncommon in early stages.
Many QOL studies have been conducted with patients with AD dementia, Mild Cognitive Impairment (MCI), and normal cognition (NC),7,23–40 but few studies have examined the relationships between cognitive complaints and QOL across the continuum from NC, to MCI, to mild stage AD dementia.35,37,41 Understanding these relationships may help characterize the patient experience in at-risk or early clinical stages. The more we understand the relationships between subjective cognitive complaints and QOL domains, the better we can explain the symptom patterns early in the disease course. Problems in some domains – such as depression and impairments in instrumental activities of living (IADLs) – can reflect some of the earliest symptoms of dementia to be reported by patients.10,11,17–21,42,43
QOL domains are distinct but given that impairments in one could influence another, it’s important to understand how cognitive complaints might relate to a QOL domain independent of that domain’s relationships to other QOL domains. This type of analysis, referred to as a “multivariate” analysis, may help inform assessment by showing how domains are or are not associated with cognitive complaints while statistically adjusting for relationships among domains. This simultaneous analysis of multiple outcomes can also help minimize spurious findings that can result from multiple comparisons of individual outcomes.
The purpose of this study was to understand relationships between self-reported cognitive complaints and QOL in older adults with NC, MCI, or mild stage AD dementia. Based on prior studies of samples with AD and MCI,7,23–31,33–35,44 we anticipated strong negative relationships between cognitive complaints and QOL. A prior study found lower QOL among older adults with MCI compared to those with either NC or AD.40 This suggests that both cognitive complaints and self-reported QOL do not follow a pattern of linear change with declining cognition. We hypothesized therefore, that in moderator analyses, the relationships between cognitive complaints and QOL would be stronger for persons with a diagnosis of MCI compared to those with either a diagnosis of AD or NC. In a multivariate analysis, we sought to understand whether these relationships would persist after controlling for associations among QOL domains. Understanding these associations may help inform the choice and use of patient reported outcomes in AD prevention trials and help guide management and intervention in clinical populations.
Methods
Study Participants
The sample of 259 older adults with mild stage probable AD dementia (n=68), MCI (n=92), and NC (n=99) was recruited from the Penn Memory Center (PMC) cohort study. Eligible adults were: age ≥65, native English speakers, able to read from a handheld visual acuity card, able to hear conversational speech, completed at least the 6th grade, scored 20 or higher on the Mini-Mental Status Exam (MMSE), and lived within one-hour drive of the PMC. Individuals with AD and MCI were required to participate with a knowledgeable informant. Individuals with NC did not meet clinical criteria for either MCI or AD dementia and demonstrated performance on neuropsychological testing commensurate with similarly aged and educated peers. All participants provided written informed consent or, as appropriate, assent with the written informed consent of their knowledgeable informant.
Participant Interviews
All participants completed a pair of face-to-face interviews. Interviews were conducted over two sessions to avoid fatigue and were completed within three months of the most recent cohort assessment. Each session lasted 1 to 1.5 hours. Participants received a $20 gift card after each visit.
Measures
Three cognitive complaints were measured. Cognitive difficulties were assessed with the Cognitive Difficulties Scale (CDS), which estimates how many and how often cognitive symptoms impact daily life.6,12 Distress due to cognitive problems was assessed using the Global Distress Index (GDI), which was adapted from the Memorial Symptom Assessment Scale Short Form.45 Belief that one experienced more memory problems than most others was obtained from a single item on the Geriatric Depression Scale (GDS).
QOL was appraised across: physical functioning, social interactions, mood, mental wellbeing, and functioning in activities of daily life.22 Physical and mental wellbeing were assessed with the Physical Composite Scale (PCS) and Mental Composite Scale (MCS) from the Short Form Health Survey (SF-12).46 Depression, anxiety, and subjective stress were assessed using the Geriatric Depression Scale (GDS),47 Beck Anxiety Inventory (BAI),48 and Perceived Stress Scale (PSS).49 Physical functioning was assessed via the Lawton-Brody Basic and Instrumental Activities of Daily Living scales (B/IADLs).50
Multiple validated measures captured distinct aspects of QOL in daily life. Difficulty in daily life related to health, well-being, cognitive functioning, social relationships, daily activities, and self-concept was measured by the DEM-QOL.51 Satisfaction about physical health, living situation, family, marriage, and finances was assessed with the QOL-AD.52 Health-related QOL linked to mobility, self-care, usual activity, pain, and anxiety was measured by the Euro-QOL (EQ-5D), which included a single visual analogue scale for overall “health state”(EQ-VAS).53
Cognitive functioning was appraised with a battery of performance-based assessments. Global cognitive functioning was measured with the MMSE54 and Montreal Cognitive Assessment (MoCA).55 Verbal and non-verbal memory were measured with the Philadelphia Verbal Learning Task (PVLT)56 and Biber Figure Learning Task (BFLT).57 Executive function was assessed with Graphic Pattern Generation (GPG) test.58 Cognitive reserve and premorbid crystalized intelligence were assessed via the Wechsler Test of Adult Reading (WTAR)59 and Wechsler Adult Intelligence Scale third edition (WAIS-III) Information subtest.60
Standard demographics were collected directly from NC participants and from knowledgeable informants of AD and MCI participants. All procedures were approved by the local Institutional Review Board. A fuller description of the methods is available elsewhere.40
Statistical Analysis
We used descriptive statistics to characterize the sample based on the report of three cognitive complaints. Cognitive complaints were analyzed as dichotomies as this aided interpretation and utility of the results. Because subjective memory complaints are common,61 dichotomizing the measures at the median values of the NC group also helped mitigate ceiling effects. CDS and GDI scores were dichotomized so that the higher category represented endorsement above that of 50% of NC individuals. For cognitive difficulties, the median was a score of 72 whereas for distress it was 18.
We assessed relationships between cognitive complaints and QOL domains in older adults with mild AD, MCI, and NC using bivariate and multivariable regression analyses. We report results from only the multivariable analyses as the bivariate results were similar. The multivariable models controlled statistically for participant characteristics that were unbalanced between study groups. Adjustment for imbalances in cognitive performance was determined by the cognitive measure that contributed to the best model fit of all potential candidates.62 In multivariable analyses of cognitive difficulties, models controlled for diagnostic category and Cognitive Composite Score (CCS), which was an objective measure of memory performance calculated as the average of the z-scores from the PVLT immediate memory, PVLT long-term memory, BFLT immediate memory, and BFLT long-term memory standardized to the NC group (mean=0, SD=1). Multivariable analyses of cognitive distress included global cognitive impairment (MMSE), college education, and diagnostic category. Multivariable analyses of more memory problems than most others controlled for global cognitive impairment (MMSE) and diagnostic category.
To understand the clinical significance of differences in the multivariable analyses, we examined the means and mean differences of measures with interpretable clinical thresholds: anxiety (BAI), depression (GDS), mental wellbeing (SF-12 MCS) and physical wellbeing (SF-12 PCS). Additionally, we examined how diagnosis modified these relationships using interaction effects. We did this for cognitive difficulties and distress from cognitive problems but not More memory problems than most others because of small cell size. We used multivariate analysis of variance (MANOVA) to assess whether associations retained their significance controlling for the variance shared among QOL domains. We report the variance explained in these models either as the reciprocal of Wilks’ lambda or R2 as appropriate.
In all analyses B/IADL scales were entered as binary variables. The cut point for these variables separated one-third of the sample into the high group. We used a log transformation on the GDS to improve model fit. All analyses were conducted in Stata 14.
Results
In a sample of 259 older adults with NC, MCI, and AD dementia, we examined three self-reported cognitive complaints: cognitive difficulties, distress due to cognitive problems, and belief of experiencing more memory problems than most others. Older adults reporting high cognitive difficulties – that is endorsement above half of similarly aged peers with normal cognition – were similar to those endorsing low cognitive difficulties on all assessed demographic characteristics (all p≥0.13; Table 1). But, those reporting high distress from cognitive symptoms were more likely to be college educated and to have a diagnosis of MCI than to have a diagnosis of AD or to have NC (both p<0.05). This pattern was similar for those who believed they had more memory problems than most others (both p<0.05).
Table 1.
Demographic Characteristics and Diagnostic Label by Cognitive Complaint (N=259)
| Variable | High | Low | P Value |
|---|---|---|---|
| Cognitive Difficulties (CDS)a, n (%) | 137 (52.9) | 122 (47.1) | |
| Age (years), mean (SD) | 78.5 (6.6) | 78.6 (6.9) | 0.99 |
| Female, n (%) | 81 (59.1) | 76 (62.3) | 0.60 |
| White, n (%) | 128 (93.4) | 108 (88.5) | 0.36 |
| College graduate, n (%) | 90 (65.7) | 69 (56.6) | 0.13 |
| Right handed, n (%) | 128 (93.4) | 112 (91.8) | 0.68 |
| Alzheimer’s Disease (AD), n (%) | 35 (25.6) | 33 (27.0) | 0.23 |
| Mild Cognitive Impairment (MCI), n (%) | 55 (40.1) | 37 (30.3) | |
| Cognitively Normal (CN), n (%) | 47 (34.3) | 52 (42.6) | |
| Cognitive Symptom Distress (GDI)b, n (%) | 147 (56.8) | 112 (43.2) | |
| Age (years), mean (SD) | 78.4 (6.9) | 78.7 (6.5) | 0.91 |
| Female, n (%) | 85 (57.8) | 72 (64.3) | 0.29 |
| White, n (%) | 138 (93.9) | 98 (87.5) | 0.19 |
| College graduate, n (%) | 99 (67.3) | 60 (53.6) | 0.02 |
| Right handed, n (%) | 137 (93.2) | 103 (92.0) | 0.81 |
| Alzheimer’s Disease (AD), n (%) | 34 (23.1) | 34 (30.4) | 0.04 |
| Mild Cognitive Impairment (MCI), n (%) | 62 (42.2) | 30 (26.8) | |
| Cognitively Normal (CN), n (%) | 51 (34.7) | 48 (42.9) | |
| More memory problems than most othersc, n (%) | 71 (27.4) | 187 (72.2) | |
| Age (years), mean (SD) | 77.5 (7.0) | 78.9 (6.6) | 0.12 |
| Female, n (%) | 39 (54.9) | 117 (62.6) | 0.26 |
| White, n (%) | 68 (95.8) | 167 (89.3) | 0.24 |
| College graduate, n (%) | 48 (67.6) | 110 (58.8) | 0.20 |
| Right handed, n (%) | 63 (88.7) | 176 (94.1) | 0.20 |
| Alzheimer’s Disease (AD), n (%) | 24 (33.8) | 43 (23.0) | <0.001 |
| Mild Cognitive Impairment (MCI), n (%) | 41 (57.7) | 51 (27.3) | |
| Cognitively Normal (CN), n (%) | 6 (8.5) | 93 (49.7) |
Note. High = at or above median. Low = below median.
Endorsed greater cognitive difficulties than most individuals who were known to have normal cognition (Cognitive Difficulties Scale (CDS); cut point ≥ 72 score).
Endorsed greater distress from cognitive difficulties than most individuals who were known to have normal cognition (General Distress Index (GDI); cut point ≥18 score).
Single “yes” or “no” item from Geriatric Depression Scale (GDS).
<.05
<.01
≤.001
Associations among self-reported cognitive complaints and QOL domains
In multivariable analyses, we assessed how each of the three self-reported cognitive complaints related to QOL domains. All three complaints were related to lower satisfaction in daily life (QOL-AD), more difficulties in daily life (DEM-QOL), greater depression (GDS), more anxiety (BAI), higher perceived stress (PSS), and lower mental wellbeing (SF-12 MCS; all p<0.05; Table 2).
Table 2.
Multivariable Regression Analyses of Self-Reported Cognitive Complaints Associations to Domains of Quality of Life (n=259)
| Outcome | Cognitive Difficultiesa,b | Cognitive Symptom Distressc,d | More memory problems than most otherse,f | |
|---|---|---|---|---|
|
|
||||
| Mean Difference | Mean Difference | Mean Difference | ||
| Quality of Life (QOL) | EQ−5D | −0.05** | −0.04* | −0.03 |
| EQ−VAS | −3.68 | −3.91* | −2.62 | |
| QOL−AD | −2.33*** | −3.64*** | −2.73*** | |
| DEM−QOL | −7.60*** | −8.72*** | −7.19*** | |
|
| ||||
| Mood & Wellbeing | GDSg | 1.60*** | 1.74*** | 2.08*** |
| BAI | 3.95*** | 3.50** | 2.64*** | |
| PSS | 4.81*** | 5.37*** | 3.73*** | |
| SF−12 MCS | −2.67** | −3.57*** | −4.96*** | |
|
| ||||
| Physical Wellbeing | IADL Scaleh | 0.18** | 0.13* | 0.08 |
| BADL Scaleh | 0.08 | 0.01 | 0.04 | |
| SF−12 PCS | −3.42** | −2.52* | 0.39 | |
Note. BADL = Basic Activities of Daily Living, BAI = Beck Anxiety Inventory, EQ= Euro-QOL, GDS = Geriatric Depression Scale, MCI = Mild Cognitive Impairment, IADL=Instrumental Activities of Daily Living, MCS = Mental Composite Scale, PCS = Physical Composite Scale, PPS = Perceived Stress Scale, QOL = Quality of Life, SF-12 = Short Form Health Survey, VAS = Visual Analog Scale.
Endorsed greater cognitive difficulties than most individuals who were known to have normal cognition (Cognitive Difficulties Scale (CDS); cut point ≥ 72 score).
Model statistically controls for memory impairment (CCS).
Endorsed greater distress from cognitive difficulties than most individuals who were known to have normal cognition (General Distress Index (GDI); cut point ≥18 score).
Model statistically controls for global cognitive impairment (MMSE), college education, and diagnostic group (AD or MCI).
Single “yes” or “no” item from Geriatric Depression Scale (GDS).
Model statistically controls for global cognitive impairment (MMSE) and diagnostic group (AD or MCI).
Log link back transformed.
Entered as binary variables. The cut point for these variables separated one-third of the sample into the high group.
<.05
≤.01
≤.001
High cognitive difficulties and high distress from cognitive problems were related to lower ratings of general physical health (EQ-5D), physical wellbeing (SF-12 PCS), and instrumental functioning (IADLs; all p<0.05). High distress from cognitive problems was additionally associated with lower ratings of global health (EQ-VAS, p<0.05). No other statistically significant differences were observed (all p>0.05).
Assessment of Clinical Differences
On average, older adults reporting high cognitive difficulties endorsed mild anxiety (BAI=8.0), which was substantively higher than that of those reporting low cognitive difficulties (BAI=4.1; Table 3). About 43% (n=52 of 122) who reported high cognitive difficulties endorsed severe anxiety (BAI≥10). Differences between the two groups on other measures appeared clinically unremarkable.
Table 3.
Mean Scores and Mean Differences in Anxiety (BAI), Depression (GDS), Mental Wellbeing (SF-12 MCS) and Physical Wellbeing (SF-12 PCS) by High and Low Endorsement of Cognitive Complaints
| Variable | High Mean (SD) |
Low (Mean SD) |
Mean Difference (SD) |
|---|---|---|---|
| Cognitive Difficulties (CDS)a, % (n) | 52.9 (137) | 47.1 (122) | |
| BAI | 8.0 (6.3) | 4.1 (4.0) | 4.0 (0.7)*** |
| GDS | 6.4 (5.0) | 4.0 (3.9) | 2.4 (0.6)*** |
| SF-12 Mental | 53.4 (7.3) | 55.2 (6.3) | −2.8 (0.9)*** |
| SF-12 Physical | 46.1 (10.1) | 49.3 (9.9) | −3.2 (1.2)** |
| Cognitive Symptom Distress (GDI)b, % (n) | 56.8 (147) | 43.2 (112) | |
| BAI | 7.6 (6.2) | 4.3 (4.4) | 3.3 (0.7)*** |
| GDS | 6.4 (5.0) | 3.8 (3.7) | 2.6 (0.6)*** |
| SF-12 Mental | 55.7 (6.2) | 52.2 (7.2) | 3.5 (0.8)*** |
| SF-12 Physical | 49.0 (10.2) | 46.5 (10.0) | 2.5 (1.3)* |
| More memory problems than most othersc, % (n) | 27.4 (71) | 72.2 (187) | |
| BAI | 8.2 (6.1) | 5.4 (5.4) | 2.8 (0.8)*** |
| GDS | 8.5 (5.1) | 4.1 (3.9) | 4.5 (0.6)*** |
| SF-12 Mental | 50.0 (7.1) | 55.1 (6.5) | −5.1 (0.9)*** |
| SF-12 Physical | 48.4 (9.9) | 47.2 (10.3) | 1.2 (0.4) |
Note. High = at or below median. Low = below median
Endorsed greater cognitive difficulties than most individuals who were known to have normal cognition (Cognitive Difficulties Scale (CDS); cut point ≥ 72 score).
Endorsed greater distress from cognitive difficulties than most individuals who were known to have normal cognition (General Distress Index (GDI); cut point ≥18 score).
Single “yes” or “no” item from Geriatric Depression Scale (GDS).
<.05
<.01
<.001
On average, older adults reporting high distress from cognitive difficulties showed clinically similar endorsement of anxiety and depression, which fell within normal limits. However, on average, endorsement of anxiety among those reporting high distress (BAI=7.6) was almost twice that of individuals reporting low distress (BAI=4.3). This pattern was similar for depression, where average endorsement of depression among those who reported high distress was 6.4 while among individuals who reported low distress it was 3.8.
On average, older adults who believed they had more memory problems than most others reported mild depression (GDS=8.5), which was substantively higher than that endorsed by those who did not believe this about themselves (GDS=4.1). About 22% (n=27 of 122) of those who believed they had more memory problems than most endorsed severe depression (GDS≥10). Mental wellbeing (SF-12 MCS) and physical wellbeing (SF-12 PCS) appeared clinically similar between those who endorsed high versus low symptoms.
MCI and AD Dementia Diagnoses Effects on Relationships between Self-Report Symptoms and QOL
Among older adults reporting high cognitive difficulties, those with MCI reported more difficulties in daily life (DEM-QOL), greater depression (GDS), greater subjective stress (PSS), and greater impairment in instrumental functioning (IADLs) than those who were cognitively normal (all p<0.05; Figure 1). Those with a diagnosis of AD dementia reported more difficulties in daily life (DEM-QOL) and greater subjective stress than those with this symptom but cognitively normal (PSS, both p<0.05). No other statistically discernible interactions were observed based on cognitive difficulties or distress from cognitive symptoms (all p>0.05).
Figure 1.
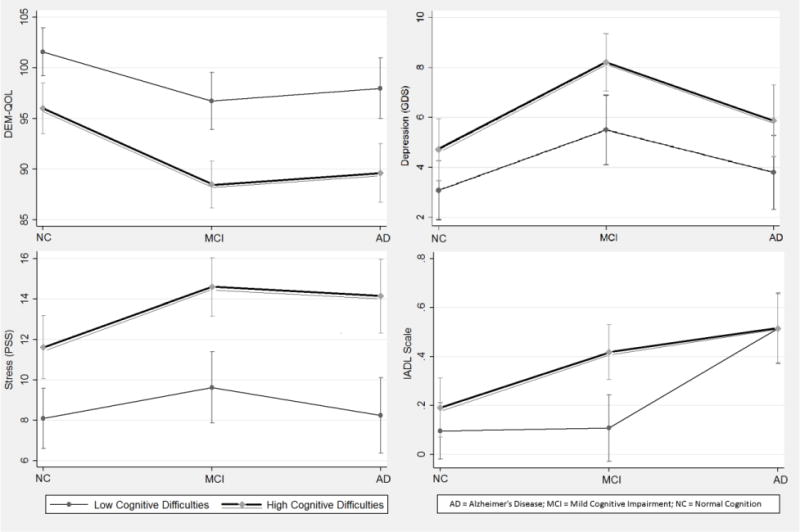
Comparisons of QOL domains by older adults reporting high versus low cognitive difficulties. All models statistical control for memory impairment (CCS).
Error bars denote 95% confidence intervals. Intervals that do not overlap represent statistically significant between-group differences (p<0.05).
Associations among self-reported cognitive complaints and QOL domains statistically adjusting for relationships among QOL domains
In multivariate analysis of cognitive difficulties, the model was not significant for explaining health-related QOL (EQ-5D; Table 4). MCI diagnosis was associated with lower satisfaction in daily life (QOL-AD), greater difficulty in daily life (DEM-QOL), greater depression (GDS), more subjective stress (PSS), and lower mental wellbeing (SF-12 MCS; p<0.05). AD dementia was associated with lower satisfaction in daily life (QOL-AD), greater difficulties in daily life (DEM-QOL), and greater impairments in instrumental activities of daily living (IADLs; all p<0.05).
Table 4.
Multivariate Analyses of Mean Differences in Domains of Quality of Life Based on Self-Reported Cognitive Complaints, Diagnosis, and Cognitive Impairment (n=259)
| Outcome | R2 | F | Cognitive Difficultiesa | MCIb | ADb | MMSE |
|---|---|---|---|---|---|---|
| EQ-5D | 0.04 | 2.33 | −.05** | −0.02 | −0.01 | −0.004 |
| QOL-AD | 0.07 | 5.02*** | −2.24*** | −1.87* | −2.16* | −0.12 |
| DEM-QOL | 0.24 | 19.57*** | −7.26*** | −6.42*** | −5.42** | −0.09 |
| GDSc | 0.14 | 10.61*** | 2.17*** | 3.16*** | 1.54 | 0.13 |
| BAI | 0.13 | 9.54*** | 3.89*** | 1.15 | 1.10 | 0.02 |
| PSS | 0.19 | 14.87*** | 4.66*** | 2.31** | 1.46 | 0.03 |
| SF-12 MCS | 0.10 | 7.02*** | −2.43** | −3.48*** | −1.18 | 0.20 |
| IADL Scaled | 0.15 | 11.34*** | 0.14** | 0.07 | 0.21* | −0.03** |
| SF-12 PCS | 0.05 | 3.34** | −3.37** | 1.28 | 2.34 | −0.30 |
|
| ||||||
| Cognitive Symptom Distresse | MCIb | ADb | MMSE | |||
|
| ||||||
| EQ-5D | 0.02 | 1.36 | −0.03 | −0.02 | −0.01 | −0.003 |
| EQ-VAS | 0.02 | 1.20 | 3.80 | −0.29 | 1.48 | −0.10 |
| QOL-AD | 0.11 | 7.93*** | −3.10*** | −1.62* | −2.20* | −0.10 |
| DEM-QOL | 0.26 | 22.65*** | −8.08*** | −5.89*** | −5.51** | −0.03 |
| GDSc | 0.14 | 11.07*** | 2.31*** | 3.01*** | 1.56 | 0.11 |
| BAI | 0.09 | 6.61*** | 3.26*** | 1.04 | 1.12 | −0.02 |
| PSS | 0.21 | 16.87*** | 5.04*** | 2.00* | 1.51 | 0.02 |
| SF-12 MCS | 0.12 | 8.39*** | −3.09*** | −3.24** | −1.22 | 0.22 |
| IADL Scaled | 0.15 | 11.06*** | 0.13* | 0.06 | 0.21* | −0.04** |
| SF-12 PCS | 0.04 | 2.50* | −2.55* | 1.34 | 2.32 | −0.27 |
|
| ||||||
| More Memory Problems than Mostf | MCIb | ADb | MMSE | |||
|
| ||||||
| QOL-AD | 0.07 | 4.73*** | −2.61*** | −1.02 | −1.13 | −0.05 |
| DEM-QOL | 0.17 | 13.14*** | −6.34*** | 4.54** | 3.05 | 0.07 |
| GDSc | 0.21 | 17.04*** | 3.98*** | 1.73* | 0.06 | 0.05 |
| BAI | 0.05 | 3.29** | 2.53** | 0.51 | 0.21 | 0.06 |
| PSS | 0.09 | 6.54*** | 3.34*** | 1.40 | 0.05 | −0.07 |
| SF-12 MCS | 0.14 | 9.89*** | −4.35*** | −1.92 | 0.49 | 0.29 |
Note. AD = Alzheimer’s Disease, BADL = Basic Activities of Daily Living, BAI = Beck Anxiety Inventory, EQ= Euro-QOL, GDS = Geriatric Depression Scale, MCI = Mild Cognitive Impairment, IADL=Instrumental Activities of Daily Living, MCI = Mild Cognitive Impairment, MCS = Mental Composite Scale, MMSE = Mini Mental Status Exam, PCS = Physical Composite Scale, PPS = Perceived Stress Scale, QOL = Quality of Life, SF-12 = Short Form Health Survey, VAS = Visual Analog Scale.
Endorsed greater cognitive difficulties than most individuals who were known to have normal cognition (Cognitive Difficulties Scale (CDS); cut point ≥ 72 score).
Reference is cognitively normal (CN) group.
Log link back transformed.
Entered as binary variable. The cut point separated one-third of the sample into the high group.
Endorsed greater distress from cognitive difficulties than most individuals who were known to have normal cognition (General Distress Index (GDI); cut point ≥18 score).
Single “yes” or “no” item from Geriatric Depression Scale (GDS).
<.05
≤.01
≤.001
In multivariate analysis of cognitive distress, the model was not significant for explaining health-related QOL (EQ-5D) or ratings of global health (EQ-VAS). MCI diagnosis was associated with lower satisfaction in daily life (QOL-AD), greater difficulty in daily life (DEM-QOL), greater depression (GDS), more subjective stress (PSS), and lower mental wellbeing (SF-12 MCS; p<0.05). AD dementia was associated with lower satisfaction in daily life (QOL-AD), more difficulties in daily life (DEM-QOL), and greater impairment in instrumental activities of daily living (IADLs; all p<0.05).
In multivariate analysis of one’s belief about the severity of memory problems, MCI diagnosis was associated with greater difficulty in daily life (DEM-QOL) and greater depression (GDS; both p<0.05). AD dementia was not independently associated with domains of QOL (all p>0.05).
Discussion
This study investigated relationships between cognitive complaints and QOL in 259 older adults with varying degrees of cognitive decline. Based on prior studies in samples with AD and MCI,7,23–31,33–35,44 we anticipated strong negative relationships between cognitive complaints and QOL. We found all three cognitive complaints were related to relatively lower quality of daily life (QOL-AD, DEM-QOL) and worse psychological outcomes – depression (GDS), anxiety (BAI), stress (PSS), and mental wellbeing (SF-12 MCS).
Studies comparing QOL in patients with MCI and mild stage AD dementia have found those with MCI report more difficulties in daily life (DEM-QOL), more mood disturbances, worse general health, and lower vitality.7,35 In a prior study that compared patients with MCI to both those with NC and AD dementia, those with MCI reported more stress, more difficulties, and lower satisfaction with daily life than those with NC and worse mental wellbeing and greater depression than those with AD.40 Thus, we expected a diagnosis of MCI would strengthen inverse relationships between cognitive complaints and QOL. We found support for this hypothesis for depression (GDS), subjective stress (PSS), difficulties in daily life (DEM-QOL), and problems in instrumental functioning (IADLs). We also found that a diagnosis of AD dementia strengthened the relationship between cognitive complaints and both difficulties in daily life (DEM-QOL) and worse mental wellbeing (SF-12 MCS).
Our results suggest that relationships between cognitive complaints and impairments in QOL are amplified for those diagnosed with MCI and AD and that how these relationships shift differs by diagnosis. These findings were independent of performance-based cognitive impairment. The underlying reasons for these findings warrant study. Understanding whether these relationships might correspond to underlying neuropathology or whether they may reflect psychosocial reactions or a combination of the two may be helpful for understanding how early diagnosis may affect individuals.
The conceptual framework for assessing QOL is often multidimensional – cognitive, physical, social, and mental.22 Fairly consistent documentation of concurrent impairment in domains, like depression and quality of daily life(e.g., DEM-QOL, QOL-AD)23,27,28,30,35 have raised the question of whether all domains are useful.7 We found QOL domains to be independent of one another. In fact, the magititude of some relationships, such as that between cognitive difficulties and depression, appeared stronger in the multivariate analysis that adjusted for relationships shared among domains.
Together, results from the multivariable and multivariate analyses show that associations between cognitive complaints and QOL can vary based on both the specific symptom and QOL domain. Broadly, our findings show older adults with cognitive complaints tended to report lower QOL than their respective counterparts with NC, MCI, or AD dementia. This underscores the import of addressing subjective symptoms during routine clinical practice. Specific findings suggest that cognitive complaints may have clinical value in helping guide providers’ assessments. That is, our findings suggest that older patients who report cognitive complaints may also be experiencing problems in other areas of functioning, such as anxiety, depression, and activities in daily life. The degree of impairment, particularly in anxiety and depression, suggests patients reporting significant cognitive complaints may benefit from screening for mood disorders and, as appropriate, psychological treatment. Given our results show patients with MCI or AD who endorsed fewer cognitive symptoms reported, on average, statistically similar levels of situational stress and anxiety as older adults with normal cognition, they raise the question of whether clinical interventions that improve symptom management may help restore or preserve QOL. In addition, AD prevention trials may benefit from including self-report measures of functioning in daily life (DEM-QOL) and subjective stress (PSS) in addition to the already commonly built-in metrics for depression (GDS), mental wellbeing (SF-12 MCS), and instrumental activities of daily living (IADLs). This may help appraise the breadth of effects of an experimental treatment, help measure disease progression, and help characterize effects in sample subgroups.
Prior studies examining relationships between dementia symptoms and QOL have focused largely on neuropsychiatric symptoms.7,23–27,30 However, self-report symptom data are gaining importance as advances in AD diagnosis are leading to identifying persons ever earlier in the disease process.3–5 Our findings show these symptoms that emerge earlier in the disease course may be helpful in identifying treatable problems. Moreover, they suggest that addressing self-report symptoms may aid in mitigating substantive differences in QOL that are observed among individuals with MCI, AD, and NC. Further study of these symptom-related patient reported outcomes is needed.
We analyzed three self-report symptoms as binary variables. To do this, we dichotomized scores on the CDS and GDI. We defined the cut between high and low categories using the respective median value for each that was obtained from our group of older adults who were known to be cognitively normal. For cognitive difficulties, scores of 72 and above on the CDS represented high levels of cognitive difficulties. In the low group, scores ranged from 41 to 71 in our sample. In the general public, the average CDS score is 32 (SD 15).12 This suggests that cognitive difficulties in our sample, high or low, was well above what most members of the general public report. The third symptom, believing one had more memory problems than most others, was a single binary item adopted from the GDS. Because of its relationship to the GDS, associations between this item and the full GDS scale should be cautiously interpreted.
We carefully constructed the statistical models to limit the risk of overfitting, and all models passed a test of goodness-of-fit. We found several factors differed between older adults who did and did not believe they experienced more memory problems than most others. These were executive function (GPG unique designs), crystalized intelligence (WAIS IS), memory (CCS), and global cognition (MMSE). Because of the multicollinearity among these factors, model variance was inflated when we added them together. In these models, all effects were larger than those we report. Because of this, our results may underestimate actual effects. In addition, it was not possible to statistically control for all potential confounders, including that our study was conducted at a single site. The results may not generalize to populations with other characteristics. Nonetheless our findings offer information that may be helpful to the design of future studies that can test the generalizability and causality of the relationships that we report.
Acknowledgments
Funding
This work was supported by The Marian S. Ware Alzheimer Program, National Institute of Aging (P30-AG-010124), the Centers for Disease Control and Prevention Healthy Brain Research Center (U48-DP-005053), the Diane Eisen Memorial Neurodegenerative Disease Research Fund and a grant from the Alzheimer’s Association (AARF-17-528934).
Footnotes
Conflicts of Interest
None.
Author Contributions
J. Karlawish and K. Harkins aided in the design and implementation of the study. S. Stites conceptualized the analyses and wrote the initial draft of the article. All authors contributed to the conceptualization and writing of the article.
Contributor Information
Shana D. Stites, Department of Medical Ethics and Health Policy, Perlman School of Medicine, University of Pennsylvania.
Kristin Harkins, Penn Memory Center, Department of Medicine, University of Pennsylvania.
Jonathan D. Rubright, National Board of Medical Examiners.
Jason Karlawish, Penn Memory Center, Departments of Medicine, Medical Ethics and Health Policy, and Neurology, University of Pennsylvania.
References
- 1.US Department of Health and Human Services. National Plan to Address Alzheimer’s Disease 2015 Update. 2015 http://aspe.hhs.gov/national-plan-address-alzheimer%E2%80%99s-disease-2015-update. Accessed September 17, 2015.
- 2.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nature Reviews Neurology. 2013;9(1):54–58. doi: 10.1038/nrneurol.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niti M, Yap K-B, Kua E-H, Ng T-P. APOE-ε4, Depressive Symptoms, and Cognitive Decline in Chinese Older Adults: Singapore Longitudinal Aging Studies. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A(2):306–311. doi: 10.1093/gerona/gln013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavretsky H, Ercoli L, Siddarth P, Bookheimer S, Miller K, Small G. Apolipoprotein ε4 Allele Status, Depressive Symptoms, and Cognitive Decline in Middle-Aged and Elderly Persons Without Dementia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2003;11(6):667–673. doi: 10.1176/appi.ajgp.11.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curiel AR, Miller KJ, Pollard K, Kim J, Kravitz J, Small GW. Anxiety and verbal memory performance in APOE-4 carriers and noncarriers aged 50 years and above. Aging health. 2012;8(1):99–104. doi: 10.2217/ahe.11.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank L, Lenderking WR, Howard K, Cantillon M. Patient self-report for evaluating mild cognitive impairment and prodromal Alzheimer’s disease. Alzheimer’s Research & Therapy. 2011;3:35. doi: 10.1186/alzrt97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berwig M, Leicht H, Hartwig K, Gertz HJ. Self-rated quality of life in mild cognitive impairment and Alzheimer’s disease: The problem of affective distortion. GeroPsych: The Journal of Gerontopsychology and Geriatric Psychiatry. 2011;24(1):45–51. [Google Scholar]
- 8.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl A-M, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? doi: 10.1159/000076354. (1420-8008(Print)) [DOI] [PubMed] [Google Scholar]
- 9.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. doi: 10.1002/gps.1367. (0885-6230 (Print)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 11.Pires C, Silva D, Maroco J, et al. Memory Complaints Associated with Seeking Clinical Care. International Journal of Alzheimer’s Disease. 2012;2012:5. doi: 10.1155/2012/725329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derouesne C, Dealberto MJ, Boyer P, et al. Empirical evaluation of the ’cognitive difficulties scale’ for assessment of memory complaints in general practice: A study of 1628 cognitively normal subjects aged 45-75 years. International Journal of Geriatric Psychiatry. 1993;8:599–607. [Google Scholar]
- 13.Grand JHG, Caspar S, MacDonald SWS. Clinical features and multidisciplinary approaches to dementia care. Journal of Multidisciplinary Healthcare. 2011;4:125–147. doi: 10.2147/JMDH.S17773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohman TJ, Beason-Held LL, Resnick SM. Cognitive Complaints, Depressive Symptoms, and Cognitive Impairment: Are They Related? Journal of the American Geriatrics Society. 2011;59(10):1908–1912. doi: 10.1111/j.1532-5415.2011.03589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner MD, Harvey RT, Denier CA. Self-report and objective measures of cognitive deficit in patients entering substance abuse treatment. Psychiatry research. 1999;86(2):155–161. doi: 10.1016/s0165-1781(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 16.Smith D, Lovell J, Weller C, et al. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLOS ONE. 2017;12(2):e0170651. doi: 10.1371/journal.pone.0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabin LA, Wang C, Katz MJ, Derby CA, Buschke H, Lipton RB. Predicting Alzheimer’s disease: neuropsychological tests, self-reports, and informant reports of cognitive difficulties. 2012 doi: 10.1111/j.1532-5415.2012.03956.x. (1532-5415(Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamkwalala A, Hulgan T, Newhouse P. Subjective memory complaints are associated with poorer cognitive performance in adults with HIV. AIDS care. 2017;29(5):654–659. doi: 10.1080/09540121.2016.1248348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gifford KA, Liu D, Hohman TJ, et al. A Mutual Self- and Informant-Report of Cognitive Complaint Correlates with Neuropathological Outcomes in Mild Cognitive Impairment. PLoS One. 2015 doi: 10.1371/journal.pone.0141831. (1932-6203 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howieson DB, Mattek N, Dodge HH, Erten-Lyons D, Zitzelberger T, Kaye JA. Memory Complaints in Older Adults: Prognostic Value and Stability in Reporting over Time. SAGE Open Med. 2015 doi: 10.1177/2050312115574796. (2050-3121 (Linking)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva D, Guerreiro M, Faria C, Maroco J, Schmand BA, Mendonça Ad. Significance of Subjective Memory Complaints in the Clinical Setting. Journal of Geriatric Psychiatry and Neurology. 2014;27(4):259–265. doi: 10.1177/0891988714532018. [DOI] [PubMed] [Google Scholar]
- 22.Whitehouse PJ, Orgogozo JM, Becker RE, et al. Quality-of-life assessment in dementia drug development. Position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer disease and associated disorders. 1997;11(Suppl 3):56–60. [PubMed] [Google Scholar]
- 23.Naglie G, Hogan DB, Krahn M, et al. Predictors of patient self-ratings of quality of life in Alzheimer disease: cross-sectional results from the Canadian Alzheimer’s disease quality of life study. Am J Geriatr Psychiatry. 2011;19 doi: 10.1097/JGP.0b013e3182006a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(6):469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- 25.Hongisto K, Hallikainen I, Selander T, et al. Quality of Life in relation to neuropsychiatric symptoms in Alzheimer’s disease: 5-year prospective ALSOVA cohort study. International Journal of Geriatric Psychiatry. 2017:n/a–n/a. doi: 10.1002/gps.4666. [DOI] [PubMed] [Google Scholar]
- 26.Beer C, Flicker L, Horner B, et al. Factors Associated with Self and Informant Ratings of the Quality of Life of People with Dementia Living in Care Facilities: A Cross Sectional Study. PLOS ONE. 2010;5(12):e15621. doi: 10.1371/journal.pone.0015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang S, Wu J, Wang C, Pan L. Cognitive function, depressive symptoms, function level, and quality of life in mild dementia and amnestic-mild cognitive impairment. Journal of Medical Sciences. 2016;36(1):14. [Google Scholar]
- 28.Woods RT, Nelis SM, Martyr A, et al. What contributes to a good quality of life in early dementia? awareness and the QoL-AD: a cross-sectional study. Health and Quality of Life Outcomes. 2014;12(1):94. doi: 10.1186/1477-7525-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heggie M, Morgan D, Crossley M, et al. Quality of life in early dementia: Comparison of rural patient and caregiver ratings at baseline and one year. Dementia. 2011;11(4):521–541. doi: 10.1177/1471301211421085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoe J, Hancock GA, Livingston G, Orrell M. Quality of life of people with dementia in residential care homes. Br J Psychiatry. 2006;188 doi: 10.1192/bjp.bp.104.007658. [DOI] [PubMed] [Google Scholar]
- 31.Sapra M, Deyoung N, Shenal B. Quality of life in mild cognitive impairment (MCI) and Alzheimer’s dementia (AD): Patient versus caregiver perspective and predictors. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 11(7):739. [Google Scholar]
- 32.Pusswald G, Tropper E, Kryspin-Exner I, et al. Health-Related Quality of Life in Patients with Subjective Cognitive Decline and Mild Cognitive Impairment and its Relation to Activities of Daily Living. Journal of Alzheimer’s Disease. 2015;47(2):479–486. doi: 10.3233/JAD-150284. [DOI] [PubMed] [Google Scholar]
- 33.Bárrios H, Narciso S, Guerreiro M, Maroco J, Logsdon R, de Mendonça A. Quality of life in patients with mild cognitive impairment. Aging & Mental Health. 2013;17(3):287–292. doi: 10.1080/13607863.2012.747083. [DOI] [PubMed] [Google Scholar]
- 34.Teng E, Tassniyom K, Lu P. Reduced quality of life ratings in mild cognitive impairment: Analyses of subject and informant responses. American Journal of Geriatric Psychiatry. 2012;20(12):1016–1024. doi: 10.1097/JGP.0b013e31826ce640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss EM, Papousek I, Fink A, Matt T, Marksteiner J, Deisenhammer EA. Lebensqualität bei älteren Personen mit unterschiedlichem Schweregrad von kognitiver Beeinträchtigung. neuropsychiatrie. 2012;26(2):72–77. doi: 10.1007/s40211-012-0016-8. [DOI] [PubMed] [Google Scholar]
- 36.Kurz X, Scuvee-Moreau J, Vernooij-Dassen M, Dresse A. Cognitive impairment, dementia and quality of life in patients and caregivers. Acta Neurol Belg. 2003;103 (0300-9009 (Print)) [PubMed] [Google Scholar]
- 37.Missotten P, Squelard G, Ylieff M, Di Notte D, et al. Quality of life in older Belgian people: comparison between people with dementia, mild cognitive impairment, and controls. doi: 10.1002/gps.1981. (1099-1166 (Electronic)) [DOI] [PubMed] [Google Scholar]
- 38.Ready RE, Ott Br, Grace J. Patient versus informant perspectives of Quality of Life in Mild Cognitive Impairment and Alzheimer’s disease. doi: 10.1002/gps.1075. (0885-6230 (Print)) [DOI] [PubMed] [Google Scholar]
- 39.Lapid MI, Rummans TA, Boeve BF, McCormick JK, et al. What is the quality of life in the oldest old? (1741-203X (Electronic)) [Google Scholar]
- 40.Stites SD, Karlawish J, Harkins K, Rubright JD, Wolk D. Awareness of Mild Cognitive Impairment and Mild Alzheimer’s Disease Dementia Diagnoses Associated with Lower Self-Ratings of Quality of Life in Older Adults. The Journals of Gerontology: Series B. 2017;72(6):974–985. doi: 10.1093/geronb/gbx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurz X, Scuvee-Moreau J, Vernooij-Dassen M, Dresse A. Cognitive impairment, dementia and quality of life in patients and caregivers. Acta Neurol Belg. 2003;103(1):24–34. [PubMed] [Google Scholar]
- 42.Robert PH, Verhey FR, Byrne EJ, et al. Grouping for behavioral and psychological symptoms in dementia: clinical and biological aspects. Consensus paper of the European Alzheimer disease consortium. European psychiatry : the journal of the Association of European Psychiatrists. 2005;20(7):490–496. doi: 10.1016/j.eurpsy.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 43.Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2005;13(6):460–468. doi: 10.1176/appi.ajgp.13.6.460. [DOI] [PubMed] [Google Scholar]
- 44.Brubaker SW, Sapra M, Kim K, Bradford L. Factors Impacting Quality of Life in Patients with Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD): A Cross-Sectional, Prospective Study in an Ambulatory Care Setting. The American Journal of Geriatric Psychiatry. 24(3):S82–S83. [Google Scholar]
- 45.Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT. The Memorial Symptom Assessment Scale Short Form (MSAS-SF) Cancer. 2000;89(5):1162–1171. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 46.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 50.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 51.Smith SC, Lamping DL, Banerjee S, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess. 2005;9(10):1–93. iii–iv. doi: 10.3310/hta9100. [DOI] [PubMed] [Google Scholar]
- 52.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 53.The EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. The EuroQol group. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 54.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 55.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MOCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 56.Libon DJ, Mattson RE, Glosser G, et al. A nine-word dementia version of the California verbal learning test. The Clinical Neuropsychologist. 1997;3:237–244. [Google Scholar]
- 57.Glosser G, Cole L, Khatri U, DellaPietra L, Kaplan E. Assessing nonverbal memory with the Biber Figure Learning Test-Extended in temporal lobe epilepsy patients. Arch Clin Neuropsychol. 2002;17(1):25–35. [PubMed] [Google Scholar]
- 58.Glosser G, Goodglass H. Disorders in executive control functions among aphasic and other brain-damaged patients. J Clin Exp Neuropsychol. 1990;12(4):485–501. doi: 10.1080/01688639008400995. [DOI] [PubMed] [Google Scholar]
- 59.Wechsler D. Wechsler Test of Adult Reading (WTAR) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 60.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 61.Tobiansky R, Blizard R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychological medicine. 1995;25(4):779–786. doi: 10.1017/s0033291700035029. [DOI] [PubMed] [Google Scholar]
- 62.Vuong QH. Likelihood Ratio Tests for Model Selection and Non-Nested Hypotheses. Econometrica. 1989;57(2):307–333. [Google Scholar]


