Abstract
Introduction.
Roughly 4–23% of the population embody stress prone personality and other traits characterizing a subclinical “broad autism phenotype” (BAP). Subjective cognitive impairment (SCI) among healthy elderly is associated with psychological distress leading us to predict BAP would be associated with SCI.
Methods.
The Autism Spectrum Quotient, a self-administered 50 item questionnaire, was completed by 419 consecutive members of the Arizona APOE Cohort who underwent neuropsychological testing every 2 years. SCI was assessed with self and informant versions of the Multidimensional Assessment of Neurodegenerative Symptoms questionnaire (MANS).
Results.
45 individuals scored in the BAP range, designated BAP+, and the rest were BAP−. At entry, both MANS-self and informant scores were higher in the BAP+ group (p<0.0001). After age 60, the BAP+ group had greater annual increases in MANS-self scores (0.05 vs 0.02; diff = 0.03; 95%CI: 0.004, 0.05; p=0.02) yet there was no difference between groups in memory decline. Over approximately 10 years 33 individuals developed mild cognitive impairment (MCI): 4 in the BAP+ group (8.9%) and 29 in the BAP− group (7.8%), p=0.77.
Discussion.
Individuals who meet criteria for the BAP have escalating SCI with age, but no greater rate of memory decline or clinical progression to MCI.
Introduction
According to the most recent prevalence estimates, 1.5% of young children have an autism spectrum disorder (ASD1). Not included in such estimates are individuals with a subclinical “broad autism phenotype” (BAP). Following Kanner’s original 1943 description of autism2, allusion to behavioral traits that were less severe but qualitatively similar to those which characterize ASD date to Kanner and Eisenberg in 19573. In 1977 Folstein and Rutter first proposed that a genetic liability for autism would be expressed as milder behavioral traits in non-autistic relatives of autistic probands4, a finding subsequently confirmed in 1994 by Bolton et al5. Most studies have sought such traits in first degree relatives of ASD children with prevalence estimates of 9–23% of mothers and 9–40% of fathers, while prevalence rates in control parents have been around 9–22% in fathers and 4–23% in mothers6–8.
Characteristics of the BAP have been debated, but include personality, language, and other cognitive traits qualitatively resembling, but milder than those that characterize individuals with an ASD9–13. Behavioral BAP traits include reduced social and communication skills9,12,13; personality characteristics that most consistently include higher Neuroticism12–16, reduced Extraversion15,16, and greater rigidity or reduced openness9,12,14,16; and higher levels of anxiety and depression9,14,17. Purported intellectual traits of the BAP phenotype have most consistently included less efficient executive skills including planning tasks such as the Tower of Hanoi/London18–20, ideational fluency21,22, and set shifting19,22, although other studies found no differences from non-BAP controls13,23.
Several questionnaires have been developed to screen for ASD related traits in adults including two that are self-administered24,25. If the observed prevalence rate of 4–23% in ASD study control groups is representative of the general population, then individuals with the BAP are likely to have been included in most if not all studies of cognitive aging and dementia. Included in such studies are individuals expressing concern that they are experiencing cognitive decline, yet in whom no objective evidence of decline exists. Studies of subjective cognitive decline have shown that psychological distress is a major factor26, and individuals with the BAP are characterized by stress prone personalities and elevated levels of trait anxiety and depression suggesting they may be at higher risk for subjective cognitive impairment (SCI).
To explore the possible influence of the BAP on subjective and objective measures of age-related memory decline and dementia risk, we administered the Autism Spectrum Quotient (AQ), a self-administered screening questionnaire24 that has been successfully utilized in population studies of ASD in adults27 to members of the Arizona APOE Cohort, a study of cognitive aging in cognitively normal individuals at genetically defined risk for Alzheimer’s disease (AD) to determine 1) the prevalence of individuals scoring in the BAP range, 2) whether such individuals fit a previously described BAP profile, and 3) how the BAP might impact subjective and objective measures of age-related memory decline and incident mild cognitive impairment (MCI) rates.
Methods
Study Participants. From January 1, 1994 through December 31 2013, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media ads, underwent APOE genotyping and longitudinal neuropsychological assessment every two years. All individuals gave their written, informed consent to participate in the study and have the results of the APOE test withheld from them which was approved by the Mayo Clinic Institutional Review Board. Determination of APOE genotype was performed using Taqman Single Nucleotide Polymorphism assays.
All identified e4 homozygotes (HMZ) were matched by age, sex, and education to one e4 heterozygote (HTZ; all with the e3/4 genotype) and two e4 non-carriers. Many additional heterozygous persons and non-carriers who were otherwise eligible for enrollment were also recruited. Occupational background was quantified using the Dictionary of Occupational Titles28. Intellectual requirements were determined by summing the components of General Educational Development (Reasoning, Mathematical, Language) (GED) for each participant’s specific occupation. Household income was estimated based on census data and home addresses and conformed to one of five income ranges from $0 to 15,000 to over $100,000 a year).
Each participant had screening tests that included a neurological examination, the Folstein Mini-Mental Status Exam (MMSE), Hamilton Depression (Ham-D) Rating Scale, Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for the Diagnostic and Statistical Manual of Mental Disorders-III-Revised (DSM-III-R). We excluded anyone with potentially confounding medical, neurologic, or psychiatric problems. None met published criteria for MCI29, AD30, other forms of dementia, or major depressive disorder31. Participants were not separately evaluated for an ASD in addition to the above screening at entry into the parent study, but based on their generally normal neuropsychiatric status did not meet DSM-5 criteria31 that require persistent deficits in social communication and interaction, psychiatric history of restricted/repetitive patterns of behavior, stereotyped movements, or related problems. Entry criteria included scores of at least 27 on the MMSE (with at least 1 of 3 on the recall subtest), 10 or less on the Ham-D, and perfect scores on the FAQ and IADL. Data were reviewed at each visit by a neurologist (RJC) and neuropsychologist (DECL).
The Autism Spectrum Quotient (AQ).
The AQ was added in 2012. It is a self-administered 50 item questionnaire whose questions are categorized into five domains comprised of 10 questions each: social skills, attention switching, attention to detail, communication, and ideas24. Answer choices for each question are definitely agree, slightly agree, slightly disagree, and definitely disagree, but scoring treats the answers dichotomously as either agree or disagree. Each item is scored as a “1” if the individual endorses the autistic trait so that scores can range from 0–50. Scores of 23–28 (1–2 standard deviations [SD] above the mean of a large normative sample) define the BAP. Scores of 29–34 (2–3 SD above the mean) define the medium autism phenotype (MAP). Scores of 35 and higher (>3 SD above the mean) define the narrow autism phenotype (NAP)7. For the purpose of this study, AQ scores were treated as both a continuous variable and a dichotomized one with scores of 23 and higher defining the BAP.
Neuropsychological Testing.
To assess the cognitive profile of the BAP in our cohort we administered a previously described comprehensive neuropsychological battery32. The neuropsychological tests within our battery encompass four broadly defined cognitive domains including executive skills (Wisconsin Card Sorting Test, Paced Auditory Serial Attention Task, Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Span, Mental Arithmetic, and Digit Symbol Substitution) , memory (Auditory Verbal Learning Test, Buschke Free and Cued Selective Reminding Test, Rey-Osterrieth Complex Figure Test Recall, and the Benton Visual Retention Test), language (Boston Naming Test, Controlled Oral Word Association Test, Token Test, and WAIS-R Vocabulary and Similarities subtests), and visuospatial skills (Judgment of Line Orientation, Facial Recognition Test,WAIS-R Block Design subtest, and CFT copy) as well as behavioral domains (Beck Depression Inventory, Geriatric Depression Scale, Neuropsychiatric Inventory Questionnaire, Personality Assessment Inventory). Administration details are comprehensively described elsewhere33. The scores used are summarized in supplementary Table 1.
Subjective Cognition was assessed with the Multidimensional Assessment of Neurodegenerative Symptoms Questionnaire Self (MANS-Self) and Informant (MANS-Informant) versions34. The MANS are paired self- and informant-based questionnaires comprised of 87 questions that assess changes over the preceding year in daily habits, personality, and motor functioning. They use a quantitative scale for rating the frequency of a symptom from 0 point (never) to 4 points (routinely), with intermediate values of 1 point (once), 2 points (occasionally), and 3 points (more than monthly); scores can range from 0 to 344 points, with higher scores indicating more frequent and severe symptoms. Those with MANS scores greater than zero were considered to have endorsed cognitive impairment.
Personality was assessed with the Neuroticism, Extraversion, and Openness Personality Inventory-Revised (NEO-PI-R) which defines personality according to five factors: Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness35. It was added to our battery in 2006. Neuroticism is a tendency to feel anxiety and other negative emotions, Extraversion is a tendency to be outgoing and lead in social contexts, Openness is a tendency to be receptive to new ideas and experiences, Agreeableness is a tendency to be trusting and deferential, and Conscientiousness is a tendency to be organized and rule abiding. Each factor is comprised of six facets. For example, the six facets of the Neuroticism factor all reflect reactivity to stress and include the tendency to experience anxiety, anger, depression, and self-consciousness; the ability to resist temptations and cravings (impulsivity), and a general ability to cope with stress (vulnerability)35.
Primary Outcome Measures.
Our main goal was to contrast longitudinal changes in memory with subjective memory impairment in the BAP+ and BAP− groups. Based upon prior experience32, we selected the long term memory score of the AVLT as our primary memory outcome measure and the MANS-self score as the primary SCI measure. We also examined clinical outcomes. Those who experienced cognitive decline and met published criteria for MCI29 had evident neuropsychological decline in memory performance relative to baseline, as well as corroboration by their informant of symptomatic decline.
Statistical Analysis.
Study group demographics and baseline neuropsychological measures reflect data collected at the time of the first AQ administration (presented in Tables 1 and 2). Subjects with baseline AQ scores ≥ 23 were considered within the BAP range (BAP+ group) and those with AQ scores < 23 were considered below the BAP range (BAP− group). Baseline behavioral measures including the NEO-PI-R, PAI, and others reflect data collected at the time of the first NEO-PI-R administration (presented in Table 3). Cross-sectional study group characteristics are presented as means with standard deviation (SD) or frequencies. Mean values and frequency distributions were compared between BAP groups using t-tests and chi-square tests, respectively. Adjusted comparisons between groups controlling for age, gender, and education level were made using multiple linear regression for continuous measures and multiple logistic regression for items measuring proportions.
Table 1.
Demographics and Social Status at Entry
| BAP + (n=45) |
BAP − (n=374) |
p | Adjusted p | Adjusted effect size (95%CI) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean years (SD) | 67.9 (14.6) | 65.7 (9.7) | 0.4 a | - | - |
| Followup duration, mean months (SD) | 119.9 (78.8) | 121.9 (78.7) | 0.87 a | - | - |
| Men, % | 48.90% | 24.30% | <0.001b | - | - |
| Education, mean years (SD) | 16.4 (2.3) | 16.3 (2.5) | 0.79 a | - | - |
| White, % | 82.20% | 83.90% | 0.77 b | - | - |
| Righthanders, % | 86.70% | 88.80% | 0.68 b | - | - |
| APOE e4 carrier, % | 48.80% | 44.00% | 0.55 b | - | - |
| Autism Spectrum Quotient, mean (SD) | |||||
| % Social Skills | 22.0 (6.4) | 12.2 (9.9) | <0.001 a | <0.001 c | 9.36 (6.27, 12.44) † |
| % Attention Switching | 22.2 (6.1) | 23.4 (11.6) | 0.52 a | 0.5 c | −1.23 (−4.82, 2.36) † |
| % Attention to Detail | 21.7 (7.7) | 32.1 (16.6) | <0.001 a | <0.001 c | −9.46 (−14.53, −4.39) † |
| % Communication | 17.1 (4.9) | 11.8 (9.7) | <0.001 a | <0.001 c | 5.31 (2.30, 8.31) † |
| % Ideas | 16.9 (6.8) | 20.5 (11.5) | 0.047 a | 0.03 c | −3.97 (−7.51, −0.43) † |
| Subjective Cognitive Impairment | |||||
| MANS-self, mean (SD) | 29.7 (36.6) | 12.6 (24.1) | <0.0001a | <0.0001 c | 16.25 (8.19, 24.31) † |
| MANS-self score > 0, % | 57.80% | 45.30% | 0.11 b | 0.096 d | 1.74 (0.91, 3.32) * |
| MANS informant, mean (SD) | 23.1 (35.5) | 7.0 (18.6) | <0.0001a | <0.0001 c | 14.31 (7.29, 21.33) † |
| MANS informant score > 0, % | 48.60% | 27.50% | <0.01 b | 0.02 d | 2.31 (1.14, 4.68) * |
| Social Status | |||||
| Married currently, % | 62.20% | 74.30% | 0.08 b | 0.05 d | 0.51 (0.26, 1.00) * |
| Multiple divorces, % | 24.40% | 11.30% | 0.01 b | 0.02 d | 2.60 (1.20, 5.63) * |
| Number of children, mean (SD) | 1.9 (1.6) | 1.7 (1.3) | 0.4 a | 0.49 c | 0.14 (−0.27, 0.55) † |
| With biological children, % | 68.90% | 76.30% | 0.28 b | 0.23 d | 0.64 (0.32, 1.31) * |
| zip code median income, mean (SD) | $59,892 ($24,850) | $59,901 ($25,675) | 0.99 a | 0.76 c | $−1,281.40 ($−9,696.78, $7,133.98) † |
| Occupation SVP, mean (SD) | 6.9 (1.4) | 6.9 (1.3) | 0.99 a | 0.41 c | −0.15 (−0.52, 0.21) † |
| Occupation GED, mean (SD) | 12.8 (2.6) | 12.9 (2.4) | 0.87 a | 0.45 c | −0.26 (−0.93, 0.41) † |
Demographic and social status results reflect data gathered from the visit of the patients’ first Autism Spectrum Quotient screening.
BAP = Broad Autism Phenotype; SD = standard deviation; MANS = Multidimensional Assessment of Neurodegenerative Symptoms; SVP = Specific Vocational Preparation; GED = General Educational Development; CI = confidence interval;
T-test;
Chi-squared test;
Linear regression model adjusted for age, sex, and education;
Logistic regression model adjusted for age, sex, and education;
Odds ratio of BAP+ vs BAP− adjusted for age, sex, and education;
Least squares mean difference between BAP+ and BAP− adjusted for age, sex, and education (BAP+ minus BAP−)
Table 2.
Neuropsychological Data at Entry
| mean (SD) | BAP + (n=45) |
BAP − (n=374) |
p a | Adjusted p b | Adjusted effect size (95%CI) † |
|---|---|---|---|---|---|
| General Intellect/Language | |||||
| MMSE | 29.6 (0.7) | 29.6 (0.8) | 0.98 | 0.65 | 0.06 (−0.19, 0.30) |
| WAIS-R-info | 12.9 (2.2) | 12.5 (2.0) | 0.25 | 0.75 | 0.09 (−0.47, 0.65) |
| DRS | 139.6 (3.7) | 140.4 (3.1) | 0.11 | 0.37 | −0.44 (−1.40, 0.53) |
| WAIS-R-vocab | 12.8 (2.0) | 13.0 (1.9) | 0.51 | 0.31 | −0.30 (−0.88, 0.28) |
| WAIS-R-similarities | 13.2 (2.1) | 13.3 (1.9) | 0.73 | 0.61 | −0.15 (−0.72, 0.42) |
| BNT | 54.9 (3.7) | 56.1 (3.9) | 0.04 | 0.03 | −1.32 (−2.50, −0.14) |
| Token Test | 42.8 (2.3) | 43.1 (1.7) | 0.16 | 0.24 | −0.32 (−0.87, 0.22) |
| Visuospatial Skills | |||||
| JLO | 25.0 (3.9) | 24.6 (3.7) | 0.53 | 0.88 | 0.09 (−1.06, 1.24) |
| Facial Recognition Test | 44.6 (5.8) | 45.9 (4.2) | 0.06 | 0.08 | −1.20 (−2.56, 0.15) |
| WAIS-R-Block Design | 12.5 (3.1) | 12.7 (2.7) | 0.72 | 0.51 | −0.28 (−1.13, 0.56) |
| CFT copy | 34.2 (2.6) | 34.5 (2.2) | 0.3 | 0.19 | −0.46 (−1.15, 0.23) |
| Arithmetic/Working Memory | |||||
| WAIS-R digit span | 11.8 (3.0) | 12.0 (2.8) | 0.72 | 0.51 | −0.29 (−1.17, 0.59) |
| WAIS forward digit span | 6.7 (1.1) | 7.0 (1.1) | 0.11 | 0.09 | −0.30 (−0.63, 0.04) |
| WAIS backward digit span | 5.0 (1.1) | 5.0 (1.2) | 0.95 | 0.91 | 0.02 (−0.35, 0.40) |
| WAIS-R-arithmetic | 11.2 (2.7) | 11.7 (2.6) | 0.21 | 0.04 | −0.81 (−1.60, −0.03) |
| PASAT-3 | 42.2 (16.2) | 48.1 (12.5) | 0.005 | 0.01 | −5.45 (−9.58, −1.32) |
| PASAT-2 | 33.3 (15.7) | 39.5 (12.1) | 0.003 | 0.002 | −6.41 (−10.48, −2.33) |
| Mental Speed | |||||
| WAIS-R DSS | 12.4 (2.5) | 13.7 (2.3) | <0.001 | 0.001 | −1.15 (−1.85, −0.44) |
| TMT-A (seconds) | 28.5 (9.1) | 25.1 (9.1) | 0.02 | 0.04 | 2.82 (0.13, 5.50) |
| TMT-B (seconds) | 80.5 (46.5) | 66.7 (29.7) | 0.006 | 0.02 | 11.62 (2.27, 20.97) |
| COWA | 44.5 (9.7) | 48.3 (11.1) | 0.03 | 0.049 | −3.48 (−6.94, −0.02) |
| Animal fluency | 19.4 (4.9) | 21.5 (5.2) | 0.01 | 0.03 | −1.72 (−3.27, −0.17) |
| Vegetable fluency | 13.5 (3.7) | 15.6 (4.1) | 0.001 | 0.03 | −1.34 (−2.53, −0.16) |
| Problem Solving | |||||
| WCST errors | 41.4 (20.0) | 33.9 (20.4) | 0.02 | 0.05 | 5.90 (−0.09, 11.90) |
| WCST Categories | 4.1 (2.2) | 4.7 (1.9) | 0.047 | 0.1 | −0.47 (−1.04, 0.10) |
| WCST Persev Errors | 19.2 (10.8) | 16.9 (12.0) | 0.23 | 0.46 | 1.34 (−2.22, 4.89) |
| IGT-T Score | 51.0 (9.4) | 49.1 (9.1) | 0.48 | 0.39 | 2.34 (−2.98, 7.65) |
| Memory | |||||
| CFT recall | 20.3 (7.0) | 20.5 (6.9) | 0.86 | 0.82 | −0.24 (−2.38, 1.90) |
| AVLT-TL | 45.0 (9.0) | 49.3 (10.4) | 0.009 | 0.13 | −2.24 (−5.11, 0.64) |
| AVLT-LTM | 8.4 (3.5) | 9.5 (3.6) | 0.07 | 0.45 | −0.40 (−1.42, 0.62) |
| AVLT % recall | 71.5 (24.0) | 74.8 (21.5) | 0.34 | 0.89 | −0.47 (−6.95, 6.01) |
| Visual Retention Test | 6.6 (2.3) | 7.0 (2.0) | 0.22 | 0.24 | −0.35 (−0.94, 0.24) |
| SRT immediate free recall | 71.2 (25.1) | 77.2 (12.7) | 0.009 | 0.04 | −4.74 (−9.27, −0.21) |
| SRT delayed free recall | 14.7 (7.3) | 13.9 (2.5) | 0.1 | 0.04 | 1.11 (0.07, 2.15) |
| Logical memory immediate | 13.1 (4.1) | 14.1 (3.6) | 0.08 | 0.27 | −0.65 (−1.82, 0.52) |
| Logical memory delayed | 12.1 (4.4) | 13.0 (4.0) | 0.17 | 0.57 | −0.37 (−1.68, 0.93) |
| Intelligence Quotient | |||||
| WAIS R VC | 116.8 (10.1) | 116.5 (8.9) | 0.83 | 0.69 | −0.52 (−3.11, 2.06) |
| WAIS FFD | 108.4 (13.1) | 110.3 (12.2) | 0.33 | 0.1 | −3.16 (−6.95, 0.62) |
| WAIS R PO | 113.9 (17.1) | 114.5 (14.7) | 0.81 | 0.59 | −1.28 (−5.94, 3.38) |
Neuropsychological results reflect data gathered from the visit of the patients’ first Autism Spectrum Quotient screening.
BAP = Broad Autism Phenotype; SD = standard deviation; CI = confidence interval;
T-test;
Linear regression model adjusted for age, sex, and education;
Least squares mean difference between BAP+ and BAP− adjusted for age, sex, and education (BAP+ minus BAP−)
Table 3.
Behavioral Measures at Baseline
| mean (SD) | BAP + (n=40) |
BAP − (n=341) |
p a | Adjusted p b | Adjusted effect size (95%CI) † |
|---|---|---|---|---|---|
| Depression/Anxiety | |||||
| Hamilton Depression Scale | 3.2 (3.3) | 2.0 (2.4) | 0.007 | 0.001 | 1.41 (0.57, 2.24) |
| Beck Depression Inventory | 6.1 (6.3) | 3.9 (4.0) | 0.003 | 0.001 | 2.41 (0.96, 3.86) |
| Geriatric Depression Scale | 6.8 (4.9) | 2.5 (3.1) | <0.001 | <0.001 | 4.27 (2.91, 5.63) |
| Childhood sleep problem | 25.0% | 11.0% | 0.01 c | 0.007 d | 3.15 (1.37,7.24) * |
| Bruxism | 50.0% | 29.8% | 0.009 c | 0.002 d | 2.96 (1.47,5.96) * |
| PAI-Somatization | 51.2 (9.2) | 47.4 (7.4) | 0.003 | 0.002 | 4.04 (1.45, 6.62) |
| PAI-Anxiety | 50.4 (10.6) | 44.6 (6.6) | <0.001 | <0.001 | 6.78 (4.42, 9.15) |
| PAI-Anxiety Related Disorders | 50.2 (11.7) | 42.9 (7.4) | <0.001 | <0.001 | 7.47 (4.79, 10.15) |
| -ARD-Obsessive Compulsive | 52.2 (11.2) | 45.5 (9.2) | <0.001 | <0.001 | 6.33 (3.14, 9.53) |
| PAI-Depression | 53.4 (13.5) | 46.1 (7.2) | <0.001 | <0.001 | 7.58 (4.83, 10.34) |
| PAI-Mania | 47.1 (9.0) | 43.2 (8.4) | 0.007 | 0.009 | 3.79 (0.97, 6.62) |
| PAI-Paranoia | 49.1 (7.2) | 42.7 (6.0) | <0.001 | <0.001 | 5.81 (3.75, 7.87) |
| PAI-Schizophrenia | 54.1 (11.3) | 44.2 (5.9) | <0.001 | <0.001 | 9.57 (7.30, 11.83) |
| PAI-Borderline | 47.0 (11.5) | 41.8 (6.2) | <0.001 | <0.001 | 5.33 (3.01, 7.65) |
| PAI-Antisocial | 47.5 (9.5) | 44.3 (5.5) | 0.002 | 0.01 | 2.40 (0.48, 4.33) |
| PAI-Alcoholism | 46.5 (5.0) | 47.3 (6.0) | 0.4 | 0.09 | −1.70 (−3.66, 0.25) |
| PAI-Drugs | 48.7 (9.6) | 47.8 (6.6) | 0.43 | 0.52 | 0.77 (−1.61, 3.15) |
| PAI-Aggression | 48.5 (11.2) | 43.3 (6.5) | <0.001 | <0.001 | 4.79 (2.38, 7.20) |
| PAI-Suicide | 49.1 (7.5) | 46.4 (5.7) | 0.008 | 0.02 | 2.36 (0.36, 4.36) |
| PAI-Stress | 45.9 (9.2) | 43.8 (7.4) | 0.11 | 0.05 | 2.52 (−0.01, 5.06) |
| PAI-Nonsupport | 52.6 (8.9) | 45.6 (7.5) | <0.001 | <0.001 | 5.83 (3.27, 8.38) |
| PAI-Treatment rejection | 54.3 (9.5) | 57.8 (7.6) | 0.007 | 0.002 | −4.13 (−6.76, −1.51) |
| PAI-Dominance | 48.2 (10.8) | 52.7 (8.8) | 0.004 | 0.002 | −4.77 (−7.81, −1.73) |
| PAI-Warmth | 40.9 (11.4) | 53.6 (8.9) | <0.001 | <0.001 | −11.28 (−14.34, −8.22) |
| Personality (NEO-PI-R) | |||||
| Neuroticism | 52.0 (12.1) | 41.9 (8.3) | <0.001 | <0.001 | 9.90 (6.96, 12.84) |
| Extraversion | 41.4 (9.3) | 50.3 (8.6) | <0.001 | <0.001 | −8.38 (−11.23, −5.53) |
| Openness | 49.0 (11.4) | 53.1 (9.8) | 0.02 | 0.05 | −3.23 (−6.46, 0.00) |
| Agreeableness | 50.4 (10.6) | 53.6 (8.5) | 0.03 | 0.02 | −3.51 (−6.45, −0.58) |
| Conscientiousness | 52.2 (12.7) | 50.7 (8.9) | 0.36 | 0.56 | 0.93 (−2.21, 4.06) |
Behavioral measure results reflect data gathered from the visit of the patients’ first NEO-PI-R screening.
BAP = Broad Autism Phenotype; SD = standard deviation; CI = confidence interval; PAI = Personality Assessment Inventory; ARD = Anxiety Related Disorders; NEO-PI-R = Neuroticism, Extraversion, Openness Personality Inventory-Revised;
T-test;
Linear regression model adjusted for age, sex, and education;
Chi-squared test;
Logistic regression model adjusted for age, sex, and education;
Least squares mean difference between BAP+ and BAP− adjusted for age, sex, and education (BAP+ minus BAP−);
Odds ratio of BAP+ vs BAP− adjusted for age, sex, and education;
To isolate the effect of longitudinal change in objective (AVLT LTM) and subjective (MANS-self) neuropsychological measures, a piecewise linear mixed effects regression model was constructed to isolate cognitive change overtime while simultaneously accounting for baseline cognitive performance36. Briefly, the piecewise component of the model was used to assess early- and late-age effects (before and after age 60) and allow for comparison of annual change (i.e., slopes) between BAP groups. Age 60 was chosen because it was approximately equal to the median entry age. Neuropsychological scores were standardized to a normal distribution with mean 0 and standard deviation 1. Therefore, annual change results may be interpreted as units of standard deviation. The longitudinal models also adjusted for sex and education level. Estimates of the difference in annual change between BAP groups are presented with 95% confidence intervals (95%CI). Figures 1 and 2 illustrate the longitudinal trajectories divided into 5-year intervals. Given the exploratory nature of this study, p values were not corrected for multiple comparisons. Statistical modeling was executed using the PROC MIXED procedure of SAS software version 9 (SAS Institute, Inc., Cary, NC).
Figure 1.
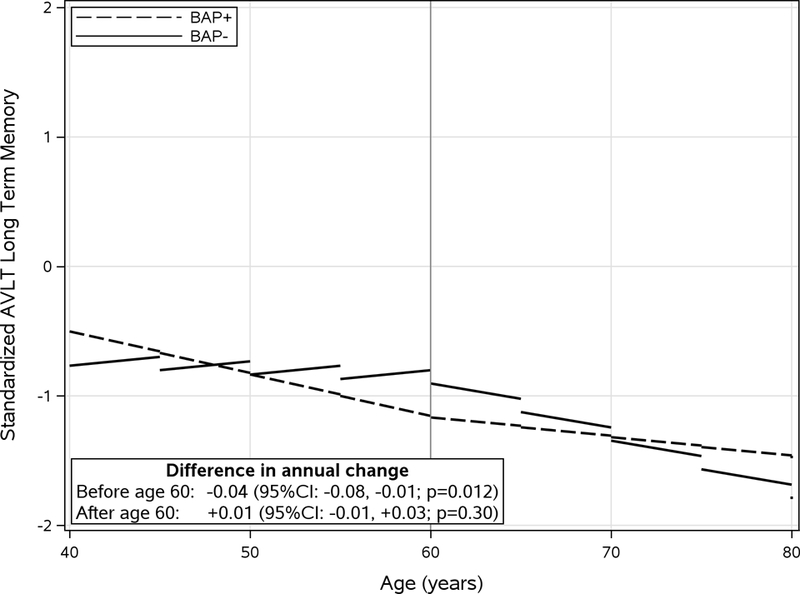
Standardized longitudinal trajectory of AVLT long term memory score by BAP group before and after age 60. Linear pricewise predicted mean scores divided into 5-year intervals illustrate longitudinal scores (annual change). Models were adjusted for sex and education level. Difference in annual change was calculated as the difference between BAP group slopes (BAP+ minus BAP−). CI: Confidence interval.
Figure 2.
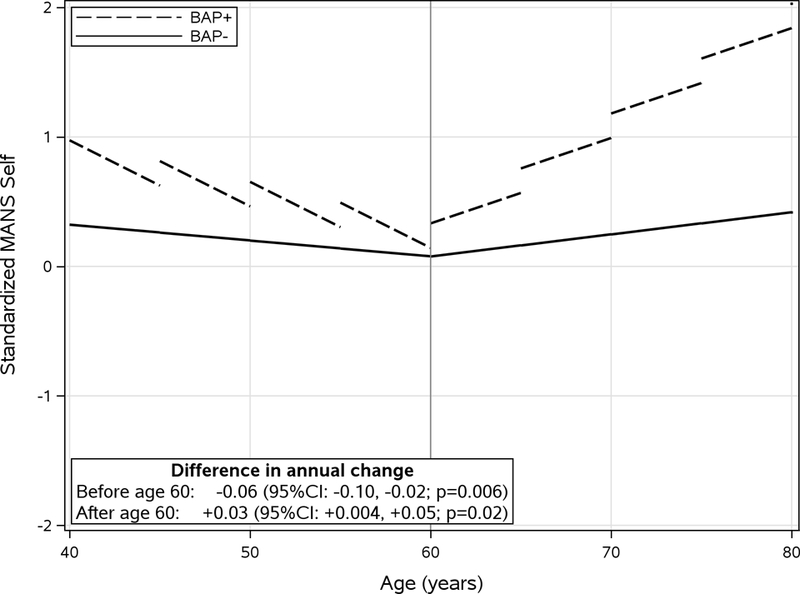
Standardized longitudinal trajectory of MANS-self score by BAP group before and after age 60. Linear pricewise predicted mean scores divided into 5-year intervals illustrate longitudinal scores (annual change). Models were adjusted for sex and education level. Difference in annual change was calculated as the difference between BAP group slopes (BAP+ minus BAP−). CI: Confidence interval.
Results
The AQ was completed by 419 participants. 45 obtained a score of 23 or higher placing them into the BAP range including 13 scoring in the MAP and 1 scoring in the NAP range (BAP+ group), and 374 scored below 23 (BAP−). Baseline demographic and MANS scores of the two groups are summarized in Table 1. At entry, there was no difference in the proportion of BAP+ individuals self-endorsing cognitive decline (57.8% v 45.3%, p=.096), but informants endorsed higher rates of decline in the BAP+ group (48.6% v 27.5%, p=0.02). BAP+ members nonetheless had higher scores on the MANS-self (29.7 SD 36.6 v 12.6 SD 24.1, p<0.0001) as well as the MANS-informant (23.1 SD 35.5 v 7.0 SD 18.6, p<0.0001). While there were no differences in age, education, racial background, or APOE e4 carrier status, there was a higher proportion of men in the BAP+ group (p<0.001). BAP+ group members were less likely to be married (62.2% v 74.3%, p=0.05) and had a higher rate of multiple divorces (24.4% v 11.3%, p=0.02), but in other aspects of social functioning including number of children, occupational status, and zip code-based estimated income stratum there were no differences between the groups. On the AQ, in addition to having higher overall scores and subscores, the distribution of responses differed. The BAP+ group endorsed trouble with social skills (22.0% v 12.2%, p<0.0001) and communication (17.1% v 11.8%, p<0.001) more often.
Baseline neuropsychological scores are summarized in Table 2. The BAP+ group did slightly but consistently less well on mental arithmetic tests (e.g., PASAT-3 42.2 SD 16.2 v 48.1 SD 12.5, p=0.01) and tests of mental speed (e.g., TMT-A seconds 28.5 SD 9.1 v 25.1 SD 9.1, p=0.04) although longitudinally the only difference between the groups was found on the WAIS-R digit symbol substitution task after age 60 on which the BAP+ group performance increased slightly less than the BAP− group (difference in annual change −0.03 [−0.05, −0.01], p=0.001). The BAP+ group also did slightly less well on a language (BNT), and memory (SRT) measure, but without consistent differences on other measures in these respective domains.
Behavioral characteristics of the BAP+ group are summarized in Table 3. Overall, the BAP+ group met the characteristic profile previously described for the BAP. Personality differences on the NEO-PI-R reflected higher Neuroticism (p<0.0001), lower Extraversion (p<0.0001), lower Openness (p=0.05), and lower Agreeableness (p=0.02). On the PAI, the BAP+ group endorsed higher levels of nearly all domains of psychopathology surveyed including, but not limited to somatization, anxiety, and depression (all p<0.01).
Longitudinal results are summarized in Figures 1 and 2. For the MANS-self, adjusting for education and gender was similar to the unadjusted results. Before age 60, the BAP+ group had a greater annual decrease in MANS than the BAP− group (−0.07 vs −0.01; diff = −0.06; 95%CI: −0.10, −0.02; p=0.006). After age 60, the BAP+ group had a greater annual rate of increase in MANS scores (0.05 vs 0.02; diff = 0.03; 95%CI: 0.004, 0.05; p=0.02). In contrast there was no difference between the groups in the longitudinal trajectory of the MANS-informant (supplementary figure). For the AVLT long term memory score, adjusting for education and gender was similar to the unadjusted results. Before age 60, the BAP+ group showed greater annual decease in AVLT score (−0.03 vs 0.01 diff = −0.04; 95%CI: −0.08, −0.01; p=0.012). However, after age 60 there was no difference in annual change between groups (−0.02 vs −0.01; diff = 0.01; 95%CI: −0.01, 0.03; p=0.30). In summary, relative to the BAP− group, the BAP+ group has more longitudinal subjective decline (MANS self) after age 60 in absence similar longitudinal objective memory decline (AVLT LTM). Over an average of 10 years of followup, 33 individuals progressed to MCI (7.9% overall) including 4 in the BAP+ group (8.9%) and 29 in the BAP− group (7.8%), p=0.77.
Discussion
There are two main findings from this study. The first is that the BAP, as identified by the AQ questionnaire, is not rare, and those individuals as a group who met the AQ criterion closely resembled descriptions generally obtained in parents of autistic children. Most prior studies have focused on much younger adults as well as children (siblings of autistic probands), but a more recent study explored phenotypic characteristics of 20 older adults (7 with a relative with an ASD) scoring above a diagnostic threshold on the self-administered Broad Autism Phenotype Questionnaire37 and found results very similar to ours. Ours is the first to describe neuropsychological profiles in an older BAP population and to examine the possible influence of the BAP longitudinally on cognitive aging. Second, individuals with the BAP as a group had increasing subjective cognitive impairment with advancing age in the absence of accelerated memory or other cognitive decline, and no difference in clinical outcomes. And even though, prior to age 60, the BAP+ group MANS-self and MANS-informant informant scores declined at a higher rate than in the BAP− group, they remained higher at all times. Previous studies of SCI have not considered the possible influence of the BAP, and it seems possible that at least some of the psychological distress associated with SCI in previous studies may be due to the inclusion of individuals with the BAP.
The individuals in our BAP+ group were not mildly autistic or autistic individuals who simply evaded clinical diagnosis, but were normally functioning adults. Regarding real life social achievements, the only differences identified between the groups were that the BAP+ group had a higher rate of multiple divorces and a modestly lower proportion of currently married members. There were no differences in years of education, occupational difficulty, estimated income, or childbearing status confirming that these are normally functioning individuals. Yet our BAP+ sample resembled previously described phenotypic characteristics of the BAP. There was a higher proportion of men, and the BAP+ group had higher Neuroticism and lower Extraversion in particular. Openness and Agreeableness were also lower. In terms of social skills, the BAP+ group’s pattern of answers on the AQ differed from the BAP− group in that the BAP+ group’s score was comprised of a higher proportion of social skill and communication difficulty. On the PAI the BAP+ group had much higher mean scores for paranoia, antisocial behavior, aggression, feelings of nonsupport, obsessive-compulsive tendencies, and much lower scores for feelings of warmth and social dominance. In terms of mood related characteristics, they endorsed much higher levels of depression and anxiety, had a higher rate of childhood sleep disorders and current bruxism which may be symptoms of chronic stress. Psychometrically, executive skills have been consistently shown to be impaired in patients with ASD38,39. This has been less consistent in individuals with the BAP18–23, although some questionnaire based studies of “real world” executive skills have shown differences from controls37,38,40. We found that while some broadly defined executive domain test scores were lower in our BAP group, these tended to be concentrated in two main areas: mental arithmetic and mental speed rather than problem solving or abstraction abilities. Regarding language, only the BNT modestly differed between the groups. Notably memory, which is the most sensitive neuropsychological indicator of preclinical AD32, did not differ between the groups on any of the tests either at entry or over time.
Several limitations of this study must be considered to keep the findings in perspective. First is the concept of BAP itself. As with many other psychiatric constructs, there is no tangible biomarker to prove its existence. However, it is part of a continuum with ASD that itself has been linked to neuroanatomical and genetic bases. Further, as a psychiatric construct it is behaviorally defined and we have shown that our BAP+ group, defined by elevated AQ scores meets the recognized BAP profile. Second, we did not have information about possible family members with ASD given that this study has been focused on cognitive aging and AD, though it is not clear how much that matters. We used the AQ to define the BAP group and were able to show its resemblance to previously described BAP features. Finally, the number of individuals progressing to MCI was small and it is possible a larger study might yield different results.
In summary, we found that as a group, individuals with the BAP have increasing levels of SCI in the absence of any observable difference in either age-related memory decline or clinical outcomes. The BAP may be a source of SCI and may account in part for the degree of psychological distress previously reported to underlie most cases of SCI.
Supplementary Material
Acknowledgments
Funding support: NIA P30AG019610, R01AG031581, and Arizona Alzheimer’s Research Consortium
References
- 1.Centers for Disease Control. Prevalence of autism spectrum disorders among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summaries 2014; 63(2): 1–22. [PubMed] [Google Scholar]
- 2.Kanner L Autistic disturbance of affective contact. Nerv Child 1943; 2: 217–250. [Google Scholar]
- 3.Kanner L, Eisenberg L. Early infantile autism, 1945–1955: “Psychiatric Research Reports.” Washington DC: American Psychiatric Association, pp 55–65 (cited in Piven J et al, Am J Med Genet [Neuropsychiatric Genetics] 1997; 74: 398–411). [DOI] [PubMed] [Google Scholar]
- 4.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin paits. J Child Psychol Psychiatry 1977; 18: 297–321. [DOI] [PubMed] [Google Scholar]
- 5.Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Utter M. A case-control family history study of autism. J Child Psychol Psychiatry 1994; 35: 877–900. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell CR, Parish-Morris J, Hsin O, Bush JC, Schultz RT. The broad autism spectrum phenotype predicts child functioning in autism spectrum disorders. J Neurodev Disord 2013; 5: 25 (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheelwright S, Auyeung B, Allison C, Baron-Cohen S. Defining the broader, medium, and narrow autism phenotype among parents using the Autism Spectrum Quotient (AQ). Mol Autism 2010; 1: 10 (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasson NJ, Lam KSL, Childress D, Parlier M, Daniels JL, Piven J. The Broad Autism Phenotype Questionnaire: prevalence and diagnostic classification. Autism Res 2013; 6(2): 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. Am J Med Genet (Neuropsychiatric Genet) 1997; 74: 398–411. [PubMed] [Google Scholar]
- 10.Baron-Cohen S, Hammer J. Parents of children with Asberger syndrome: what is the cognitive phenotype? J Cogn Neurosci 1997; 9(4): 548–554. [DOI] [PubMed] [Google Scholar]
- 11.Murphy M, Bolton PF, Pickles A, Fombonne E, Piven J, Rutter M. Personality traits of the relatives of autistic probands. Psychol Med 2000; 30: 1411–1424. [DOI] [PubMed] [Google Scholar]
- 12.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype. Am J Med Genet Part B (Neuropsychiatric Genet) 2008; 147B: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baraneck GT, Piven J. Neuropsychological profile of autism and the Broad Autism Phenotype. Arch Gen Psychiatry 2009; 66(5): 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruparelia K, Manji K, Abubakar A, Newton CR. Investigating the evidence of behavioral, cognitive, and psychiatric endophenotypes in autism: a systematic review. Autism Res Treat 2017; 10.1155/2017/6346912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin EJ. Personality correlates of the broader autism phenotype as assessed by the autism spectrum quotient (AQ). Personality Indiv Diff 2005; 38(2): 451–460. [Google Scholar]
- 16.Wakabayashi A, Baron-Cohen S, Wheelwright S. Are autistic traits an independent personality dimension? A study of the autism-spectrum quotient (AQ) and the NEO-PI-R. Personality Indiv Diff 2006; 41(5): 873–883. [Google Scholar]
- 17.Murphy M, Bolton PF, Pickles A, Fombonne E, Piven J, Rutter M. Personality traits of the relatives of autistic probands. Psychol Med 2000; 30: 1411–1424. [DOI] [PubMed] [Google Scholar]
- 18.Ozonoff S, Rogers SJ, Farnham JM, Pennington BF. Can standard measures identify subclinical markers of autism? J Autism Dev Disord 1993; 23(3): 429–441. [DOI] [PubMed] [Google Scholar]
- 19.Hughes C, Leboyer M, Bouvard M. Executive function in parents of children with autism. Psychol Med 1997; 27: 209–220. [DOI] [PubMed] [Google Scholar]
- 20.Delorme R, Gousse, Roy I, et al. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. Eur Psychiatry 2007; 22: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes C, Plumet MH, Leboyer M. Towards a cognitive phenotype for autism: inceased prevalence of executive dysfunction and superior spatial span amongst siblings of children with autism. J Child Psychol Psychiat 1999; 40(5): 705–718. [PubMed] [Google Scholar]
- 22.Wong D, Maybery M, Bishop DVM, Maley A, Hallmayer J. Profiles of executive function in parents and siblings of individuals with autism spectrum disorders. Genes Brain Behav 2006; 5: 561–576. [DOI] [PubMed] [Google Scholar]
- 23.Bolte S, Poustka F. The broader cognitive phenotype of autism in parents: how specific is the tendency for local processing and executive dysfunction? J Child Psychol Psychiatry 2006; 47(6): 639–645. [DOI] [PubMed] [Google Scholar]
- 24.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The Autism Spectrum Quotient (AQ): evidence from Aspberger Syndrome/high functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 2001; 31: 5–17. [DOI] [PubMed] [Google Scholar]
- 25.Hurley RSE, Losh M, Parlier M, Reznick JS, Piven J. The Broad Autism Phenotype Questionnaire. J Autism Dev Disord 2007; 37: 1679–1690. [DOI] [PubMed] [Google Scholar]
- 26.Caselli RJ, Chen K, Locke DE, Lee W, Roontiva A, Bandy D, Fleisher AS, Reiman EM. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement 2014. January; 10: (1)93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, Bebbington P, Jenkins R, Meltzer H. Epidemiology of Autism Spectrum Disorders in adults in the community in England. Arch Gen Psychiatry 2011; 68(5): 459–466. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Labor. Dictionary of Occupational Titles, 4th Ed., Revised Washington, DC: U.S. Department of Labor, Employment and Training Administration, 1991. [Google Scholar]
- 29.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Autism Spectrum Disorder. Diagnostic and Statistical Manual of Mental Disorders 5th Edition. American Psychiatric Association, 2013, pp.50–51. [Google Scholar]
- 32.Caselli RJ, Locke DE, Dueck AC, Knopman DS, Woodruff BK, Hoffman-Snyder C, Rademakers R, Fleisher AS, Reiman EM. The neuropsychology of normal aging and preclinical Alzheimer’s disease. Alzheimers Dement 2014. January; 10(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 34.Locke DE, Dassel KB, Hall G, Baxter LC, Woodruff BK, Hoffman Snyder C, Miller BL, Caselli RJ. Assessment of patient and caregiver experiences of dementia-related symptoms: development of the Multidimensional Assessment of Neurodegenerative Symptoms questionnaire. Dement Geriatr Cogn Disord 2009; 27(3):260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa PT Jr., & McCrae RR (1992). NEO-PI-R PI-R professional manual Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- 36.Ware JH, Dockery DW, Louis TA, et al. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiology 1990; 132:685–700. [DOI] [PubMed] [Google Scholar]
- 37.Wallace GL, Budgett J, Charlton RA. Aging and autism spectrum disorder: evidence from the Broad Autism Phenotype. Autism Res 2016; 9: 1294–1303. [DOI] [PubMed] [Google Scholar]
- 38.McLean RL, Johnson AJ, Zimak E, Joseph RM, Morrow EM. Executive function in autism probands with average intellectual ability and their unaffected first-degree relatives. J Am Acad Child Adolesc Psychiatry 2014; 53(9): 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braden BB, Smith CJ, Thompson A, et al. Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Res 2017. (in press). [DOI] [PubMed]
- 40.Wallace GL, Kenworthy L, Pugliese CE, et al. Real-world executive functions in adults with autism spectrum disorders: profiles of impairment and associations with adaptive functioning and co-morbid anxiety and depression. J Autism Dev Disord 2016; 46: 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


