Abstract
Background
The availability of direct-acting antiviral (DAA) therapy might have impacted use of HCV-infected (HCV+) deceased-donor kidneys for transplantation.
Methods
We used 2005-2018 SRTR data to identify 18 936 candidates willing to accept HCV+ kidneys and 3348 HCV+ recipients of HCV+ kidneys. We compared willingness to accept, utilization, discard, and posttransplant outcomes associated with HCV+ kidneys between 2 treatment eras (Interferon [IFN] era: 1/1/2005-12/5/2013 versus DAA era: 12/6/2013-8/2/2018). Models were adjusted for candidate, recipient, and donor factors where appropriate.
Results
In the DAA era, candidates were 2.2-times more likely to list as willing to accept HCV+ kidneys (aOR: 2.072.232.41, p<0.001), and HCV+ recipients were 1.95-times more likely to have received an HCV+ kidney (aOR: 1.761.952.16, p<0.001). Median KDPI of HCV+ kidneys decreased from 77 (IQR 59-90) in 2005 to 53 (40-67) in 2017. KDPI of HCV- kidneys remained unchanged from 45 (21-74) to 47 (24-73). After adjustment, HCV+ kidneys were 3.7-times more likely to be discarded than HCV- kidneys in the DAA era (aRR 3.363.674.02, p<0.001); an increase from the IFN era (aRR: 2.783.023.27, p<0.001). HCV+ kidney use was concentrated within a subset of centers; 22.5% of centers performed 75% of all HCV+ kidney transplants in the DAA era. Mortality risk associated with HCV+ kidneys remained unchanged (aHR: 1.071.191.32 in both eras).
Conclusions
Given the elevated risk of death on dialysis facing HCV+ candidates, improving quality of HCV+ kidneys, and DAA availability, broader utilization of HCV+ kidneys is warranted to improve access in this era of organ shortage.
INTRODUCTION
In the United States, the prevalence of hepatitis C virus (HCV) among patients with end-stage-renal disease (ESRD) is estimated to be between 7-14% (1–3), and HCV increases the risk of death on dialysis for these patients (4–7). Kidney transplantation (KT) improves long term survival of HCV-infected (HCV+) ESRD patients, even for those who receive marginal donor kidneys (8–11). In the current kidney transplant setting where deceased-donor availability has declined (12), HCV+ deceased donor organs could provide a potential source of organs for candidates with HCV. While studies have shown worse outcomes associated with HCV+ kidneys (13, 14), candidates are not faced with simultaneous offers of HCV+ or HCV- kidneys, in which case the choice would be clear, candidates are faced with the decision to accept an available HCV+ kidney or to wait for an HCV- kidney, which might never come. Studies have demonstrated that HCV-infected candidates experience a survival benefit from kidney transplantation (7, 15, 16) and have reduced waiting times when accepting HCV+ kidneys (14, 17).
At the same time, the therapy available for HCV treatment has improved. In the past, HCV treatment required PEGylated interferon (IFN), which was associated with low rates of sustained virologic response (SVR) and high rates of kidney injury, rejection, and graft loss among KT recipients (18–20). IFN-free direct-acting antiviral (DAA) combinations for HCV are now widely available, well tolerated, and have cure rates greater than 95%, including among ESRD patients and KT recipients (21–25). In December 2013, 2 FDA-approved DAAs (sofosbuvir and simeprevir) were available and allowed providers to prescribe DAA curative regimens for patients. Since then, more than 8 distinct DAA regimens have been FDA-approved and made available for use (26). While the decision to treat HCV+ ESRD patients pre or posttransplant remains controversial (27–30), these therapeutic breakthroughs might have changed clinical practice with respect to HCV+ kidneys. Finally, HCV+ deceased donors are now younger and healthier (31), a change likely driven by the drug overdose epidemic (32, 33).
We hypothesized that DAA availability, coupled with improving quality of HCV+ donors, might have influenced practices related to HCV+ donor kidneys. We used national registry data to characterize changes in willingness to accept, utilization, discard, and posttransplant outcomes associated with HCV+ donor kidneys in the era of highly effective IFN-free DAAs.
METHODS
Data sources
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (34). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Study population
We identified 442 501 adult (≥18) kidney transplant candidates listed between 1/1/2005 and 3/2/2018 of whom 18 936 (4.3%) were willing to accept HCV+ donor kidneys. To characterize receipt of HCV+ kidneys, we identified 11 409 adult HCV-antibody positive (HCV+) DDKT recipients of whom 3348 (29.4%) had been transplanted with an HCV+ donor kidney between 1/1/2005 and 3/2/2017. To compare changes in practice before and after the introduction of IFN-free DAAs, hereafter referred to as DAAs, we defined 2 eras (IFN era: 1/1/2005-12/5/2013 vs DAA era: 12/5/2013-3/2/2018). We compared characteristics of candidates willing to accept HCV+ kidneys and of HCV+ recipients of HCV+ kidneys in each era using χ-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. HCV+ recipients with missing donor HCV (n=4) were excluded.
Changes in willingness to accept HCV+ kidneys and receipt of HCV+ kidneys
We used multilevel logistic regression to determine whether candidates were more likely to list as willing to accept HCV+ kidneys in the DAA versus IFN era, adjusting for candidate age, sex, race, diagnosis, insurance, and time on dialysis. We used multilevel logistic regression to determine whether HCV+ recipients were more likely to have received an HCV+ kidney in the DAA versus IFN era, adjusting for recipient age, sex, race, diagnosis, insurance, and time on dialysis. The multilevel logistic regression models include a random intercept for center, thereby allowing underlying rates of listing and HCV+ kidney utilization to vary across centers. The changes observed in the recent era can be interpreted as independent of center-level variation. To determine whether observed changes in willingness or receipt of HCV+ kidneys in the DAA era were beyond what we would expect given existing trends, we modeled listing and utilization as a function of time and used spline terms to determine whether there was a statistically significant change in existing trends after the introduction of DAAs.
Changes in center-level use of HCV+ kidneys
To determine whether an increase in use of HCV+ kidneys was driven by a few aggressive centers or occurring across centers, we identified 173 kidney transplant programs that had performed at least 1 transplant per year over the study period and that were not pediatric centers (defined as performing >70% pediatric transplants). To estimate center-level clustering of HCV+ to HCV+ kidney transplants, we calculated the Gini coefficient per era, a dimensionless statistic between 0 and 1 that indicates clustering. In the context of this study, a Gini coefficient closer to 1 indicates that fewer centers performed the majority of HCV+ to HCV+ kidney transplants.
Posttransplant outcomes associated with HCV+ kidneys
We identified 121 480 adult DDKT recipients transplanted between 1/1/2005 and 7/31/2017 and used Cox proportional hazards regression to estimate changes in posttransplant mortality associated with HCV+ deceased-donor kidneys in the recent era. We tested the interaction between era and donor HCV-status to characterize changes in the recent era that were above and beyond any changes observed overall among DDKT recipients. The final model was adjusted for recipient age, sex, race, time on dialysis, diagnosis, insurance, previous malignancy, vascular disease, panel reactive antibody status, transplant type, HLA mismatch, and BMI, and donor age, sex, race, BMI, cause of death, increased infectious risk, hypertension, diabetes, creatinine, regional sharing, cold ischemia time, prerecovery diuretics, Hepatitis B virus, lung infection, history of cancer, cigarette use, and donation after cardiac death.
Changes in quality and discard of HCV+ kidneys
To characterize changes in quality (determined using Kidney Donor Profile Index (KDPI)) and discard (defined as recovered and not transplanted) of HCV+ kidneys, we identified 195 202 deceased-donor kidneys recovered between 01/01/2005 and 3/2/2018, of which 8088 (4.14%) were recovered from HCV+ donors. We estimated the crude and adjusted relative rates of discard of HCV+ kidneys using modified Poisson regression accounting for clustering at the Donation Service Area (DSA) level. The crude relative rate indicates the proportion of the HCV+ kidneys that were discarded relative to the proportion of HCV- kidneys that were discarded, while the adjusted relative rate accounts for changes in donor quality occurring among HCV+ deceased donors (31). The final model was adjusted for donor age, race, sex, increased-infectious risk, BMI, donation after cardiac death, cause of death, blood type, creatinine>1.5, history of hypertension, and history of diabetes (35). To determine whether the association between HCV status and discard changed in the recent era, we tested the interaction between donor HCV and era. Deceased-donors with missing or unknown HCV-antibody status (0.1%) or missing BMI (0.43%) were excluded.
Sensitivity analysis
The first line of DAAs, introduced in December 2013, were contraindicated for renal transplant candidates. To determine whether inferences were dependent on this definition, we performed a sensitivity analysis and estimated the odds of having received an HCV+ kidney in the era of FDA-approved DAA therapy (after 10/1/2014) among HCV+ recipients.
Statistical analysis
Confidence intervals were reported according to the method of Louis and Zeger (36). Analyses were performed using Stata 14.2/MP for Windows (College Station, TX). We used a 2-sided α of 0.05 to indicate a statistically significant difference.
RESULTS
Willingness to accept HCV+ kidneys
The number of incident candidates listed as willing to accept an HCV+ kidney increased from 1308 in 2005 (4.6% of incident candidates) to 2756 in 2017 (7.8%) (Figure 1a). In the IFN and DAA eras, 3.3% and 6.1% of candidates listed as willing to accept HCV+ kidneys, respectively. After accounting for center variation and candidate characteristics, candidates listed in the DAA era were 2.2-times more likely to list as willing to accept HCV+ kidneys than those listed in the IFN era (adjusted odds ratio (aOR): 2.072.232.41, p<0.001). After modeling the trend as a function of calendar time, the per-year increase observed in the DAA era was marginally higher than expected given the trend observed between 2010-2013 (per-year increase from 2010-2013: 1.171.211.24 and from 2013-2018: 1.231.251.28, p value of interaction between spline terms=0.047). Candidates listed as willing to accept HCV+ kidneys in the DAA era had slightly higher EPTS scores at listing, were more likely to be female, more likely to be Caucasian, more likely to have diabetes, and slightly less likely to have been on dialysis (Table 1a).
Figure 1.
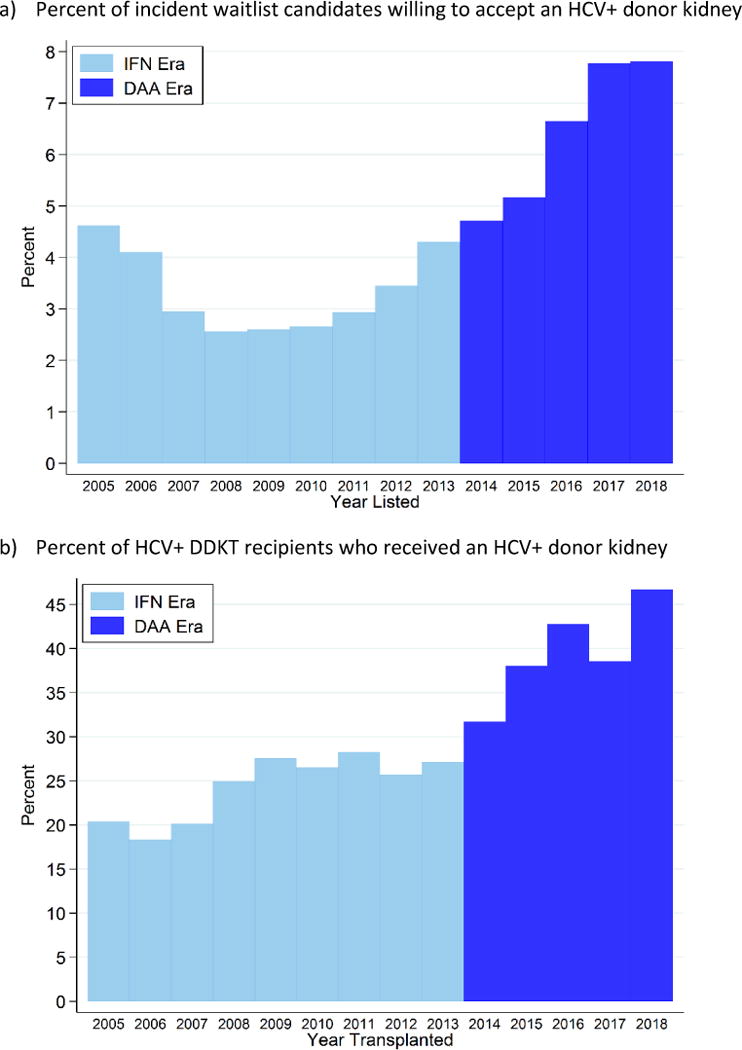
Percent of a) incident kidney transplant candidates who were willing to accept an HCV+ kidney and b) HCV+ kidney transplant recipients who received an HCV+ kidney; 2005-2018.
Table 1a.
Characteristics of candidates willing to accept an HCV+ kidney before and after the introduction of DAA therapy (n=18 936).
| Candidate characteristic | 2005-2013 (N=9733) |
2014-2018 (N=9203) |
p value |
|---|---|---|---|
| EPTS score at listing (Med (IQR)) | 55 (34, 76) | 57 (33, 79) | <0.001 |
| Age at listing (Med (IQR)) | 55 (48, 60) | 56 (47, 63) | <0.001 |
| Female (%) | 30.5% | 34.3% | 0.01 |
| Race/Ethnicity (%) | <0.001 | ||
| Caucasian | 39.9% | 41.7% | |
| African American | 43.8% | 36.7% | |
| Latino/Hispanic | 12.4% | 15.8% | |
| Other | 3.9% | 5.8% | |
| Primary indication for ESRD (%) | <0.001 | ||
| Glomerulonephritis | 13.9% | 17.0% | |
| Diabetes | 28.8% | 33.3% | |
| Hypertension | 26.2% | 23.1% | |
| Other | 31.1% | 26.6% | |
| Blood type (%) | 0.03 | ||
| A | 32.0% | 31.5% | |
| AB | 3.6% | 2.8% | |
| B | 15.3% | 15.8% | |
| O | 49.1% | 50.0% | |
| cPRA≥80% (%) | 2.1% | 2.7% | 0.5 |
| Diabetes | 43.5% | 44.8% | <0.001 |
| Dialysis (%) | 81.2% | 74.4% | <0.001 |
| Dialysis time (yr; Med (IQR)) | 0.8 (0.0, 2.1) | 0.8 (0.0, 1.9) | <0.001 |
Use of HCV+ kidneys for HCV+ recipients
The number of HCV+ recipients who received HCV+ kidneys increased from 154 in 2005 (20.4% of HCV+ recipients) to 384 in 2017 (38.6%) (Figure 1b). In the IFN and DAA eras overall, 24.4% and 38.3% of HCV+ recipients were transplanted with HCV+ kidneys, respectively. After accounting for center variation and recipient characteristics, HCV+ recipients transplanted in the DAA era were 1.95-times more likely to have received an HCV+ kidney than those in the IFN era (aOR: 1.761.952.16, p<0.001). After modeling the trend as function of time, the per-year increase observed during the DAA era was statistically significantly higher than expected given the 2010-2013 trend (per-year increase from 2010-2013: 0.910.991.07 and from 2013-2018: 1.071.151.23, p-interaction of spline terms=0.006). HCV+ recipients of HCV+ kidneys in the DAA era had higher EPTS scores, were older, more likely to be female, more likely to be Caucasian, more likely to have diabetes, and less likely to have been on dialysis (Table 1b).
Table 1b.
Characteristics of HCV+ recipients who received an HCV+ kidney before and after the introduction of DAA therapy (n=3348).
| Recipient characteristic | 2005-2013 (N=1799) |
2014-2018 (N=1549) |
p value |
|---|---|---|---|
| EPTS score at transplant (Med (IQR)) | 64 (44, 80) | 72 (55, 87) | <0.001 |
| Age at transplant (Med (IQR)) | 56 (51, 60) | 60 (56, 64) | <0.001 |
| Female (%) | 16.2% | 22.2% | <0.001 |
| Race/Ethnicity (%) | <0.001 | ||
| Caucasian | 21.4% | 28.0% | |
| African American | 67.8% | 56.7% | |
| Latino/Hispanic | 8.7% | 11.4% | |
| Other | 2.2% | 3.9% | |
| Primary indication for ESRD (%) | <0.001 | ||
| Glomerulonephritis | 10.6% | 10.4% | |
| Diabetes | 33.4% | 40.5% | |
| Hypertension | 40.6% | 29.1% | |
| Other | 15.4% | 20.0% | |
| Blood type (%) | 0.4 | ||
| A | 29.1% | 29.3% | |
| AB | 3.7% | 3.6% | |
| B | 15.5% | 14.3% | |
| O | 51.6% | 52.8% | |
| cPRA≥80% (%) | 0.6% | 1.0% | 0.06 |
| Diabetes | 48.3% | 53.9% | <0.01 |
| Pretransplant dialysis (%) | 91.3% | 87.2% | <0.001 |
| Dialysis time (yr; Med (IQR)) | 2.3 (1.0, 4.3) | 2.0 (0.7, 3.8) | <0.001 |
Center-level use of HCV+ kidneys for HCV+ recipients
Among the 173 transplant centers, all had performed at least 1 transplant among an HCV-infected recipient, 146 (84.3%) had performed at least 1 HCV+ donor to HCV+ recipient DDKT, and 106 (61.3%) used HCV+ kidneys among >20% of their HCV+ recipients over the study period. Ninety-three centers (53.8%) increased, 29 (16.7%) had no change in the percent, and 51 (29.5%) decreased the percent of HCV+ recipients transplanted with HCV+ kidneys in the DAA era (Figure 2). Centers that increased use of HCV+ kidneys in the DAA era had similar annual DDKT volume to centers that decreased use (median annual DDKT of 52 [33-78] vs 51 [33-73], respectively) and a slightly higher percent of recipients that were HCV-antibody positive (median HCV+ recipient prevalence 6.0% [4.1-7.6] vs 4.8% [3.2-7.0], respectively). Of the 106 centers that used HCV+ kidneys for >20% of HCV+ recipients, 65 centers (61%) increased and 41 (38.7%) decreased HCV+ kidney use in the DAA era.
Figure 2.
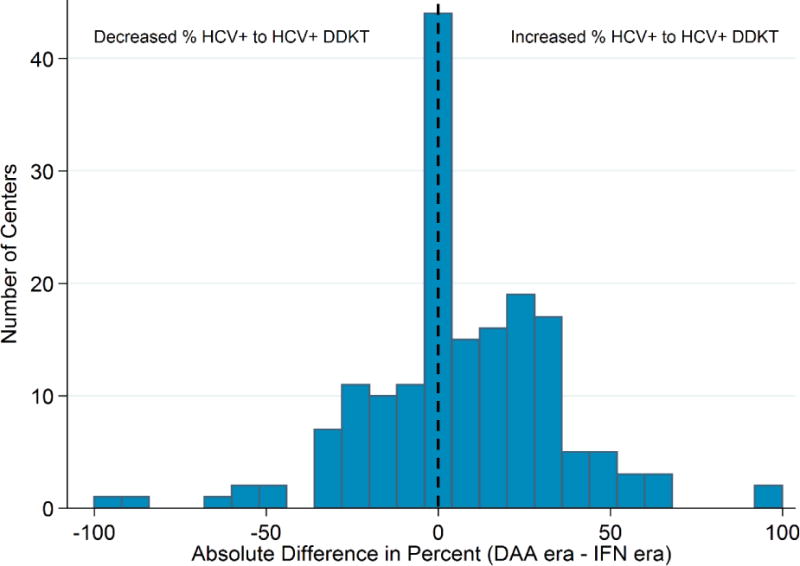
Center-level changes in the percent of HCV+ recipients who received an HCV+ kidney among 173 centers that performed at least 1 DDKT per year. After the introduction of DAAs, 93 (54%) increased, 51 (29%) decreased, and 16.7% remained unchanged in the percent of HCV+ recipients transplanted with HCV+ kidneys.
Clustering of HCV+ to HCV+ transplants was highly localized within a subset of centers in both the IFN and DAA eras (Gini coefficients: IFN era 0.68 vs DAA era: 0.69). In the IFN era, 43 of the 173 centers (24.9%) performed 75% of the HCV+ to HCV+ transplants, and in the DAA era, 39 of the 173 centers (22.5%) performed 75% of the HCV+ to HCV+ transplants. For reference, 86 centers (49.7% of 173) performed 75% of the total number of DDKTs (irrespective of HCV) over the study period (Gini=0.36).
Posttransplant outcomes associated with HCV+ kidneys
Among HCV+ recipients, receiving an HCV+ deceased-donor kidney was associated with 1.19-fold higher mortality when compared to receiving an HCV- kidney (aHR: 1.071.191.32). This 19% increased risk translated to 1-year survival of 94.9% and 95.3% for HCV+ recipients of HCV+ and HCV- kidneys, respectively (Figure 3). Three-year survival was 86.9% and 88.4% for HCV+ recipients of HCV+ and HCV- recipients. The risk associated with being an HCV+ recipient (1.36-fold higher mortality when compared to HCV- recipients [aHR:1.281.361.46]), and the risk associated with receiving an HCV+ kidney were not statistically significantly different in the recent era (p value of interaction terms = 0.07 and 0.08, respectively).
Figure 3.
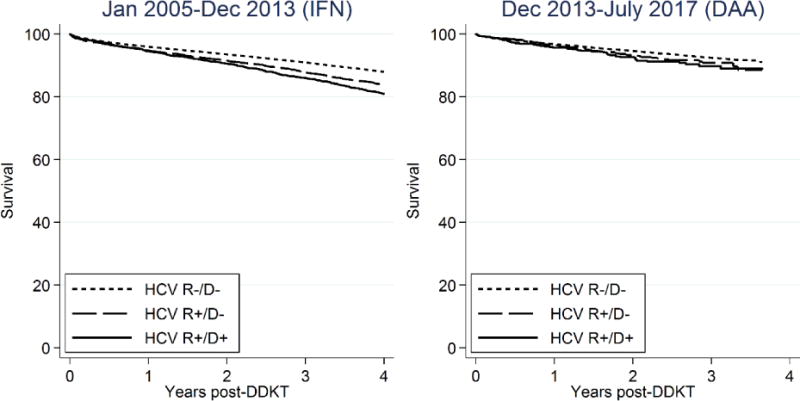
Posttransplant survival among HCV- recipients (R−/D−), HCV+ recipients of HCV- kidneys (R+/D−), and HCV+ recipients of HCV+ kidneys (R+/D+).
Quality of HCV+ kidneys
The prevalence of HCV+ donor kidneys increased from 3.6% in 2005 to 5.7% in 2017 and 7.1% in the first 2 months of 2018. Median (IQR) KDPI of HCV+ kidneys decreased from 78 (59-90) in 2005 to 53 (40-67) in 2017 (Figure 4). Median KDPI of HCV- kidneys remained unchanged between 45 (21-74) in 2005 to 47 (24-73) in 2017. Median KDPI of HCV- and HCV+ kidneys increased slightly to 53 (26-91) and 57 (46-81) in the first 2 months of 2018. KDPI of HCV+ kidneys was statistically significantly higher than that of HCV- kidneys in each year (Wilcoxon p values<0.001).
Figure 4.
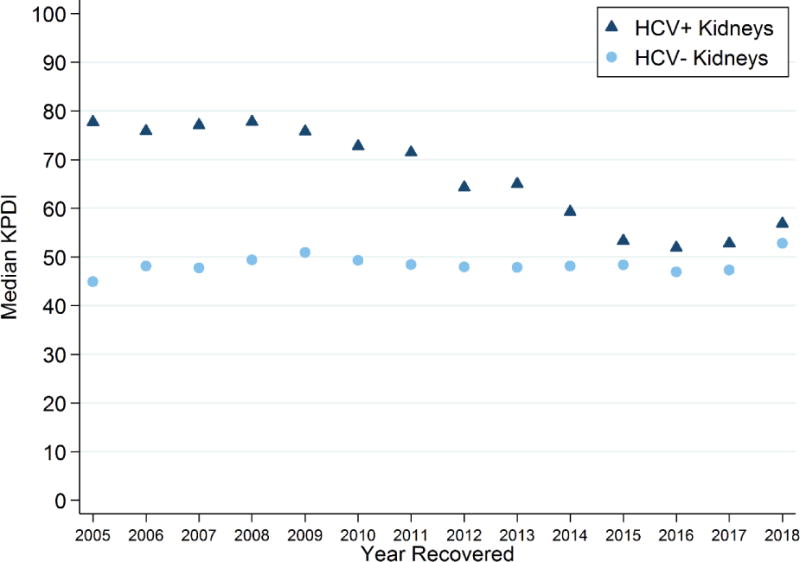
Median KDPI of HCV+ and HCV- deceased-donor kidneys each year. Median KDPI of recovered HCV+ donor kidneys decreased from 77 (IQR 59-90) in 2005 to 52 (IQR 39-67) to 2017, while KDPI of HCV- donor kidneys remained unchanged at 45 (21-74) and 47 (24-73), respectively. In the first 2 months of 2018, KDPI of HCV- and HCV+ kidneys increased to 53 (26-76) and 57 (46-81), respectively.
Discard of HCV+ kidneys
During the IFN era, 52.3% of HCV+ (n=2382) and 16.7% of HCV- (n=20 031) kidneys were discarded, and discarded HCV+ and HCV- kidneys had median (IQR) KDPIs of 82 (65-94) and 84 (65-94). During the DAA era, 42.8% of HCV+ (n=1513) and 18.0% of HCV- (n=12 100) kidneys were discarded, and discarded HCV+ and HCV- kidneys had median KDPIs of 64 (49-81) and 82 (65-92). The percent of discarded HCV+ kidneys decreased from 52.9% to 37.6% between 2014-2017 (Figure 5a).
Figure 5.
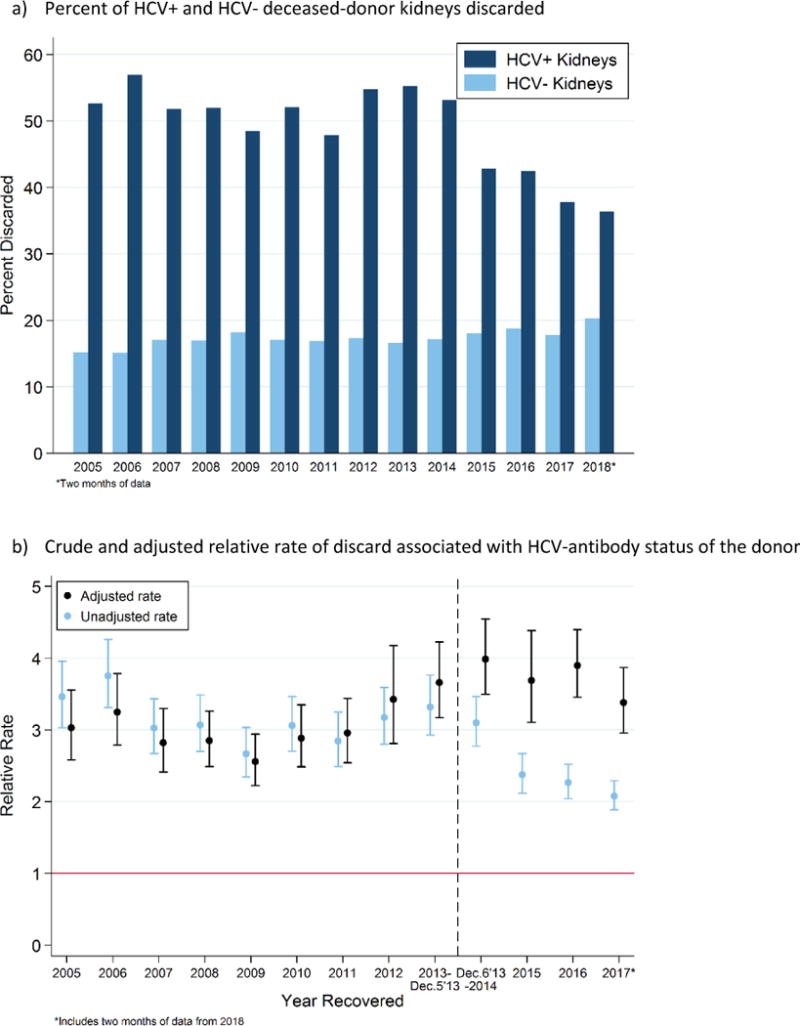
a) Percent HCV+ and HCV- deceased-donor kidneys that were discarded and b) crude and adjusted relative rates of discard associated with HCV-antibody status; 2005-2018.
In an unadjusted model, HCV+ kidneys were 3.1- and 2.4-times more likely to be discarded than HCV- kidneys during the IFN (RR: 3.033.123.22, p<0.001) and DAA eras (RR: 2.272.372.47, p<0.001): a statistically significant decrease (p-interaction<0.001). The unadjusted rate varied from year-to-year, such that HCV+ kidneys were discarded at a 3.75-folder high rate in 2006 (RR: 3.313.754.26, p<0.001), a 2.67-fold higher rate in 2009 (RR: 2.342.673.03, p<0.001), and a 3.3-fold higher rate in 2013 (RR: 2.933.323.76, p<0.001) (Figure 5b). After 2013, the unadjusted relative rate decreased to a 2.1-fold higher rate in the 2017 (RR: 1.892.082.29, p<0.001).
After adjustment, HCV+ kidneys were 3.0- and 3.7-times more likely to be discarded than comparable HCV- kidneys in the IFN (aRR: 2.783.023.27, p<0.001) and DAA eras (aRR: 3.363.67-4.02, p<0.001): a statistically significant increase (p value of the interaction<0.001). In 2014, HCV+ kidneys were 3.99-times more likely to be discarded than comparable HCV- kidneys (aRR: 3.503.994.54, p<0.001). The relative rate declined slightly in 2017, such that HCV+ kidneys were 3.38-times more likely to be discarded than comparable HCV- kidneys (aRR: 2.963.383.87, p<0.001).
Sensitivity analysis
We found that HCV+ recipients transplanted after the introduction of FDA-approved DAAs were 2.0-times more likely to have received an HCV+ kidney than those transplanted before (aOR: 1.812.012.23,p<0.001). These findings were consistent with the original analysis.
DISCUSSION
In this national registry study, we characterized changes in the use of HCV+ kidneys for HCV+ recipients after the introduction of DAA therapy. In the recent DAA era, candidates were 2.2-times more likely to list as willing to accept HCV+ kidneys, and HCV+ recipients were 1.95-times more likely to have been transplanted with an HCV+ kidney. While 54% of transplant centers increased use of HCV+ kidneys in the DAA era, the practice remained highly concentrated within a few centers. In the DAA era, 22.5% of centers performed 75% of HCV+ to HCV+ transplants, with no improvement since the IFN era. Discard of HCV+ kidneys decreased from 52.3% in the IFN era to 42.8% in the DAA era; however, after adjustment for donor characteristics, the relative rate of discard of HCV+ kidneys actually increased from a 3.0-fold higher rate in the IFN era to a 3.7-fold higher rate in the DAA era. Finally, posttransplant mortality associated with HCV+ kidneys (1.19-fold higher risk) remained unchanged in the recent era, although this translated to a 1.5% difference in survival between HCV+ recipients of HCV+ and HCV- kidneys 3 years posttransplant.
Possible explanations for the increasing willingness to accept HCV+ kidneys include increased prevalence of HCV-infection among patients with ESRD, shorter waiting times for candidates who accepted HCV+ kidneys (14, 17, 37), and the introduction of DAAs, which could be used to cure HCV infection after transplant. While we observed an increase in willingness to accept HCV+ kidneys in the DAA era, it is important to note that the trend began prior to the introduction of IFN-free DAA therapy. The increase we observed in 2011 might have been driven by the introduction of telaprevir, the first DAA used in combination with IFN which foreshadowed the IFN-free DAA era. The increased availability of HCV-infected deceased-donor organs driven by the opioid overdose epidemic might have also contributed to increased willingness (33). Our finding that willingness decreased from 2005 to 2009 was consistent with the only other study on willingness to accept HCV+ kidneys (38). In this study, Massie et al also observed homogeneity in willingness to accept HCV+ kidneys within centers, and thus it might be possible that the increase was not driven by individual decision-making, but by shifts in center-wide protocols (38).
We report that HCV+ recipients transplanted in the recent era were more likely to receive an HCV+ kidney than those transplanted during the IFN era. Possible explanations might include the improving quality of HCV+ kidneys driven by the opioid overdose epidemic (33), the increasing prevalence of HCV in the deceased-donor pool (from 3.6% in 2005 to 5.6% in June 2017), evidence of acceptable posttransplant outcomes associated with HCV+ kidneys (17, 39), survival benefit associated with transplantation(7, 15, 16), and the availability of DAA therapy. Recent changes to allocation policy might have increased the prioritization of HCV-infected recipients willing to accept HCV+ kidneys, leading to an increase in the proportion that were transplanted with HCV+ kidneys. However, we saw no significant changes in the panel reactive antibody of recipients of HCV+ kidneys in the recent era.
Center utilization of HCV+ kidneys remained highly clustered during the DAA era. While 54% of centers increased use of HCV+ kidneys, only 39 of 173 centers (22.5%) performed 75% of the total of HCV+ to HCV+ transplants in the DAA era. Kucirka et al found similar clustering of HCV+ kidney to HCV+ recipient transplants between 1995 and 2009, indicating minimal improvement in the spread of these transplants across programs (14).
Multiple studies have reported 2- to 6-fold higher rates of discard of HCV+ kidneys using modified Poisson regression and logistic regression models (14, 35, 40–42). During the IFN era, HCV+ donors had higher KDPI driven by older age and additional comorbidities; discard of these organs was likely not only due to HCV-infection, but also related to overall poorer quality of the kidney. Although the percent of discarded HCV+ kidneys decreased from 52% in 2005 to 37.6% in 2017, the adjusted relative rate of HCV+ kidneys actually increased from a 3.0-fold higher rate to 3.7-fold higher rate of discard than comparable HCV- kidneys. This suggests that discard of HCV+ kidneys was driven by the presence of HCV-infection. Concerns about posttransplant outcomes or a small increased risk of other infections such as HIV might also be driving increased discard in recent years (43). While we observed 19% higher risk of mortality among those who received an HCV+ kidney, this translated to a 1.5% percentage point difference in survival 3 years posttransplant. These findings are consistent with those of Kucirka (14) and Heo (44). However, candidates with HCV+ are not faced with the decision to accept an HCV+ or HCV- kidney on the same day, in which the choice would be clear. Candidates are more likely to face the decision to accept an HCV+ kidney today or remain on dialysis, where risk of death is higher. These elevated rates of discard are concerning given the risk of death on dialysis that HCV+ patients face and the survival benefit of kidney transplantation even when the donor possesses an increased risk of disease transmission (8, 45).
We report that 27 of 173 active centers performed zero HCV+ kidney to HCV+ recipient transplants in the DAA era, and yet, the median KDPI of discarded HCV+ kidneys was 64 (49-81) indicating that 25% of these kidneys had a KDPI below 49. Low competition surrounding HCV-infected donor kidneys might be allowing participating centers to select optimal HCV+ kidneys while transplantable HCV+ kidneys are discarded. To maximize the use of HCV+ kidneys, 2 centers have initiated pilot trials in which HCV-negative transplant candidates receive an HCV+ kidney transplant with either DAA prophylaxis (46) or preemptive DAA treatment (47). While this approach is attractive, DAAs are expensive and access to DAAs for transplant recipients outside of clinical trials is uncertain (48). A recent study from the UK showed that transplantation with DAAs was cost-neutral with continued hemodialysis after 5 years (49). Thus, the use of HCV+ kidneys in HCV- recipients may prove cost-effective and become standard practice in the future (50). In the meantime, maximizing utilization of HCV+ kidneys for HCV+ recipients could improve outcomes for HCV+ candidates.
The limitations of our study merit consideration. HCV-antibody status of transplant candidates is not available through OPTN data, and thus, we used willingness to accept HCV+ kidneys to characterize waitlist behaviors with respect to HCV+ kidneys. The prevalence of willingness to accept HCV+ kidneys does not represent the prevalence of HCV-infection among dialysis patients, and we also expect that HCV+ candidates willing to accept HCV+ kidneys were different from HCV+ candidates unwilling to accept them. Unfortunately, we were unable to make this distinction. However, the incident waitlist candidates willing to accept HCV+ kidneys indicate that there remains a growing and unmet need for HCV+ kidneys. We did not have access to recipient or donor HCV RNA status or treatment history, factors which might directly affect the decision to use an HCV+ kidney (51). Without treatment history, is it likely that we underestimated the proportion of actively infected HCV+ recipients who received HCV+ kidneys, as patients treated on the waitlist would still appear as HCV-antibody positive in the OPTN data. We defined drug eras based on the availability of IFN-Free DAAs, however it is important to note that studies demonstrating the safety and efficacy of DAAs in kidney transplant recipients were not published until later. Finally, we were limited in our ability to control for unmeasured confounding that might have been present in our model comparisons. More granular kidney-related factors, such as biopsy findings or cold ischemia time, were not available for adjustment in the discard analysis.
We observed encouraging increases in willingness to accept HCV+ kidneys and use of HCV+ in the era of DAA therapy. However, we also observed that the number of centers performing HCV+ kidney transplants remains low, and might be driving the disproportionately high discard rates of HCV+ kidneys, despite the otherwise high quality of these organs. Broader utilization of HCV+ kidneys could improve outcomes for HCV+ candidates and reduce unnecessary discard of high quality HCV+ kidneys.
Acknowledgments
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, UNOS/OPTN, or the US Government. This work was originally presented in preliminary form at the American Transplant Congress in May 2016.
FUNDING
C.M. Durand is supported by grant K23CA177321-01A1 from the National Cancer Institute. M.S. is supported by National Institute of Allergy and Infectious Disease by grant K24DA034621. D.L.S, L.M.K., A.A.S., and A.B.M. are supported by the National Institute of Diabetes and Digestive and Kidney Diseases by grants K24DK101828 (Segev), F30DK095545 (Kucirka), F30DK116658 (Shaffer) and K23DK101677 (Massie), respectively.
ABBREVIATIONS
- aRR
adjusted relative rate
- BMI
body mass index
- DA
Adirect-acting antiviral therapy
- DDKT
deceased-donor kidney transplant(ation)
- DSA
Donation Service Area
- EPTS
estimated posttransplant survival score
- ESRD
end stage renal disease
- HCV
hepatitis C virus
- HRSA
Health Resources and Services Administration
- INF
pegylated interferon
- IQR
interquartile range
- KDPI
Kidney Donor Profile Index
- KT
kidney transplant(ation)
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
AUTHORSHIP
M.G.B. participated in research design, statistical analysis, and writing and revising the manuscript. L.M.K. and A.B.M participated in research design, statistical analysis, and revising the manuscript. T.I. and S.B. participated in independent statistical analysis and results replication. A.A.S., J.G.W., M.S., and N.D. participated in research design and revising the manuscript. D.L.S. and C.M.D. participated in research design, analytical support, and writing and revising the manuscript.
DISCLOSURES
C.M.D has received research grants from Gilead Sciences and Bristol Meyers Squibb, served as a scientific advisor for Gilead Sciences, Bristol Meyers Squibb, and Merck Pharmaceuticals, and served as a speaker for Roche Diagnostics. C.M.D. also received funds from Gilead for grant review. M.S. served as scientific advisor for AbbVie, Gilead Sciences, Cocrystal, Janssen, Merck Pharmaceuticals, and Trek. M.S. also received research grants from AbbVie, Gilead Sciences, and Merck Pharmaceuticals. The remaining authors of this manuscript have nothing to disclose as described by Transplantation.
References
- 1.Fissell RB, Bragg-Gresham JL, Woods JD, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–42. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 2.Saab S. Hepatitis C virus transmission in the hemodialysis community. Am J Kidney Dis. 2001;37:1052–5. doi: 10.1016/s0272-6386(05)80024-1. [DOI] [PubMed] [Google Scholar]
- 3.Saab S, Brezina M, Gitnick G, Martin P, Yee HF., Jr Hepatitis C screening strategies in hemodialysis patients. Am J Kidney Dis. 2001;38:91–7. doi: 10.1053/ajkd.2001.25199. [DOI] [PubMed] [Google Scholar]
- 4.Butt AA, Skanderson M, McGinnis KA, et al. Impact of hepatitis C virus infection and other comorbidities on survival in patients on dialysis. J Viral Hepat. 2007;14:688–96. doi: 10.1111/j.1365-2893.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang SM, Liu JH, Chou CY, Huang CC, Shih CM, Chen W. Mortality in hepatitis C-positive patients treated with peritoneal dialysis. Perit Dial Int. 2008;28:183–7. [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–93. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 7.Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR. Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant. 2005;5:139–44. doi: 10.1111/j.1600-6143.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 8.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–33. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 9.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14:2310–6. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 10.Roth D, Gaynor JJ, Reddy KR, et al. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152–60. doi: 10.1681/ASN.2010060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis c-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant. 2004;4:2032–7. doi: 10.1046/j.1600-6143.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 12.Saidi RF, Markmann JF, Jabbour N, et al. The faltering solid organ donor pool in the United States (2001-2010) World J Surg. 2012;36:2909–13. doi: 10.1007/s00268-012-1748-0. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JB, Eddinger KC, Shelton B, Locke JE, Forde KA, Sawinski D. Effect of kidney donor hepatitis C virus serostatus on renal transplant recipient and allograft outcomes. Clin Kidney J. 2017;10:564–72. doi: 10.1093/ckj/sfx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10:1238–46. doi: 10.1111/j.1600-6143.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 15.Knoll GA, Tankersley MR, Lee JY, Julian BA, Curtis JJ. The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis. 1997;29:608–14. doi: 10.1016/s0272-6386(97)90345-0. [DOI] [PubMed] [Google Scholar]
- 16.Sezer S, Ozdemir FN, Akcay A, Arat Z, Boyacioglu S, Haberal M. Renal transplantation offers a better survival in HCV-infected ESRD patients. Clin Transplant. 2004;18:619–23. doi: 10.1111/j.1399-0012.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 17.Scalea JR, Barth RN, Munivenkatappa R, et al. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation. 2015;99:1192–6. doi: 10.1097/TP.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 18.Carbognin SJ, Solomon NM, Yeo FE, et al. Acute renal allograft rejection following pegylated IFN-alpha treatment for chronic HCV in a repeat allograft recipient on hemodialysis: a case report. Am J Transplant. 2006;6:1746–51. doi: 10.1111/j.1600-6143.2006.01374.x. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa Y, Kyo M, Hanafusa T, et al. A 20-year case study of a kidney transplant recipient with chronic active hepatitis C: clinical course and successful treatment for late acute rejection induced by interferon therapy. Transplantation. 1998;65:134–8. doi: 10.1097/00007890-199801150-00026. [DOI] [PubMed] [Google Scholar]
- 20.Weclawiack H, Kamar N, Mehrenberger M, et al. Alpha-interferon therapy for chronic hepatitis C may induce acute allograft rejection in kidney transplant patients with failed allografts. Nephrol Dial Transplant. 2008;23:1043–7. doi: 10.1093/ndt/gfm678. [DOI] [PubMed] [Google Scholar]
- 21.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–45. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 22.Colombo M, Aghemo A, Liu H, et al. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Ann Intern Med. 2017;166:109–17. doi: 10.7326/M16-1205. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez I, Munoz-Gomez R, Pascasio JM, et al. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol. 2017;66:718–23. doi: 10.1016/j.jhep.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Chen K, Lu P, Song R, et al. Direct-acting antiviral agent efficacy and safety in renal transplant recipients with chronic hepatitis C virus infection: A PRISMA-compliant study. Medicine (Baltimore) 2017;96:e7568. doi: 10.1097/MD.0000000000007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin MV, Sise ME, Pavlakis M, et al. Efficacy and Safety of Direct Acting Antivirals in Kidney Transplant Recipients with Chronic Hepatitis C Virus Infection. PLoS One. 2016;11:e0158431. doi: 10.1371/journal.pone.0158431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Available from: http://www.hcvguidelines.org.
- 27.Sawinski D, Bloom RD. Novel Hepatitis C Treatment and the Impact on Kidney Transplantation. Transplantation. 2015;99:2458–66. doi: 10.1097/TP.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 28.Kusnir J, Roth D. Direct-Acting Antiviral Agents for the Hepatitis C Virus-Infected Chronic Kidney Disease Population: The Dawn of a New Era. Semin Dial. 2016;29:5–6. doi: 10.1111/sdi.12456. [DOI] [PubMed] [Google Scholar]
- 29.Sawinski D, Wyatt CM, Locke JE. Expanding the use of hepatitis C-viremic kidney donors. Kidney Int. 2017;92:1031–3. doi: 10.1016/j.kint.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Choi G, Lee KG, Wu C, Saab S. Kidney transplantation threshold in patients with hepatitis C: a decision analysis model. Transplantation. 2015;99:829–34. doi: 10.1097/TP.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 31.Bowring MG, Kucirka LM, Massie AB, et al. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. Am J Transplant. 2017;17:519–27. doi: 10.1111/ajt.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving Organ Utilization to Help Overcome the Tragedies of the Opioid Epidemic. Am J Transplant. 2016;16:2836–41. doi: 10.1111/ajt.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durand CM, Bowring MG, Thomas AG, et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med. 2018;168:702–11. doi: 10.7326/M17-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723–30. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massie AB, Desai NM, Montgomery RA, Singer AL, Segev DL. Improving distribution efficiency of hard-to-place deceased donor kidneys: Predicting probability of discard or delay. Am J Transplant. 2010;10:1613–20. doi: 10.1111/j.1600-6143.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- 36.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10:1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sageshima J, Troppmann C, McVicar JP, Santhanakrishnan C, de Mattos AM, Perez RV. Impact of Willingness to Accept Hepatitis C Seropositive Kidneys Among Hepatitis C RNA-positive Waitlisted Patients. Transplantation. doi: 10.1097/TP.0000000000002096. Published online January 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massie AB, Stewart DE, Dagher NN, Montgomery RA, Desai NM, Segev DL. Center-level patterns of indicated willingness to and actual acceptance of marginal kidneys. Am J Transplant. 2010;10:2472–80. doi: 10.1111/j.1600-6143.2010.03294.x. [DOI] [PubMed] [Google Scholar]
- 39.Morales JM, Campistol JM, Dominguez-Gil B, et al. Long-term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients. Am J Transplant. 2010;10:2453–62. doi: 10.1111/j.1600-6143.2010.03280.x. [DOI] [PubMed] [Google Scholar]
- 40.Marrero WJ, Naik AS, Friedewald JJ, et al. Predictors of Deceased Donor Kidney Discard in the United States. Transplantation. 2017;101:1690–7. doi: 10.1097/TP.0000000000001238. [DOI] [PubMed] [Google Scholar]
- 41.Singh SK, Kim SJ. Epidemiology of Kidney Discard from Expanded Criteria Donors Undergoing Donation after Circulatory Death. Clin J Am Soc Nephrol. 2016;11:317–23. doi: 10.2215/CJN.07190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting Hepatitis C-Positive Kidneys. N Engl J Med. 2015;373:303–5. doi: 10.1056/NEJMp1505074. [DOI] [PubMed] [Google Scholar]
- 43.Kucirka LM, Sarathy H, Govindan P, et al. Risk of window period HIV infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant. 2011;11:1176–87. doi: 10.1111/j.1600-6143.2010.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heo NY, Mannalithara A, Kim D, Udompap P, Tan JC, Kim WR. Long-term Patient and Graft Survival of Kidney Transplant Recipients With Hepatitis C Virus Infection in the United States. Transplantation. 2018;102:454–60. doi: 10.1097/TP.0000000000001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowring MG, Holscher CM, Zhou S, et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transplant. 2018;18:617–24. doi: 10.1111/ajt.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durand CM, Brown D, Wesson R, et al. EXPANDER-1: Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients. [abstract] Am J Transplant. 2017;17(suppl 3) [Google Scholar]
- 47.Goldberg DS, Abt PL, Blumberg EA, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376:2394–5. doi: 10.1056/NEJMc1705221. [DOI] [PubMed] [Google Scholar]
- 48.Axelrod DA, Schnitzler MA, Alhamad T, et al. The Impact of Direct-Acting Antiviral Agents on Liver and Kidney Transplant Costs and Outcomes. Am J Transplant. doi: 10.1111/ajt.14895. Published online January 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trotter PB, Summers DM, Ushiro-Lumb I, et al. Use of Organs From Hepatitis C Virus-Positive Donors for Uninfected Recipients: A Potential Cost-Effective Approach to Save Lives? Transplantation. 2018;102:664–72. doi: 10.1097/TP.0000000000002033. [DOI] [PubMed] [Google Scholar]
- 50.Holscher CM, Durand CM, Desai NM. Expanding the Use of Organs From Hepatitis C-Viremic Donors: The Evidence Continues to Build. Transplantation. 2018;102:546–7. doi: 10.1097/TP.0000000000002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kling CE, Perkins JD, Landis CS, Limaye AP, Sibulesky L. Utilization of Organs From Donors According to Hepatitis C Antibody and Nucleic Acid Testing Status: Time for Change. Am J Transplant. 2017;17:2863–68. doi: 10.1111/ajt.14386. [DOI] [PubMed] [Google Scholar]


