Abstract
Signal Amplification By Reversible Exchange (SABRE) is a new and rapidly developing hyperpolarization technique. The recent discovery of Spin-Lock Induced Crossing SABRE (SLIC-SABRE) shows that high field hyperpolarization transfer techniques developed so far were optimized for singlet spin order that does not coincide with the experimentally produced spin state. Here, we investigate the SLIC-SABRE approach and the most advanced quantitative theoretical SABRE model. It is the goal to achieve the highest possible polarization with SLIC-SABRE at high field using the standard SABRE system, IrIMes catalyst with pyridine. We demonstrate the accuracy of SABRE model describing the effects of various physical parameters such as the amplitude and frequency of the radio-frequency field, and the effects of chemical parameters such as the exchange rate constants. The combined use of experiments and theory allows to determine the effective lifetime of SABRE-complex. Furthermore, the entropy and enthalpy of the SABRE-complex dissociation reaction based on the temperature dependence of SLIC-SABRE signal can be accessed. We show, for the first time, that this SLIC-SABRE model can be useful for the evaluation of the chemical exchange parameters that are very important for the production of highly polarized contrast agents via SABRE.
Graphical Abstract
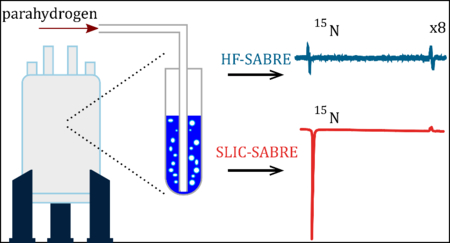
I. Introduction
Nuclear Magnetic Resonance (NMR) is a versatile tool that is extensively applied in science and medicine. Different approaches to increase the sensitivity of the method were proposed during the last decades1–10. One of these methods is Signal Amplification By Reversible Exchange4, SABRE, a ParaHydrogen Induced Polarization (PHIP) technique11,12, that has shown quite interesting developments13–24. SABRE hyperpolarization can be “spontaneously” generated at low (mT)25–28, ultra-low (<μT)21,29–32 and high (T) magnetic fields33,34. High-field SABRE offers an advantage of producing and detecting hyperpolarization at the same location, and therefore, mitigates sample transfer requirements, and offers advantages of signal averaging35 and permanent addition of pH236. For the high-field regime, several polarization transfer sequences were developed in order to make SABRE more robust and efficient: 1H radio-frequency pulse induced SABRE (1H-RF-SABRE)37, Low Irradiation Generation of High-Tesla SABRE (LIGHT-SABRE)38, a method based on the application of adiabatic RF-pulses39, SABRE-INEPT40,41, Alternating Delays Achieve Polarization Transfer SABRE (ADAPT-SABRE)22, SABRE COSY and Sepp-HoSQC35 and Spin-Lock Induced Crossing SABRE (SLIC-SABRE)42,43. SLIC was pioneered by Rosen and co-workers44. The sequence has been shown to be very efficient for transformation of nascent parahydrogen-derived singlet spin order into observable magnetization38,45–48.
High-field SABRE not only provides hyperpolarized samples without a need of external polarizers, but allows as well to use all conventional NMR methods to elucidate chemical reaction parameters under fully controlled reaction conditions. For example, even before SABRE was established, modification of INEPT sequence was employed to study the reactivity of different metal-organic complexes49.
Spin-order transfer (SOT) RF-pulse sequences are typically developed for a specific molecular system. In theory, most of these methods provide a polarization close to the absolute maximum50. However, these theoretical values are challenging to reach experimentally. Moreover, the same high-field SOT sequences often provide a much higher polarization level for systems where pH2 was added permanently51 as compared to systems undergoing the reversible exchange39. The main reason for this is that the chemical exchange between the catalyst-bound and free states of pH2 and substrate, S, is continuous, while the duration of a SOT sequence is finite40. As a result, only a fraction of all complexes are subjected to the entire SOT sequence. For some pulse sequences, e.g., PH-INEPT40,41,52, it is crucial that complex does not dissociate during the entire duration of the SOT sequence, otherwise there will be no hyperpolarization.
The substrate polarization in SABRE experiments at high and low fields strongly depends on the experimental conditions: concentration of the catalyst, substrate, pH2 flow rate, pressure and sample temperature13,21,40,53–55.
Using a simplified approach to describe the polarization transfer, a “SABRE formula” was introduced56, encompassing various chemical properties of the SABRE chemical exchange. This model attempts in a simplified manner to explain an effect of chemical exchange on the resulting SABRE polarization level.
Another, more advanced quantitative theory was introduced recently which provides the most accurate theoretical description of SABRE phenomenon to date53. This method, however, is restricted to a maximum of 6–7 spins in the SABRE-complex because of computational limitations.
In this work, we investigate the properties of SLIC-SABRE experiments42, and demonstrate a significant improvement of hyperpolarization level. As it was shown in the original work42,43, SLIC-SABRE is more tolerant with respect to the initial spin state of the system. At least half of the maximum polarization is retained even when fast S-T0 mixing occurs. This is different compared to other methods, where the entire signal can be lost (e.g., LIGHT-SABRE38). As a result, SLIC-SABRE is a method robustly applicable for a wide range of different metal complexes.
An additional advantage of SLIC-SABRE over other polarization pulse sequences was identified in this work: by fitting a model to experimental SLIC-SABRE data, the effective exchange rate (or mean lifetime) of the SABRE complex can be derived. Only during this time period, polarization transfer occurs before the SABRE complex dissociates57. This parameter is critical for achieving high polarization levels. However, as one will find in the Results and discussion section, the current state of the art quantitative SABRE model53 used in this work does not give a perfect explanation of the experimental results and further improvement of the theory (and experimental protocols) is necessary.
II. Materials and methods
Materials.
All experiments were carried out using a 0.1 M solution of 15N-pyridine (15N-Py) and 5 mM of IrIMes iridium N-heterocyclic carbene complex13 (IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazole-2-ylidene) in methanol-d4 (purchased from Carl Roth GmbH, CAS: 811-98-3). 15N-Py and IrIMes were synthesized according to earlier published procedures13,58. The initial IrIMes complex was activated by flushing pH2 through the mixture for 20 minutes, resulting in three molecules of substrate (Py) binding to the complex. The resulting solution consisted of SABRE-active complex [IrH2Imes(Py)3] and free Py, fPy, with 1 to 17 concentration ratio.
Equipment.
pH2 was prepared using commercially available setup with a conversion temperature of 38 K that provides ≈ 90 % enrichment of the para fraction (Bruker, BPHG 90). Experiments were carried out inside a 300 MHz NMR spectrometer (Bruker AVANCE). The reaction chamber for pH2 supply was described before38. pH2 is delivered in a standard 5 mm NMR tube (Wilmad) via a thin 1/16” capillary. pH2 flow rate was 7 scm3/min with 1.7 bar overpressure with respect to an ambient pressure.
Pulse sequences.
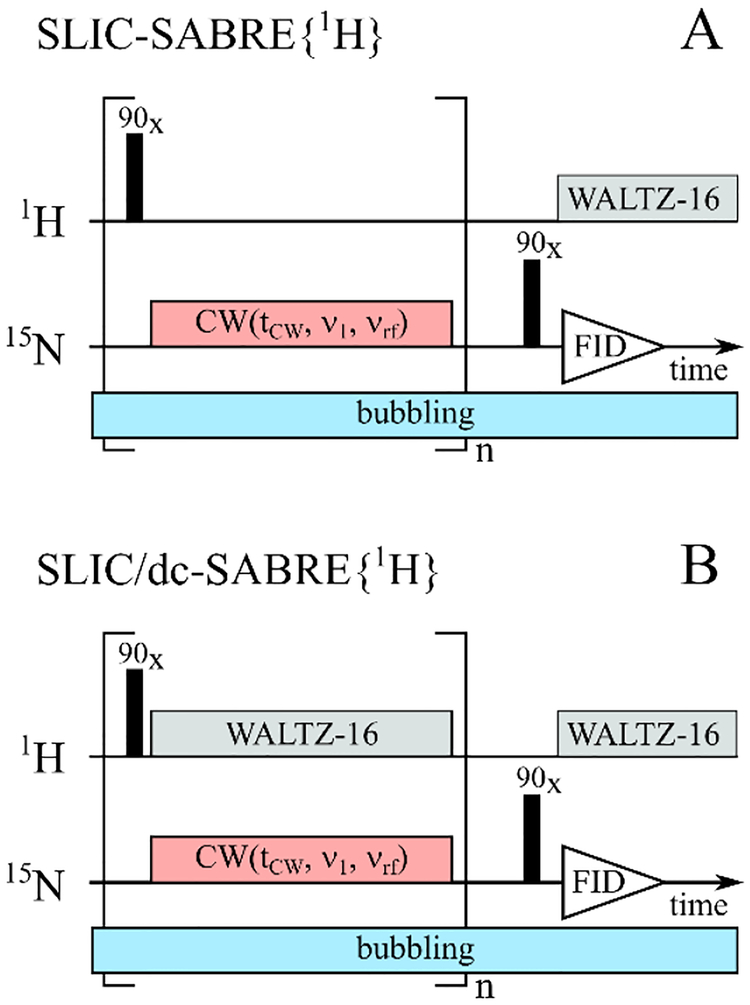
We used the previously published SLIC-SABRE pulse sequence42 with the following modifications (Figure 1): during the acquisition, a 1H WALTZ-1659 block was added to decouple the pyridine protons to increase SNR and to eliminate an anti-phase spin order. As a result, net polarization of free 15N-pyridine was observed. Hereafter, 15Nbound stands for the 15N signal of bound equatorial 15N-Py, and 15Nfree is for the 15N signal of free 15N-Py. During the experiment, pH2 was constantly supplied by bubbling. No significant distortion of the 15N resonances was observed.
Figure 1.
Schematic view of the original SLIC-SABRE{1H} (A) and modified SLIC/dc-SABRE{1H} (B) sequences, without and with 1H-decoupling during pH2 bubbling, respectively. Typical parameters of the CW field were v1 = 5 Hz, vrf ≅ 255.5 ppm with duration tCW = 1.17 s, the amplitude of WALTZ-16 decoupling block was 300 Hz and carrier frequency was set at 8.3 ppm, durations of 90o 1H and 15N RF-pulses were 26.7 μs and 21 μs, respectively. The SLIC block in brackets was repeated n = 20 times.
Also, a selective 1H WALTZ-16 decoupling block was added during the continuous wave radio frequency (CW RF) pulse (sequence SLIC/dc-SABRE) to decouple 15N from 1H nuclei of pyridine. The signal of the two hydride protons should not be affected. The decoupling of pyridine protons and 15N significantly simplified the spin system such that the quantitative SABRE theory53 was used for the simulations of the experiment.
Quantitative SABRE theory.
For a quantitative description of SABRE, the chemical reaction process has to be combined with the spin evolution. The chemical exchange reaction in SABRE can be described with the following set of equations. Association and dissociation of substrate:
| (eq1) |
Here, IrH2S represents the Ir-complex where H2 and substrate (S) are coordinated and mutual J-couplings exist. IrH2 is a transient Ir-complex. kd and ka are dissociation and association rate constants, respectively.
Exchange of hydrogen:
| (eq2) |
Loss of free hydrogen to exhaust/atmosphere:
| (eq3) |
Supply of fresh pH2 () in solution:
| (eq4) |
The simulation of this set of equations is rather complicated because they are nonlinear. To address this issue, the following simplifications were proposed in the quantitative SABRE theory53:
A1.
The reservoir of pH2 in solution is large: an unlimited amount of pH2 is always available, at a constant enrichment of 100 %.
A2.
The chemical exchange of H2 with Ir is fast: H2 in IrH2 is always pure pH2 (we will denote that complex as below).
From these simplifications, the following set of equations follows:
| (eq5) |
| (eq6) |
| (eq7) |
where the primed association and dissociation rate constants indicate that these are the constants in the simplified models. In general, they are not the same as the constants in the full model (eq 1).
When pH2 is supplied to the system for a long period of time, a “steady-state” association rate, Wa, can be introduced:
| (eq8) |
Since the ratio [IrH2S]/[S] = complex/substrate is known from the experiment and is the only variable parameter in the model. The meaning of is the effective dissociation rate constant and is the mean lifetime of IrH2S complex. In the SI we discuss how lifetime of the complex and dissociation constant rate are related as well as the effect of lifetime of the complex on polarization level.
It is expected that polarization level scales linearly with pH2 enrichment, so that polarization level for 90% pH2 differs from that for 100 % pH2 by a factor of about 0.8723. The detailed description of how to solve this set of equations was given in the original publication53. Here, we used the in-house software to calculate SABRE experiments using this model, where chemical exchange, spin Hamiltonian and relaxation superoperators are implemented and only parameters like T1-relaxation, chemical shifts, constants of J-coupling and and Wa need to be specified. A detailed description of the software will be published elsewhere.
Calculation demands.
In this model, relaxation was included using Redfield relaxation theory60, i.e., using relaxation superoperators that increase computational efforts. We can calculate SABRE complexes for a system with up to 6 spins-½. A single superoperator for a 6 spin-1/2 system is composed of 46 × 46 ≈ 16777216 ≅ 1.7 × 107 numbers. Adding two more protons would increase the size of the superoperators and hence the complexity of computation by a factor of 42 × 42 = 256; in this case, the calculation of SLIC-SABRE with one set of parameters on a PC with 4.2 GHz processor would require about 180 hours. Consequently, we did not attempt to model the experimental data obtained with SLIC-SABRE (>8 coupled spins-½); instead the SLIC/dc-SABRE data is modeled with the quantitative SABRE theory (4 coupled spins-½).
III. Results and discussion
3.1. SLIC-SABRE: theoretical analysis
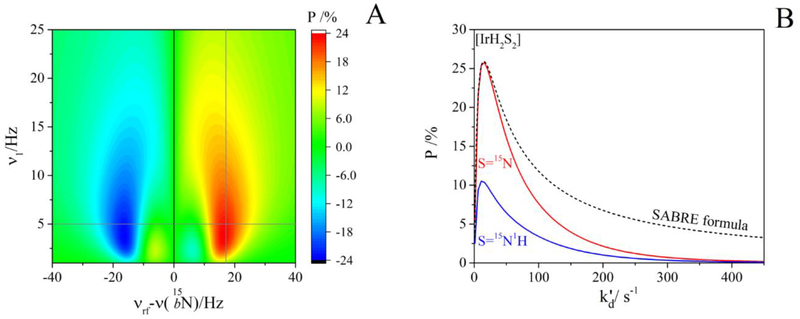
In order to determine the optimal conditions for SLIC-SABRE, we calculated the 15N-polarization of the free substrate (15Nfree) as a function of the frequency and amplitude P(vrf, v1) of the CW-pulse.
The computation was performed for a 4-spin-½ SABRE complex [IrH2S2], where S was a single 15N nucleus (Figure 2A, simulations details are provided in the caption). P(vrf, v1) was found to be antisymmetric with respect to the frequency of the resonance of a bound substrate, vrf = v(15Nbound): two polarization extrema with different signs were observed.
Figure 2.
(A) Simulated 15Nfree polarization as a function of CW RF field amplitude, v1, and frequency offset from 15Nbound resonance, vrf − v(15Nbound) for a [IrH2S2] SABRE complex, where S = 15N. Note that two distinct extrema occur, symmetric with respect to the frequency of the bound substrate, v(15Nbound). The maximum polarization for , , tCW = 1.17 s and n = 20 occurs at v1=5 Hz and vrf − v(15Nbound)=17 Hz. (B) Polarization of SLIC-SABRE as a function of for an [IrH2S2] complex with S=1H (red) and S=15N1H (blue) and the “simple analytical SABRE formula” (dashed line).
The maximum polarization ≈ 24% was found at v1=5 Hz and vrf − v(15Nbound)=17 Hz (for IrIMes, 15N-Py with decoupled protons in methanol, see J-coupling constants in Table S1).
Similar simulations were already performed in the original SLIC-SABRE work. However, it is important to note that P(vrf, v1) strongly depends on the chosen (see supplementary video for P(vrf, v1,) and Figures S4 and S6).
Next, was simulated for fixed v1=5 Hz and vrf − v(15Nbound)=17 Hz for a 4-spin-½ and 6-spin-½ SABRE complex [IrH2S2]. Here, the substrate was assumed to be either a single 15N spin or 15N with 1H (i.e., a substrate with one 15N nucleus and one ortho-1H hydrogen of Py). Both systems exhibited a similar that rises quickly to one maximum and descends slowly to zero. Some of the features are reproduced by the analytical “SABRE formula” from Ref. (56) too. The similar peak function was predicted by analytical equation for SABRE at ultra-low magnetic fields (SABRE-SHEATH)61, however it did not provide satisfactory agreement with the experiments and adaptation for high-field SLIC-SABRE experiment is necessary, therefore we will not use that method and “SABRE formula” in the work.
Although, addition of 1H to the substrate does not change the shape of , the absolute polarization is reduced by a factor of 2.5. Thus, a reduced spin system appears to be quite beneficial for an increased hyperpolarization yield. Experimentally, we realized that by applying frequency selective 1H WALTZ-16 decoupling on the Py-protons (see Figure 1, 3, 4 and discussion below). Before, attempts were made to simplify the SABRE system using e.g., substitution of 1H by 2H33,39, however, deuterons also have J-couplings with 15N and often 2H exchanges with 1H2 in the solution as a result of pH2 bubbling in the presence of an Ir-complex. Unfortunately, we were not able to simulate the system where S = (15N1H1H), i.e., with both ortho-protons of pyridine taken into account, because of our current computational power limitations.
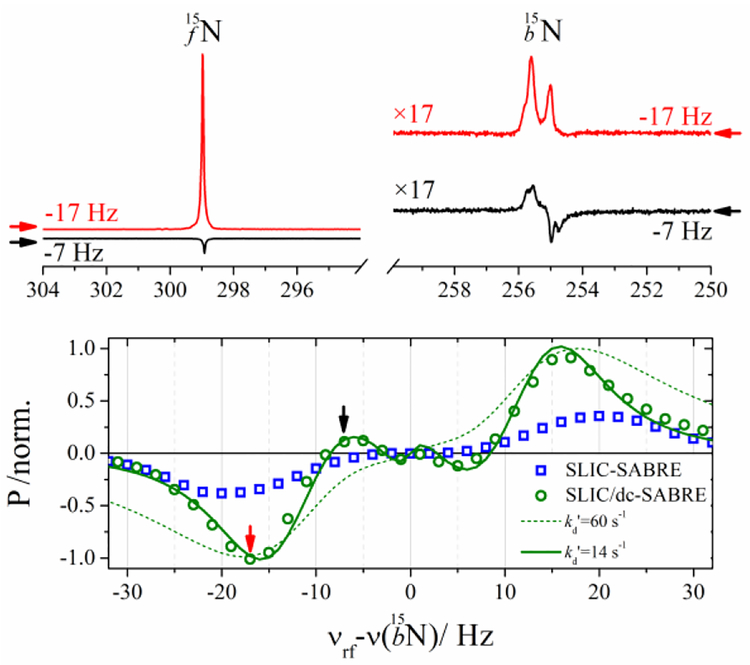
Figure 3.
(Top) High-resolution 15N NMR spectra of free (15Nfree) and bound (15Nbound) 15N-Py signals after application of SLIC/dc-SABRE{1H} with v1 = 5 Hz, n=20 and vrf − v(15Nbound)=−17 Hz (red) or −7 (black) at T = 15 oC,. (Bottom) Normalized polarization level of SLIC/dc-SABRE{1H} (green circles) and SLIC-SABRE{1H} (blue squares) as a function of vrf − v(15Nbound). Green lines are the result of quantitative SABRE model for [IrH2S2] complex with S = 15N and (solid line) or 60 s−1 (dashed line). Arrows indicate the offsets corresponding to the spectra demonstrated above.
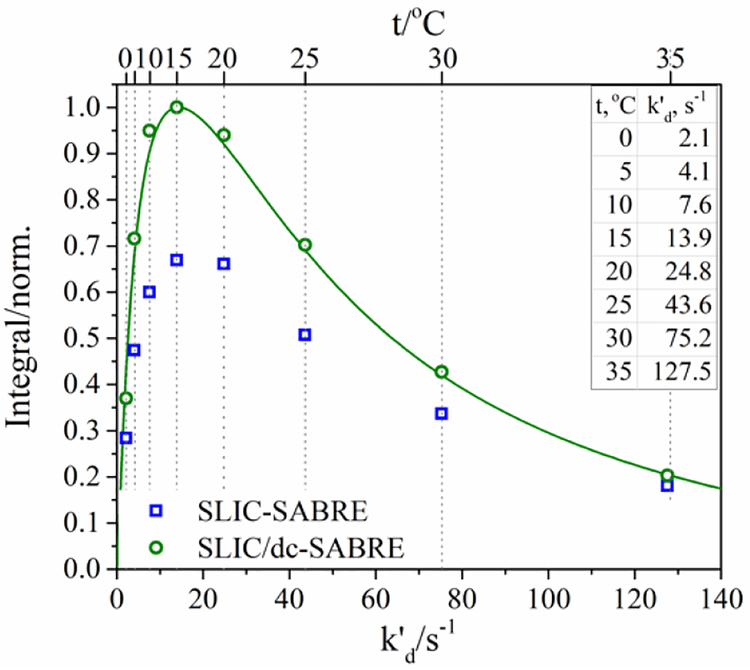
Figure 4.
Experimentally measured SLIC-SABRE{1H} (blue squares) and SLIC/dc-SABRE{1H} (green circles) signal amplitudes as a function of temperature and effective dissociation constant rate, . Corresponding quantitative SABRE theory simulations (solid lines) for a [IrH2S2] complex with S=15N. To interrelate temperature and dissociation rate, Eyring equation with entropy ΔS‡ = 52.9 J/mol·K and enthalpy ΔH‡ = 79.4 kJ/mol of activation was used.
To summarize, for an optimization of polarization yield, it is required to find the appropriate values of v1, vrf and tCW. Furthermore, the polarization is strongly dependent on (see supplementary video or Figure 6S), which, in turn, depends on the temperature, the choice of substrate, solvent and catalyst.
3.2. SLIC-SABRE RF-field matching
Using the optimal parameters deduced from simulation, v1 = 5 Hz and vrf − v(15Nbound)=17 Hz, the maximal signal was experimentally observed for a CW duration tCW = 1.17 s. After n=20 repetitions of SLIC, a constant polarization level was achieved. Holding v1 = 5 Hz and tCW constant, we varied the frequency offset of the CW RF-pulse, vrf − v(15Nbound), at T = 15 °C for both variants of the sequence (Figure 3).
For SLIC/dc-SABRE, the two main extrema at frequencies ±17 Hz and two minor extrema at ±8 Hz were observed. These were well reproduced by the simulations with . For SLIC-SABRE, the main extrema were shifted to ±20 Hz and no other extrema were observed.
This experimental data proves that the matching conditions for SLIC-SABRE and SLIC/dc-SABRE are different (because the effective spin systems are different). Note that both ortho-protons effectively contribute and enlarge the spin system in SLIC-SABRE. The polarization is 2.6 times larger in the effectively smaller spin system.
To demonstrate the effect of , the simulations were repeated with (Figure 3). This calculation fails to reproduce experimentally observed SLIC/dc-SABRE data. For more examples of simulated frequency/amplitude/ dependences we refer the reader to Figure S6 (SI) or supplementary video.
3.3. SLIC-SABRE temperature dependence
To investigate the effect of , we performed experiments at different temperatures with fixed CW RF-field amplitude v1 = 5 Hz and frequency offset, vrf − v(15Nbound)=17 Hz (SLIC/dc-SABRE) and 20 Hz (SLIC-SABRE) (see Figure 4 and Figure S4 in the SI), tCW = 1.17 s and n = 20. The experimental SLIC/dc-SABRE results were simulated using quantitative SABRE theory for a [IrH2S2] complex with S = 15N. We also think that it is interesting to mention that temperature variation was used before for optimization of low field 1H-SABRE54.
To correlate simulated with experimental P(T), we used conventional Eyring equation in the form that couples rate constant, , and temperature, T. A step-by-step procedure of fitting experimental data is given in SI (Figure S7).
The same system has been investigated before and data sets for kd (T) are available, measured with EXSY13 and SABRE-INEPT40. The corresponding entropy, ΔS‡, and enthalpy, ΔH‡, of activation are given in Table 1. Beside EXSY and SABRE-INEPT methods one can use e.g., variation of line-width with temperature to determine the average lifetime of the SABRE complex61, however, this data for IrIMes with Py are not available and is very hard to obtain, because field homogeneity is disturbed by the bubbling system and a simulation of spectrum of SABRE complex is necessary61.
Table 1.
Within the error margin the enthalpy, ΔH‡, obtained with SLIC-SABRE method coincides with the results reported in Ref. 40 (SABRE-INEPT). Both SABRE-INEPT and SLIC-SABRE data deviate from the data obtained with EXSY.
Matching the dissociation rate parameters with the previously reported data support the validity of quantitative SABRE theory for describing not only various SABRE polarization transfer methods but also chemical exchange processes responsible for the production of hyperpolarization.
3.4. SLIC-SABRE polarization level
We simulated SLIC/dc-SABRE experiment for various experimental parameters: v1, vrf, tCW and for different values of (see Figure 2, S6 and supplementary video). The high level of 15N-polarization, ~25 %, was found for the following model parameters: tCW = 1.17 s, n = 20, v1=5 Hz,|vrf −v(15Nbound)| in the range from 10 Hz to 20 Hz, , T1 relaxation times of IrH2, 15N in the complex and in the bulk equal to 1 s, 6 s and 60 s respectfully and J-coupling constants given in Table 1S. However, the maximum level of polarization experimentally achieved here with SLIC/dc-SABRE is about 0.45 %, which is far from the theoretical maximum.
We have compared the performance of SLIC-SABRE and LIGHT-SABRE reported in Refs. (38,42,43) with our results (see Table 2S in SI). In all cases, the levels of 15N polarization are moderate and fall in the range from 0.0067% to 0.545%. However, for application not only the level of polarization but the total intensity of the polarized signal is very important. To characterize total intensity, we introduce the quantity S which is a product of polarization level (in %) and the concentration of free substrate (in mM). This parameter characterizes the available NMR signal, therefore its value is a measure of efficiency of hyperpolarized agents production.
The achieved values of parameter S are in the range from 0.134 to 45. It is noteworthy that the optimized SLIC-SABRE43 and the original LIGHT-SABRE38 experiments give almost the same values (10.9 and 9.7, respectively), while with our optimization and experimental settings the value of 45 was achieved.
The data collected in Table 2S demonstrate clearly that not only the experimental settings used in various studies are different (e.g., duration of some RF pulses) but also the concentration of substrates, catalyst, pH2 pressure and pH2 flow differ as well. SABRE experimental setups are not unified and therefore it is hard to compare the results obtained in different laboratories. However, we are certain that decoupling of substrate protons will always be beneficial for direct and efficient polarization transfer to 15N nuclei in high field SABRE experiments.
At the same time, we note that here the theory is employed for a qualitative estimation of the real system, because several simplifications were used. The main simplification is neglecting the chemical exchange with H2. Theoretically, one can say that an unlimited supply of pH2 in the bulk is available, however, by SABRE-SHEATH experiment it was perfectly demonstrated that it is not the case: 15N polarization in SABRE-SHEATH experiment increases together with increase of pH2 pressure and a flow rate21. Our current theoretical approach is more descriptive of the experimental condition of significantly higher pH2 concentration and gas pressure and therefore, future modifications of the theory are necessary and will be attempted. We believe that including pH2 exchange will be the next step in understanding moderate performance of high field SABRE.
Moreover, the vast majority of modern high-field high-resolution NMR spectrometers have significant experimental and fundamental shortcomings in the context of maximizing the efficiency of SLIC-SABRE. First, and foremost, only a small fraction of the sample is encompassed by the RF coil – as a result, RF pulses often cover only a fraction of the sample. Moreover, the irradiated sample fraction is constantly changing due to continuous pH2 bubbling. Second, significant RF field gradients exist at the ends of the RF coil resulting in sub-optimal sample irradiation. Third, parahydrogen bubbling creates some susceptibility gradients, which may additionally contribute to lower than expected SLIC pulse efficiency. Last but not least, 15N chemical shift anisotropy decreases the 15N T1 at high magnetic fields62, thereby potentially limiting the maximum attainable 15N polarization due to disproportionately greater T1-associated losses compared to those at lower magnetic fields. Most of these experimental and fundamental limitations can be overcome through the use of lower field magnets (e.g. 1.5 T or lower MRI scanners), which in principle can be equipped with RF coils encompassing the entire SABRE sample with better RF homogeneity and reduced susceptibility induced gradients. Moreover, 15N T1 times are longer at lower magnetic fields than at higher magnetic fields in the context of SABRE62. We also point out that these hardware advances have already been enjoyed by conventional hydrogenative PHIP technique enabling higher polarization values36,63.
Conclusion
We have analyzed and optimized SLIC-SABRE polarization transfer technique for IrIMes SABRE catalyst with pyridine in methanol. Under optimal conditions we obtained the polarization level of 0.45% for 100 mM Py which corresponds to about 1900-fold enhancement at 7 T. When parameter S which is equal to the product of polarization and concentration is compared, the signal intensity achieved in this work is higher than in the original SLIC-SABRE and LIGHT-SABRE experiments. We have shown that quantitative SABRE model is valid for the description of SABRE experiments at high fields even though for calculations performed on a personal computer the SABRE complex can comprise only up to 6 spins ½, whereas for bigger spin systems more powerful computational facilities are required. Alternatively, one can use a 1H decoupling strategy proposed in this work. Moreover, proton decoupling during the pulse sequence not only simplifies the computation procedure but also increases the level of achievable polarization. Using quantitative SABRE model approach, we have shown that SLIC-SABRE method allows one to determine the effective lifetime of the complexes that varies from 8 ms to 476 ms with temperature variation from 35 °C down to 0 °C, and that is similar to the previously published INEPT-based analysis40. When one determines an effective lifetime of the complex, the quantitative model can be used to predict the most appropriate low or high field conditions for any SABRE polarization transfer procedure. However, for evaluation of kinetic parameters the use of conventional EXSY experiments may still be more reliable. We believe that this work is important in the context that current elaborate SABRE theory give a hint but does not quantitatively reproduce experimental results, and further improvement of the theory is necessary.
Supplementary Material
ACKNOWLEDGMENT
A.P. and J.-B.H. acknowledge support by the Emmy Noether Program of the DFG (HO 4604/2–1). Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as core facility for imaging in vivo. MOIN CC was founded by a grant of the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053). E.Y.C. thanks the following for funding: NSF CHE-1416268 and 1836308, NIH 1R21EB020323 and 1R21CA220137, DOD CDMRP W81XWH-12-1-0159/BC112431. IVK and IVS thank Federal Agency for Scientific Organizations (#0333-2017-0002) for support of the parahydrogen activation studies. KVK, AIS, NVC and LMK thank the grant from the Russian Science Foundation (17-73-20030) for the support of SABRE catalyst synthesis and its testing with pyridine.
Footnotes
ASSOCIATED CONTENT
Supporting Information
This information is available free of charge via the Internet at http://pubs.acs.org.
Analysis of the average lifetime of SABRE complex, simple SABRE formula, RF-field frequency/amplitude/temperature/ dependences (.avi) and step-by-step description of dissociation rate constant fitting (.PDF).
REFERENCES
- (1).Kaptein R Chemically Induced Dynamic Nuclear Polarization in Five Alkyl Radicals. Chem. Phys. Lett 1968, 2 (4), 261–267. [Google Scholar]
- (2).Eisenschmid TC; Kirss RU; Deutsch PP; Hommeltoft SI; Eisenberg R; Bargon J; Lawler RG; Balch AL Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc 1987, 109 (26), 8089–8091. [Google Scholar]
- (3).Maly T; Debelouchina GT; Bajaj VS; Hu K-N; Joo C-G; Mak–Jurkauskas ML; Sirigiri JR; van der Wel PCA; Herzfeld J; Temkin RJ; et al. Dynamic Nuclear Polarization at High Magnetic Fields. J. Chem. Phys 2008, 128 (5), 052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Adams RW; Aguilar JA; Atkinson KD; Cowley MJ; Elliott PIP; Duckett SB; Green GGR; Khazal IG; López-Serrano J; Williamson DC Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323 (5922), 1708–1711. [DOI] [PubMed] [Google Scholar]
- (5).Griesinger C; Bennati M; Vieth HM; Luchinat C; Parigi G; Höfer P; Engelke F; Glaser SJ; Denysenkov V; Prisner TF Dynamic Nuclear Polarization at High Magnetic Fields in Liquids. Prog. Nucl. Magn. Reson. Spectrosc 2012, 64, 4–28. [DOI] [PubMed] [Google Scholar]
- (6).Hirsch ML; Kalechofsky N; Belzer A; Rosay M; Kempf JG Brute-Force Hyperpolarization for NMR and MRI. J. Am. Chem. Soc 2015, 137 (26), 8428–8434. [DOI] [PubMed] [Google Scholar]
- (7).Khurana D; Mahesh TS Bang-Bang Optimal Control of Large Spin Systems: Enhancement of 13C–13C Singlet-Order at Natural Abundance. J. Magn. Reson 2017, 284, 8–14. [DOI] [PubMed] [Google Scholar]
- (8).Buckenmaier K; Rudolph M; Back C; Misztal T; Bommerich U; Fehling P; Koelle D; Kleiner R; Mayer HA; Scheffler K; et al. SQUID-Based Detection of Ultra-Low-Field Multinuclear NMR of Substances Hyperpolarized Using Signal Amplification by Reversible Exchange. Sci. Rep 2017, 7 (1), 13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nelson SJ; Kurhanewicz J; Vigneron DB; Larson PEZ; Harzstark AL; Ferrone M; van Criekinge M; Chang JW; Bok R; Park I; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med 2013, 5 (198), 198ra108–198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cunningham CH; Lau JY; Chen AP; Geraghty BJ; Perks WJ; Roifman I; Wright GA; Connelly KA Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res 2016, CIRCRESAHA.116.309769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bowers CR; Weitekamp DP Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett 1986, 57 (21), 2645–2648. [DOI] [PubMed] [Google Scholar]
- (12).Bowers CR; Weitekamp DP Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc 1987, 109 (18), 5541–5542. [Google Scholar]
- (13).Cowley MJ; Adams RW; Atkinson KD; Cockett MCR; Duckett SB; Green GGR; Lohman JAB; Kerssebaum R; Kilgour D; Mewis RE Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from Para-Hydrogen. J. Am. Chem. Soc 2011, 133 (16), 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Glöggler S; Colell J; Appelt S Para-Hydrogen Perspectives in Hyperpolarized NMR. J. Magn. Reson 2013, 235, 130–142. [DOI] [PubMed] [Google Scholar]
- (15).Franzoni MB; Buljubasich L; Spiess HW; Münnemann K Long-Lived 1H Singlet Spin States Originating from Para-Hydrogen in Cs-Symmetric Molecules Stored for Minutes in High Magnetic Fields. J. Am. Chem. Soc 2012, 134 (25), 10393–10396. [DOI] [PubMed] [Google Scholar]
- (16).Pravdivtsev AN; Yurkovskaya AV; Vieth H-M; Ivanov KL; Kaptein R Level Anti-Crossings Are a Key Factor for Understanding Para-Hydrogen-Induced Hyperpolarization in SABRE Experiments. ChemPhysChem 2013, 14 (14), 3327–3331. [DOI] [PubMed] [Google Scholar]
- (17).Eshuis N; van Weerdenburg BJA; Feiters MC; Rutjes FPJT; Wijmenga SS; Tessari M Quantitative Trace Analysis of Complex Mixtures Using SABRE Hyperpolarization. Angew. Chem. Int. Ed 2015, 54 (5), 1481–1484. [DOI] [PubMed] [Google Scholar]
- (18).Theis T; Ortiz GX; Logan AWJ; Claytor KE; Feng Y; Huhn WP; Blum V; Malcolmson SJ; Chekmenev EY; Wang Q; et al. Direct and Cost-Efficient Hyperpolarization of Long-Lived Nuclear Spin States on Universal 15N2-Diazirine Molecular Tags. Sci. Adv 2016, 2 (3), e1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kovtunov KV; Kovtunova LM; Gemeinhardt ME; Bukhtiyarov AV; Gesiorski J; Bukhtiyarov VI; Chekmenev EY; Koptyug IV; Goodson BM Heterogeneous Microtesla SABRE Enhancement of 15N NMR Signals. Angew. Chem. Int. Ed 2017, 56 (35), 10433–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Schmidt AB; Berner S; Schimpf W; Müller C; Lickert T; Schwaderlapp N; Knecht S; Skinner JG; Dost A; Rovedo P; et al. Liquid-State Carbon-13 Hyperpolarization Generated in an MRI System for Fast Imaging. Nat. Commun 2017, 8, ncomms14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Truong ML; Theis T; Coffey AM; Shchepin RV; Waddell KW; Shi F; Goodson BM; Warren WS; Chekmenev EY 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119 (16), 8786–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Roy SS; Stevanato G; Rayner PJ; Duckett SB Direct Enhancement of Nitrogen-15 Targets at High-Field by Fast ADAPT-SABRE. J. Magn. Reson 2017, 285, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hovener J-B; Pravdivtsev AN; Kidd B; Bowers CR; Glöggler S; Kovtunov KV; Plaumann M; Katz-Brull R; Buckenmaier K; Jerschow A; et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed 2018, 57, 11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kovtunov KV; Pokochueva E; Salnikov O; Cousin S; Kurzbach D; Vuichoud B; Jannin S; Chekmenev E; Goodson B; Barskiy D; et al. Hyperpolarized NMR: D-DNP, PHIP, and SABRE. Chem. Asian J 2018, 13, 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dücker EB; Kuhn LT; Münnemann K; Griesinger C Similarity of SABRE Field Dependence in Chemically Different Substrates. J. Magn. Reson 2012, 214, 159–165. [DOI] [PubMed] [Google Scholar]
- (26).Hövener J-B; Schwaderlapp N; Lickert T; Duckett SB; Mewis RE; Highton LAR; Kenny SM; Green GGR; Leibfritz D; Korvink JG; et al. A Hyperpolarized Equilibrium for Magnetic Resonance. Nat. Commun 2013, 4, ncomms3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pravdivtsev AN; Ivanov KL; Yurkovskaya AV; Petrov PA; Limbach H-H; Kaptein R; Vieth H-M Spin Polarization Transfer Mechanisms of SABRE: A Magnetic Field Dependent Study. J. Magn. Reson 2015, 261, 73–82. [DOI] [PubMed] [Google Scholar]
- (28).Hövener J-B; Schwaderlapp N; Borowiak R; Lickert T; Duckett SB; Mewis RE; Adams RW; Burns MJ; Highton LAR; Green GGR; et al. Toward Biocompatible Nuclear Hyperpolarization Using Signal Amplification by Reversible Exchange: Quantitative in Situ Spectroscopy and High-Field Imaging. Anal. Chem 2014, 86 (3), 1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Barskiy DA; Shchepin RV; Coffey AM; Theis T; Warren WS; Goodson BM; Chekmenev EY Over 20% 15N Hyperpolarization in Under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J. Am. Chem. Soc 2016, 138 (26), 8080–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Shchepin RV; Goodson BM; Theis T; Warren WS; Chekmenev EY Toward Hyperpolarized 19F Molecular Imaging via Reversible Exchange with Parahydrogen. ChemPhysChem 2017, 18 (15), 1961–1965. [DOI] [PubMed] [Google Scholar]
- (31).Barskiy DA; Shchepin RV; Tanner CPN; Colell JFP; Goodson BM; Theis T; Warren WS; Chekmenev EY The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem 2017, 18 (12), 1493–1498. [DOI] [PubMed] [Google Scholar]
- (32).Kiryutin AS; Yurkovskaya AV; Zimmermann H; Vieth H-M; Ivanov KL Complete Magnetic Field Dependence of SABRE-Derived Polarization. Magn. Reson. Chem 2017, 56, 651–662. [DOI] [PubMed] [Google Scholar]
- (33).Barskiy DA; Kovtunov KV; Koptyug IV; He P; Groome KA; Best QA; Shi F; Goodson BM; Shchepin RV; Coffey AM; et al. The Feasibility of Formation and Kinetics of NMR Signal Amplification by Reversible Exchange (SABRE) at High Magnetic Field (9.4 T). J. Am. Chem. Soc 2014, 136 (9), 3322–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pravdivtsev AN; Yurkovskaya AV; Petrov PA; Vieth H-M; Ivanov KL Analysis of the SABRE (Signal Amplification by Reversible Exchange) Effect at High Magnetic Fields. Appl. Magn. Reson 2016, 47 (7), 711–725. [Google Scholar]
- (35).Hermkens NKJ; Aspers RLEG; Feiters MC; Rutjes FPJT; Tessari M Trace Analysis in Water-Alcohol Mixtures by Continuous p-H2 Hyperpolarization at High Magnetic Field. Magn. Reson. Chem 2018, 56, 633–640. [DOI] [PubMed] [Google Scholar]
- (36).Schmidt AB; Berner S; Braig M; Zimmermann M; Hennig J; von Elverfeldt D; Hövener J-B In Vivo 13C-MRI Using SAMBADENA. PLOS ONE 2018, 13 (7), e0200141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pravdivtsev AN; Yurkovskaya AV; Vieth H-M; Ivanov KL RF-SABRE: A Way to Continuous Spin Hyperpolarization at High Magnetic Fields. J. Phys. Chem. B 2015, 119 (43), 13619–13629. [DOI] [PubMed] [Google Scholar]
- (38).Theis T; Truong M; Coffey AM; Chekmenev EY; Warren WS LIGHT-SABRE Enables Efficient in-Magnet Catalytic Hyperpolarization. J. Magn. Reson 2014, 248, 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Pravdivtsev AN; Yurkovskaya AV; Zimmermann H; Vieth H-M; Ivanov KL Transfer of SABRE-Derived Hyperpolarization to Spin-1/2 Heteronuclei. RSC Adv 2015, 5 (78), 63615–63623. [Google Scholar]
- (40).Pravdivtsev AN; Yurkovskaya AV; Zimmermann H; Vieth H-M; Ivanov KL Enhancing NMR of Insensitive Nuclei by Transfer of SABRE Spin Hyperpolarization. Chem. Phys. Lett 2016, 661, 77–82. [Google Scholar]
- (41).Knecht S; Kiryutin AS; Yurkovskaya AV; Ivanov KL Re-Polarization of Nuclear Spins Using Selective SABRE-INEPT. J. Magn. Reson 2018, 287, 10–14. [DOI] [PubMed] [Google Scholar]
- (42).Knecht S; Kiryutin AS; Yurkovskaya AV; Ivanov KL Efficient Conversion of Anti-Phase Spin Order of Protons into 15N Magnetization Using SLIC-SABRE. arXiv 2018. [Google Scholar]
- (43).Knecht S; Kiryutin AS; Yurkovskaya AV; Ivanov KL Efficient Conversion of Anti-Phase Spin Order of Protons into 15N Magnetisation Using SLIC-SABRE. Mol. Phys 2018, (DOI: 10.1080/00268976.2018.1515999). [Google Scholar]
- (44).DeVience SJ; Walsworth RL; Rosen MS Preparation of Nuclear Spin Singlet States Using Spin-Lock Induced Crossing. Phys. Rev. Lett 2013, 111 (17), 173002. [DOI] [PubMed] [Google Scholar]
- (45).Kovtunov KV; Truong ML; Barskiy DA; Koptyug IV; Coffey AM; Waddell KW; Chekmenev EY Long-Lived Spin States for Low-Field Hyperpolarized Gas MRI. Chem. Eur. J 2014, 20 (45), 14629–14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kovtunov KV; Truong ML; Barskiy DA; Salnikov OG; Bukhtiyarov VI; Coffey AM; Waddell KW; Koptyug IV; Chekmenev EY Propane-D6 Heterogeneously Hyperpolarized by Parahydrogen. J. Phys. Chem. C 2014, 118 (48), 28234–28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Barskiy DA; Salnikov OG; Shchepin RV; Feldman MA; Coffey AM; Kovtunov KV; Koptyug IV; Chekmenev EY NMR SLIC Sensing of Hydrogenation Reactions Using Parahydrogen in Low Magnetic Fields. J. Phys. Chem. C 2016, 120 (51), 29098–29106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Barskiy DA; Salnikov OG; Romanov AS; Feldman MA; Coffey AM; Kovtunov KV; Koptyug IV; Chekmenev EY NMR Spin-Lock Induced Crossing (SLIC) Dispersion and Long-Lived Spin States of Gaseous Propane at Low Magnetic Field (0.05T). J. Magn. Reson 2017, 276, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Atkinson KD; Cowley MJ; Duckett SB; Elliott PIP; Green GGR; López-Serrano J; Khazal IG; Whitwood AC Para-Hydrogen Induced Polarization without Incorporation of Para-Hydrogen into the Analyte. Inorg. Chem 2009, 48 (2), 663–670. [DOI] [PubMed] [Google Scholar]
- (50).Goldman M; Jóhannesson H Conversion of a Proton Pair Para Order into 13C Polarization by Rf Irradiation, for Use in MRI. C. R. Phys 2005, 6 (4), 575–581. [Google Scholar]
- (51).Pravdivtsev AN; Yurkovskaya AV; Lukzen NN; Ivanov KL; Vieth H-M Highly Efficient Polarization of Spin-1/2 Insensitive NMR Nuclei by Adiabatic Passage through Level Anticrossings. J. Phys. Chem. Lett 2014, 5 (19), 3421–3426. [DOI] [PubMed] [Google Scholar]
- (52).Bär S; Lange T; Leibfritz D; Hennig J; von Elverfeldt D; Hövener J-B On the Spin Order Transfer from Parahydrogen to Another Nucleus. J. Magn. Reson 2012, 225, 25–35. [DOI] [PubMed] [Google Scholar]
- (53).Knecht S; Pravdivtsev AN; Hövener J-B; Yurkovskaya AV; Ivanov KL Quantitative Description of the SABRE Process: Rigorous Consideration of Spin Dynamics and Chemical Exchange. RSC Adv 2016, 6 (29), 24470–24477. [Google Scholar]
- (54).Zeng H; Xu J; Gillen J; McMahon MT; Artemov D; Tyburn J-M; Lohman JAB; Mewis RE; Atkinson KD; Green GGR; et al. Optimization of SABRE for Polarization of the Tuberculosis Drugs Pyrazinamide and Isoniazid. J. Magn. Reson 2013, 237, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Appleby KM; Mewis RE; Olaru AM; Green GGR; Fairlamb IJS; Duckett SB Investigating Pyridazine and Phthalazine Exchange in a Series of Iridium Complexes in Order to Define Their Role in the Catalytic Transfer of Magnetisation from Para-Hydrogen. Chem. Sci 2015, 6 (7), 3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).A. Barskiy D; N. Pravdivtsev A; L. Ivanov K; V. Kovtunov K; V. Koptyug I A Simple Analytical Model for Signal Amplification by Reversible Exchange (SABRE) Process. Phys. Chem. Chem. Phys 2016, 18 (1), 89–93. [DOI] [PubMed] [Google Scholar]
- (57).Hövener J-B; Knecht S; Schwaderlapp N; Hennig J; von Elverfeldt D. Continuous Re-Hyperpolarization of Nuclear Spins Using Parahydrogen: Theory and Experiment. ChemPhysChem 2014, 15 (12), 2451–2457. [DOI] [PubMed] [Google Scholar]
- (58).Whaley TW; Ott DG Syntheses with Stable Isotopes: Pyridine-15N. J. Label. Compd 10 (2), 283–286. [Google Scholar]
- (59).Shaka AJ; Keeler J; Frenkiel T; Freeman R An Improved Sequence for Broadband Decoupling: WALTZ-16. Journal of Magnetic Resonance (1969) 1983, 52 (2), 335–338. [Google Scholar]
- (60).Kowalewski J; Mäler L Nuclear Spin Relaxation in Liquids: Theory, Experiments, and Applications; Chemical Physics; Taylor&Francis, 2006. [Google Scholar]
- (61).Colell JFP; Logan AWJ; Zhou Z; Shchepin RV; Barskiy DA; Ortiz GX; Wang Q; Malcolmson SJ; Chekmenev EY; Warren WS; et al. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J. Phys. Chem. C 2017, 121 (12), 6626–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Shchepin RV; Jaigirdar L; Chekmenev EY Spin–Lattice Relaxation of Hyperpolarized Metronidazole in Signal Amplification by Reversible Exchange in Micro-Tesla Fields. J. Phys. Chem. C 2018, 122 (9), 4984–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Waddell KW; Coffey AM; Chekmenev EY In Situ Detection of PHIP at 48 MT: Demonstration Using a Centrally Controlled Polarizer. J. Am. Chem. Soc 2011, 133 (1), 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.