Abstract
Cardiovascular disease (CVD) due to atherosclerosis is a disease of chronic inflammation at both the systemic and the tissue level. CD44 has previously been implicated in atherosclerosis in both humans and mice. This multi-faceted receptor plays a critical part in the inflammatory response during the onset of CVD, though little is known of CD44’s role during the latter stages of the disease. This review focuses on the role of CD44-dependent HA-dependent effects on inflammatory cells in several key processes, from disease initiation throughout the progression of atherosclerosis. Understanding how CD44 and HA regulate inflammation in atherogenesis is key in determining the utility of the CD44-HA axis as a therapeutic target to halt disease and potentially promote disease regression.
Keywords: CD44, hyaluronic acid, atherosclerosis, inflammation
Introduction
CVD due to atherosclerosis is the number one cause of death worldwide [1]. Lesion formation is triggered by endothelial dysfunction and subendothelial accumulation of oxidized low-density lipoprotein (oxLDL), which promotes the adhesion and extravasation of leukocytes and a sustained inflammatory response. Leukocytes may consume oxLDL to become foam cells, a cell type found in early lesions (fatty streaks) through the later stages of disease. High inflammatory cell content in atherosclerotic lesion correlates with an increase in susceptibility to rupture, which triggers thrombosis and myocardial infarction, whereas a paucity of inflammation combined with the - generation of lesional fibrotic caps is associated with lesion stabilization. It therefore follows that inflammation is a key process that should be targeted to halt the progression of atherosclerosis and prevent acute catastrophic events associated with this chronic disease [2]. A great body of evidence suggests that local tissue inflammation drives atherogenesis, however the molecular mechanisms that govern this are only partly understood [3]. It is crucial to gain a thorough understanding of the mechanisms that govern atherogenesis to support the rational design of novel therapies to reduce risk for a myocardial event [2]. It is important to consider signaling pathways that may be targeted to modulate inflammation in atherosclerosis, including but not limited to pathways involving NFκB, PPARγ, endothelial nitric oxide synthase (eNOS), and iNOS ; all of which are discussed in more detail later. These important signaling pathways may modulate the inflammatory milieu of lesion, and have previously been targeted to halt the progression of atherosclerosis [4].
While these are well-established pathways involved in the progression of atherosclerosis, there is continued interest in the discovery of novel therapeutic targets to combat CVD. In recent years CD44 has emerged as a potential therapeutic target that may provide insights into local inflammation at the site of lesion. CD44 is a type 1 transmembrane receptor and its standard form is widely expressed on multiple cell types [5]. Under inflammatory conditions, CD44 is upregulated and functionally activated on vascular endothelial, smooth muscle and inflammatory cells [5]. CD44 modulates leukocyte adhesion, migration, and functional phenotype. It is a multi-faceted receptor, which exists in multiple activation states, variant isoforms, as well as intracellular and soluble forms [6]. CD44 is regulated with respect to the affinity for its primary ECM ligand, hyaluronan (HA). This regulation occurs via multiple mechanisms (alternative splicing of variant exons, post-translational modifications including glycosylation, sulfation, phosphorylation and clustering) all of which can modulate CD44’s capacity to bind HA [7,8].
HA is a polymeric non-sulfated glycosaminoglycan comprised of repeating units of n-acetyl-glucosamine and d-glucuronic acid. HA is a key biophysical component of extracellular matrices due to its hydrating viscoelastic physical properties. Furthermore, HA exists over a broad range of different molecular weights (MW) that have contrasting effects on cell behavior. Generally speaking, low molecular weight HA (LMW HA) tends to be pro-inflammatory, whereas high molecular weight HA (HMW HA) tends to have anti-inflammatory properties [9]. Control of HA MW occurs at both its anabolic and catabolic stages of metabolism. Significant research has gone into understanding the metabolic, biophysical, and signaling roles that HA exerts during homeostasis [10–13]. HA synthases (HAS 1-3) are responsible for de novo synthesis of HA of different MW. These synthases have been implicated in a variety of inflammatory conditions including rheumatoid arthritis, asthma, atherosclerosis and murine neointimal hyperplasia [14,15]. HA is degraded by multiple different processes; enzymatic digestion by hyaluronidases (HYALs) or by non-enzymatic oxidative processes in response to reactive oxygen or nitrogen species (ROS or RNS) that generate a pool of HA fragments of various size. Interestingly, increased HYAL activity is often a hallmark of pro-inflammatory conditions [9].
Both CD44 and HA play critical roles in atherogenesis. CD44 has been implicated in both human and in murine models of atherosclerosis. Gene co-expression analysis has revealed that CD44 is a critical mediator of murine atherosclerosis susceptibility [16]. It is expressed on all key cells that contribute to the progression of disease and is both upregulated and functionally activated in atheroprone regions of the vasculature [17–20]. Furthermore, CD44 alters gene expression in these areas prior to lesion development and even in the absence of any difference in cholesterol levels. This suggests that CD44 exerts its effects in response to the local inflammatory environment, not at the systemic level [20]. Since vascular CD44 is upregulated in response to both altered shear stress and hyperlipidemia (two key risk factors for CVD) [21,22] it implies that CD44 might be an early indicator of risk for the development of atherosclerosis. Such findings led to a focus on a potential role for CD44 in the initiation of CVD and paved the way for studies examining the role of CD44 in lesion burden in vivo. Global CD44 deletion resulted in a significant decrease in lesion burden compared to wild-type control mice on an atherogenic background (apoE−/− mice) [18]. In order to delve into the cell types involved and the mechanism by which CD44 controls atherogenesis, bone marrow chimeras were generated using WT and CD44-null donor bone marrow transplantation into apoE−/− and apoE−/− CD44−/− mice. CD44 expression in both the vascular and the bone marrow-derived compartments contributed to lesion development in the apoE−/− mouse model, implying that CD44 on both resident as well as recruited cells may be required for CD44 to exert its full pro-atherogenic affect in vivo [17]. Furthermore, CD44 deletion also promoted an increase in fibrotic content in apoE−/−CD44−/− compared to apoE−/− mice demonstrating that CD44 also regulates the composition of lesion [23].
Interestingly, global CD44 deletion on an alternative atherogenic LDLR−/− background did not result in decreased lesion burden compared to wild-type littermate controls [24]. This apparent discrepancy between the two studies may be due to the difference in diets used in the two models. Specifically, development of atherosclerosis in LDLR−/− requires a high fat diet (HFD), whereas disease develops spontaneously in apoE−/− mice even if maintained on a regular chow diet, as was used in the studies in the model described above. Although both these mouse models develop hypercholesterolemia, cholesterol levels are remarkably higher in HFD-fed LDLR−/− mice than chow-fed apoE−/− mice and plasma lipid profiles are also distinct between the two models [25,26]. In support of the concept that differences in diet and lipid profiles might underlie the differences in CD44 deletion between these two models, maintenance of apoE−/− mice on a HFD overwhelmed the impact of CD44 deletion on lesion burden (Zhao and Puré, unpublished data). This implies that the overwhelming inflammatory environment associated with a HFD can overcome any differences between CD44 wild-type and CD44-null mice regardless of model.
HA is a modulator of atherosclerotic lesion stability. It is highly upregulated at atheroprone regions in mouse models of atherosclerosis and may regulate smooth muscle cell migration, proliferation, leukocyte activation and lipid accumulation within the plaque to alter atherosclerotic plaque stability [17,20,27]. In human atheroma HA is present in both early and late lesion and an increase in HA accumulation correlates with lesion progression [28,29]. In addition, increased hyaluronic acid metabolism correlates with increased plaque destabilization in human atheroma [30]. Furthermore, both HA and its receptor CD44 accumulate at sites of plaque erosion in human ruptured lesions [31,32]. Since LMW HA and HMW HA often exert contrasting effects on diverse cell types in lesion, it is difficult to determine the net result of total HA content on lesion progression and therefore represents an important area for future study. There is also evidence that both HASs and HYALs may contribute to the progression of atherosclerosis. There is an increase in HYAL1 expression in complex human lesions compared to fibrous plaque, and one report observed that overexpression of HAS2 in smooth muscle cells promotes the development of atherosclerosis, potentially by altering vascular stiffness [33,34]. Moreover, it has been suggested that plasma HYAL activity may serve as a biomarker for patients with atherosclerosis [35].
There is a body of evidence showing crucial roles for both CD44 and/or HA in CVD [16–18,20,24,36–47]. Several studies have separately examined the role of CD44 and HA on inflammation in other pathological settings [48–71]. However, the specific roles of the CD44-HA axis on inflammation in atherosclerosis have not yet been comprehensively elucidated. CD44 exerts a prominent role in the onset of CVD, regulates lesion composition and is itself regulated by the inflammatory environment. In addition, CD44 on multiple-interacting cells may be required to exert a pro-atherogenic effect. Generally, previous work has focused on the role of CD44 as an adhesion receptor during the initiation of disease. Interestingly, the role of CD44 in the processes that govern mid- and advanced-stage disease has been under-explored. Particularly intriguing is the potential role that CD44 may regulate the balance between inflammation and fibrosis in plaque composition, which ultimately dictates risk for a myocardial event. Herein, we examine the potential impact of CD44 throughout the progression of disease, which may provide insights into the mechanisms by which CD44 affects atherogenesis and whether targeting the CD44-HA axis may be utilized to achieve disease regression.
Initiation of Disease Endothelial Dysfunction
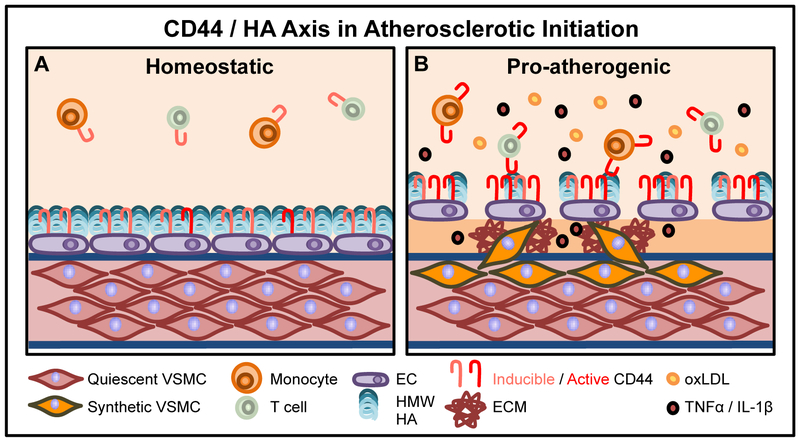
Local tissue inflammation promotes the initiation and progression of disease, which can lead to lesion rupture and myocardial infarction [3]. Under homeostatic conditions, laminar flow and low LDL levels maintain the HA-rich glycocalyx, a protective carbohydrate network that prevents leukocyte adhesion to the endothelium (Figure 1A). However under atherogenic conditions (Figure 1B), hyperlipidemia and disturbed blood flow at curved areas and branched points in the vasculature together promote increased endothelial permeability and activation to promote leukocyte adhesion [2]. oxLDL may compromise glycocalyx integrity by increasing leukocyte adhesion, endothelial permeability, and pro-inflammatory gene expression, all of which support the progression of disease. oxLDL may also promote leukocyte adhesion by upregulating key adhesion molecules via LOX-1-CD40 dependent mechanisms [72,73] or increase the permeability of the endothelial cell layer via LDLR and caveolin 1-dependent pathways [74]. oxLDL may also directly degrade the glycocalyx since it has been shown that administration of oxLDL reduces the thickness of the EC layer and promotes leukocyte adhesion. This phenotype is reversed in response to superoxide dismutase (SOD), implying that the damage to the EC layer may be dependent on the presence of reactive oxygen species [75]. Since the glycocalyx also directly modulates the inflammatory micro-environment by docking with key enzymes including protective SOD (which quenches oxygen radicals and promotes the availability of protective NO), it follows that NO is one of the key factors that maintains and prevents destruction of the glycocalyx [76]. In contrast, several factors lead to alterations in glycocalyx integrity, including low shear stress, oxidized LDL, pro-inflammatory mediators (IL1-β, TNFα, LPS), matrix metalloproteinases and elastase [76].
Figure 1. The CD44 / HA axis in the initiation of atherosclerosis.
1A: During homeostasis, an intact, HMW HA-rich EC glycocalyx prevents adhesion of circulating leukocytes (monocytes and T cells). CD44 is inducible, but inactive. VSMCs are quiescent and contractile. 1B: Under pro-atherogenic conditions, high circulating levels of oxLDL promote EC activation and increased endothelial permeability by degrading the protective glycocalyx. EC adhesion molecule expression, including CD44, is increased, supporting adhesion of blood-borne leukocytes. Incoming active leukocyte CD44 bridges with its ligand HA on EC CD44 to facilitate leukocyte adhesion. The release of pro-inflammatory cytokines and chemokines (TNFα and IL-1β) also promote monocyte and T cell CD44 expression, as well as cell adhesion and extravasation into the sub-endothelium. VSMCs adopt a synthetic phenotype characterized by production of pro-inflammatory stimuli and increased ECM production.
Disruption of the glycocalyx barrier allows more LDL to access the subendothelial space to initiate the inflammatory response. Both HA and CD44 are critical to sustain robust vascular endothelial integrity. In the LDLR−/− model of atherosclerosis, administration of 4-methylumbellieferone, an inhibitor of HA synthesis, resulted in increased leukocyte recruitment to the carotid artery by intravital imaging, increased macrophage recruitment to lesion and increased atherosclerosis, highlighting the critical anti-atherogenic role of an intact HA-rich glycocalyx [77]. LMW HA promotes endothelial permeability by inducing HABP2 activity and subsequent protease activated receptor (PAR) activation on endothelial cells [78]. In contrast, HMW HA-CD44 binding protects murine pulmonary endothelium from LPS-induced permeability [79]. In addition, endothelial HAS2 appears to be anti-atherogenic and is upregulated under atheroprotective conditions of high shear stress [80]. However, pro-inflammatory, pro-atherogenic cytokines may also induce endothelial cell HAS2 synthesis and subsequent HMW HA production, promoting CD44-HA mediated adhesion of monocytes to human activated endothelium under static conditions [81]. These conflicting studies highlight the need for additional studies to better ascertain the net effect of endothelial HAS2 on the disease to better-determine whether this enzyme may serve as a potential therapeutic target to promote endothelial cell integrity at the initiation of atherosclerosis.
One of the critical signaling pathways that alters inflammation of ECs during the initiation of disease is the NFκB pathway. NFκB is known be critical for promotion of pro-inflammatory mediator production in immune cells - T cells, macrophages and vascular endothelial cells [82]. The NFκB signaling pathway is comprised of NFκB heterodimers or homodimers that contain the Rel homology domain containing polypeptides and their inhibitor proteins, the IκBs. NFκB activation requires proteasomal degradation of the IκBs. The IκB Kinase (IKK) complex phosphorylates and thus inactivates IκB [83]. In vascular endothelial cells NFκB signaling promotes pro-inflammatory cytokine production and upregulation of critical adhesion molecules, both of which promote atherogenesis [84]. In vivo, it has been shown that deletion of NEMO (IKKγ) in endothelial cells inhibits monocyte recruitment and lesion burden in apoE−/− mice on a HFD [84].
Leukocyte Adhesion to Activated Endothelium
Concomitant with endothelial dysfunction, a pro-inflammatory environment promotes the production of cytokines/chemokines and the subsequent upregulation of key adhesion molecules ICAM-1, VCAM-1, P-selectin, E-selectin and CD44 to support leukocyte adhesion and extravasation into the subendothelial space [2]. The role of CD44 in lesion initiation is in part attributed to its ability to mediate the interaction between incoming leukocytes and activated endothelial cells. Specifically, HA associated with the luminal surface via association with endothelial CD44 serves as a bridge binding to CD44 expressed on activated leukocytes to facilitate leukocyte adhesion and transmigration (Figure 1B).
Progression of Disease
While the role of CD44 in the initiation of disease is well established, there have been relatively few studies examining CD44 during the later stages of atherosclerosis. Atherosclerosis progresses due to the actions of multiple cells types working inconcert to promote a sustained pro-inflammatory environment (Figure 2) [2]. eNOS is also a critical anti-atherogenic target in atherogenesis since NO regulates arterial tone and suppresses proliferation of vascular smooth muscle cells (VSMCs) [85]. It is important to understand how to maintain eNOS activity. Dysregulation of eNOS results in decreased NO synthesis/activity, which enhances the progression of atherosclerosis [86]. It has also been shown that administration of L-arginine, a precursor of nitric oxide, may ameliorate the severity of atherosclerosis [87].
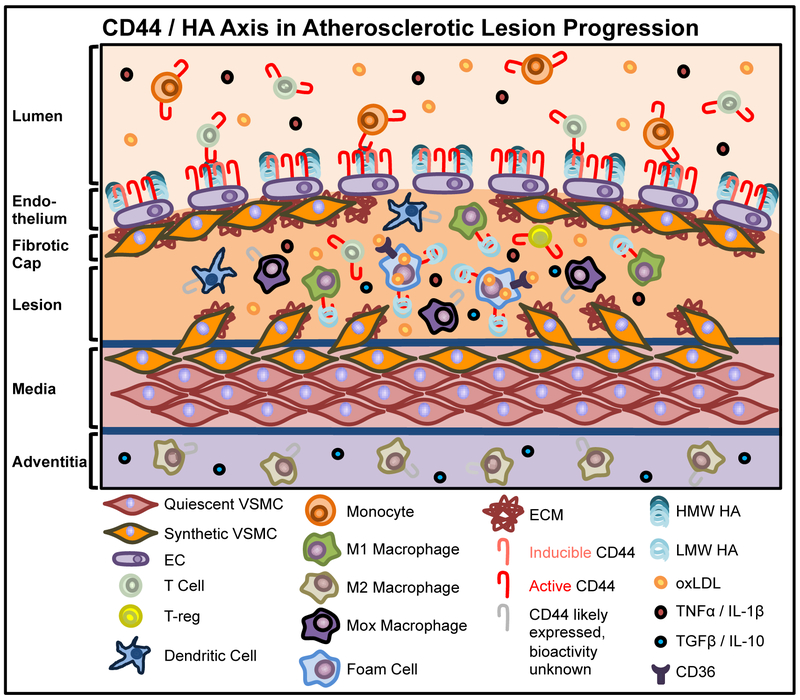
Figure 2. Role of CD44 in the inflammatory response and impact on the progression of atherosclerosis.
The impact of CD44 on the progression of the atherosclerosis remains under-explored. CD44 may influence the balance between prolonged inflammation versus potential anti-inflammatory, disease-resolving processes through varying mechanisms on different cell types. Pro-inflammatory cytokines TNFα and IL-1β increase M1 macrophage CD44 bioactivity and LMW HA binding, leading to increased pro-inflammatory gene expression. M2 macrophages, localized predominantly in the adventitia, contribute to the resolution of inflammation via the production of anti-inflammatory cytokines, although the bioactivity of CD44, or the affect of HMW HA versus LMW HA binding in these cells is unknown. The expression, activity and role of CD44/HA on Mox macrophages (a separate anti-inflammatory macrophage subset in lesions) has not been investigated. TNFα and IL-1β rapidly induce expression and bioactivity on T cells, in turn prolonging the inflammatory response. Conversely, T-reg CD44 expression is critical for the production of the anti-inflammatory cytokines TGFβ and IL-10. CD44-LMW HA binding promotes foam cell formation and consequent CD36 expression and increased uptake of oxLDL. In turn, this facilitates foam cell release of pro-inflammatory cytokines TNFα and IL-1β. CD44 activity on dendritic cells is required for dendritic cell-induced T cell activation, although this has not yet been confirmed in the context of atherosclerosis.
Since NO production is a key factor in modulating the inflammatory environment, it is important to consider how CD44 interacts with these known targets to combat atherosclerosis. CD44 is known to associate with cholesterol-rich microdomains termed lipid rafts. It has been reported that HA binding to endothelial CD44v10 promotes engagement with ankyrin and IP3 receptor into lipid rafts, which promotes enhanced NO production which may be due to eNOS activity[88]. This therefore represents a potential avenue to modulate endothelial cell phenotype, and subsequent effects on VSMCs in the inflammatory micro-environment.
Regulating the Lesion Milieu: Pro-inflammatory versus pro-fibrotic functions of infiltrating leukocytes
Macrophages are key leukocytes that drive atherogenesis as they exert critical affects throughout the course of disease. An abundance of macrophages is associated with lesions containing thin fibrotic caps relative to more stable, complex fibrotic lesions [89]. After blood-borne monocytes firmly adhere and extravasate into the subendothelium, these cells differentiate into mature tissue macrophages in the presence of macrophage colony stimulated factor (MCSF) produced by the activated endothelium [90]. Macrophages express receptors for oxLDL that mediate signal transduction to regulate cell behavior and gene expression and function to internalize oxLDL leading to the formation of lipid-laden foam cells. Macrophages within lesion are heterogeneous, representing cells along a spectrum ranging from pro-inflammatory M1-like macrophages to anti-inflammatory M2-like macrophages. Notably, cells along this spectrum have been shown to exhibit plasticity in response to factors in the milieu which opens the intriguing possibility of therapeutically driving reprogramming of macrophages in the context of lesions [91,92]. The details of macrophage biology in atherosclerosis has been covered extensively elsewhere [93]. Briefly, M1 macrophages are characterized by the production of ROS and RNS, as well as pro-inflammatory cytokines including IL-1β, TNFα and IL-12 all of which have been shown to promote atherogenesis. Each of these has also been implicated in CD44 activation and/or downstream of CD44 to influence inflammatory gene expression, or both. IL-12 is of particular interest in this context as it has been shown that the CD44/HA axis is a potent inducer of IL-12 production that in turn drives the generation of type 1 inflammation and the production of interferon-γ – a potent macrophage activating and anti-fibrogenic factor whose function in part attributed to inhibition of VSMC proliferation and macrophage production of ECM degrading enzymes [94]. This may further amplify a pro-atherogenic inflammatory circuit that may potentially prevent the formation or cause the degradation of fibrotic caps that would tip the balance toward plaque destabilization [94]. M2-like macrophages are generated in response to IL-4, glucocorticoids, or IL-10 and TGFβ. M2 macrophages promote healing and contribute to the resolution of inflammation through production of anti-inflammatory cytokines [95]. Another anti-inflammatory macrophage subset (Mox) has been implicated in atherosclerosis and is generated in response to oxLDL administration in a Nrf2-dependent manner. These cells have distinct gene expression profiles compared to both the M1 and M2 macrophages and represent an intriguing avenue of future study [96]. Relatively-high M1 lesion content correlates with increased lesion vulnerability whereas relatively-high M2 lesion content correlates with lesion stability lesion in both human and mouse models of atherosclerosis [97]. In addition, different macrophage subsets exhibit distinct spatial localization within lesion - M1 macrophages populate the vulnerable shoulder region of lesion, M2s are found to be prominent in the adventitia, and Mox macrophages are found throughout lesion, as shown in Figure 2 ([98–100] and Monslow and Puré, unpublished data). Increasing the preponderance of M2 or Mox macrophages relative to M1s or decreasing the abundance of M1s at vulnerable shoulder regions of lesion may promote lesion stability. Thus, understanding how to regulate macrophage abundance, localization and functional phenotype by reprogramming macrophage subsets may serve as a key therapeutic opportunity to regulate disease progression.
NFκB signaling is critical for the development of the pro-inflammatory M1 macrophage phenotype [101]. Deletion of myeloid IκBα increases lesion burden in LDLR−/− mice, while deletion of myeloid IκBβ reduces lesion burden in the LDLR−/− mice fed a HFD. Thus, targeting the NFκB pathway may alter macrophage phenotype and regulate the inflammatory micro-environment [102,103].
As stated earlier, while eNOS is a potent anti-atherogenic target, other sources of NO may have pro-atherogenic effects. It has been shown that iNOS deletion decreases lesion burden in both male and female apoE−/− iNOS−/− mice compared to apoE−/− mice[104]. iNOS is a well-established marker for M1 pro-inflammatory macrophages in both human and mouse lesion, a cell subset that as stated previously, is increased in unstable lesions [97,98,105]. This contrasting impact of NOS isoform on the progression of disease is complex and may reflect the low levels of basal NO produced by eNOS relative to the transient but markedly greater levels of NO produced by activation of iNOS. In any case, iNOS represents a pro-atherogenic target to potentially impede the progression of disease.
Peroxisome proliferator activated receptors (PPARs) also play a critical role in atherogenesis often by modulating macrophage phenotype. PPARs are lipid-derived nuclear hormone receptors that may regulate dyslipidemia and thus the course of atherosclerosis. Of particular note is PPARγ, which forms heterodimeric transcription factor with the retinoid X receptor (RXR), binding to distinct peroxisome proliferator elements (PPRE) in the promoter region of their target genes to alter lipid metabolism. It is expressed on multiple cell types - adipocytes, macrophages, hepatocytes, and skeletal muscle [106,107]. While scavenger receptor CD36 is one of the key target genes of PPARγ, PPARγ agonists may inhibit macrophage foam cell formation in vitro and in vivo [108–110]. PPARγ also inhibits monocyte production of key pro-inflammatory cytokines - IL-6, IL-1β, and TNFα, and thus may directly alter the lesional micro-environment [111,112]. In vivo the PPARγ agonist troglitazone has been shown to decrease carotid artery intimal thickness [113]. Thus, it is important to keep these signaling pathways in mind with regard to how they may specifically intersect with CD44.
As stated previously, ROS is a strong pro-atherogenic mediator. It degrades protective nitric oxide, leading to endothelial dysfunction and VSMC proliferation. It is thus critical to fully understand the role that ROS generation has on the CD44-HA axis. NADPH oxidase is a membrane-bound enzyme that produces ROS. It has been shown that CD44 immunostaining is significantly decreased in apoE−/− mice with global NADPH oxidase deletion (apoE−/−ph47phox−/− mice) [47]. This decrease in CD44 staining was localized to macrophages in lesion. Coupled with the observation that apoE−/− ph47phox−/− mice had decreased lesion burden compared to apoE−/− mice, this implies a potential regulatory role for NADPH on macrophage CD44 expression in an atherogenic setting, total macrophage abundance, and the progression of disease.
CD44 is critical for maximal macrophage recruitment and/or retention in atherosclerosis and other inflammatory conditions [17,18,114,115]. It may also regulate the pro-inflammatory state of monocytes and macrophages. Freshly isolated human monocytes required stimulation with either IFNy or LPS in short-term culture to increase both CD44 expression and to induce the high affinity state of CD44 [116]. Additional pro-atherogenic stimuli may also induce CD44 activity, including, IL-1α, IL-1β, IL-2, GMCSF, TNFα, and IL-4. These include a few of the key stimuli that govern macrophage differentiation and thus it will be interesting to determine whether different macrophage subsets have differential CD44 expression and/or activity under normal conditions as well as under pro-atherogenic conditions of atherosclerosis [117–119].
The balance between M1, M2 and Mox macrophages may influence disease progression, since the balance between inflammation and fibrosis is a critical determinant of plaque fate. The key M1 pro-inflammatory stimuli - IFNγ and TNFα as well as the M2 anti-inflammatory stimuli (IL-4, IL-10) alter CD44 activity of murine bone marrow-derived macrophages (levels of CD44 on Mox macrophages are currently unknown). CD44 activity can be further modified by post-translational modification of the receptor in a chondroitin sulfate-specific manner [119–121]. Thus, CD44 activity can be regulated on diverse macrophage subsets, and is thus a viable therapeutic target. This study paves the way for an in-depth examination of whether macrophage CD44 expression and regulation alters function of the subsets in the context of atherosclerosis.
Since HA promotes inflammatory cell retention and is also an important component of eroded atherosclerotic lesion, it is important to understand HA bioactivity and its impact on the key inflammatory cells in lesion. [32,54]. LMW HA promotes pro-inflammatory gene expression in murine macrophages in a CD44-dependent manner [122–125]. There is also evidence that suggests that this interaction is due to the signaling properties of LMW HA, and not solely due to its physical viscoelastic properties [123]. In contrast, HMW HA promotes anti-inflammatory gene expression profiles in both human and murine macrophages [125,126]. Thus, HA modulates macrophage inflammatory state and the status of macrophage activation can modulate CD44 activity. In addition, in macrophages NFκB signaling is downstream of CD44 binding and thus represents an important area for future study. CD44-LMW HA ligation leads to degradation of IκB and subsequent activation of NFκB and pro-inflammatory gene expression and iNOS activity [124,127–129]. Nevertheless, several questions remain regarding the impact of CD44 and HA on macrophages and how they may regulate lesion fate. Do CD44 and/or HA alter macrophage differentiation capacity in lesion? Can CD44 and/or HA be used to reprogram macrophage phenotype? Is CD44 expression or activity differentially regulated between macrophage phenotypes (and does this alter their functions in a subtype-specific manner)?
While macrophages are important inflammatory cells in lesion, T cells also modulate progression of disease. Macrophages recruit T cells to lesion by releasing TNFα. CD4+ T cells can then differentiate to a variety of subsets in response to different cytokines, found in both human and murine atherosclerosis. Th1 cells, driven in part by macrophage-derived IL-12, are pro-atherogenic; T-reg cells are atheroprotective, while Th2 cells (driven by the pro-fibrotic cytokines IL-4 and IL-13) may exert both pro-atherogenic and anti-atherogenic effects [130,131].
Cytokines and chemokines critical for atherogenesis can also rapidly induce increased expression of CD44 and its transition from a high-to-low affinity state for HA binding on T cells. This occurs under both static and shear stress conditions and may promote T cell adhesion and migration into inflamed sites in vivo [132–134]. In addition, CD44 on both bone marrow and non-bone marrow derived cells regulates T cell recruitment and retention in both the apoE−/− and the LDLR−/− models [23,24]. It has also been shown that deletion of HAS3 in apoE−/− mice reduced Th1 cell polarization and atherosclerosis, supporting a pro-atherogenic role for HAS3 [135].
T cells can also adopt both pro-inflammatory and anti-inflammatory phenotypes and thus may influence the lesion microenvironment. One previous study reported that Th1 T cells produce a variety of pro-inflammatory cytokines, including IFNγ and TNFα, while T-regs produce anti-inflammatory, anti-atherogenic cytokines such as IL-10 and TGFβ [131]. T-regs exert a dominant role in atherogenesis relative to the other T cell subtypes, and CD44 is necessary for T-regs to produce TGFβ and IL-10 in wild-type mice [130,136]. In addition, CD44 deletion has been reported to decrease the production of IL-10 and IL-5 in a highly inflammatory milieu (LDLR−/− mice on a HFD), suggesting that CD44 may exert an anti-atherogenic role on the dominant T cell population in lesion [24]. This contradictory anti-atherogenic role may in fact be dwarfed by the overwhelming pro-inflammatory role of CD44 on other key cells in lesion, resulting in a net pro-atherogenic role for CD44.
In contrast, VSMCs play seemingly paradoxical roles on lesion fate. Under basal conditions VSMCs exist in a contractile, low proliferative state in the media where they control the vascular tone of blood vessels [137]. During atherogenesis pro-inflammatory cytokines and oxLDL promote VSMC migration into the intima where they adopt a synthetic phenotype, as shown in Figure 1B [138,139]. Synthetic VSMCs produce large amounts of pro-inflammatory cytokines (MCP-1, IL-6) and increased extracellular matrix (ECM), notably collagen and fibronectin, which may help stabilize the plaque [70,140–143]. However, these ECM components and any derived matrix protein fragments may also exert pro-atherogenic effects. Macrophage adherence to type-I collagen induces macrophage phagocytosis of modified LDL; this interaction also increases MMP9 expression which degrades the protective fibrotic cap in lesion [144–146]. There is also some evidence that lesional VSMCs can phagocytose cholesterol to become foam cells in lesion [147]. Understanding the net role that these VSMC-derived foam cells play is complicated, since the increased ECM production and pro-inflammatory cytokine production from these cells may support both stabilization and weakening of the fibrotic cap, respectively.
Vascular CD44 gene expression is increased and CD44 is functionally activated in atheroprone regions of the vasculature in apoE−/− mice relative to wild-type mice [20]. LMW HA regulates VSMC migration and phenotypic de-differentiation to the synthetic state in a CD44-dependent manner [17]. In addition, LMW HA-CD44 ligation enhances aortic smooth muscle migration in an ERK 1-2 dependent manner [148], and it has been shown that LMW HA-CD44 promotes thrombin-induced VSMC proliferation [46]. NADPH-deficient apoE−/− mice demonstrated decreased CD44 and HA expression in both plasma and in atherosclerotic lesion compared to apoE−/− mice, implying a role for NADPH at both the systemic and the local level. Administration of a Nox4-NADPH inhibitor reduced both ROS generation, atherosclerotic lesion burden, and CD44-HA expression in human aortic VSMCs [46]. Thus, it is critical to consider the role of NADPH as a potential therapeutic target which may impact the CD44-HA axis to ameliorate the progression of atherosclerosis.
Since synthetic VSMCs exhibit contradictory functions - pro-inflammatory cytokine production and increased MMP1 and MMP9 expression (which degrade type-I and type-IV collagen) versus ECM production and potential plaque stabilization, it is difficult to ascertain the net atherogenic effect of these cells on lesion stability. Although HAS2 is responsible for the majority of VSMC-derived HA production, both HAS1 and HAS3 contribute to VSMC HA production [65]. VSMC production of HA may promote either a pro- or anti-inflammatory environment depending on the size of HA (LMW or HMW) due to differential processing by HYALs and/or ROS, which influences its signaling capabilities, structure and interestingly, collagen architecture. HAS1-derived HA may form anti-atherogenic cables that trap leukocytes to prevent their active intralesional migration [149]. It has also been shown that oxLDL-LOX1 ligation on human aortic VSMCs may promote HAS2 and HAS3 overexpression, increased HA deposition, and subsequent enhanced monocyte adhesion in vitro [150]. Thus, it will be of interest to explore how different VSMC phenotypes regulate HA metabolism as well as the effect of HA on VSMC phenotype.
Foam Cell Formation:
One of the hallmarks of atherosclerotic lesion is the formation of lipid-laden foam cells, which may originate from macrophages, VSMCs and potentially dendritic cells [140,151–155]. Foam cells produce a variety of factors that promote necrotic cell death and the formation of a necrotic core. Of note, VSMC lineage tracing using Myh11-CreERT2-ROSA-floxed-STOP-eYFP apoE−/− mice has highlighted a novel insight that lesional VSMC-derived cells may adopt a macrophage-like phenotype [156]. In addition, these cells may be modulated in a KLF4-dependent manner, since VSMC-specific knock-out of KLF4 resulted in decreased lesion size and an increase in fibrotic cap area. There is evidence that CD44 and its pro-inflammatory ligand LMW HA both promote the formation of macrophage-derived foam cells by enhancing scavenger receptor CD36 expression and promoting the in vitro uptake of oxLDL in a PKC-dependent manner [157,158]. It would be interesting to determine whether CD44 alters the foam cell formation capacity of different foam cell precursors (VSMCs, dendritic cells, macrophages) using a similar mechanism in vivo. It would also be of interest to examine whether CD44 alters foam cell migration and subsequent egress from lesion back into the blood or via the lymphatic system.
Necrotic Cell Death and Efferocytosis:
Dead cell accumulation contributes to the formation of a necrotic core and the progression of atherosclerosis due to increased apoptosis or defective efferocytosis (clearance of apoptotic cells) [159]. These apoptotic cells are primarily macrophages/VSMCs/foam cells, but the apoptosis of any cells in lesion promotes a sustained pro-inflammatory milieu that is pro-atherogenic [160]. In vitro it has been shown that HMW HA prevents 4-MU-induced apoptosis in human VMSCs in a CD44/TLR4-dependent manner, highlighting the anti-inflammatory potential of HMW HA to reduce the sustained chronic inflammatory micro-environment of established lesion [161].
In both human and rabbit atherosclerosis lesional macrophages co-localize with classic apoptosis and oxidative stress markers - p53, cJun-API, and MnSOD. These highly-stressed cells also co-localize with TUNEL-positive nuclei, marking DNA strand breakage [162]. A follow up study revealed that CD44+ macrophages also co-localize with pro-inflammatory markers such as iNOS, TNFα and MHC class 1 and 2 implying that CD44 may contribute to pro-inflammatory M1 cell death in lesion [162,163]. If CD44 preferentially promotes M1 macrophage apoptosis in lesion, this again provides a pro-atherogenic role for the receptor. A pertinent extension of this study would be to examine the co-localization of macrophage subset specific markers such as heme oxygenase 1 for the Mox subset or CD206 for the M2 subset with apoptotic markers and CD44, which would provide a more complete picture of CD44’s role in apoptosis on different macrophage subsets.
CD44 regulates the general phagocytic capacity, as well as the efferocytic capacity of macrophages under inflammatory conditions. CD44 appears to be necessary for efficient phagocytosis of apoptotic leukocytes, specifically neutrophils [164–167]. Thus, a loss of CD44 on macrophages may in fact cause defective efferocytosis and a sustained pro-inflammatory environment. To answer this it would be pertinent to stain cells for markers of distinct scavenger receptors such as MERTK, which is the primary receptor responsible for efferocytosis in lesion [168].
Future Research Questions and Directions CD44 and Atherosclerosis Regression
The ideal therapeutic intervention for atherosclerosis would not only halt the progression of disease, but also enable lesion stabilization as defined as the alteration in plaque morphology that tilts the balance of lesion content to a decrease in lipid and inflammatory content and an increase in fibrotic content and/or regression [169]. Atherosclerosis regression has been studied in several different models - arguably the most direct approach so far involved the transplantation of lesion-filled aortas into wild-type mice which resulted in reduced lesion burden [170]. Other compelling evidence comes from studies using the Reversa transgenic mouse (LDLR−/−Apob100/100Mttpfl/flMx1Cre+/+) in which the severe hypercholesterolemia induced by HFD in LDLR−/− mice can be normalized by conditionally knocking-out microsomal triglyceride transfer protein (Mttp) [169]. In the Reversa model, aged mice with advanced lesion and hypercholesterolemia were given polyinosinicpolyctidylic acid (pIpC) to induce the Mx1-Cre transgene to enable deletion of Mttp. This resulted in the normalization of plasma cholesterol levels in these mice. Analysis of plaque content revealed that mice with normalized lipid profiles had decreased total CD68+ macrophage content and lipid content. In addition, the group found that lipid normalization also increased both collagen content and classic M2 macrophage gene expression (Arg 1, CD163, Fizz-1, Mannose Receptor) in CD68+ laser captured cells from lesion relative to untreated mice. This implies that one of the key forces that promotes lesion regression is a decrease in total macrophage content combined with an increase in the relative amount of M2 macrophages [169].
One study has examined the role of CD44 in atherosclerosis regression. In the apo3Leiden atherosclerosis model, administration of an LXR agonist resulted in atherosclerosis regression. This regression was accompanied by decreased vascular CD44 expression in treated mice compared to untreated mice, as determined by mRNA and staining of aortic roots [171]. Co-staining with CD44 and cell-specific markers was not performed, so it is unknown whether this staining is localized to specific cell types, but it provides the basis for a more in-depth analysis of CD44’s role in regression. Co-staining aortic roots for CD44 and MERTK, the classic critical marker for efferocytosis in these models will provide a clearer understanding of the mechanism by which CD44 influences lesion regression.
Lesion regression may be influenced by many of the key processes that drive atherogenesis by directly influencing the inflammatory microenvironment of lesion. Since CD44 is necessary for efficient macrophage efferocytosis, the receptor may possess the capacity to promote lesion regression. However, given CD44’s pro-atherogenic role in the apoE−/− mouse model as well as the previously mentioned regression study in the apo3Leiden model, any CD44 ‘pro-regression’ roles may prove complex to dissect. It is possible that an anti-atherogenic effect exerted by macrophage CD44 in efferocytosis is not sufficient to overcome the overwhelming pro-inflammatory milieu that CD44 promotes by influencing the other processes in lesion. It is also possible that CD44’s positive effect on regression is not sufficient to overcome CD44’s initial pro-inflammatory role by supporting leukocyte recruitment. Thus, it will be necessary to examine the impact of deleting or blocking the function of CD44 at multiple time points over the course of disease on atherogenesis in mouse models in order to fully delineate the temporal role that CD44 plays during key processes that govern regression. Co-staining for classic markers of apoptosis, efferocytosis, foam cell formation and cell-types will give a deeper understanding of how each of these cells works in concert to promote disease, and ultimately whether CD44 may be utilized to turn the tide of atherogenesis to favor disease regression.
Cellular Cross-Talk
Co-culture of T-regs with macrophages can decrease the capacity of macrophages to become foam cells in a IL-10 and TGFβ-dependent manner [172]. Conversely, CD11c+ antigen presenting cells (APCs) can activate Th1 cells to produce IFNγ and TNFα, which in turn promote macrophage-derived foam cell formation [173]. Thus, T cell subsets produce pro-inflammatory cytokines that may regulate monocyte CD44 activity and alter macrophage polarization, which may tip the balance in lesion to favor inflammation compared to fibrosis [119]. It is important to be cognizant of how CD44 expressed on one particular cell-type may indirectly regulate the actions of adjacent cells in lesion. This concept opens an additional avenue of study that may be illuminated by examining the effect of wild-type versus CD44−/− cells in co-culture experiments (i.e. examining the role of wild-type versus CD44−/− macrophages on wild-type or CD44−/− VSMC and T cell function).
Role of CD44 on Other Myeloid cells in Lesion
This review has focused on key inflammatory players in lesion — endothelial cells, macrophages, T cells, and VSMCs. While these cells play key roles in atherosclerosis, it is essential to consider the roles of other inflammatory cells, such as dendritic cells (DCs) and neutrophils. DCs influence atherogenesis by activating T cells and phagocytosing oxLDL to form foam cells [173,174]. There is some evidence that CD44 is critical for DC-induced T cell activation but the function of CD44 in this regard has not been studied in the context of atherosclerosis [175–177]. Neutrophils contribute to both the initiation of disease by disrupting endothelial permeability and enhancing recruitment of blood-borne monocytes, but also weaken advanced fibrotic lesion by degrading ECM components, and increasing the expression of proteases MMP8 and MMP13 [178]. Although CD44 has been implicated in these processes in other contexts, the role that CD44 plays on neutrophil cell recruitment, phenotype, and survival in the context of atherosclerosis has not yet been elucidated. Since both DC and neutrophils can contribute to lesion morphology, understanding the impact of DC and neutrophil CD44 on atherogenesis remains an exciting avenue for the field either by tissue-specific deletion in vivo, or using in vitro models for the key processes mentioned above - inflammatory functional phenotype, foam cell formation, proliferation and efferocytosis.
CD44 Regulation: Post-Translational Modification and Variant Expression
While this review has focused on CD44 and HA in atherosclerosis there are, of course, several complexities that have not been discussed in detail. As already briefly mentioned, CD44 exists in several states (inducible, active, and inactive). Examining CD44 expression is not sufficient to fully-understand the role that CD44 exerts on atherogenesis. CD44’s activity may be regulated differently on multiple cell types (via alternative splicing of variant exons, post-translational modifications, glycosylation, sulfation, phosphorylation, clustering, receptor activation status) [7,8]. CD44 has multiple variants, which may be targets for regulation of the receptor’s activity. The standard form of CD44 and its variants are highly expressed in macrophage-rich advanced atheromatous lesion [179], which hints that macrophage-specific CD44 variant expression may correlate with lesion severity - another intriguing avenue of future study.
CD44-Dependent HA-Independent, vs. CD44-lndependent HA-Dependent Effects
This review has mainly discussed CD44-dependent HA-dependent effects on inflammation in atherosclerosis. However, it is important to briefly note that while CD44 is the principal receptor for HA, HA binds other target proteins including LYVE-1, TSG-6 and RHAMM, all of which are expressed in atherosclerotic lesions and may alter lesion burden [33,180,181]. In addition, CD44 may also have independent effects divorced from HA. CD44’s other potential ligands - osteopontin, fibronectin and collagen all contribute to atherogenesis [182–184]. Thus, it is imperative to take into account CD44-dependent HA-dependent effects, but also CD44-independent HA-dependent effects in order to fully dissect out the role that the CD44/HA axis has on atherosclerosis.
Conclusions
Lesion progression is governed by a constant tug-of-war between pro-inflammatory and disease-resolving processes, and CD44 regulates these pathways on multiple cell types to alter the progression of atherosclerosis. CD44 and HA are intriguing potential therapeutic targets for atherosclerosis as they play critical roles that influence the key processes that govern atherogenesis - maintenance of a pro-inflammatory milieu, foam cell formation, necrotic cell death, and efferocytosis. By analyzing the entire course of disease instead of just the end state of advanced lesion, it may be possible to identify critical checkpoints and dominant CD44-positive cell types that will allow us to alter the course of disease to halt the progression of disease and potentially enable lesion regression.
Highlights.
Cardiovascular disease (CVD) due to atherosclerosis is a disease of chronic inflammation
The CD44-HA axis has been implicated in CVD and thus is an attractive therapeutic target to halt the course of atherosclerosis
While the role of CD44 and HA in the initiation of the inflammatory response in atherosclerosis is well-established, the role of CD44 and HA during the progression of the disease remains relatively unexplored
We review the role of CD44 and HA in the key processes and multiple cell types throughout atherosclerosis to determine the possibilities of targeting the CD44-HA axis to halt disease progression and enable lesion regression
Acknowledgements
This original research was supported by the American Heart Association Predoctoral Fellowship [16PRE30960039] and National Institutes of Health grant [RO1-AG-047373].
Abbreviations used
- CVD
cardiovascular disease
- VSMC
vascular smooth muscle cell
- ECM
extracellular matrix
- DC
dendritic cell
- oxLDL
oxidized LDL
- HA
hyaluronan
- LMW HA
low molecular weight HA
- HMW HA
high molecular weight HA
- MERTK
proto-oncogene tyrosine-protein kinase MER
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- T-reg
regulatory T cell
- APC
antigen presenting cell
- NO
nitric oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association, Circulation. (2017). 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaperonis EA, Liapis CD, Kakisis JD, Dimitroulis D, Papavassiliou VG, Inflammation and atherosclerosis, Eur.J.Vasc.Endovasc.Surg 31 (2006) 386–393. 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- [3].Galkina E, Ley K, Immune and Inflammatory Mechanisms of Atherosclerosis, Annu. Rev. Immunol 27 (2009)165–197. 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yash Prashar NSG, Ritu, Souravh Bais, Emerging role of various signaling pathways in the pathogenesis and therapeutics of atherosclerosis, Vasc. Med 10-11 (2017) 1–12. [Google Scholar]
- [5].Johnson P, Ruffell B, CD44 and its Role in Inflammation and Inflammatory Diseases, Inflamm. Allergy - Drug Targets. 8 (2009) 208– 220. 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- [6].Puré E, Cuff CA, A crucial role for CD44 in inflammation, Trends Mol. Med 7 (2001) 213–221. 10.1016/S1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- [7].Cichy J, Puré E, Cytokines regulate the affinity of soluble CD44 for hyaluronan, FEBS Lett 556 (2004) 69–74. 10.1016/S0014-5793(03)01370-X. [DOI] [PubMed] [Google Scholar]
- [8].Goodison S, Urquidi V, Tarin D, CD44 cell adhesion molecules., Mol. Pathol 52 (1999) 189–96. 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Monslow J, Govindaraju P, Puré E, Hyaluronan - a functional and structural sweet spot in the tissue microenvironment, Front. Immunol 6(2015). 10.3389/fimmu.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wight TN, Provisional matrix: A role for versican and hyaluronan, Matrix Biol 60-61 (2017) 38–56. 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, Vigetti D, Hanson RW, Hart GW, The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc, Matrix Biol 35 (2014) 14–17. 10.1016/j.matbio.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, Passi A, Hyaluronan: Biosynthesis and signaling, Biochim. Biophys. Acta - Gen. Subj 1840 (2014) 2452–2459. 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [13].Viola M, Vigetti D, Genasetti A, Rizzi M, Karousou E, Moretto P, Clerici M, Bartolini B, Pallotti F, De Luca G, Passi A, Molecular control of the hyaluronan biosynthesis., Connect. Tissue Res 49 (2008) 111–4. 10.1080/03008200802148405. [DOI] [PubMed] [Google Scholar]
- [14].Siiskonen H, Oikari S, Pasonen-Seppänen S, Rilla K, Hyaluronan synthase 1: A mysterious enzyme with unexpected functions, Front. Immunol 6 (2015). 10.3389/fimmu.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kiene LS, Homann S, Suvorava T, Rabausch B, Müller J, Kojda G, Kretschmer I, Twarock S, Dai G, Deenen R, Hartwig S, Lehr S, Köhrer K, Savani RC, Grandoch M, Fischer JW, Deletion of Hyaluronan Synthase 3 Inhibits Neointimal Hyperplasia in Mice, Arterioscler. Thromb. Vasc. Biol 36 (2016) e9–e16. 10.1161/ATVBAHA.115.306607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Noble Paul JD, Liang Jiurong, Genetic network identifies novel pathways contributing to atherosclerosis susceptibility in the innominate artery., Physiol. Rev 91 (2011) 221–264. 10.1186/1755-8794-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao L, Lee E, Zukas AM, Middleton MK, Kinder M, Acharya PS, a Hall J, Rader DJ, Puré E, CD44 expressed on both bone marrow-derived and non-bone marrow-derived cells promotes atherogenesis in ApoE-deficient mice., Arterioscler. Thromb. Vasc. Biol 28 (2008) 1283–9. 10.1161/ATVBAHA.108.165753. [DOI] [PubMed] [Google Scholar]
- [18].Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Puré E, The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation, J. Clin. Invest 108 (2001) 1031–1040. 10.1172/JCI200112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hägg D, Sjöberg S, Hultén LM, Fagerberg B, Wiklund O, Rosengren A, Carlsson LMS, Borén J, Svensson P-A, Krettek A, Augmented levels of CD44 in macrophages from atherosclerotic subjects: a possible IL-6-CD44 feedback loop?, Atherosclerosis. 190 (2007) 291–7. 10.1016/j.atherosclerosis.2006.03.020. [DOI] [PubMed] [Google Scholar]
- [20].Zhao L, Hall JA, Levenkova N, Lee E, Middleton MK, Zukas AM, Rader DJ, Rux JJ, Puré E, CD44 regulates vascular gene expression in a proatherogenic environment, Arterioscler. Thromb. Vasc. Biol 27 (2007)886–892. 10.1161/01.ATV.0000259362.10882.c5. [DOI] [PubMed] [Google Scholar]
- [21].Dattilo JB, Dattilo MPM, Yager DR, Makhoul RG, Hypercholesterolemia alters the gene expression of novel components of the extracellular matrix in experimental vein grafts, Ann. Vasc. Surg 12 (1998) 168–173. 10.1007/s100169900136. [DOI] [PubMed] [Google Scholar]
- [22].Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Davies PF, Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta., Proc. Natl. Acad. Sci. U. S. A 101 (2004)2482–7. 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhao L, Lee E, Zukas AM, Middleton MK, Kinder M, Acharya PS, Hall JA, Rader DJ, Pur E??, CD44 expressed on both bone marrow-derived and non-bone marrow-derived cells promotes atherogenesis in ApoE-deficient mice, Arterioscler. Thromb. Vasc. Biol 28 (2008) 1283–1289. 10.1161/ATVBAHA.108.165753. [DOI] [PubMed] [Google Scholar]
- [24].Sjöberg S, Eriksson EE, Tivesten Å, Carlsson A, Klasson A, Levin M, Borén J, Krettek A, CD44-deficiency on hematopoietic cells limits T-cell number but does not protect against atherogenesis in LDL receptor-deficient mice, Atherosclerosis. 206 (2009)369–374. 10.1016/j.atherosclerosis.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [25].Getz GS, Reardon CA, Animal Models of Atherosclerosis, in: Anim. Model. Study Hum. Dis Second Ed., 2017:pp.205–217. 10.1016/B978-0-12-809468-6.00008-5. [DOI] [Google Scholar]
- [26].Lee YT, Lin HY, Chan YWF, Li KHC, To OTL, Yan BP, Liu T, Li G, Wong WT, Keung W, Tse G, Mouse models of atherosclerosis: A historical perspective and recent advances, Lipids Health Dis 16 (2017). 10.1186/s12944-016-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vigetti D, Viola M, Karousou E, Genasetti A, Rizzi M, Clerici M, Bartolini B, Moretto P, De Luca G, Passi A, Vascular pathology and the role of hyaluronan., Sci. World J 8 (2008)1116–8. 10.1100/tsw.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Papakonstantinou E, Roth M, Block LH, Mirtsou-Fidani V, Argiriadis P, Karakiulakis G, The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and migration., Atherosclerosis. 138 (1998)79–89.http://www.ncbi.nlm.nih.gov/pubmed/9678773. [DOI] [PubMed] [Google Scholar]
- [29].Lévesque H, Girard N, Maingonnat C, Delpech a, Chauzy C, Tayot J, Courtois H, Delpech B, Localization and solubilization of hyaluronan and of the hyaluronan-binding protein hyaluronectin in human normal and arteriosclerotic arterial walls., Atherosclerosis. 105 (1994)51–62.http://www.ncbi.nlm.nih.gov/pubmed/7512338. [DOI] [PubMed] [Google Scholar]
- [30].Bot PT, Pasterkamp G, Goumans MJ, Strijder C, Moll FL, De Vries JP, Pals ST, De Kleijn DP, Piek JJ, Hoefer IE, Hyaluronic acid metabolism is increased in unstable plaques, Eur. J. Clin. Invest 40 (2010) 818–827. 10.1111/j.1365-2362.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- [31].Kolodgie FD, Burke AP, Wight TN, Virmani R, The accumulation of specific types of proteoglycans in eroded plaques: A role in coronary thrombosis in the absence of rupture, Curr. Opin. Lipidol 15 (2004) 575–582. 10.1097/00041433-200410000-00012. [DOI] [PubMed] [Google Scholar]
- [32].Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R, Differential accumulation of proteoglycans and hyaluronan in culprit lesions: Insights into plaque erosion, Arterioscler. Thromb. Vasc. Biol 22 (2002) 1642–1648. 10.1161/01.ATV.0000034021.92658.4C. [DOI] [PubMed] [Google Scholar]
- [33].Krupinski J, Ethirajan P, Font MA, Turu MM, Changes in Hyaluronan Metabolism and RHAMM Receptor Expression Accompany Formation of Complicated Carotid Lesions and May be Pro-Angiogenic Mediators of Intimal Neovessel Growth, (2007) 361–367. [PMC free article] [PubMed] [Google Scholar]
- [34].Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L, Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis, Circ. Res 96 (2005) 583–591. 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- [35].Kucur M, Karadag B, Isman FK, Ataev Y, Duman D, Karadag N, Ongen Z, Vural VA, Plasma hyaluronidase activity as an indicator of atherosclerosis in patients with coronary artery disease, Bratislava Med. J 110 (2009) 21–26. [PubMed] [Google Scholar]
- [36].Krettek A, Sjöberg S, CD44 - a new cardiovascular drug target or merely an innocent bystander?, Cardiovasc. & Hematol. Disord. Drug Targets. 9 (2009) 293–302. http://www.benthamdirect.org/pages/content.php?CHDDT/2009/00000009/00000004/0008X.SGM. [DOI] [PubMed] [Google Scholar]
- [37].Viola M VD, Karousou E, D’Angelo ML, Moretto P, Caon I, Luca G, Passi A, Extracellular Matrix in Atherosclerosis: Hyaluronan and Proteoglycan Insights, Curr. Med. Chem 23 (2016) 2958–2971. [DOI] [PubMed] [Google Scholar]
- [38].Moretto P, Karousou E, Viola M, Caon I, D’Angelo ML, De Luca G, Passi A, Vigetti D, Regulation of hyaluronan synthesis in vascular diseases and diabetes, J. Diabetes Res 2015 (2015). 10.1155/2015/167283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sadowitz B, Seymour K, Gahtan V, Maier KG, The role of hyaluronic acid in atherosclerosis and intimal hyperplasia, J. Surg. Res 173 (2012). 10.1016/j.jss.2011.09.025. [DOI] [PubMed] [Google Scholar]
- [40].Wight TN, Arterial remodeling in vascular disease: a key role for hyaluronan and versican, Front Biosci 13 (2008) 4933–4937. 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- [41].Karangelis DE, Kanakis I, Asimakopoulou AP, Karousou E, Passi A, Theocharis AD, Triposkiadi F, Tsilimingas NB, Karamanos NK, Glycosaminoglycans as Key Molecules in Atherosclerosis: The Role of Versican and Hyaluronan, Curr. Med. Chem 17 (2012) 4018–4026. 10.2174/092986710793205354. [DOI] [PubMed] [Google Scholar]
- [42].Riessen R, Wight TN, Pastore C, Henley C, Isner JM, Distribution of Hyaluronan During Extracellular Matrix Remodeling in Human Restenotic Arteries and Balloon-Injured Rat Carotid Arteries, Circ. 93 (1996)1141–1147. 10.1161/01.CIR.93.6.1141. [DOI] [PubMed] [Google Scholar]
- [43].Bot PT, Hoefer IE, Piek JJ, Pasterkamp G, Hyaluronic acid: targeting immune modulatory components of the extracellular matrix in atherosclerosis., Curr. Med. Chem 15 (2008) 786–91. 10.2174/092986708783955554. [DOI] [PubMed] [Google Scholar]
- [44].Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S, Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways, Matrix Biol 26 (2007) 58–68. 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- [45].Sakr SW, Potter-Perigo S, Kinsella MG, Johnson PY, Braun KR, Goueffic Y, Rosenfeld ME, Wight TN, Hyaluronan accumulation is elevated in cultures of low density lipoprotein receptor-deficient cells and is altered by manipulation of cell cholesterol content, J. Biol. Chem 283 (2008) 36195–36204. 10.1074/jbc.M807772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS, NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis, J. Biol. Chem 285 (2010) 26545–26557. 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vendrov AE, Madamanchi NR, Hakim ZS, Rojas M, Runge MS, Thrombin and NAD(P)H oxidase-mediated regulation of CD44 and BMP4-Id pathway in VSMC, restenosis, and atherosclerosis, Circ. Res 98 (2006) 1254–1263. 10.1161/01.RES.0000221214.37803.79. [DOI] [PubMed] [Google Scholar]
- [48].Wang A, De La Motte C, Lauer M, Hascall V, Hyaluronan matrices in pathobiological processes, FEBS J 278 (2011)1412–1418. 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Karousou E, Misra S, Ghatak S, Dobra K, Götte M, Vigetti D, Passi A, Karamanos NK, Skandalis SS, Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer, Matrix Biol 59 (2017) 3–22. 10.1016/j.matbio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- [50].Hascall V, Karamanos N, Regulatory roles of hyaluronan in health and disease, FEBS J 278 (2011) 1411 10.1111/j.1742-4658.2011.08068.x. [DOI] [PubMed] [Google Scholar]
- [51].Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN, Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration, Matrix Biol 31 (2012) 90–100. 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN, Intracellular hyaluronan: A new frontier for inflammation?, Biochim. Biophys. Acta - Gen. Subj 1673 (2004) 3–12. 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- [53].Evanko SP, Angello JC, Wight TN, Formation of hyaluronan-and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells, Arterioscler. Thromb. Vasc. Biol 19(1999)1004–13. http://atvb.ahajournals.org/cgi/content/abstract/19/4/1004. [DOI] [PubMed] [Google Scholar]
- [54].Day AJ, De La Motte CA, Hyaluronan cross-linking: A protective mechanism in inflammation?, Trends Immunol 26 (2005)637–643. 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [55].Misra S, Hascall VC, Markwald RR, Ghatak S, Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer, Front. Immunol 6 (2015). 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Noble PLJJD, Hyaluronan as an Immune Regulator in Human Diseases, Physiol. Rev 91 (2011) 221–264. 10.1152/physrev.00052.2009.Hyaluronan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Theocharis AD, Karamanos NK, Proteoglycans remodeling in cancer: Underlying molecular mechanisms, Matrix Biol (2017). 10.1016/j.matbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [58].Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P, Genasetti A, Clerici M, Hascall VC, De Luca G, Passi A, Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging, J Biol Chem 283 (2008) 4448–4458. 10.1074/jbc.M709051200. [DOI] [PubMed] [Google Scholar]
- [59].Toole BP, Wight TN, Tammi MI, Hyaluronan-cell interactions in cancer and vascular disease, J. Biol. Chem 277 (2002) 4593–4596. 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- [60].Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S, Hyaluronan-CD44 interactions as potential targets for cancer therapy., FEBS J 278 (2011) 1429–43. 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vigetti D, Rizzi M, Viola M, Karousou E, Genasetti A, Clerici M, Bartolini B, Hascall VC, De Luca G, Passi A, The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells, Glycobiology. 19 (2009) 537–546. 10.1093/glycob/cwp022. [DOI] [PubMed] [Google Scholar]
- [62].Vigetti D, Moretto P, Viola M, Genasetti A, Rizzi M, Karousou E, Clerici M, Bartolini B, Pallotti F, De Luca G, Passi A, Aortic Smooth Muscle Cells Migration and the Role of Metalloproteinases and Hyaluronan, Connect. Tissue Res 49 (2008) 189–192. 10.1080/03008200802143141. [DOI] [PubMed] [Google Scholar]
- [63].Bollyky PL, Bogdani M, Bollyky JB, Hull RL, Wight TN, The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation, Curr. Diab. Rep 12(2012)471–480. 10.1007/s11892-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN, Bollyky PL, 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer, Front. Immunol 6 (2015). 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wilkinson TS, Bressler SL, Evanko SP, Braun KR, Wight TN, Overexpression of hyaluronan synthases alters vascular smooth muscle cell phenotype and promotes monocyte adhesion, J. Cell. Physiol 206 (2006)378–385. 10.1002/jcp.20468. [DOI] [PubMed] [Google Scholar]
- [66].Lemire JM, Merrilees MJ, Braun KR, Wight TN, Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro, J. Cell. Physiol 190 (2002) 38–45. 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- [67].Karousou E, D’Angelo ML, Kouvidi K, Vigetti D, Viola M, Nikitovic D, De Luca G, Passi A, Collagen VI and hyaluronan: The common role in breast cancer, Biomed Res. Int 2014 (2014). 10.1155/2014/606458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vigetti D, Viola M, Karousou E, Deleonibus S, Karamanou K, De Luca G, Passi A, Epigenetics in extracellular matrix remodeling and hyaluronan metabolism, FEBS J 281 (2014)4980–4992. 10.1111/febs.12938. [DOI] [PubMed] [Google Scholar]
- [69].Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC, Passi A, Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB) pathway., J. Biol. Chem 285 (2010) 24639–45. 10.1074/jbc.M110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu J, Ren Y, Kang L, Zhang L, Oxidized low-density lipoprotein increases the proliferation and migration of human coronary artery smooth muscle cells through the upregulation of osteopontin, Int. J. Mol. Med 33 (2014) 1341–1347. 10.3892/ijmm.2014.1681. [DOI] [PubMed] [Google Scholar]
- [71].Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW, Reprint of: A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease, Matrix Biol 35 (2014) 162–173. 10.1016/j.matbio.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Li D, Mehta JL, Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells, Circulation. 101 (2000) 2889–2895. 10.1161/01.CIR.101.25.2889. [DOI] [PubMed] [Google Scholar]
- [73].Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL, LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells, Circulation. 107 (2003) 612–617. 10.1161/01.CIR.0000047276.52039.FB. [DOI] [PubMed] [Google Scholar]
- [74].Magalhaes A, Matias I, Palmela I, Brito MA, Dias S, LDL-cholesterol increases the transcytosis of molecules through endothelial monolayers, PLoS One. 11 (2016). 10.1371/journal.pone.0163988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vink H, Constantinescu AA, Spaan JAE, Oxidized Lipoproteins Degrade the Endothelial Surface Layer: Implications for Platelet-Endothelial Cell Adhesion, Circulation. 101 (2000) 1500–1502. 10.1161/01.CIR.101.13.1500. [DOI] [PubMed] [Google Scholar]
- [76].Kolàřová H, Ambrůzovà B, Švihalkovà Šindlerovà L, Klinke A, Kubala L, Modulation of endothelial glycocalyx structure under inflammatory conditions, Mediators Inflamm 2014 (2014). 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nagy N, Freudenberger T, Melchior-Becker A, Röck K, Ter Braak M, Jastrow H, Kinzig M, Lucke S, Suvorava T, Kojda G, Weber AA, Sörgel F, Levkau B, Ergün S, Fischer JW, Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: Novel insights into the role of hyaluronan synthesis, Circulation. 122 (2010) 2313–2322. 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- [78].Mambetsariev N, Mirzapoiazova T, Mambetsariev B, Sammani S, Lennon FE, Garcia JGN, Singleton PA, Hyaluronic acid binding protein 2 Is a novel regulator of vascular integrity, Arterioscler. Thromb. Vasc. Biol 30 (2010)483–490. 10.1161/ATVBAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JGN, High molecular weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness, Am J Physiol Lung Cell Mol Physiol (2010) ajplung.00405.2009 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Maroski J, Vorderw??Ibecke BJ, Fiedorowicz K, Da Silva-Azevedo L, Siegel G, Marki A, Pries AR, Zakrzewicz A, Shear stress increases endothelial hyaluronan synthase 2 and hyaluronan synthesis especially in regard to an atheroprotective flow profile, Exp. Physiol 96 (2011) 977–986. 10.1113/expphysiol.2010.056051. [DOI] [PubMed] [Google Scholar]
- [81].Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC, Passi A, Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-κB (NF-κB) pathway, J. Biol. Chem 285 (2010) 24639–24645. 10.1074/jbc.M110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yu XH, Zheng XL, Tang CK, Nuclear Factor-κΒ Activation as a Pathological Mechanism of Lipid Metabolism and Atherosclerosis, Adv. Clin. Chem 70 (2015) 1–30. 10.1016/bs.acc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- [83].Oeckinghaus A, Ghosh S, The NF-κB Family of Transcription Factors and Its Regulation, Cold Spring Harb. Perspect. Biol 1 (2009) a000034 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJJ, Kardakaris R, Polykratis A, Kollias G, de Winther MPJ, Pasparakis M, Endothelial Cell-Specific NF-κB Inhibition Protects Mice from Atherosclerosis, Cell Metab 8 (2008) 372–383. 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- [85].Lockette W, Otsuka Y, Carretero O, The loss of endothelium-dependent vascular relaxation in hypertension., Hypertension. 8 (1986) 1161–6. 10.1161/01.HYP.8.6. [DOI] [PubMed] [Google Scholar]
- [86].Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P, Systemic nature of endothelial dysfunction in atherosclerosis, Am. J. Cardiol 75 (1995). 10.1016/0002-9149(95)80017-M. [DOI] [PubMed] [Google Scholar]
- [87].Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ, Nitric oxide and atherosclerosis: An update, Nitric Oxide - Biol. Chem 15 (2006) 265–279. 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [88].Singleton PA, Bourguignon LYW, CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation, Exp. Cell Res 295 (2004)102–118. 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- [89].Virmani R, Burke AP, Kolodgie FD, Farb A, Vulnerable Plaque: The Pathology of Unstable Coronary Lesions, J. Interv. Cardiol 15 (2002) 439–446. 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- [90].Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M, Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E., Proc. Natl. Acad. Sci. U. S. A 92 (1995) 8264–8. 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mantovani A, Locati M, Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization lessons and open questions, Arterioscler. Thromb. Vasc. Biol 33 (2013) 1478–1483. 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- [92].Noble Paul JD, Jiurong Liang, Macrophage plasticity in experimental atherosclerosis., Physiol. Rev 91 (2011) 221–264. 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Moore KJ, Sheedy FJ, Fisher EA, Macrophages in atherosclerosis: a dynamic balance, Nat. Rev. Immunol 13 (2013) 709–721. 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Puré E, Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages., J. Immunol 159 (1997)2492–2500. [PubMed] [Google Scholar]
- [95].Cybulsky MI, Cheong C, Robbins CS, Macrophages and Dendritic Cells: Partners in Atherogenesis, Circ. Res 118 (2016) 637–652. 10.1161/CIRCRESAHA.115.306542. [DOI] [PubMed] [Google Scholar]
- [96].Marques L, Negre-Salvayre A, Costa L, Canonne-Hergaux F, Iron gene expression profile in atherogenic Mox macrophages, Biochim. Biophys. Acta - Mol. Basis Dis 1862 (2016) 1137–1146. 10.1016/j.bbadis.2016.03.004. [DOI] [PubMed] [Google Scholar]
- [97].de Gaetano M, Crean D, Barry M, Belton O, M1- and M2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis, Front. Immunol 7 (2016). 10.3389/fimmu.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Stöger JL, Gijbels MJJ, van der Velden S, Manca M, van der Loos CM, Biessen E. a L., Daemen M.J. a P., Lutgens E, de Winther MPJ, Distribution of macrophage polarization markers in human atherosclerosis., Atherosclerosis. 225 (2012) 461–8. 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- [99].Hoeksema MA, Stöger JL, De Winther MPJ, Molecular pathways regulating macrophage polarization: Implications for atherosclerosis, Curr. Atheroscler. Rep 14 (2012) 254–263. 10.1007/s11883-012-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, Haulon S, Zawadzki C, Jude B, Staels B, Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways, Circ. Res 108 (2011)985–995. 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang N, Liang H, Zen K, Molecular mechanisms that influence the macrophage M1-M2 polarization balance, Front. Immunol 5 (2014). 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Park SH, Sui Y, Gizard F, Xu J, Rios-Pilier J, Helsley RN, Han SS, Zhou C, Myeloid-specific IkappaB kinase beta deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice, Arter. Thromb Vasc Biol 32 (2012) 2869–2876. doi:ATVBAHA.112.254573 [pii]\r10.1161/ATVBAHA.112.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Goossens P, Vergouwe MN, Gijbels MJJ, Curfs DMJ, van Woezik JHG, Hoeksema MA, Xanthoulea S, Leenen PJM, Rupec RA, Hofker MH, de Winther MPJ, Myeloid IκBα deficiency promotes atherogenesis by enhancing leukocyte recruitment to the plaques, PLoS One. 6 (2011). 10.1371/journal.pone.0022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].a Detmers P, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, Weikel R, Bergstrom JD, Shevell DE, Hermanowski-Vosatka a, Sparrow CP, Chao YS, Rader DJ, Wright SD, Puré E, Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice., J. Immunol 165 (2000)3430–3435. 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- [105].Huang H, Koelle P, Fendler M, Schröttle A, Czihal M, Hoffmann U, Conrad M, Kuhlencordt PJ, Induction of inducible nitric oxide synthase (iNOS) expression by oxLDL inhibits macrophage derived foam cell migration, Atherosclerosis. 235 (2014) 213–222. 10.1016/j.atherosclerosis.2014.04.020. [DOI] [PubMed] [Google Scholar]
- [106].Janani C, Ranjitha Kumari BD, PPAR gamma gene – A review, Diabetes Metab. Syndr. Clin. Res. Rev 9 (2015) 46–50. 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- [107].Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G, PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties, Cell Metab 6 (2007) 137–143. 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- [108].Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B, PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway, Nat. Med 7 (2001) 53–58. 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- [109].Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, Milstone DS, Mortensen RM, Spiegelman BM, Freeman MW, The role of PPAR-gamma in macrophage differentiation and cholesterol uptake., Nat. Med 7 (2001) 41–47. 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- [110].Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, a Davis R, Willson TM, Witztum JL, Palinski W, Glass CK, Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma., J. Clin. Invest 114 (2004) 1564–76. 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mahamuni SP, Khose RD, Menaa F, Badole SL, Therapeutic approaches to drug targets in hyperlipidemia, BioMedicine. 2 (2012) 137–146. 10.1016/j.biomed.2012.08.002. [DOI] [Google Scholar]
- [112].Jiang C, Ting AT, Seed B, PPAR-γ agonists inhibit production of monocyte inflammatory cytokines, Nature. 391 (1998) 82–86. 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- [113].Law RE, Meehan WP, Xi XP, Graf K, a Wuthrich D, Coats W, Faxon D, a Hsueh W, Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia., J. Clin. Invest 98 (1996) 1897–905. 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hollingsworth JW, Li Z, Brass DM, Carantziotis S, Timberlake SH, Kim A, Hossain I, Savani RC, Schwartz DA, CD44 regulates macrophage recruitment to the lung in lipopolysaccharide- induced airway disease, Am. J. Respir. Cell Mol. Biol 37 (2007)248–253. 10.1165/rcmb.2006-03630C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Leemans JC, Florquin S, Heikens M, Pals ST, Van der Neut R, Van der Poll T, CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates macrophage recruitment and protective immunity against tuberculosis, J. Clin. Invest 111 (2003)681–689. 10.1172/JCI200316936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Weiss JM, Renkl AC, Ahrens T, Moll J, Mai BH, Denfeld RW, Schöpf E, Ponta H, Herrlich P, Simon JC, Activation-dependent modulation of hyaluronate-receptor expression and of hyaluronate-avidity by human monocytes, J. Invest. Dermatol Ill (1998) 227–232. 10.1046/j.1523-1747.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- [117].Levesque MC, Haynes BF, Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNF-alpha and is inhibited by IL-4 and IL-13., J. Immunol 159 (1997) 6184–6194. [PubMed] [Google Scholar]
- [118].Levesque MC, Haynes BF, Activated T lymphocytes regulate hyaluronan binding to monocyte CD44 via production of IL-2 and IFN-gamma., J. Immunol 166 (2001) 188– 196. 10.4049/jimmunol.166.1.188. [DOI] [PubMed] [Google Scholar]
- [119].Levesque MC, Haynes BF, TNFalpha and IL-4 regulation of hyaluronan binding to monocyte CD44 involves posttranslational modification of CD44., Cell. Immunol 193 (1999)209–18. 10.1006/cimm.1999.1456. [DOI] [PubMed] [Google Scholar]
- [120].Ruffell B, Poon GFT, Lee SSM, Brown KL, Tjew SL, Cooper J, Johnson P, Differential use of chondroitin sulfate to regulate hyaluronan binding by receptor CD44 in inflammatory and interleukin 4-activated macrophages, J. Biol. Chem 286 (2011) 19179–19190. 10.1074/jbc.M110.200790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Brown KL, a Maiti, Johnson P, Role of sulfation in CD44-mediated hyaluronan binding induced by inflammatory mediators in human CD14(+) peripheral blood monocytes., J. Immunol 167 (2001) 5367–74. http://www.ncbi.nlm.nih.gov/pubmed/11673554. [DOI] [PubMed] [Google Scholar]
- [122].McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW, Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages: The role of HA size and CD44, J. Clin. Invest 98 (1996) 2403–2413. 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA, High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation, ACS Biomater. Sci. Eng 1 (2015) 481–493. 10.1021/acsbiomaterials.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AMK, Noble PW, Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor κB-dependent mechanism, J. Biol. Chem 272 (1997)8013–8018. 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- [125].Yamawaki H, Hirohata S, Miyoshi T, Takahashi K, Ogawa H, Shinohata R, Demircan K, Kusachi S, Yamamoto K, Ninomiya Y, Hyaluronan receptors involved in cytokine induction in monocytes, Glycobiology. 19 (2009) 83–92. 10.1093/glycob/cwn109. [DOI] [PubMed] [Google Scholar]
- [126].He H, Zhang S, Tighe S, Son J, Tseng SCG, Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide- activated macrophages toward m2 phenotype, J. Biol. Chem 288 (2013) 25792– 25803. 10.1074/jbc.M113.479584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Noble HSS, Paul W McKee Charlotte Cowman Mary M, Hyaluronan fragments activate an NFKB/IKBa autoregulatory loop in murine macrophages, J Exp Med 183 (1996)2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Steffan NM, Bren GD, Frantz B, Tocci MJ, O’Neill EA, V Paya C, Regulation of IκB alpha phosphorylation by PKC- and Ca(2+)-dependent signal transduction pathways., J. Immunol 155 (1995) 4685–91. http://www.ncbi.nlm.nih.gov/pubmed/7594468. [PubMed] [Google Scholar]
- [129].Oertli B, Beck-Schimmer B, Fan X, Wuthrich RP, Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubullar epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-kappa B and activat, J.Immunol 161 (1998) 3431–3437. [PubMed] [Google Scholar]
- [130].Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT, CD44 Costimulation Promotes FoxP3+ Regulatory T Cell Persistence and Function via Production of IL-2, IL-10, and TGF-, J. Immunol 183 (2009) 2232–2241. 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tse K, Tse H, Sidney J, Sette A, Ley K, T cells in atherosclerosis, Int. Immunol 25 (2013) 615–622. 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Baaten BJG, Li CR, Bradley LM, Multifaceted regulation of T cells by CD44, Commun. Integr. Biol 3 (2010) 508–512. 10.4161/cib.3.6.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Siegelman MH, Stanescu D, Estess P, The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion, J. Clin. Invest 105 (2000) 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bonder CS, Clark SR, Norman MU, Johnson P, Kubes P, Use of CD44 by CD4+ Th1 and Th2 lymphocytes to roll and adhere, Blood. 107 (2006) 4798–4806. 10.1182/blood-2005-9-3581. [DOI] [PubMed] [Google Scholar]
- [135].Homann S, Grandoch M, Kiene LS, Podsvyadek Y, Feldmann K, Rabausch B, Nagy N, Lehr S, Kretschmer I, Oberhuber A, Bollyky P, Fischer JW, Hyaluronan synthase 3 promotes plaque inflammation and atheroprogression, Matrix Biol (2017). 10.1016/j.matbio.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z, Natural regulatory T cells control the development of atherosclerosis in mice, Nat. Med 12 (2006) 178–180. 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- [137].Chistiakov DA, Orekhov AN, Bobryshev YV, Vascular smooth muscle cell in atherosclerosis, Acta Physiol. (Oxf) 214 (2015) 33–50. 10.1111/apha.12466. [DOI] [PubMed] [Google Scholar]
- [138].Foster LC, Arkonac BM, Sibinga NES, Shi CW, Perrella MA, Haber E, Regulation of CD44 gene expression by the proinflammatory cytokine interleukin-1 beta in vascular smooth muscle cells, J. Biol. Chem 273 (1998) 20341–20346. 10.1074/jbc.273.32.20341. [DOI] [PubMed] [Google Scholar]
- [139].Rudijanto A, The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis., Acta Med. Indones 39 (2007) 86–93. 10.1159/000108992. [DOI] [PubMed] [Google Scholar]
- [140].Allahverdian S, Pannu PS, Francis GA, Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation, Cardiovasc. Res 95 (2012) 165–172. 10.1093/cvr/cvs094. [DOI] [PubMed] [Google Scholar]
- [141].Chistiakov DA, Orekhov AN, Bobryshev YV, Vascular smooth muscle cell in atherosclerosis, Acta Physiol 214(2015)33–50. 10.1111/apha.12466. [DOI] [PubMed] [Google Scholar]
- [142].Lao KH, Zeng L, Xu Q, Endothelial and smooth muscle cell transformation in atherosclerosis, Curr. Opin. Lipidol 26 (2015) 1 10.1097/MOL.0000000000000219. [DOI] [PubMed] [Google Scholar]
- [143].Cecchettini A, Rocchiccioli S, Boccardi C, Citti L, Vascular Smooth-Muscle-Cell Activation. Proteomics Point of View, Int. Rev. Cell Mol. Biol 288 (2011) 43–99. 10.1016/B978-0-12-386041-5.00002-9. [DOI] [PubMed] [Google Scholar]
- [144].Lepidi S, Kenagy RD, Raines EW, Chiu ES, Chait A, Ross R, Clowes AW, MMP9 production by human monocyte-derived macrophages is decreased on polymerized type I collagen, J. Vasc. Surg 34 (2001) 1111–1118. 10.1067/mva.2001.119401. [DOI] [PubMed] [Google Scholar]
- [145].Wesley RB, Meng X, Godin D, Galis ZS, Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro., Arterioscler. Thromb. Vasc. Biol 18 (1998)432–440. 10.1161/01.ATV.18.3.432. [DOI] [PubMed] [Google Scholar]
- [146].Kaplan G, Gaudernack G, In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen., J. Exp. Med 156 (1982) 1101–14. 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Rong JX, Shapiro M, Trogan E, Fisher EA, Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading., Proc. Natl. Acad. Sci. U. S. A 100 (2003) 13531–6. 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P, Genasetti A, Clerici M, Hascall VC, De Luca G, Passi A, Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging, J.Biol.Chem 283 (2008) 4448–4458. http://www.jbc.org/cgi/content/abstract/283/7/4448. [DOI] [PubMed] [Google Scholar]
- [149].Grandoch M, Hoffmann J, Röck K, Wenzel F, Oberhuber A, Schelzig H, Fischer JW, Novel effects of adenosine receptors on pericellular hyaluronan matrix: Implications for human smooth muscle cell phenotype and interactions with monocytes during atherosclerosis, Basic Res. Cardiol 108 (2013). 10.1007/s00395-013-0340-6. [DOI] [PubMed] [Google Scholar]
- [150].Viola M, Bartolini B, Vigetti D, Karousou E, Moretto P, Deleonibus S, Sawamura T, Wight TN, Hascall VC, De Luca G, Passi A, Oxidized low density lipoprotein (LDL) affects hyaluronan synthesis in human aortic smooth muscle cells., J. Biol. Chem 288 (2013)29595–603. 10.1074/jbc.M113.508341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Bobryshev YV, Monocyte recruitment and foam cell formation in atherosclerosis, Micron 37 (2006) 208–222. 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [152].Yin YW, Liao SQ, Zhang MJ, Liu Y, Li BH, Zhou Y, Chen L, Gao CY, Li JC, Zhang LL, TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression., Cell Death Dis 5 (2014) e1574 10.1038/cddis.2014.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Chaabane C, Coen M, Bochaton-Piallat M-L, Smooth muscle cell phenotypic switch: implications for foam cell formation., Curr. Opin. Lipidol 25 (2014) 374–9. 10.1097/MOL.0000000000000113. [DOI] [PubMed] [Google Scholar]
- [154].Chellan B, Reardon CA, Getz GS, Bowman MAH, Enzymatically modified low-density lipoprotein promotes foam cell formation in smooth muscle cells via macropinocytosis and enhances receptor-mediated uptake of oxidized low-density lipoprotein, Arterioscler. Thromb. Vasc. Biol 36 (2016) 1101–1113. 10.1161/ATVBAHA.116.307306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R, Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis, Circ. Res 115 (2014) 662–667. 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- [156].Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AAC, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK, KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis, Nat. Med 21 (2015) 628–637. 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Tabata T, Mine S, Okada Y, Tanaka Y, Low molecular weight hyaluronan increases the Uptaking of oxidized LDL into monocytes., Endocr.J 54 (2007) 685–93. 10.1507/endocrj.K05-120. [DOI] [PubMed] [Google Scholar]
- [158].Kishikawa H, Mine S, Kawahara C, Tabata T, Hirose A, Okada Y, Tanaka Y, Glycated albumin and cross-linking of CD44 induce scavenger receptor expression and uptake of oxidized LDL in human monocytes, Biochem. Biophys. Res. Commun 339 (2006) 846–851. 10.1016/j.bbrc.2005.11.091. [DOI] [PubMed] [Google Scholar]
- [159].Schrijvers DM, De Meyer GRY, Kockx MM, Herman AG, Martinet W, Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis, Arterioscler. Thromb. Vasc. Biol 25 (2005) 1256–61. 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- [160].Bauriedel G, Hutter R, Welsch U, Bach R, Sievert H, Lüderitz B, Role of smooth muscle cell death in advanced coronary primary lesions: Implications for plaque instability, Cardiovasc. Res 41 (1999) 480–488. 10.1016/S0008-6363(98)00318-6. [DOI] [PubMed] [Google Scholar]
- [161].Vigetti D, Rizzi M, Moretto P, Deleonibus S, Dreyfuss JM, Karousou E, Viola M, Clerici M, Hascall VC, Ramoni MF, De Luca G, Passi A, Glycosaminoglycans and Glucose Prevent Apoptosis in 4-Methylumbelliferone-treated Human Aortic Smooth Muscle Cells, J. Biol. Chem 286 (2011) 34497–34503. 10.1074/jbc.M111.266312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kinscherf R, Deigner HP, Usinger C, Pill J, Wagner M, Kamencic H, Hou D, Chen M, Schmiedt W, Schrader M, Kovacs G, Kato K, Metz J, Induction of mitochondrial manganese superoxide dismutase in macrophages by oxidized LDL: its relevance in atherosclerosis of humans and heritable hyperlipidemic rabbits., FASEB J 11 (1997) 1317–28. http://www.ncbi.nlm.nih.gov/pubmed/9409551. [DOI] [PubMed] [Google Scholar]
- [163].Kinscherf R, Wagner M, Kamencic H, Bonaterra GA, Hou D, Schiele RA, Deigner HP, Metz J, Characterization of apoptotic macrophages in atheromatous tissue of humans and heritable hyperlipidemic rabbits, Atherosclerosis. 144 (1999) 33–39. 10.1016/S0021-9150(99)00037-4. [DOI] [PubMed] [Google Scholar]
- [164].Vivers S, Dransfield I, Hart SP, Role of macrophage CD44 in the disposal of inflammatory cell corpses., Clin. Sci (Lond) 103 (2002) 441–449. 10.1042/CS20020115. [DOI] [PubMed] [Google Scholar]
- [165].Hart SP, Dougherty GJ, Haslett C, Dransfield I, CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages., J. Immunol 159 (1997)919–25. http://www.ncbi.nlm.nih.gov/pubmed/9218612. [PubMed] [Google Scholar]
- [166].Hart SP, Rossi AG, Haslett C, Dransfield I, Characterization of the effects of cross-linking of macrophage CD44 associated with increased phagocytosis of apoptotic PMN, PLoSOne. 7 (2012). 10.1371/journal.pone.0033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Teder P, Resolution of Lung Inflammation by CD44, Science (80-.) 296 (2002) 155–158. 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- [168].Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG, Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis., Circ. J 80 (2016) 2259–2268. 10.1253/circj.CJ-16-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA, Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques, Circulation. 123 (2011) 989–998. 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA, Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein e-deficient mouse in a novel transplantation model, J. Vasc. Surg 34 (2001) 541–547. 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- [171].Verschuren L, de Vries-van der Weij J, Zadelaar S, Kleemann R, Kooistra T, LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoE*3Leiden mice: time course and mechanisms., J. Lipid Res 50 (2009) 301–11. 10.1194/jlr.M800374-JLR200. [DOI] [PubMed] [Google Scholar]
- [172].Lin J, Li M, Wang Z, He S, Ma X, Li D, The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation, J Lipid Res 51 (2010) 1208–1217. doi:jlr.D000497 [pii]\r10.1194/jlr.D000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, Von Vietinghoff S, Galkina E, Miller Y.l., Acton ST, Ley K, Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis, J. Clin. Invest 122 (2012) 3114–3126. 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Cho HJ, Shashkin P, a Gleissner C, Dunson D, Jain N, Lee JK, Miller Y, Ley K, Induction of dendritic cell-like phenotype in macrophages during foam cell formation., Physiol. Genomics 29 (2007) 149–160. 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- [175].Hegde VL, Singh NP, Nagarkatti PS, Nagarkatti M, CD44 mobilization in allogeneic dendritic cell-T cell immunological synapse plays a key role in T cell activation, J. Leukoc. Biol 84 (2008) 134–142. 10.1189/jlb.1107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Do Y, Nagarkatti PS, Nagarkatti M, Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells., J. Immunother 27 (2004) 1–12. 10.1097/00002371-20040100000001. [DOI] [PubMed] [Google Scholar]
- [177].Termeer C, Averbeck M, Hara H, Eibel H, Herrlich P, Sleeman J, Simon JC, Targeting dendritic cells with CD44 monoclonal antibodies selectively inhibits the proliferation of naive CD4+ T-helper cells by induction of FAS-independent T-cell apoptosis, Immunology. 109 (2003) 32–40. 10.1046/j.1365-2567.2003.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Hartwig H, Silvestre Roig C, Daemen M, Lutgens E, Soehnlein O, Neutrophils in atherosclerosis: A brief overview, Hamostaseologie. 35 (2015) 121–127. 10.5482/HAMO-14-09-0040. [DOI] [PubMed] [Google Scholar]
- [179].Krettek A, Sukhova GK, Schönbeck U, Libby P, Enhanced expression of CD44 variants in human atheroma and abdominal aortic aneurysm: possible role for a feedback loop in endothelial cells., Am. J. Pathol 165 (2004) 1571–81. 10.1016/S0002-9440(10)63414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Watanabe R, Watanabe H, Takahashi Y, Kojima M, Konii H, Watanabe K, Shirai R, Sato K, Matsuyama T, Ishibashi-Ueda H, Iso Y, Koba S, Kobayashi Y, Hirano T, Watanabe T, Atheroprotective Effects of Tumor Necrosis FactorγStimulated Gene-6, JACC Basic to Transl. Sci 1 (2016) 494–509. 10.1016/j.jacbts.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Taher M, Nakao S, Zandi S, Melhorn MI, Hayes KC, Hafezi-Moghadam A, Phenotypic transformation of intimal and adventitial lymphatics in atherosclerosis: A regulatory role for soluble VEGF receptor 2, FASEB J 30 (2016)2490–2499. 10.1096/fj.201500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Singh M, Ananthula S, Milhorn DM, Krishnaswamy G, Singh K, Osteopontin: a novel inflammatory mediator of cardiovascular disease., Front. Biosci 12 (2007) 214–21. 10.2741/2059. [DOI] [PubMed] [Google Scholar]
- [183].Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K, Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta, J. Clin. Invest 92 (1993) 2814–2820. 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Chung EJ, Targeting and therapeutic peptides in nanomedicine for atherosclerosis., Exp. Biol. Med (Maywood) (2016) 1535370216640940 10.1177/1535370216640940. [DOI] [PMC free article] [PubMed] [Google Scholar]