Abstract
Historically, Australia was considered free of rabies and rabieslike viruses. Thus, the identification of Australian bat lyssavirus (ABLV) in 1996 in a debilitated bat found by a member of the public precipitated both public health consternation and a revision of lyssavirus taxonomy. Subsequent observational studies sought to elaborate the occurrence and frequency of ABLV infection in Australian bats. This paper describes the taxonomic diversity of bat species showing evidence of ABLV infection to better inform public health considerations. Blood and/or brain samples were collected from two cohorts of bats (wild‐caught and diagnostic submissions) from four Australian states or territories between April 1996 and October 2002. Fresh brain impression smears were tested for ABLV antigen using fluorescein‐labelled anti‐rabies monoclonal globulin (CENTOCOR) in a direct fluorescent antibody test; sera were tested for the presence of neutralising antibodies using a rapid fluorescent focus inhibition test. A total of 3,217 samples from 2,633 bats were collected and screened: brain samples from 1,461 wild‐caught bats and 1,086 submitted bats from at least 16 genera and seven families, and blood samples from 656 wild‐caught bats and 14 submitted bats from 14 genera and seven families. Evidence of ABLV infection was found in five of the six families of bats occurring in Australia, and in three of the four Australian states/territories surveyed, supporting the historic presence of the virus in Australia. While the infection prevalence in the wild‐caught cohort is evidently low, the significantly higher infection prevalence in rescued bats in urban settings represents a clear and present public health significance because of the higher risk of human exposure.
Keywords: Australia, bat, lyssavirus, public health, reservoir host
Impacts.
With Australia historically considered free of lyssaviruses, the emergence/detection of Australian bat lyssavirus (ABLV) posed a risk management challenge in a (then) naïve public and public health landscape.
Numerous bat taxa have a regular urban presence in Australia, and well‐meaning members of the public regularly “rescue” sick and injured bats for rehabilitation through an established carer network.
While the ABLV infection prevalence in wild‐caught bats is low, the significantly higher infection prevalence in rescued sick and injured bats represents a clear and present public health danger because of the higher risk of human exposure.
1. INTRODUCTION
Australian bat lyssavirus (ABLV) was serendipitously identified in 1996 (Fraser et al., 1996) during active surveillance of pteropodid bats (flying foxes) for Hendra virus, a novel zoonotic paramyxovirus which dramatically emerged in 1994 in Australia (Murray et al., 1995). Lyssaviruses belong to the family Rhabdoviridae, and prior to the description of ABLV, there were six recognised species/genotypes: classical rabies virus (genotype 1), Lagos bat virus (genotype 2), Mokola virus (genotype 3), Duvenhage virus (genotype 4), European bat lyssavirus 1 (genotype 5) and European bat lyssavirus 2 (genotype 6). The description of ABLV precipitated a revision of lyssavirus taxonomy, with genotype 7 defined to accommodate it (Badrane, Bahloul, Perrin, & Tordo, 2001; Gould et al., 1998). Sequence analysis of the ABLV nucleocapsid protein gene (used as the basis for genotypic classification) showed marked nucleotide and amino acid homology (73%–74% and 92%, respectively) with classical rabies viruses (CRV), and the ABLV phosphoprotein, matrix protein and glycoprotein genes also show a closer sequence homology to CRV than to the other lyssaviruses (Gould et al., 1998). Further, ABLV and CRV share the same serotype (1), with cross‐neutralisation evident against 19 of a panel of 21 monoclonal antibodies (Gould et al., 1998). Rabies vaccine and anti‐rabies immunoglobulin protect laboratory animals from ABLV infection (Hooper et al., 1997) and are evidently equally effective in humans, although Brookes, Parsons, Johnson, McElhinney, and Fooks (2005) suggest that maximum vaccine efficacy against ABLV (and other non‐RABV lyssaviruses) may require a higher virus‐neutralising antibody threshold. Two variants of ABLV have been described, with isolates from pteropodid bats (Pteropus spp.) and yellow‐bellied sheathtail bats (Saccolaimus flaviventris) falling into two distinct clades by sequence analysis (Gould, Kattenbelt, Gumley, & Lunt, 2002; Gould, Kattenbelt, Hyatt, Gumley, & Lunt, 1999; Warrilow, Smith, Harrower, & Smith, 2002).
Historically, Australia was considered free of rabies and rabieslike viruses. Rhabdoviruses from the genus Ephemerovirus were known to occur (e.g.,Bovine ephemeral fever, Adelaide River virus), but none from the genus Lyssavirus had been described. Prophetically, St George (1989), postulating the origins of Adelaide River virus, suggested the possibility of an undiscovered rabieslike virus in Australian bats. St. George went further, suggesting that the typically low prevalence of lyssavirus infections in bats meant that an Australian bat lyssavirus might not become evident unless active surveillance was undertaken or unless a human or a domestic animal was infected by a bat.
Within 6 months of the identification of ABLV in flying foxes, the zoonotic capability of the virus became evident when a wildlife carer in central Queensland developed a fatal rabies illness in October 1996 (Allworth, Murray, & Morgan, 1996). A second fatal human case occurred in December 1998, 27 months after a bite from a flying fox (Hanna et al., 2000). These cases precipitated an aggressive public heath campaign promoting avoidance of bat contact and rabies vaccination for vocationally at‐risk people. No further human cases were reported until 2012 when a young boy contracted a fatal infection following unsolicited contact with a bat (Francis et al., 2014). This tragic case reignited public and media interest in ABLV, which was further promoted by two related equine cases in 2013 (Shinwari et al., 2014).
This paper presents the findings of observational studies which investigated the occurrence and frequency of ABLV infection in Australian bats. The primary aim was to scope the taxonomic diversity of bat species showing evidence of infection to inform public health considerations.
2. METHODS
2.1. Animal ethics
Fieldwork was approved under the (then) Queensland Government Department of Primary Industries and the Western Australian Government Department of Conservation and Land Management Animal Ethics Committee permits Bribie/31/00 and CAEEC11/98, respectively. All capture events and all methods (detailed below) were specifically approved under the permits. All capture, handling, anaesthesia and euthanasia were undertaken by or directly supervised by an experienced veterinarian.
2.2. Sample characteristics
Blood and/or brain samples were collected from bats in the Australian states of Queensland, New South Wales and Western Australia, and the Northern Territory between April 1996 and October 2002 (Field, 2005). Samples were obtained from two cohorts of bats: actively sampled and evidently healthy free‐living bats (hereafter referred to as “wild‐caught” bats) and sick, injured or recently dead bats submitted to the Queensland Government veterinary laboratory in Brisbane for diagnostic testing (“submitted” bats). Wild‐caught bats were typically captured in mist nets or harp traps between dusk and dawn, either as they left or returned to their roost or as they foraged (Epstein & Field, 2011). Bats from which only blood was taken were typically sampled under inhalation anaesthetic (Jonsson, Johnston, Field, Jong, & Smith, 2004) with individuals released at the point of capture within four hours. Bats from which brain (or brain and blood) samples were taken were humanely euthanised immediately prior to sampling: larger bats (Pteropodidae and Megadermatidae) by a lethal dose of barbiturate (Lethabarb®, 325 mg/ml pentobarbitone sodium, Virbac) intravenously or (diluted 1:3) intraperitoneally and smaller bats (Hipposideridae and Vespertilioniformes) by the inhalation of CO2. Event and individual animal data including location, date, genus/species, sex and age were recorded.
2.3. Test characteristics
Fresh brain impression smears were tested for ABLV antigen at the Queensland Government veterinary laboratory using fluorescein‐labelled anti‐rabies monoclonal globulin (CENTOCOR) in a direct fluorescent antibody test (FAT). Smears were made from at least three sites (medulla, cerebellum and hippocampus) on the cut brain surface. Antigen detection provides direct evidence of current infection.
Sera were forwarded to the Australian Animal Health Laboratory (AAHL) in Geelong for testing for the presence of neutralising antibodies using the rapid fluorescent focus inhibition test (RFFIT), as reported by Smith, Yager, and Baer (1973). Briefly, the method entails infecting BHK‐21 cells with a tissue culture‐adapted rabies virus (CVS‐11). Subsequently, an equal volume of challenge virus is added to serum dilutions, and after 24 hr of incubation, the number of fluorescing fields counted. The criterion for positivity was a 50% reduction in infectious centres relative to a positive (NIH reference serum) control. Given the absence of classical rabies or other serotype 1 lyssaviruses in Australia, this assay provided a practical screening test while a specific ABLV RFFIT was being developed. Antibody detection provides indirect evidence of past exposure or infection.
2.4. Analysis
Descriptive summary statistics are used to describe the data. Within sample cohorts, 95% confidence intervals are used to indicate significant difference. Sparse data precluded definitive statistical analyses.
3. RESULTS
A total of 3,217 brain and/or blood samples were collected from 2,633 bats (Figure 1). Brain samples were collected from 2,547 bats, being 1,461 wild‐caught bats (Table 1) and 1,086 submitted bats (Table 2). Individuals from at least 16 genera and seven families (44 submitted bats were unidentified) and both chiropteran suborders were sampled, with a median genus sample size of 26.5 (range 2–902). Antigen was not detected in any wild‐caught bat, but was detected in 74 (6.8%) submitted bats from two genera and four species: Pteropus alecto, Pteropus poliocephalus, Pteropus scapulatus and S. flaviventris (Table 3).
Figure 1.
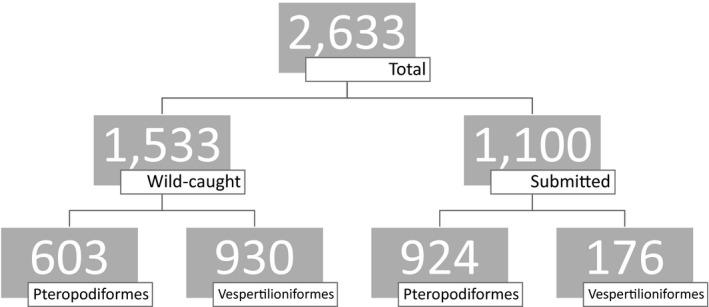
Composition of a sample of 2,633 bats screened for ABLV antigen and/or antibody
Table 1.
Australian bat lyssavirus surveillance in 1,5331 wild‐caught bats in Queensland, Western Australia and the Northern Territory between April 1996 and October 2002
| Suborder | Family | Genus | Antigen detection (FAT) | Antibody detection (RFFIT) | ||
|---|---|---|---|---|---|---|
| Number tested | Number (%, 95% CI) positive | Number tested | Number (%, 95% CI) positive | |||
| Pteropodiformes | Pteropodidae | Pteropus | 475 | 0 | 266 | 8 (3.0, 1.5–5.8) |
| Megadermatidae | Macroderma | 0 | 0 | 68 | 1 (1.5, 0.3–7.9) | |
| Hipposideridae | Hipposideros | 30 | 0 | 30 | 1 (3.3, 0.6–16.7) | |
| Vespertilioniformes | Mollosidae | Chaerophon | 4 | 0 | 2 | 1 (50.0, 9.5–90.6) |
| Mormopterus | 236 | 0 | 3 | 0 | ||
| Tadarida | 45 | 0 | 45 | 1 (2.2, 0.4–11.6) | ||
| Vespertilionidae | Chalinolobus | 61 | 0 | 55 | 2 (3.6, 1.0–12.3) | |
| Myotis | 34 | 0 | 14 | 0 | ||
| Nyctophilus | 2 | 0 | 1 | 0 | ||
| Scotorepens | 64 | 0 | 2 | 0 | ||
| Vespedalus | 51 | 0 | 45 | 1 (2.2, 0.4–11.6) | ||
| Miniopteridae | Miniopterus | 393 | 0 | 60 | 0 | |
| Emballonuridae | Saccolaimus | 26 | 0 | 24 | 3 (12.5, 4.4–31.0) | |
| Taphozous | 40 | 0 | 41 | 0 | ||
| Total | 1,461 | 0 | 656 | 18 (2.7, 1.7–4.3) | ||
Some bats (266 Pteropodiformes and 318 Vespertilioniformes) yielded both brain and blood samples, reflected in the total number of 2,117 tests.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Australian bat lyssavirus surveillance in 1,100 submitted bats in Queensland and New South Wales between June 1996 and March 2002
| Suborder | Family | Genus | Antigen detection (FAT) | Antibody detection (RFFIT) | ||
|---|---|---|---|---|---|---|
| Number tested | Number (%, 95% CI) positive | Number tested | Number (%, 95% CI) positive | |||
| Pteropodiformes | Pteropodidae | Pteropus a | 902 | 69 (7.7, 6.1–9.6) | 14 | 4b (28.6, 11.7–54.7) |
| Nyctimene | 2 | 0 | 0 | 0 | ||
| Syconycteris | 6 | 0 | 0 | 0 | ||
| Vespertilioniformes | Mollosidae | Mormopterus | 19 | 0 | 0 | 0 |
| Vespertilionidae | Chalinolobus | 22 | 0 | 0 | 0 | |
| Myotis | 2 | 0 | 0 | 0 | ||
| Nyctophilus | 24 | 0 | 0 | 0 | ||
| Scotorepens | 22 | 0 | 0 | 0 | ||
| Vespedalus | 4 | 0 | 0 | 0 | ||
| Miniopteridae | Miniopterus | 29 | 0 | 0 | 0 | |
| Emballonuridae | Saccolaimus c | 8 | 5 (62.5, 30.6–86.3) | 0 | 0 | |
| Taphozous | 2 | 0 | 0 | 0 | ||
| Unidentified | 44 | 0 | 0 | 0 | ||
| Total | 1,086 | 74 (6.8, 5.5–8.5) | 14 | 4 (28.6, 11.7–54.7) | ||
Antigen detection in Pteropus alecto = 37/481 (7.7%), P. conspicillatus = 1/95 (1%), Pteropus poliocephalus = 9/200 (4.5%), Pteropus scapulatus = 22/126 (17.4%).
Two bats tested positive for both antibody and antigen.
Saccolaimus flaviventris.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Characteristics of 96 bats of the total 2,633 surveyed that showed evidence of Australian bat lyssavirus infection either by FAT or by RFFIT
| Suborder | Family | Genus | No. of bats tested positive | |
|---|---|---|---|---|
| by FAT | by RFFIT | |||
| Pteropodiformes | Hipposideridae | Hipposideros | 1 | |
| Megadermatidae | Macroderma | 1 | ||
| Pteropodidiae | Pteropus | 69 | 12 | |
| Vespertilioniformes | Emballonuridae | Saccolaimus | 5 | 3 |
| Molossidae | Chaerophon | 1 | ||
| Tadarida | 1 | |||
| Vespertilionidae | Vespedalus | 1 | ||
| Chalinolobus | 2 | |||
| Sex | ||||
| Male | 34 | 7 | ||
| Female | 32 | 14 | ||
| Unknown | 8 | 1 | ||
| Age | ||||
| Immature | 12 | 4 | ||
| Mature | 51 | 11 | ||
| Unknown | 11 | 7 | ||
| Sample method | ||||
| Wild‐caught | 18 | |||
| Submitted | 74 | 4 | ||
| Sample location | ||||
| Northern Territory | 5 | |||
| Queensland | 74 | 4 | ||
| Western Australia | 13 | |||
| Sample year | ||||
| 1996 | 6 | 2 | ||
| 1997 | 24 | 1 | ||
| 1998 | 23 | 6 | ||
| 1999 | 4 | |||
| 2000 | 9 | |||
| 2001 | 5 | 13 | ||
| 2002 | 3 | |||
| Total | 74 | 22 | ||
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Blood samples were collected from 670 bats, being 656 wild‐caught bats (Table 1) and 14 submitted bats (Table 2). Individuals from 14 genera, seven families and both suborders were sampled (Table 2), with a median genus sample size of 27 (range 1–266). Antibody was detected in 18 (2.7%) wild‐caught bats and four (28.6%) submitted bats from eight genera, six families and both suborders (Table 3). Antibody prevalence in genera in which antibody was detected ranged from 1.5% to 50% (median = 3.3%). Two submitted bats (one P. alecto and one P. poliocephalus) from which brain and blood were sampled tested positive for both antibody and antigen (Table 2).
4. DISCUSSION
Despite its first description in 1996, there has been limited investigation of the eco‐epidemiology of ABLV in Australian bats (Barrett, 2004; Field, 2005). This report shows that ABLV is taxonomically widespread in Australian bats, with evidence of infection found in five of the six families present. The detection of either antigen or antibody in bats in three of the four states/territories surveyed further supports the historic presence of the virus in Australia, as the taxonomic and geographical scale of detections is epidemiologically inconsistent with recent introduction. The lack of detections in NSW likely reflects the limited number of samples from that state. However, the findings indicate that at the population level, infection prevalence is low, with viral antigen not detected in the wild‐caught cohort notwithstanding reasonably large sample sizes in some taxa known (from submitted samples) to be susceptible to infection. In Pteropus spp. for example, with a sample size of 475, this translates to an estimated “background” infection prevalence in wild populations of <0.5%. That ABLV causes clinical disease, debilitation and death in bats means that infected bats are over‐represented in the submitted bat cohort, with such individuals more likely to be found and submitted than healthy bats. With many such submissions being debilitated bats found in urban backyards, the higher infection prevalence in this cohort (e.g., 7.6% in Pteropus spp.) has direct public health significance because of the higher risk of human exposure.
Antigen detection was significantly higher in the genus Saccolaimus (S. flaviventris) than in all other genera, while P. scapulatus had a significantly higher antigen prevalence than other Pteropus species. From a public health perspective, these findings suggest that these two species pose a heightened exposure risk. The lack of antigen detection in Vespertilioniformes other than S. flaviventris may in part reflect their typically smaller size, which makes them less readily detected by members of the public, meaning that relatively few are submitted. The lack of a positively biased sample is a fundamental impediment to the direct detection of infection in these species and to a more complete elaboration of the ecology and phylogeny of ABLV in Australian bats. That said, it appears evident from the findings that Pteropus spp. and S. flaviventris play an important role in the ecology of ABLV. This is supported by phylogenetic analyses to date, which identify two distinct virus clades reflecting sequence variation in Pteropus spp. and S. flaviventris isolates (Warrilow et al., 2002). Given bat diversity in Australia, it is probable that targeted surveillance will reveal further diversity. Such surveillance might usefully focus on the Mollosidae and Vespertilionidae, both of which had multiple genera in which antibodies were detected.
Generically, antibodies provide indirect evidence of past exposure or infection. However, serology has been an irrelevant lyssavirus surveillance tool in nonbat species because of a near 100% case fatality rate. The putative adapted ancestral reservoir of lyssaviruses (Badrane & Tordo, 2001), bats are the only taxa in which antibodies are detected with sufficient frequency to support serosurveillance. The crude antibody prevalence in wild‐caught bats in this study was 18%. Some taxa yielded high antibody prevalence in very small sample sizes; conversely, no antibodies were detected in Miniopterus notwithstanding a reasonably large sample size across multiple locations and times. Arguin et al. (2002) reported an anti‐ABLV antibody prevalence of 9.5% (22/231) in a multigenera sample of Philippine bats (including Miniopterus) using a RFFIT with a specific ABLV antigen. The detection of only five antibody‐positive individuals in a parallel RFITT using rabies virus antigen suggests that the latter has a sensitivity of only 23% relative to the ABLV RFFIT, at least on the virus circulating in the Philippine bats. Thus, notwithstanding the different criteria for positivity in Arguin et al. (2002) (90% or greater reduction in infectious centres versus 50% in the AAHL assay), it is probable that serologic investigations in Australian bats using rabies virus RFITT underestimate ABLV antibody prevalence.
The difficulty in detecting infections with a short clinical course, high case fatality rate and low prevalence in wild populations using a cross‐sectional study design are well recognised. As discussed above, screening a positively biased sample is one approach to the challenge, but while useful to establish presence, it can constrain understanding of the characteristics of the infection at the population level. An alternative is to increase sample size, but where the test requires destructive sampling (as for lyssavirus FAT), this alternative is untenable from both an ethical and a resource standpoint; statistically, to detect an infected individual at say 0.1% infection prevalence would necessitate the capture and destruction of 1,000 individuals. In bats, serology offers a larger surveillance “window” because of the typically persistent nature of antibodies. Whether anti‐lyssavirus antibodies represent noninfectious exposure, subclinical “aborted” infection, pending clinical infection or recovered infection is unclear and contentious, but in the context of this study, it is also irrelevant. Detection of antibodies in this study is interpreted as evidence of ABLV “infection” at a population level rather than the status of an individual bat. Nonetheless, serosurveillance has limitations in that the absence of detection of antibodies cannot be interpreted as the absence of susceptibility to infection of taxa, and prevalence comparisons across taxa are not valid in the absence of relative case fatality rates. Finally, serology does not allow identification of the current infection status of an individual nor provide antigenic material to support molecular epidemiology studies.
In conclusion, there is direct or indirect evidence of ABLV infection in diverse and geographically widespread Australian bat taxa, consistent with an historic presence in the landscape. While the infection prevalence in wild populations is evidently low, the findings suggest that some species have a higher likelihood of infection, although this interpretation should be constrained by the nonrandom nature of the sampling and the varying sample sizes. Nonetheless, it is evident that the submitted bat cohort (comprising bats rescued by members of the public) poses a substantially higher ABLV exposure risk from a human health perspective. The enduring messaging from both animal and human health authorities in Australia is for members of the public to avoid direct contact with all bats, to call registered and vaccinated wildlife carers to rescue a bat and to seek immediate medical advice should direct contact occur.
CONFLICT OF INTEREST
The author has no conflict of interests.
ACKNOWLEDGEMENTS
Acknowledgement and thanks are due to colleagues at the (then) Queensland Department of Primary Industries Yeerongpilly Veterinary Laboratory and at the CSIRO Animal Health Laboratory. This publication utilises data from my doctoral thesis and, in that context, also warrants acknowledgement of my mentors Simon More (then at the University of Queensland) and the late Chris Baldock, and colleagues Russell Rogers, Kevin Dunn, Ian Douglas and Ron Glanville (then at the Queensland Government Department of Primary Industries). Finally, this publication benefitted from intellectual developments or contributions from both the PREDICT project of the US Agency for International Development (USAID) “Emerging Pandemic Threats” programme (Cooperative Agreement No. AID‐OAA‐A‐14‐00102) and the “Understanding the Risk of Bat Coronavirus Emergence” project of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Award No. R01AI110964).
Field HE. Evidence of Australian bat lyssavirus infection in diverse Australian bat taxa. Zoonoses Public Health.2018;65:742–748. 10.1111/zph.12480
REFERENCES
- Allworth, A. , Murray, K. , & Morgan, J. (1996). A human case of encephalitis due to a Lyssavirus recently identified in fruit bats. Communicable Diseases Intelligence, 20, 504. [Google Scholar]
- Arguin, P. , Murray‐Lillibridge, K. , Miranda, M. , Smith, J. , Calaor, A. , & Rupprecht, C. (2002). Serologic evidence of Lyssavirus infections among bats, the Philippines. Emerging Infectious Diseases, 8, 258–262. 10.3201/eid0803.010330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrane, H. , Bahloul, C. , Perrin, P. , & Tordo, N. (2001). Evidence of two lyssavirus phylogroups with distinct pathogenicity and immunogenicity. Journal of Virology, 75, 3268–3276. 10.1128/JVI.75.7.3268-3276.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrane, H. , & Tordo, N. (2001). Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. Journal of Virology, 75, 8096–8104. 10.1128/JVI.75.17.8096-8104.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, J. (2004). Australian bat lyssavirus . PhD thesis, The University of Queensland, Brisbane, Qld, Australia. https://espace.library.uq.edu.au/view/UQ:9486
- Brookes, S. , Parsons, G. , Johnson, N. , McElhinney, L. , & Fooks, A. (2005). Rabies human diploid cell vaccine elicits cross‐neutralising and cross‐protecting immune responses against European and Australian bat lyssaviruses. Vaccine, 23, 4101–4109. 10.1016/j.vaccine.2005.03.037 [DOI] [PubMed] [Google Scholar]
- Epstein, J. H. , & Field, H. E. (2011).Disease surveillance in free‐ranging bat populations: Challenges and logistical considerations InNewman S. H., Field H. E., Epstein J. H., & de Jong C. E. (Eds.), Investigating the role of bats in emerging zoonoses: Balancing ecology, conservation and public health interest (pp.47–62). Rome, Italy:Food and Agriculture Organization of the United Nations. [Google Scholar]
- Field, H. (2005) The ecology of Hendra virus and Australian bat lyssavirus . PhD thesis, The University of Queensland, Brisbane, Qld, Australia. https://espace.library.uq.edu.au/view/UQ:13859
- Francis, J. R. , Nourse, C. , Vaska, V. L. , Calvert, S. , Northill, J. A. , McCall, B. , & Mattke, A. C. (2014). Australian Bat Lyssavirus in a child: The first reported case. Pediatrics, 133, e1063–e1067. 10.1542/peds.2013-1782 [DOI] [PubMed] [Google Scholar]
- Fraser, G. , Hooper, P. , Lunt, R. , Gould, A. , Gleeson, L. , Hyatt, A. , … Kattenbelt, J. (1996). Encephalitis caused by a lyssavirus in flying‐foxes in Australia. Emerging Infectious Diseases, 2, 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, A. R. , Hyatt, A. D. , Lunt, R. , Kattenbelt, J. A. , Hengstberger, S. , & Blacksell, S. D. (1998). Characterisation of a novel lyssavirus isolated from Pteropid bats in Australia. Virus Research, 54, 165–187. 10.1016/S0168-1702(98)00025-2 [DOI] [PubMed] [Google Scholar]
- Gould, A. , Kattenbelt, J. , Gumley, S. , & Lunt, R. (2002). Characterisation of an Australian bat lyssavirus variant isolated from an insectivorous bat. Virus Research, 89, 1–28. 10.1016/S0168-1702(02)00056-4 [DOI] [PubMed] [Google Scholar]
- Gould, A. , Kattenbelt, J. , Hyatt, A. , Gumley, S. , & Lunt, R. (1999). Coding potential and phylogenetic analyses of the Australian bat lyssaviruses and their relationship to classic rabies In Proceedings of the XI International Congress of Virology, Sydney, Australia. [Google Scholar]
- Hanna, J. , Carney, I. , Smith, G. , Tannenberg, A. , Deverill, J. , Botha, J. , … Searle, J. (2000). Australian bat lyssavirus infection: A second human case, with a long incubation period. Medical Journal of Australia, 172, 597–599. [DOI] [PubMed] [Google Scholar]
- Hooper, P. , Lunt, R. , Gould, A. , Samaratunga, H. , Hyatt, A. , Gleeson, L. , … Murray, P. (1997). A new lyssavirus‐the first endemic rabies‐related virus recognised in Australia. Bulletin De L'institut Pasteur, 95, 209–218. [Google Scholar]
- Jonsson, N. N. , Johnston, S. D. , Field, H. , de Jong, C. , & Smith, C. (2004). Field anaesthesia of three Australian species of flying fox. Veterinary Record, 154, 664 10.1136/vr.154.21.664 [DOI] [PubMed] [Google Scholar]
- Murray, K. , Selleck, P. , Hooper, P. , Hyatt, A. , Gould, A. , Gleeson, L. , … Ketterer, P. (1995). A Morbillivirus that caused fatal disease in horses and humans. Science, 268, 94–97. 10.1126/science.7701348 [DOI] [PubMed] [Google Scholar]
- Shinwari, M. , Annand, E. , Driver, L. , Warrilow, D. , Harrower, B. , Allcock, R. , … Diallo, I. (2014). Australian bat lyssavirus infection in two horses. Veterinary Microbiology, 173, 224–231. 10.1016/j.vetmic.2014.07.029 [DOI] [PubMed] [Google Scholar]
- Smith, J. , Yager, P. , & Baer, G. (1973). A rapid reproducible test for determining rabies neutralising antibody. Bulletin of the World Health Organization, 48, 535–541. [PMC free article] [PubMed] [Google Scholar]
- St George, T. D. (1989) Are there common features in lyssavirus diseases? In Uren M. F., Blok J., & Manderson L. H. (Eds.), Proceedings of the Arbovirus research in Australia: Proceedings of the fifth symposium Brisbane, Australia (pp. 264–267). [Google Scholar]
- Warrilow, D. , Smith, I. L. , Harrower, B. , & Smith, G. A. (2002). Sequence analysis of an isolate from a fatal human infection of Australian bat lyssavirus. Virology, 297, 109–119. 10.1006/viro.2002.1417 [DOI] [PubMed] [Google Scholar]


