Abstract
Objective
Central nervous system pathways involving pain modulation shape the pain experience in patients with chronic pain. Our objectives were to understand the mechanisms underlying pain in rheumatoid arthritis (RA) and identify brain signals that may serve as imaging markers for developing targeted treatments for RA pain.
Methods
Subjects with RA and matched controls underwent functional magnetic resonance imaging, using pulsed arterial spin labeling (pASL). The imaging conditions included: 1) resting state, 2) low intensity stimulus and 3) high intensity stimulus. Stimuli consisted of mechanical pressure applied to metacarpophalangeal (MCP) joints with an automated cuff inflator. The low intensity stimulus was 30 mmHg. The high intensity stimulus was the amount of pressure required to achieve 40/100 pain intensity for each RA patient, with the same amount of pressure given to the matched control.
Results
Among RA patients, regional cerebral blood flow (rCBF) in medial frontal cortex (MFC) and dorsolateral prefrontal cortex increased during both low and high pressure stimuli. No rCBF changes were noted for pain-free controls. In region of interest analyses among RA patients, baseline rCBF in MFC was negatively correlated with pressure required for the high intensity stimulus (p<0.01) and positively correlated with pain induced by the low intensity stimulus (p<0.05). Baseline rCBF also marginally correlated with disease activity (p=0.05). rCBF during high pain was positively correlated with pain severity and interference (p’s<0.05).
Conclusion
In response to clinically-relevant joint pain evoked by MCP pressure, neural processing in MFC increases and is directly associated with clinical pain in RA.
Pain is the main reason patients seek rheumatologic care, but little is known about the mechanisms of pain in rheumatoid arthritis (RA). Most rheumatologists conceptualize pain primarily in the context of inflammation at joint sites (1). However, even with treatment of inflammation, average pain levels often do not return to general population norms (2, 3). The imperfect association between inflammation and pain intensity may be due to many factors, including differences in central nervous system (CNS) processing and modulation of joint-specific pain perception.
Historically, the need for invasive techniques to assess CNS pain mechanisms limited this area of study in humans, but the development of advanced functional magnetic resonance imaging (fMRI) techniques has enabled the non-invasive visualization of brain responses. Arterial spin labeling (ASL) is an fMRI technique that uses water in arterial blood as a freely diffusible endogenous tracer to measure blood perfusion in the brain, an indirect marker of neural activity, noninvasively (4). Using ASL, a quantifiable measurement of regional cerebral blood flow (rCBF) can be obtained by comparing images taken with and without application of the tagging magnetic pulse, that inverts the natural magnetization of water in arterial blood (5).
Several studies have applied ASL to the study of experimental pain in healthy humans (6–8). More recently, ASL has also been applied to the investigation of the neural correlates of clinical pain. For instance, in individuals with chronic low back pain, acute pain exacerbations were associated with increases in rCBF in several brain regions, including the insular and medial prefrontal cortices (9). Another study showed rCBF changes in the insular and medial prefrontal cortices, related to clinical pain from carpometacarpal osteoarthritis (10). Both studies were able to capture brain responses to ongoing pain, a signal that evolves slowly (typically over minutes or hours) and, importantly, is not easily detected by traditional blood oxygen level-dependent (BOLD) fMRI in block or event-related designs, which require multiple, brief alternations between epochs of pain and no-pain. These observations suggest that ASL imaging has the potential to be an important biomarker for pain in clinical studies. To our knowledge, no study has used ASL to measure rCBF changes associated with clinically-relevant pain in RA patients.
In this study, we used pulsed ASL (pASL) to identify changes in rCBF associated with pain provocations at the metacarpophalangeal (MCP) joints in RA patients and pain-free controls. Using a similar experimental stimulus, Schweinhardt et al. showed that brief, two second, pressure provocation of hand joint pain resulted in increases in BOLD brain signal in portions of the medial prefrontal cortex (MPFC) and pregenual anterior cingulate cortex (pgACC) (11). Because brief pain stimuli are likely to be particularly salient, we designed our study to use longer (six minute), tonic stimuli to minimize the effect of attentional reallocation associated with rapid perceptual changes. We were particularly interested in minimizing attentional responses because previous studies have reported that the startle response is altered in individuals with chronic illnesses associated with pain (12–15). As pASL is better equipped to assess brain activity for low-frequency stimuli (16), our hypothesis was that pASL would identify changes in rCBF associated with clinical, tonic exacerbations of RA pain. This finding could have an important impact by furthering our understanding of the brain mechanisms mediating RA pain, and by paving the way for the use of imaging markers to objectively assess pain in clinical trials, thereby decreasing heterogeneity and increasing the power to detect medication effects.
PATIENTS AND METHODS
Participants
RA patients were recruited from the outpatient rheumatology clinics of a single academic institution. Inclusion criteria for RA patients were: 1) age between 25 and 70 years old, 2) diagnosis of RA by a board-certified rheumatologist, 3) chronic pain ≥ 3 months with an average of ≥ 3/10 in intensity at the left MCPs, and 4) no or minimal corticosteroid use (≤ equivalent of prednisone 10 mg daily). Exclusion criteria were: 1) history of surgery at the left MCPs, 2) current opioid and/or benzodiazepine use, and 3) contraindications for MRI screening (e.g., metal in the body, cardiac pacemaker, claustrophobia, pregnancy). Age and sex-matched pain-free controls were recruited through advertisements in Craigslist and a registry of individuals interested in clinical research. Exclusion criteria for controls were the same as those for the RA group. Additional exclusion criteria for pain-free controls were: 1) history of RA and/or other systemic rheumatic diseases, 2) history of chronic pain conditions, and 3) acute pain at the time of the screening visit. All participants provided written informed consent. The Partners Institutional Review Board approved this study.
Study overview
Subjects participated in two sessions: a behavioral training visit and an imaging visit. The objectives of the training session were to: 1) familiarize participants with pressure-induced pain and rating procedures, and 2) identify the approximate pressure needed for the high intensity stimulus during the imaging session. The objective of the imaging visit was to obtain the questionnaire and neuroimaging data for analyses.
Training session
Participants were instructed to lie on an examining table, and a Velcro-adjusted vascular cuff was secured around the left MCP joints. The cuff was connected to a rapid cuff inflator (Hokanson, USA) that increases pressure to a target level over approximately two seconds. This type of cuff pressure stimulus preferentially targets deep tissue nociceptors (17) and has been used in other neuroimaging studies of chronic pain (18, 19). Testing began by inflating the cuff to 60 mmHg and increasing the pressure by 20-30 mmHg until a pain intensity rating of 70 on a 100-point scale was obtained. Pressure was then decreased by 20-30 mmHg every 15 seconds, until the participant did not feel any pain. Two trials were performed, with a six-minute rest period between trials. The average pressure required to achieve a pain intensity rating of 40 out of 100 was then applied to the left MCPs for six minutes, to simulate what the participants would experience in the MRI scanner during the imaging session.
Imaging session
The imaging session occurred within two weeks of the training session and included a physical examination, blood work, questionnaires, and MRI scanning at rest and during application of pressure stimuli.
Physical examination, blood work and questionnaires
A trained assessor performed a standardized 28-joint count on all participants to assess tenderness and swelling, and blood was obtained to assess C-reactive protein (CRP) levels. The swollen-to-tender joint count ratio was calculated as a measure of widespread, non-inflammatory pain (20). All participants completed the following questionnaires: the Brief Pain Inventory (BPI), the Hospital Anxiety and Depression Scale (HADS), the Pain Catastrophizing Scale (PCS) and the Medical Outcomes Study (MOS) Sleep Scale. The BPI is a validated, 9-question survey that assesses the sensory and reactive aspects of clinical pain (21). The HADS is a validated, 12-item questionnaire that assesses anxiety and depression in chronically ill patients (22). The MOS Sleep Scale is a validated, 12-item questionnaire developed to assess sleep quality and quantity in people with chronic illnesses (23). The PCS is a validated, 13-item questionnaire that examines catastrophic thinking about pain in people with chronic pain.
MRI scans
Using a 3T Siemens MAGNETOM Skyra, with a 32-channel head coil, six-minute pASL scans (TR/TE/TI1/TI2 = 3000/17/700/1700 ms, voxel size = 4×4×5mm, number of slices = 17) were collected with the PICORE-Q2TIPS MRI labeling method (24). Tag images were acquired by labeling a thick inversion slab (110 mm) proximal to the imaging slices (gap = 21.1. mm). Tag and control images were acquired in an interleaved pattern. At the beginning of each pASL scan, a calibration M0 scan was acquired for rCBF quantification purposes. A high-resolution anatomical volume (MPRAGE) was also collected (TR/TE = 2300/2.95 ms, voxel size = 1.1×1.1×1.2 mm, number of slices = 176) for anatomical localization purposes (9).
pASL scans were collected under three conditions: 1) baseline, 2) low intensity pressure stimulus, and 3) high intensity pressure stimulus. During all three conditions, participants were instructed to remain still with their eyes open. During the baseline scan, the vascular cuff was wrapped around the left MCPs, but no pressure stimulus was provided. During the low intensity pressure scan, the vascular cuff was inflated around the left MCPs to 30 mmHg for six minutes. The pressures used for the high intensity pressure scan were individualized to achieve a 40 out of 100 pain intensity rating for each RA patient. The required pressure was recalibrated immediately before the scan, using pressure values from the training session as the starting point. Each RA patient was age and sex-matched to a pain-free control, and the pressures used for the control subject were the same pressures used for the RA patient, with whom they were matched, as has been done in previous studies (25). The rationale behind using stimulus-matched conditions was to demonstrate that patients are hypersensitive to pressure stimuli (e.g., they exhibit hyperalgesia or allodynia), and to identify brain patterns that might contribute to explain such hypersensitivity. The order of the high intensity and low intensity pain provocation scans was randomized to minimize order effects. The high intensity and low intensity pain provocation scans were separated by at least 10 minutes to allow subjects to recover between pain provocations.
Data analysis
To characterize the RA patients and age and sex-matched controls, means and frequencies were calculated. Wilcoxon signed rank tests were used to compare responses to the pressure stimuli between RA patients and controls.
Imaging data analysis was performed using a combination of analysis packages, including FSL v.5.0.7 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl; (26)), Freesurfer v.5.3.0 (https://surfer.nmr.mgh.harvard.edu) (27), and the ASLtbx (https://cfn.upenn.edu/~zewang/ASLtbx.php) (28, 29). pASL time series were motion-corrected (by realigning tag and control images separately), co-registered to the M0 scan, spatially smoothed (FWHM = 6mm) and converted into rCBF maps in absolute values (ml/100 g of tissue/min) (30), using ASLtbx. This preliminary spatial smoothing, prior to rCBF calculation, was performed to prevent noise propagation, as recommended by the ASLtbx documentation. rCBF maps were then brain extracted using BET, registered to high resolution anatomic images using Freesurfer’s boundary-based registration tool (BBregister) (31) and spatially normalized to the MNI152 standard template. To avoid differences in brain coverage (e.g., due to difference in head size or slice placement during acquisition) that may confound group imaging results, all MNI-normalized rCBF maps were masked by an ‘intersection volume’, so that only voxels imaged in all participants were included in all analyses. The rCBF maps were then intensity-normalized by dividing each voxel by the global rCBF, computed within the intersection volume, as commonly done in ASL or PET perfusion studies (32, 33), to improve sensitivity to regional changes. Normalized rCBF maps were smoothed using a Gaussian kernel (FWHM = 8 mm) to improve between-subjects co-registration, signal-to-noise ratio and validity of statistical tests. Group differences in baseline rCBF maps, as well as the effect of stimulation (low/high intensity vs. baseline), and its group interaction were assessed using general linear models (GLM). The group level analyses were performed using a mixed-effects analysis, and corrected for multiple comparisons with a voxel-wise cluster forming threshold of Z = 2.3 and a corrected cluster significance threshold of P = 0.05. Because no subcortical effects were detected, and for ease of visualization, imaging results were visualized on the brain surface (FreeSurfer’s fsaverage).
In exploratory analyses, the cluster demonstrating significantly elevated rCBF in RA patients in the ‘high-intensity stimulation vs. baseline’ contrast was used as a region-of-interest (ROI) to probe group differences in the mean rCBF signal using GLM. To assess the relationship between rCBF in this cluster and clinical measures, Pearson’s correlations were used. ROI analyses were performed with Statistica 10.0 (StatSoft INC, Tulsa, OK), using an alpha level of .05.
RESULTS
Participant characteristics
We enrolled 16 RA patients and 16-pain free controls. One male RA patient was excluded from analysis because his large head size would excessively limit brain coverage for the pASL scans. One female RA patient was excluded from analysis because she fell asleep during the scan. The final analytic cohort included 14 RA patients and 16 pain-free controls (Table 1). Mean (±SD) age was 44.8 (±9.3) years for RA patients and 47.1 (±11.4) years for controls. RA patients differed significantly from controls in terms of average pain intensity, pain interference, depression, anxiety, pain catastrophizing and sleep problems. Among RA patients, the mean disease duration was 11.4 (±9.9) years, and the mean Disease Activity Score in 28 joints (DAS28) was 3.8 (±1.0). Of the nine (64.3%) RA patients who were taking disease-modifying antirheumatic drugs (DMARDs), three (33.3%) were taking a synthetic DMARD only; four (44.4%) were taking a biologic DMARD only; and two (22.2%) were taking both synthetic and biologic DMARDs. Eight (57.1%) RA patients were taking a non-steroidal anti-inflammatory drug (NSAID), and two (14.3%) RA patients were taking prednisone.
Table 1.
Demographic and clinical data on study subjects1
| Characteristics | RA patients (n = 14) |
Controls (n = 16) |
P-value2 |
|---|---|---|---|
| Age, years | 44.8 (9.3) | 47.1 (11.4) | 0.55 |
| % Female | 100% | 93.8% | 0.34 |
| % Seropositive | 78.6% | - | - |
| Disease duration, years | 11.4 (9.9) | - | - |
| DAS28 | 3.8 (1.0) | - | - |
| % Corticosteroid use | 14.3% | - | - |
| % DMARD use | 57.1% | ||
| % Synthetic DMARD use | 35.7% | ||
| % Biologic DMARD use | 42.9% | - | - |
| BPI | |||
| Pain average pain, 0-10 | 4.6 (1.9) | 0.6 (1.3) | <0.01 |
| Pain interference, 0-10 | 3.9 (2.1) | 0.1 (0.4) | <0.01 |
| HADS Depression, 0-21 | 5.4 (3.6) | 0.7 (1.6) | <0.01 |
| HADS Anxiety, 0-21 | 7.0 (2.6) | 2.1 (3.1) | <0.01 |
| PCS, 0-52 | 16.8 (10.5) | 6.2 (6.7) | <0.01 |
| MOS Sleep Problems II, 0-100 | 45.8 (17.7) | 16.6 (15.1) | <0.01 |
Except where indicated otherwise, values are the mean (SD). RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 Joints; DMARD = disease-modifying antirheumatic drug; BPI = Brief Pain Inventory; HADS = Hospital Anxiety and Depression Scale; PCS = Pain Catastrophizing Scale; MOS = Medical Outcomes Study
P-values from two-sample t-tests for continuous variables and from Chi-Square test for categorical variables.
Pressure pain induction in RA patients and controls
During the high intensity pain stimulus, all RA patients and 11 (68.8%) controls reported pain. Mean pain severity in response to the high intensity pain stimulus was significantly higher among RA patients compared to controls (51.6 ± 24.4 vs. 14.8 ± 22.3; P = 0.0006) (Figure 1). During the low intensity stimulus, ten (71.4%) RA patients and two (13.5%) controls reported pain. Mean pain severity in response to the low intensity pain stimulus was significantly higher among RA patients compared to controls (24.6 ± 30.1 vs. 0.6 ± 1.7; P = 0.0005). We also examined the effect of the order of high vs. low intensity pain provocation scans on patient-reported pain. While there was no order effect for patient-reported pain in response to the high intensity pain provocation, there was a significant order effect for the low intensity pain provocation. Specifically, RA patients perceived the low intensity provocation as significantly more painful when it was preceded by the high stimulus than when the low intensity provocation was given first (43.4 ± 37.7 vs. 10.6 ±14.0, P = 0.02). This observation is suggestive of sensitization after the high intensity provocation.
Figure 1.
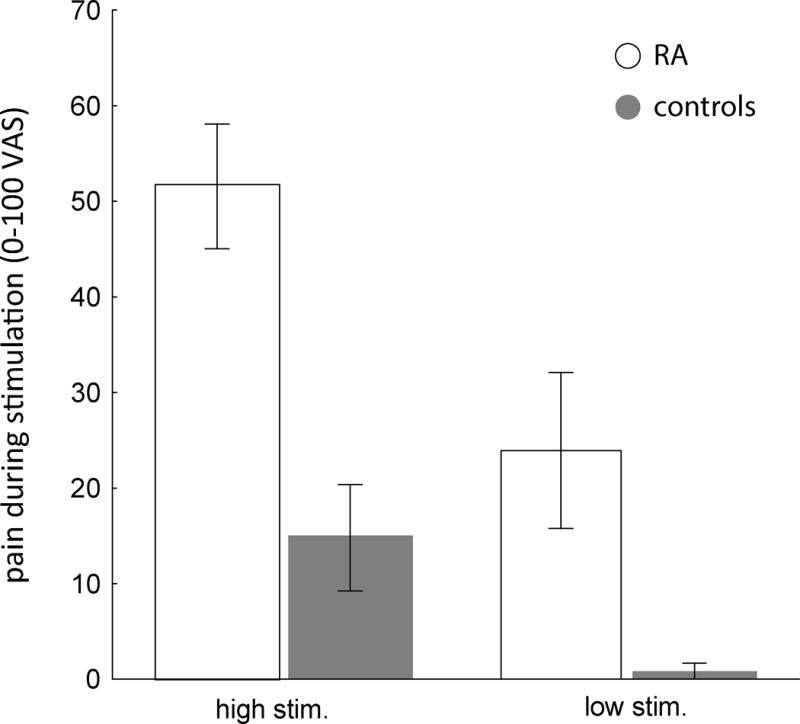
Mean pain ratings in response to the high intensity (40-418 mmHg) and low intensity (30 mmHg) stimuli at the MCP joints in rheumatoid arthritis (RA) patients vs. controls. Bars represent the standard error of the mean.
Imaging results – Group differences
At baseline, resting state whole-brain voxel-wise comparisons revealed no statistically significant differences in rCBF between RA patients and pain-free controls. In RA patients, low intensity stimulation (which was perceived as mildly painful, on average) was accompanied by a statistically significant rCBF increase in the posterior medial frontal cortex (MFC) (34); including anterior midcingulate cortex (aMCC), and supplementary and pre-supplementary motor areas (SMA/pre-SMA), as well as in the precentral gyrus, the dorsolateral prefrontal cortex (dLPFC) and underlying white matter, compared to baseline (Figure 2A; Table 2). In RA patients, high intensity stimulation (which was moderately painful on average) led to similar rCBF increases as low intensity stimulation in the posterior MFC, with the additional recruitment of more rostral portions of the MFC, expanding into the pgACC, and of the dLPFC, expanding into the frontal pole, compared to baseline. (Figure 2B; Table 2). In healthy participants (for whom cuff stimulation was only mildly painful, or not painful at all), these effects were absent. The only statistically significant effect detected was a rCBF reduction in the dorsomedial (dMPFC) and dorsolateral prefrontal cortices (dLPFC) and frontal pole for the low-intensity stimulation.
Figure 2.
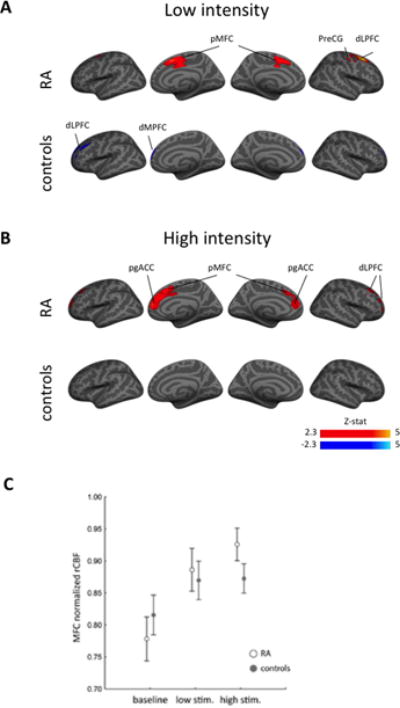
A) Regional cerebral blood flow (rCBF) in response to a low intensity pressure stimulus at the metacarpophalangeal (MCP) joints in rheumatoid arthritis (RA) patients and pain-free controls. B) rCBF in response to a high intensity pressure stimulus at the MCP joints in RA patients and pain-free controls. C) The high intensity MCP stimulus led to significant increases in rCBF in the medial frontal cortex among RA patients but not among healthy controls. Bars represent the standard error of the mean.
Table 2.
Brain regions showing significant rCBF changes in response to the stimuli.
| Group | Contrast | Cluster size (# voxels) | Cluster P-value | Local maxima
|
||||
|---|---|---|---|---|---|---|---|---|
| Z | x (mm) | y (mm) | z (mm) | Label | ||||
| RA | low stimulus > baseline | 3714 | 0.0023 | 4.96 | 22 | 12 | 52 | R superior frontal sulcus |
| 3.58 | 10 | 10 | 52 | R pre-supplementary motor area | ||||
| 3.56 | 0 | 2 | 42 | anterior middle cingulate cortex | ||||
| 3.41 | −4 | 4 | 46 | L anterior middle cingulate cortex /pre-supplementary motor area | ||||
| 3.11 | 32 | −12 | 56 | R precentral gyrus | ||||
| baseline > low stimulus | n.s. | |||||||
| high stimulus > baseline | 4078 | 0.00161 | 3.55 | 8 | 48 | 12 | R pregenual anterior cingulate cortex | |
| 3.34 | 10 | 22 | 44 | R pre-supplementary motor area | ||||
| 3.08 | −6 | 48 | 12 | L pregenual anterior cingulate cortex | ||||
| 3.04 | −16 | 22 | 46 | L superior frontal sulcus | ||||
| 2.7 | −10 | 24 | 40 | L pre-supplementary motor area | ||||
| baseline > high stimulus | n.s. | |||||||
| controls | low stimulus > baseline | n.s. | ||||||
| baseline > low stimulus | 1951 | 0.0336 | 3.34 | −38 | 58 | 14 | L frontal pole | |
| 3.22 | 2 | 58 | 10 | R medial prefrontal cortex | ||||
| 3.04 | −30 | 32 | 38 | L middle frontal gyrus | ||||
| 2.89 | 10 | 58 | 32 | R frontal pole | ||||
| high stimulus > baseline | n.s. | |||||||
| baseline > high stimulus | n.s. | |||||||
A direct comparison of high and low-intensity stimulation scans, or any group interactions, did not yield results surviving statistical thresholding in voxel-wise analyses. A follow-up ROI-based analysis confirmed the response of an effect of stimulus (levels: baseline, low intensity, high intensity) on the average rCBF extracted from the significant cluster identified in the ‘high intensity vs baseline’ contrast in RA patients (F(2,54)=9.455, P <0.001). No statistically significant condition*group interaction was observed (F(2,54)=1.689, P =0.19). However, an exploratory post-hoc decomposition of the interaction using Tukey HSD tests revealed that, while, in patients, the high stimulation vs baseline comparison was significant (replicating the result of the voxelwise analyses; P <0.01) and the low stimulation vs baseline comparison trended towards significance (P =0.053), these comparisons did not yield statistically significant results in controls (P’s>0.53).
Imaging results – Correlations with clinical measures
In patients, baseline normalized rCBF in the MFC was negatively correlated with the amount of pressure required for the high intensity pain stimulus (r = −0.76, P < 0.01). It also positively correlated with pain ratings in response to the low intensity pain stimulus (r = 0.56, P < 0.05) and with the DAS-28 score (with borderline statistical significance; r=0.53, P = 0.05) (Figure 3). In other words, the higher the rCBF in the MFC at baseline, the higher the disease activity and the sensitivity to experimental pain, as assessed in terms of both lower intensity of stimulation needed to achieve the target percept, and higher pain ratings in response to a fixed, low-intensity stimulus. Furthermore, normalized rCBF during the high intensity stimulation was significantly correlated with both BPI average pain (r = 0.53, P < 0.05) and BPI pain interference ratings (r = 0.60, P < 0.05) (Figure 4). No statistically significant associations were observed with rCBF at baseline or during the high intensity stimulation and measures of depression, anxiety, catastrophizing, sleep problems and widespread, non-inflammatory pain (swollen to tender joint count ratio).
Figure 3.
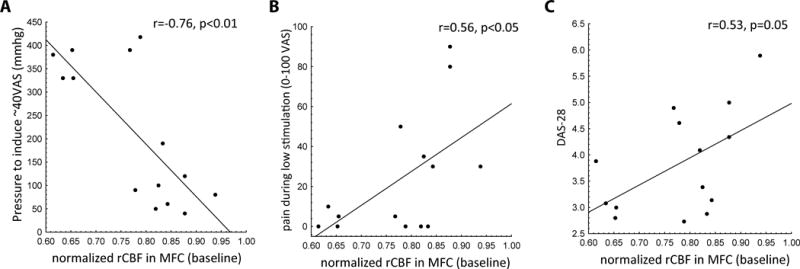
Region of interest analyses in the medial frontal cortex and associations with pain sensitivity and RA disease activity. A) The pressure required for the high intensity metacarpophalangeal (MCP) joint stimulus negatively correlated with resting regional cerebral blood flow (rCBF). B) The pain evoked by the low intensity metacarpophalangeal MCP stimulus positively correlated with resting rCBF. C) Disease Activity Score in 28 Joints (DAS28) positively correlated with resting rCBF.
Figure 4.
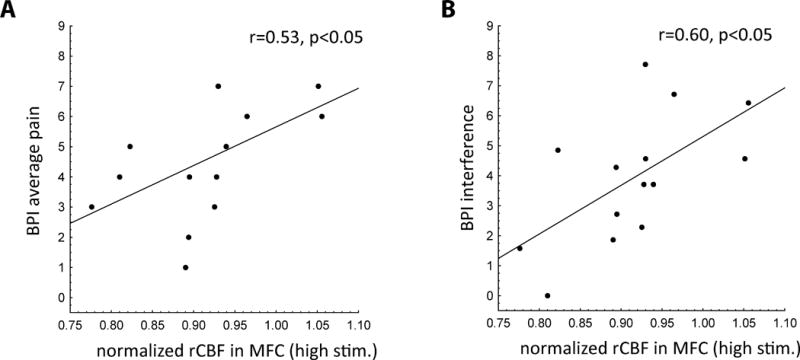
Region of interest analyses in the medial frontal cortex and associations with clinical pain measures. A) Brief Pain Inventory short form (BPI-sf) average pain level was positively correlated with regional cerebral blood flow (rCBF) in response to pain induced by a cuff wrapped around the metacarpophalangeal (MCP) joints. B) BPI-sf pain interference score was positively correlated with rCBF in response to pain induced by a cuff wrapped around the MCP joints.
DISCUSSION
Using pASL, we identified the MFC as a key area involved in the sensation and/or regulation of tonic, clinically-relevant joint pain in RA. Joint pain exacerbation was associated with increases in rCBF in the MFC, and rCBF in the MFC was significantly associated with measures of experimental pain sensitivity and clinical pain severity and interference. Moreover, the MFC was not found to be involved in pain induction for our sample of healthy controls. Based on these observations, we interpret rCBF response in MFC to represent neural processing of tonic, clinically-relevant pain in RA.
The MFC, including the medial prefrontal cortex (MPFC), aMCC and supplementary motor complex (SMA/pre-SMA) (35, 36), is consistently activated in response to pain (37) and is part of a group of regions in which activity reliably predicts experimental pain (38). Activation of the posterior region of the rostral MFC (prMFC) is thought to be associated with cognitive endeavors, such as attending and monitoring actions, whereas activation of the anterior region of the rostral MFC (arMFC) is associated with emotional undertakings, such as evaluating emotions in reaction to positive and negative images (35). Additionally, the aMCC, also referred to as dorsal anterior cingulate cortex, has been suggested to mediate the affective component of pain (39). In our study, both low- and high-intensity stimuli activated a posterior component of the MFC, but only the high-intensity stimulation significantly activated a more anterior component, possibly indicating the engagement of attentional resources in both conditions, and the recruitment of additional emotional processing in the latter (40). Furthermore, the activation of the pgACC by the high-intensity stimulation might reflect activity of the descending pain modulatory system, as this region has been extensively associated with antinociceptive functions (41–43), likely exerted through its descending projections to the periaqueductal gray matter (44).
In addition, the MFC is also a component of the default mode network, a group of brain regions associated with self-referential cognitive processing (45), which our group and others have shown to exhibit alterations in chronic pain (46–53). Using the same imaging technique employed in the present study, pASL, our group demonstrated elevations in the MFC (including the dorsomedial prefrontal cortex and pre-supplementary motor complex) in chronic low back pain patients after the experimental exacerbation of their clinical pain (9). The recruitment of the MFC across different pain disorders supports a central role for this region in chronic pain perception.
During both low and high intensity stimuli the patients also demonstrated the activation of the dLPFC, another region commonly seen activated in response to noxious stimulation (54). Interestingly, the dLPFC activation appeared stronger on the right side. A possible explanation for this finding is that the right dLPFC might be more implicated than the left side in the processing of fear and negative emotions (55), although this hypothesis would needs to be further evaluated.
In contrast to the statistically significant rCBF elevations detected in the patients in MPF and DLPFC, two sets of negative results are particularly noteworthy. First, several regions commonly observed as activated in acute pain imaging studies (e.g., primary somatosensory, insula, thalamus) (56) did not show a statistically significant elevation in rCBF in our study. We speculate that this may be due to the fact that we used tonic stimuli, whereas prior studies employed mostly brief, phasic stimuli.
Second, as opposed to the patients, the controls did not demonstrate any significant rCBF elevations, in response to either high or low stimuli. While the exact cause for this negative result remains uncertain, it is possible that the pain levels experienced by the controls, and/or the signal-to-noise ratio in our dataset, were simply too low to yield a measurable change in rCBF in our control sample, particularly in the context of a tonic stimulation. Despite these negative results, the observed patterns of stimulus-related brain changes in patients, and their relationship with clinical variables, suggests that pASL might be a promising tool to identify perfusion changes that are related to clinically-relevant pain.
Prior to this study, few studies have used fMRI to examine associations between brain function and pain in RA (11, 57). Most recently, Basu et al. examined functional connectivity between the default mode network, which includes the MPFC, and the insula among 54 RA patients with clinically significant fatigue (58). This study revealed that functional connectivity between the default mode network and insula was directly correlated with modified American College of Rheumatology Preliminary Diagnostic Criteria scores for fibromyalgia (59), suggesting that connectivity between the default mode network and insula may serve as an imaging marker for pain centralization. Because this study was cross-sectional, however, it could not provide information on causality.
Interestingly, a small longitudinal study (N = 5) reported that treatment with a infliximab, a monoclonal tumor necrosis factor-alpha (TNF-α) inhibitor, was associated with decreases in BOLD signal in the ACC, MPFC, and other brain areas involved in pain perception (e.g., thalamus, secondary somatosensory cortex, insula) within 24 hours (57). These changes were accompanied by significant decreases in pain intensity, whereas measures of inflammation (e.g., CRP, IL-6, swollen joint count, DAS28) were slower to change, with no statistically significant or clinically meaningful changes after 24 hours. These observations suggest that TNF-α may mediate nociception, independent of inflammation, among individuals with RA. Future studies with longitudinal data on psychosocial factors are needed to determine whether depression may mediate the association between TNF-α inhibition and changes in rCBF and clinical pain intensity.
In addition to fMRI, Positron Emission Tomography (PET), an invasive technique involving ionizing radiation, has also been used to assess rCBF in RA. Using PET, Jones and Derbyshire also identified the MPFC and ACC as regions in which rCBF differed in response to noxious stimuli in six RA patients vs. six age and sex-matched controls (60). In contrast to our study, which showed increases in rCBF in these areas, they noted dampened responses in the MPFC and ACC. The authors postulated that the dampened responses may reflect cognitive coping strategies, which are more developed (and thus more effective) among RA patients who experience pain on a regular basis. Responses, however, may differ depending on the type of noxious stimulus (61, 62). In the Jones and Derbyshire study, the noxious stimulus was thermal heat applied to the back of the hand, whereas, in our study, the noxious stimulus was a pressure cuff wrapped around the MCPs. RA patients may be better able to cope with an experimental noxious stimulus applied to an area not typically affected by RA. In contrast, pressure on the MCPs, which are actively inflamed due to RA, may elicit maladaptive cognitive responses, resulting in the increases in rCBF in the MPFC and ACC observed in this study.
Strengths of this study are: 1) the inclusion of age and sex-matched controls, 2) detailed clinical data on pain, disease activity and psychosocial factors, and 3) the pASL technique, which includes a 6-min continuous stimulus that minimizes contributions from attentional, salience and orienting responses.
The main limitations are the small sample size and the absence of a higher intensity pain stimulus that was universally painful for the control group. Due to the small sample size, we may not have been powered to detect modest differences between RA patients and healthy, pain-free controls. Because the study design did not include a higher intensity pain stimulus that was specifically constructed to be painful for the control group, nearly one third of controls did not find the high intensity pain stimulus to be painful. Thus, the lack of differences in rCBF in the control group may reflect that the controls did not find either the high intensity or low intensity stimulus to be significantly painful. As a result of these two limitations, ambiguity remains regarding the interpretation of our results. It is possible that the lack of significant group interaction effects in rCBF may be due to: 1) the small sample size, 2) lack of painful responses in the controls, or 3) no differences in the way noxious pressure is processed centrally. In addition, this study is inherently limited by the assumption that data from acute experimental stimuli, even directed at the peripheral source for clinical pain as in our study, accurately reflect chronic pain processing. This assumption is universal to all neuroimaging studies that require an acute on/off stimulus, but it neglects the many nuances that differ between acute and chronic pain (63).
In conclusion, our results highlight the roles of the MFC in the sensation and regulation of pain in RA. By identifying the CNS regions involved in the experience of pain, our findings contribute important information regarding the pathophysiology of pain in systemic inflammatory conditions. In addition, this information may represent an early step towards the use of imaging markers to objectively assess pain in research studies. However, before imaging markers can be used in clinical trials to assess the efficacy of interventions to treat pain, further studies are necessary to evaluate the clinical utility of these markers and to determine the populations and scenarios in which imaging marker assessment is appropriate.
SIGNIFICANCE AND INNOVATION.
This study is the first to identify the medial frontal cortex (MFC) as a key area involved in the sensation and/or regulation of tonic, clinically-relevant joint pain in rheumatoid arthritis (RA).
Joint pain exacerbation was associated with increases in regional cerebral blood flow (rCBF) in the MFC, and rCBF in the MFC was significantly associated with pain severity and interference.
The results from this study will inform the development of targeted pharmacologic and non-pharmacologic interventions for pain in systemic inflammatory conditions, such as RA.
Acknowledgments
Funding: This study was funded by Disease Targeted Research Pilot grant from the Rheumatology Research Foundation. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. Additional funding sources include NIAMS R01 AR064850 (YCL), NINDS R01 NS094306 (MLL) and R01 NS095937 (MLL).
Disclosures: Dr. Lee reports a research grant from Pfizer and stock in Express Scripts. She also served as an unpaid member of an advisory board for Eli Lilly.
Footnotes
DR. YVONNE CLAIRE LEE (Orcid ID : 0000-0002-2105-3393)
References
- 1.Borenstein D, Altman R, Bello A, Chatham W, Clauw DJ, Crofford LJ, et al. Report of the American College of Rheumatology Pain Management Task Force. Arthritis care & research. 2010;62:590–9. doi: 10.1002/acr.20005. [DOI] [PubMed] [Google Scholar]
- 2.Altawil R, Saevarsdottir S, Wedren S, Alfredsson L, Klareskog L, Lampa J. Remaining Pain in Early Rheumatoid Arthritis Patients Treated With Methotrexate. Arthritis care & research. 2016;68:1061–8. doi: 10.1002/acr.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh DA, McWilliams DF. Pain in rheumatoid arthritis. Current pain and headache reports. 2012;16:509–17. doi: 10.1007/s11916-012-0303-x. [DOI] [PubMed] [Google Scholar]
- 4.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detre JA, Rao H, Wang DJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging. 2012;35:1026–37. doi: 10.1002/jmri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen DG, Bureau Y, Thomas AW, Prato FS, St Lawrence KS. Quantification of pain-induced changes in cerebral blood flow by perfusion MRI. Pain. 2008;136:85–96. doi: 10.1016/j.pain.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, et al. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J Neurosci. 2015;35:15307–25. doi: 10.1523/JNEUROSCI.2542-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasan AD, Loggia ML, Chen LQ, Napadow V, Kong J, Gollub RL. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–74. doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard MA, Sanders D, Krause K, O’Muircheartaigh J, Fotopoulou A, Zelaya F, et al. Alterations in resting cerebral blood flow demonstrate ongoing pain in osteoarthritis: An arterial spin labelled magnetic resonance imaging study. Arthritis Rheum. 2012 doi: 10.1002/art.37685. [DOI] [PubMed] [Google Scholar]
- 11.Schweinhardt P, Kalk N, Wartolowska K, Chessell I, Wordsworth P, Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. NeuroImage. 2008;40:759–66. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Vaidyanathan U, Welo EJ, Malone SM, Burwell SJ, Iacono WG. The effects of recurrent episodes of depression on startle responses. Psychophysiology. 2014;51:103–9. doi: 10.1111/psyp.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorka SM, Liu H, Sarapas C, Shankman SA. Time course of threat responding in panic disorder and depression. Int J Psychophysiol. 2015;98:87–94. doi: 10.1016/j.ijpsycho.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comasco E, Gulinello M, Hellgren C, Skalkidou A, Sylven S, Sundstrom-Poromaa I. Sleep duration, depression, and oxytocinergic genotype influence prepulse inhibition of the startle reflex in postpartum women. Eur Neuropsychopharmacol. 2016;26:767–76. doi: 10.1016/j.euroneuro.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Rhudy JL, DelVentura JL, Terry EL, Bartley EJ, Olech E, Palit S, et al. Emotional modulation of pain and spinal nociception in fibromyalgia. Pain. 2013;154:1045–56. doi: 10.1016/j.pain.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tracey I, Johns E. The pain matrix: reloaded or reborn as we image tonic pain using arterial spin labelling. Pain. 2010;148(3):359–60. doi: 10.1016/j.pain.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain. 2002;100:19–26. doi: 10.1016/s0304-3959(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner F, Garcia RG, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67:1395–405. doi: 10.1002/art.39043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66:203–12. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen LE, Bliddal H, Christensen R, Karlsson JA, Gulfe A, Saxne T, et al. Is swollen to tender joint count ratio a new and useful clinical marker for biologic drug response in rheumatoid arthritis? Results from a Swedish cohort. Arthritis care & research. 2014;66:173–9. doi: 10.1002/acr.22107. [DOI] [PubMed] [Google Scholar]
- 21.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Hays RD, Stewart AL. Sleep Measures In: Ware ALSaJE, editor Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Durham University Press; 1992. pp. 235–59. [Google Scholar]
- 24.Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–54. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn Reson Imaging. 2012;30:1409–15. doi: 10.1016/j.mri.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–9. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404–13. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- 31.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol. 2000;83:1701–9. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- 33.Inoue M, Mikami A, Ando I, Tsukada H. Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cereb Cortex. 2004;14:106–19. doi: 10.1093/cercor/bhg109. [DOI] [PubMed] [Google Scholar]
- 34.Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- 35.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 36.Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–55. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- 37.Fomberstein K, Qadri S, Ramani R. Functional MRI and pain. Curr Opin Anaesthesiol. 2013;26:588–93. doi: 10.1097/01.aco.0000433060.59939.fe. [DOI] [PubMed] [Google Scholar]
- 38.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–97. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 40.Koban L, Jepma M, Geuter S, Wager TD. What’s in a word? How instructions, suggestions, and social information change pain and emotion. Neurosci Biobehav Rev. 2017;81(Pt A):29–42. doi: 10.1016/j.neubiorev.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1-2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia– imaging a shared neuronal network. Science. 2002;295(5560):1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 43.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 44.Vogt BA, Sikes RW, Vogt LJ. Anterior cingulate cortex and the medial pain system In: Gabriel M, editor Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook. Boston, MA: Birkhauser; 1993. pp. 313–44. [Google Scholar]
- 45.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 46.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110:18692–7. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PloS one. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–73. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neuroscience letters. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seminowicz DA, Moayedi M. The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. J Pain. 2017;18(9):1027–35. doi: 10.1016/j.jpain.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 56.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A. 2011;108:3731–6. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basu N, Kaplan CM, Ichesco E, Larkin T, Harris RE, Murray A, et al. Neurobiological features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis Rheumatol. 2018 doi: 10.1002/art.40451. [DOI] [PubMed] [Google Scholar]
- 59.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 60.Jones AK, Derbyshire SW. Reduced cortical responses to noxious heat in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:601–7. doi: 10.1136/ard.56.10.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janal MN, Glusman M, Kuhl JP, Clark WC. On the absence of correlation between responses to noxious heat, cold, electrical and ischemic stimulation. Pain. 1994;58:403–11. doi: 10.1016/0304-3959(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 62.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 63.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–68. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]


