Abstract
Currently there is a growing population of cochlear-implant (CI) users with (near) normal hearing in the non-implanted ear. This configuration is often called SSD (single-sided deafness) CI. The goal of the CI is often to improve spatial perception, so the question raises to what extent SSD CI listeners are sensitive to interaural time differences (ITDs). In a controlled lab setup, sensitivity to ITDs was investigated in 11 SSD CI listeners. The stimuli were 100-pps pulse trains on the CI side and band-limited click trains on the acoustic side. After determining level balance and the delay needed to achieve synchronous stimulation of the two ears, the just noticeable difference in ITD was measured using an adaptive procedure. Seven out of 11 listeners were sensitive to ITDs, with a median just noticeable difference of 438 μs. Out of the four listeners who were not sensitive to ITD, one listener reported binaural fusion, and three listeners reported no binaural fusion. To enable ITD sensitivity, a frequency-dependent delay of the electrical stimulus was required to synchronize the electric and acoustic signals at the level of the auditory nerve. Using subjective fusion measures and refined by ITD sensitivity, it was possible to match a CI electrode to an acoustic frequency range. This shows the feasibility of these measures for the allocation of acoustic frequency ranges to electrodes when fitting a CI to a subject with (near) normal hearing in the contralateral ear.
Keywords: cochlear implant, single-sided deafness, interaural time difference, binaural hearing
Introduction
Given the success of cochlear implants (CIs), there are many implantees with residual acoustic hearing in the non-implanted ear. While previously cochlear implantation was only considered if speech intelligibility was poor to impossible with acoustic hearing (NIDCD 2016; NIH 1995), there is currently a trend to provide a CI in addition to (near) normal hearing in the other ear, i.e., in single-sided deafness (SSD), also referred to as unilateral deafness (Arndt et al. 2011; Buechner et al. 2010; Kitterick et al. 2016; Távora-Vieira et al. 2016; Van de Heyning et al. 2008). Cochlear implantation in subjects suffering from SSD aims at enabling binaural hearing (re)habilitation and thus, improving sound source localization (Arndt et al. 2011; Buss et al. 2018; Hansen et al. 2013; Jacob et al. 2011; Litovsky et al. 2018), and enhancing speech recognition in noise particularly by providing benefit from the head shadow effect in certain spatial configurations (Arndt et al. 2011; Buechner et al. 2010; Vermeire and Van de Heyning 2009; Buss 2018). Another important motivation for CI in adults with SSD is the treatment of intractable tinnitus (Arndt et al. 2011; Van de Heyning et al. 2008; Kleine Punte et al. 2011). Given the objective of improving sound source localization, it is important to know whether such listeners are sensitive to interaural time differences (ITDs), which are important cues for sound source localization.
We previously investigated whether bimodal listeners, with severe hearing loss in the non-implanted ear, can be sensitive to ITD. We found that listeners with sufficient residual hearing could indeed be sensitive to ITDs, for electric pulse trains and acoustic filtered click trains (Francart et al. 2009), for transposed electric pulse trains and transposed acoustic tones, and multi-electrode signals and their acoustic counterpart (Francart et al. 2011), and for vowels processed by a novel sound processing strategy (Francart et al. 2014). However, we found no ITD sensitivity for low-frequency pure tones in the acoustically stimulated ear (Lenssen et al. 2011). While for many stimuli, our subjects were sensitive to ITD, just noticeable differences (JNDs) remained relatively poor compared to normal hearing, and there was much unexplained variability between subjects.
An important issue when considering ITD sensitivity in bimodal hearing is synchronization of the two modalities. Clinical devices can differ in their processing delays (which in case both ears are aided could relatively straightforwardly be equalized by delaying the faster device), but for synchronous stimulation of the two auditory nerves, the electrical stimulus needs to be additionally delayed to compensate for the acoustical traveling-wave delay in the other ear. While one would expect this delay to be frequency-dependent, we were not able to measure this with bimodal listeners with severe hearing loss (Francart et al. 2009). Zirn et al. (2015) investigated the interaural stimulation timing in SSD CI listeners using the MED-EL OPUS 2 device. They quantified the frequency-specific difference in delay between the acoustical and electrical stimulus based on the (electrically evoked) auditory brainstem potentials ((e)ABR) wave V latency and found that this latency difference largely corresponded to the frequency-specific delay in the existing signal processing path of the subjects’ CI sound processor (Zirn et al. 2015). However, for CI sound processors manufactured by other companies with a potentially larger delay that is fixed across frequencies, this may not be the case, nor if a hearing aid is used in the non-implanted ear.
Another issue is matching the place of excitation across the ears. For a certain narrowband acoustic input signal, the CI sound processor will stimulate a certain electrode in the cochlea according to its frequency-to-electrode allocation. This allocation does not exactly correspond to the intracochlear frequency-to-place map in normal hearing and is not fitted individually. Therefore, most likely different places in the two cochleae will be stimulated, which is detrimental for binaural cue perception (Francart and Wouters 2007; Nuetzel and Hafter 1981). While pitch matching has been suggested as an easy way to match places of excitation, it has some inherent problems, such as adaptation (Francart and McDermott 2013). Therefore, it has been proposed to use ITD sensitivity as a proxy for place matching for bimodal stimulation (Francart et al. 2009; Francart and McDermott 2013). Several authors have investigated the effect of interaural differences in place on ITD sensitivity with bilateral CIs (e.g., Goupell et al. 2013; Kan et al. 2013; Long et al. 2003; van Hoesel and Clark 1997) and proposed the use of ITD sensitivity as a proxy for interaural place matching (e.g., Hu and Dietz 2015; Poon et al. 2009). Recently, Bernstein et al. (2018) investigated ITD discrimination performance of SSD CI listeners as a function of acoustic carrier frequency and found a discernible peak for 17 out of the 26 electrodes tested across eight listeners, showing the potential of this method.
In most studies investigating perception with bilateral CIs, a larger than normal binaural fusion range has been found. A single electrode in one ear has been frequently reported to yield a fused auditory image when combined with one out of a larger range of electrodes in the other ear. For example, Kan et al. (2013) examined the effects of interaural electrode place mismatches up to ± 8 electrodes (± 5 mm) on fusion and localization. They found that the range of electrodes fused varied from 0 up to 16 electrodes across the array, with five of nine subjects experiencing fusion over the whole range. Similarly, Poon et al., ( 2009) found five times greater binaural fusion as a function of interaural place mismatch with bilateral CIs than in normal-hearing listeners. This means that the mismatch in place of excitation might be offset by an increased binaural fusion range.
Apart from binaural fusion, there is a growing body of work showing that users of hearing aids, bilateral cochlear implants, and bimodal devices have a much wider pitch fusion range than normal-hearing listeners, i.e., different monaural pitches are fused into a single binaural pitch (Oh and Reiss 2017; Reiss et al. 2018; Reiss et al. 2017). A similar phenomenon has been shown for vowel fusion (Reiss et al. 2016).
The availability of a number of cochlear implantees with normal hearing in the non-implanted ear allowed us to investigate a number of open questions: (1) Does normal hearing in the non-implanted ear lead to better ITD sensitivity in CI listeners with one normal-hearing ear than the severe hearing loss of the bimodal listeners tested in earlier studies? (2) How can the acoustical and electrical signals be synchronized at the auditory-nerve level, as a function of frequency? (3) What is the best match between electrodes and acoustic frequency ranges for ITD perception? We investigated this by measuring sensitivity to ITD in a number of SSD CI listeners, as a function of difference in place of stimulation between the ears, as well as the delay needed to synchronize stimuli between the ears.
Methods
Subjects
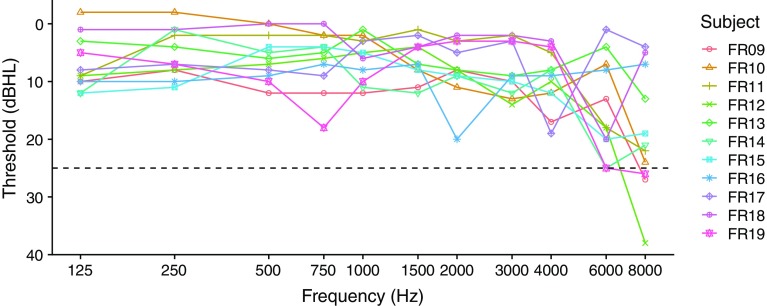
Eleven subjects with SSD were recruited from the clinical population of the University Medical Center Freiburg. This study was conducted in accordance with the guidelines of the Declaration of Helsinki. University of Freiburg ethics committee approval was obtained. Their deaf ear was implanted with a CI from Cochlear Ltd., Sydney, Australia. All subjects had had listening experience with their CI for more than 6 months. Various subject details, including etiology and reason for implantation of the deaf, ear are given in Table 1. Unaided air conduction pure-tone audiograms of the non-implanted ear are shown in Fig. 1. All thresholds were better than or equal to 25 dB HL, except for some subjects at 8 kHz. Only the threshold at 8 kHz for subject FR12 can be considered significantly worse than normal hearing (i.e., > 25 dB HL).
Table 1.
Subject details, including age at time of the experiments (in years), duration of deafness before acquiring a CI (“DD,” in months), duration of CI use at time of testing (“CI use”, in years), and etiology of deafness of the implanted ear
| Subject | DD | CI use | Age | Etiology | CI ear | Reason for implantation |
|---|---|---|---|---|---|---|
| FR09 | 10 | 7.3 | 62.2 | Acute | Left | Regain stereophonic hearing |
| FR10 | 2 | 5.7 | 48.0 | Meningitis (pneumococci) | Right | Reduce listening effort |
| FR11 | 7 | 7.8 | 47.1 | Acute | Right | Regain stereophonic hearing |
| FR12 | 16 | 0.6 | 47.9 | Acute | Right | Participation in society |
| FR13 | 24 | 6.0 | 34.2 | Acute | Left | Improve hearing in noise |
| FR14 | 3 | 7.8 | 48.9 | Labyrinthitis | Left | Regain stereophonic hearing |
| FR15 | 13 | 6.9 | 51.1 | Noise trauma | Right | Preventative (noise trauma) |
| FR16 | 6 | 8.1 | 32.0 | Trauma: bone fracture | Left | Regain stereophonic hearing |
| FR17 | 19 | 2.8 | 54.9 | Acute | Left | Improve hearing in noise |
| FR18 | 4 | 5.6 | 34.4 | Cogan-I-Syndrome | Right | Regain directional hearing |
| FR19 | 23 | 1.6 | 57.0 | Acoustic neuroma | Right | Regain directional hearing |
Fig. 1.
Unaided pure-tone thresholds of the non-implanted ear. The dashed line indicates the threshold limit of normal hearing according to our definition
Apparatus
All stimuli were presented under direct computer control using the APEX 3 program developed at ExpORL (KU Leuven) (Francart et al. 2008). For acoustic stimulation, we used a laptop with an RME Fireface sound card connected to Sennheiser HDA200 headphones. For electric stimulation, we used the Cochlear NIC v2.0 interface, connected to an L34 research processor provided by Cochlear Ltd. To ensure synchronous stimulation, the L34 was set up to start stimulating when a trigger pulse was received from the sound card. In this way, synchronous stimulation was achieved with 200 precision. The delay between the acoustic and electric signal was measured using the output of an implant-in-a-box and the electric output of the sound card visualized on an oscilloscope. The delay was calibrated such that the first edge of the electric pulse coincided with the maximum of the first peak of a broadband acoustic click. From the interaural delay values reported below, the delay of the headphone (300 μs) was removed. Note that there was no clinical sound processor in the signal path, and stimuli were synchronized at the output of the acoustical and electrical signal paths, so there were no processing delays of sound processor or hearing aid to be taken into account.
The headphone was calibrated using a G.R.A.S 43AA coupler that conforms to the ISO 389 norm. The shapes of both the electric and acoustic signals were checked using an oscilloscope. All stimuli were created using custom MATLAB (The Mathworks Inc., Natick, MA, USA) scripts.
Stimuli
All stimuli were presented bilaterally, i.e., the two ears were stimulated simultaneously. On the CI side, one of three stimulation electrodes was selected (electrode 12, 16, or 22, numbered from base to apex). For all electrodes, the stimulus was a 100-pps pulse train at most comfortable level, in monopolar mode, with a phase with of 25 μs and an inter-phase-gap of 8 μs.
The acoustic stimulus was a band-limited click train, one octave wide, with a fundamental frequency of 100 Hz. It was generated by adding the individual harmonics together (in-phase sinusoids) and ranged from 200–500 Hz to 4000–8000 Hz, depending on the subject’s sensitivity to ITD (see below).
The total stimulus duration was 1 s, and no ramping was used, in order to provide salient onset and ongoing ITD cues.
Procedures
For simultaneous stimulation of the two auditory nerves, the electrical stimulus needs to be delayed relative to the acoustic stimulus, to compensate for the traveling-wave delay of the acoustic signal through the middle and inner ear. This delay is expected to be frequency-dependent because for lower frequencies, closer to the apex, the traveling-wave delay is longer. The goal of our study was to obtain this electrical stimulation delay, which we will call D, and the JND in ITD. For the assessment of these measures, a comprehensive testing was applied including the following procedures: (1) select electrodes and corresponding acoustic frequency ranges based on binaural fusion, (2) manually obtain a rough estimate of D and the corresponding interaural level difference (ILD) for a centered percept, (3) obtain a precise estimate of D and ILD using a constant stimulus lateralization procedure, (4) obtain a precise estimate of the JND in ITD using an adaptive procedure.
For each electrode, the most comfortable level was determined for a 100-pps pulse train, and stimulation was fixed at that level. The level of the acoustically stimulated side was set according to the balancing procedures outlined below, resulting in typical sound pressure levels between 60 and 74 dB(A).
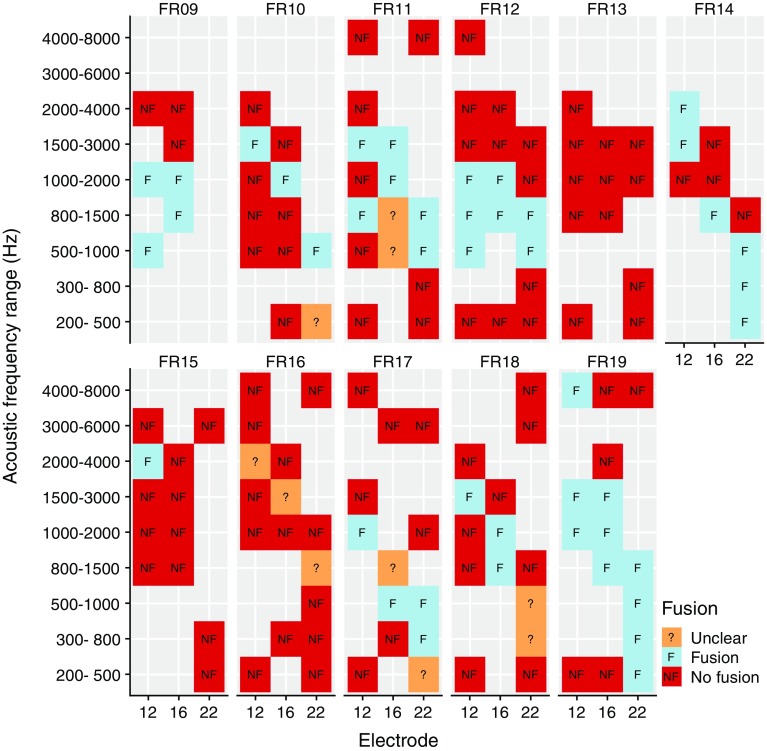
It was initially unknown which acoustic frequency range yielded the best ITD sensitivity for each electrode, but it was not feasible to measure ITD sensitivity for each combination of three electrodes and nine acoustic frequency ranges. We therefore started with a screening based on binaural fusion. We assumed that to be able to lateralize a stimulus based on ITD, the subject needed to perceive one fused sound image, rather than two separate sounds. This assumption is often made in the literature with bilateral CIs (e.g., Kan et al. 2013; Long et al. 2003; van Hoesel and Clark 1997; Steel et al. 2015; van Hoesel et al. 1993). We presented a selection of combinations of electrodes and frequency ranges to the subject, balanced them roughly in level, and asked the subject: Did you hear one single sound or two separate sounds? We then proceeded with the ITD sensitivity measurement procedures below for the combinations that yielded a fused image. The combinations included in the screening were based on the greenwood function and typical insertion depths (the most apical electrode combined with the lowest acoustic frequencies). If time allowed, other promising combinations were included as well. When time was very limited, combinations were excluded based on the subject’s feedback, for instance, if the sounds were not fused and the acoustic signal was reported to sound higher in pitch, even higher frequencies were not tried. The final selection can be seen in Fig. 3.
Fig. 3.
Fusion screening results. Combinations of electrodes and acoustic frequency ranges for which fusion was screened are indicated by colored boxes. Note that the acoustic frequency ranges overlap
We expect no effect of the initial choice of D on the fusion screening results because of the following: (1) we used a value of D informed by the objective measures (wave (e)V latencies) described below, (2) there is some evidence that sounds remain fused even for unnaturally large ITDs (Baumgärtel et al. 2017), and (3) the finally obtained D values were sufficiently close to the values used during the screening.
The difficulty in obtaining D is that the perceived laterality of a stimulus is influenced by both the ITD and ILD, and for neither the balanced value is known a priori. Therefore, we used a procedure based on the perceived extent of lateralization. From research with normal-hearing listeners, it is known that when manipulating only the ITD, extent of laterality is the largest and most symmetric when the ILD is zero (Domnitz 1973; Shepard and Colburn 1976). We first conducted a rough procedure to obtain an initial level balance and value of D. This rough procedure was controlled manually by the experimenter, informed by the objective measures described below, and only served to obtain an initial estimate of the parameters of the subsequent constant stimulus procedure. This procedure also provided the subject with the opportunity to get used to the stimuli and cues to listen for, which is essential after potentially many years without access to ITD cues.
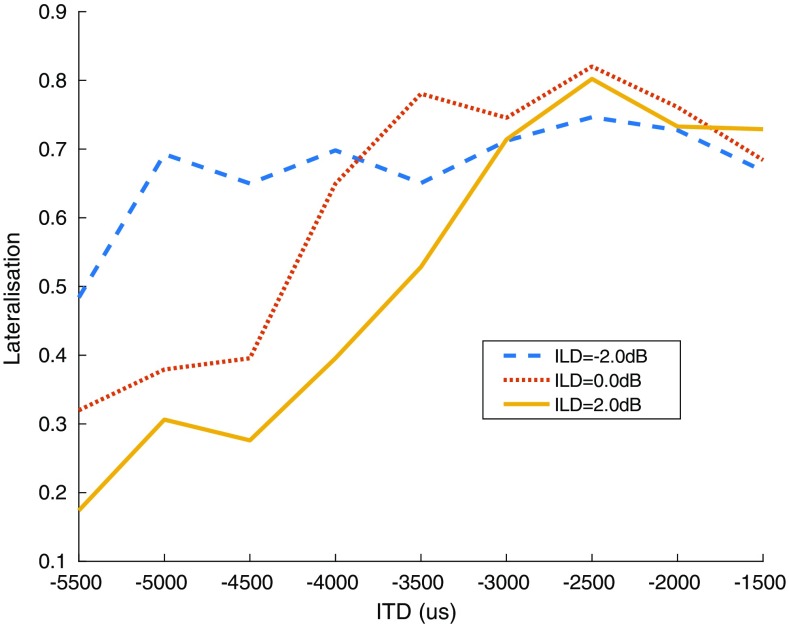
In the subsequent constant stimulus lateralization procedure, three ILDs were selected around the rough level balance, and at least seven ITDs, linearly spaced by 500 μs, centered around the rough D value. Each combination of ILD and ITD was presented five times, except for electrode 16 in subject FR09 with three presentations, in random order and the subject responded on a visual analog scale (a drawing of a head seen from above) where in the head, they perceived the sound. We then selected the laterality function with the largest and most symmetric extent of lateralization, noted its ILD, and calculated its D as the 50 %-point of a sigmoid function fitted to the laterality function. For a sigmoid defined by , with γ the guess-rate, λ the lapse-rate, and β the slope, extent of lateralization was defined as 1 − γ − λ and symmetry as (0.5 − γ)/(0.5 − λ). An example of this procedure is shown in Fig. 2. In this case, three ILDs were selected around the rough level balance, and nine ITDs around the rough D of 3500 μs. ILD = 2 dB yielded the largest extent of lateralization and symmetry, so it was selected for the next step.
Fig. 2.
Example result from the lateralization procedure, for subject FR18, electrode 11, acoustic stimulus 1500–3000 Hz. In this case, ILD 2 dB (relative to rough balance) yielded the largest extent of lateralization and was the most symmetric
Finally, the JND in ITD was determined in a 1up-2down adaptive procedure, with a start value of 2000 μs and step size 2 dB (of μs). The procedure was stopped after 16 trials and the JND was calculated as the geometric mean of the last 10 trials. Each trial consisted of two intervals, either +ITD and −ITD or −ITD and +ITD, with ITDs specified relative to D, and the subject’s task was to indicate whether the stimulus moved from the right to the left or vice versa. When time allowed, this procedure was conducted multiple times and the median result was used for further analysis. This procedure on the one hand avoids the uncertainty inherent in methods that assess sensitivity to cues in a target signal that follows a preceding reference stimulus (Hartmann and Raked 1989), and on the other hand is less sensitive to centering errors than procedures where the centered stimulus is the reference. It has been frequently used in work with bilateral CIs (e.g., van Hoesel 2007; van Hoesel et al. 2009).
Objective Measures
To obtain an initial estimate of D for the extent-of-lateralization procedure, and as an extra validation of our results, we measured auditory brainstem responses (ABRs). For practical reasons, we were limited to clinically used stimuli, so the stimuli were different from the ones described above. This means that comparison of results from the behavioral and objective measures should be done with caution.
ABRs were recorded using an Interacoustics Eclipse device, and Beyerdynamic DT 48 headphones, with a 33–1500-Hz bandpass filter applied to the EEG signal. The positive electrode was placed on the upper forehead, the negative electrode on the mastoid on the normal-hearing ear side, and the ground electrode on the lower forehead. Both acoustic and electric stimuli were set to a subjective loud level, corresponding approximately to an acoustic level of 80-dB nHL.
Electric stimuli were generated using Custom Sound EP (provided by Cochlear), consisted of 20-pps pulse trains (in contrast to the 100-pps pulse trains used for the behavioral experiments) and were presented on electrode 16 in all subjects, except electrode 12 in subject FR10. Acoustic stimuli were 20 tone bursts per second, with alternating polarity and the following parameters: 1-kHz carrier, 2-ms ramps, 1-ms plateau; 2-kHz carrier, 1-ms ramps, 0.5-ms plateau; 4-kHz carrier, 0.5-ms ramps, 0.25-ms plateau. The design and duration of the tone bursts were chosen according to the 2-1-2 paradigm endorsed by the British/Canadian test protocol (Hall 2007).
Stimulation was unilateral, and for each stimulus, wave (e)V was visually labeled, and its latency was determined.
Statistical Analysis
Statistics were computed using R (V3.4.4; using the nlme package version 3.1-137 for mixed model calculations).
Results
Fusion
The results of the initial fusion screening are shown in Fig. 3. For two out of 11 subjects (FR13, FR16), sounds were never well fused (apart from one outlier). For the other subjects, there was a clear tendency of increasing acoustic frequency paired with decreasing electrode number (closer to the base of the cochlea) eliciting fused sounds. Empty cells indicate that this combination was not tested, because it was unlikely to have yielded additional information for the subsequent procedures.
ITD Sensitivity
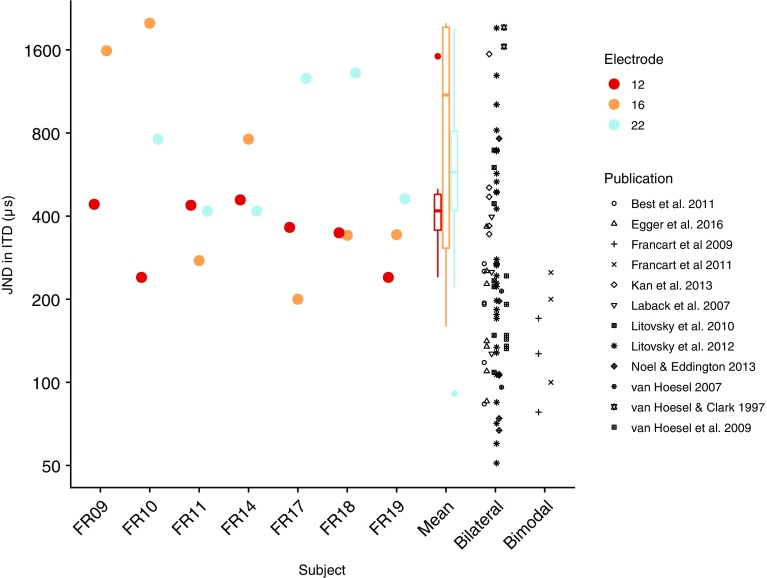
For nine out of 11 subjects, we could find electrodes and corresponding acoustic stimuli yielding a fused sound image, and JNDs in ITD could be measured in seven of these nine subjects. ITD sensitivity was only measured if sounds were fused, which makes sense because the percept of laterality, based on which the ITD task could be performed, is only defined for a single fused sound image. However, fused sounds did not automatically result in ITD sensitivity: subjects FR12 and FR15 reported fused sounds but were not sensitive to ITD. In Fig. 4, a high-level overview is shown. We plotted the JND in ITD for each subject and electrode, for the acoustic frequency range that yielded the lowest JND. JNDs across subjects, electrodes, and acoustic frequency ranges ranged from 200 to 2000 μs, with a median of 438 μs. Reference data from the literature for bilateral CI and bimodal listeners are also shown. The bilateral CI data were taken from a recent review paper (Laback et al. (2015), Fig. 2, all data with pulse rate 100 pps). If multiple ITD thresholds were available per listener, the mean was calculated. For studies in which the opposite ITD was not used as a reference (Francart et al. 2009; Francart et al. 2011; Litovsky et al. 2010), the JNDs were divided by 2. Note that other small procedural differences could have influenced the results, such as target level of the adaptive procedure. In particular, Francart et al. (2009) and Francart et al. (2011) used the 50 %-correct point from a constant stimuli procedure, which may lead to lower JNDs than estimated with the typically used target point of the adaptive procedure of around 70 %. However, Francart et al. (2014) compared the results of their constant stimuli procedure with a 1up/2down, 70 % correct, −ITD +ITD procedure, and found similar results when the thresholds obtained from the adaptive procedure were multiplied by 2.
Fig. 4.
Best JND in ITD across acoustic stimuli, for each electrode. Bilateral and bimodal data were replotted from published papers. Note that procedural differences between these studies may have influenced the results
While sometimes large intra-individual differences were present between stimulation electrodes, overall, there was no consistent effect of electrode. A Wilcoxon rank-sum test of JND between our median SSD CI data per subject and bilateral CI data from the literature (see above) indicated that our SSD CI listeners performed significantly worse (W = 406, p = 0.022). They also performed significantly worse than the limited number of bimodal listeners reported in the literature (W = 56, p = 0.001). However, this conclusion has to be considered with care due to the limited sample size and procedural differences. JNDs are much higher than the normal-hearing listeners, for whom a JND in the order of 100 μs would be expected with similar stimuli (Bernstein and Trahiotis 2002).
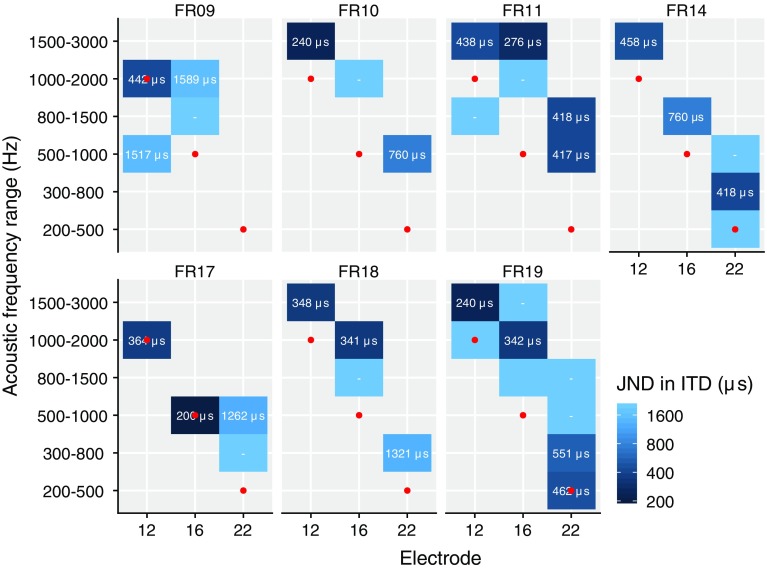
In Fig. 5, the JND in ITD is shown for each subject as a function of both electrode and acoustic frequency range. Note that only the small number of acoustic frequency ranges and electrodes was tested for which binaural fusion was found. It is clear that with increasing electrode number, i.e., more apical stimulation, the best-matching acoustic frequency range decreases. A mixed model with outcome JND and with factors electrode and acoustic frequency range, and a random effect of subject, indicated no significant effects, nor after logarithmically transforming the JND, indicating no clear effect across subjects of electrode (F(9, 2) = 0.08, p = 0.92) or acoustical frequency range (F(9, 5) = 0.71, p = 0.63) on JND in ITD.
Fig. 5.
JND in ITD for each acoustic frequency range and electrode combination. A dash indicates that the subject was not sensitive to ITD. Red dots indicate the default clinical frequency-to-electrode allocation
Synchronization
With physically synchronized electrical and acoustical stimulation as applied in our lateralization experiment, the electric stimulus reaches the auditory nerve first, due to the traveling-wave delay on the acoustic side. From our experiment, we could derive the delay required to compensate for this (D).
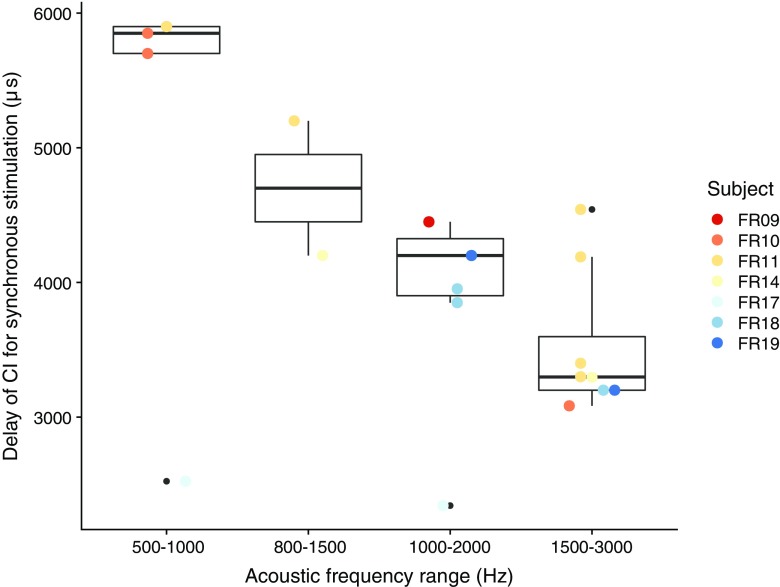
In Fig. 6, D is shown as a function of acoustic frequency range. Conditions with a JND higher than 1000 μs were removed, as the precision of the estimate of D depends on the JND in ITD. D significantly decreased with increasing acoustic center frequency (mixed model with a random effect of subject, df = 31, t = 3.90, p < 0.01), with differences between the lowest and highest center frequencies in the order of 3 ms, which is plausible according to the literature on acoustic traveling-wave delays (e.g., Ruggero and Temchin 2007; Strelcyk and Dau 2009).
Fig. 6.
Delay of the CI needed for synchronous stimulation of the auditory nerves, as a function of acoustic frequency range. Delay values for JNDs larger than 1000 μs were removed, as well as acoustic frequency ranges that yielded ITD sensitivity in only one subject
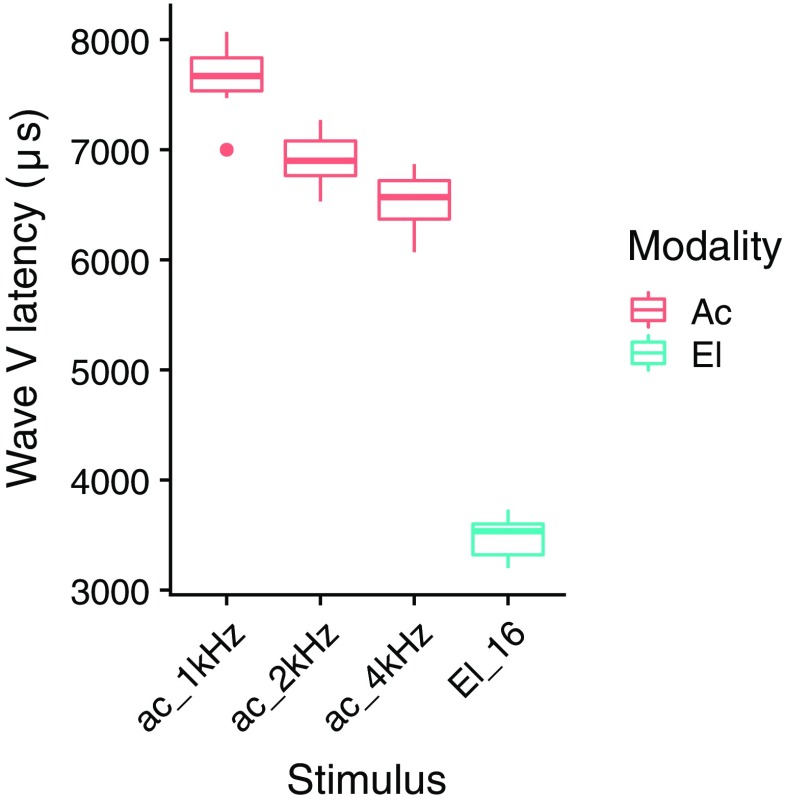
In Fig. 7, the (e)ABR wave V latencies are shown for the different stimuli. The median eABR wave V latency obtained for stimulation at electrode 16 conforms to the mean eABR wave latencies found for stimulation at two relatively neighboring electrodes of a Nucleus 22-channel cochlear-implant system: 3.82 ms for electrode 20 and 3.94 ms for electrode 12 (Shallop et al. 1990). For all frequencies, median ABR wave V latencies mostly correspond to those assessed for tone burst stimulation of the normal-hearing ear in seven SSD CI users in a recent study, reporting mean latencies of 7.9 ms for 1 kHz, 6.7 ms for 2 kHz, and 6.2 ms for 4 kHz (Zirn et al. 2015). eABR latencies were clearly shorter than ABR latencies, and we expect that for synchronous stimulation of the two auditory nerves, the electric signal needs to be delayed by the difference between the ABR and eABR latency (in clinical devices after compensation by the difference in device processing delay). The difference between (e)ABR wave V latency for electric and acoustic stimulation was significantly affected by acoustic frequency range (linear model, t = − 3.78, p < 0.01).
Fig. 7.
Acoustic tone burst and electric ABR wave V latency
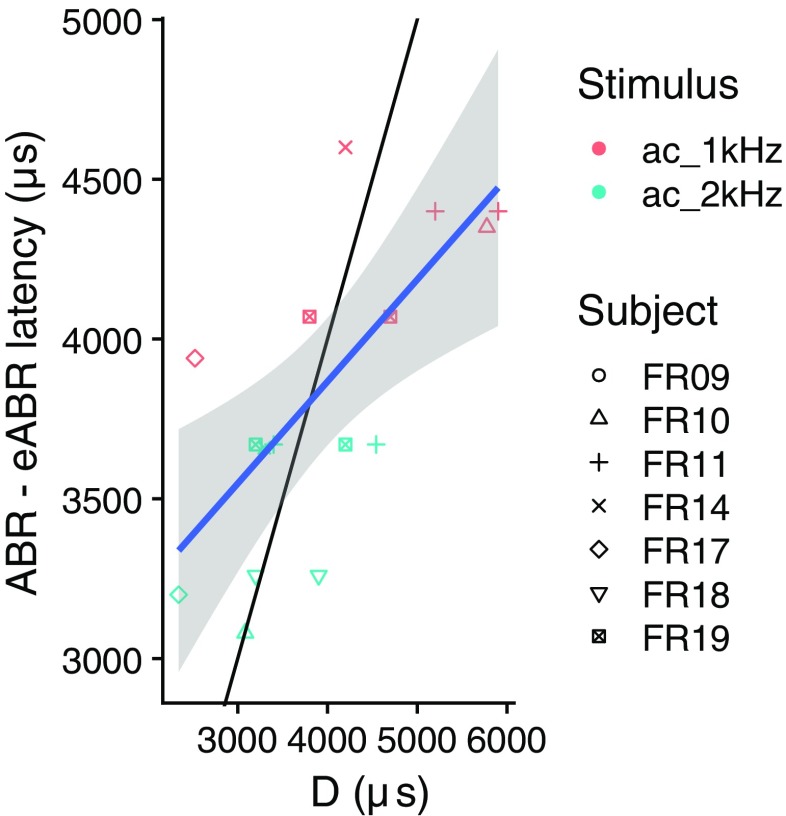
To allow rough comparison of the ABR-eABR latencies with the D values obtained in our behavioral experiment, we assigned each acoustic frequency range to the tone burst with carrier frequency closest to its center frequency and calculated the correlation between the difference in wave (e)V latency and D. This is shown in Fig. 8. This correlation was significant (ρ = 0.70, p < 0.01). The remaining difference with the diagonal (thin black line) might be due to differences in stimuli between the (e)ABR and D measurements.
Fig. 8.
Correlation between (e)ABR latency difference and D. The thin black line indicates the diagonal and the blue line and gray shading indicate a linear fit and corresponding 95 % confidence intervals
Subject Factors
To better understand differences in ITD sensitivity between subjects, we collected audiometric data that was obtained in other studies and either compared between our groups of subjects who were not sensitive to ITD (N = 4) or sensitive to ITD (N = 7), or investigated the correlation between JND in ITD and the audiometric outcome measures for all subjects (N = 11).
Audiometric outcome measures included speech intelligibility in quiet, speech intelligibility in noise with spatially separated speech and noise sources, and sound source localization. These measures were obtained monaurally (unaided) and binaurally (aided with CI).
No correlations or group comparisons yielded significant results.
Discussion
We measured sensitivity to ITD for 11 subjects with a unilateral CI and (nearly) normal hearing in the other ear. Seven subjects were sensitive to ITD, with JNDs higher than those found in subjects with bilateral CIs, and with bimodal hearing and a severe hearing loss in the non-implanted ear. Thresholds were much worse than for normal-hearing subjects. We were able to match acoustic frequency ranges to electrodes based on binaural fusion and ITD sensitivity. We measured the delay required for synchronous stimulation of the two auditory nerves, and found that it depended on the acoustic frequency range.
JND in ITD
In our previous study with bimodal listeners (Francart et al. 2009), we found that only the subjects with better residual hearing were sensitive to ITD. This prompted the hypothesis that much better residual hearing in the non-implanted ear may yield better ITD sensitivity. However, the current subjects, with (nearly) normal hearing in the non-implanted ear, were on average less sensitive to ITD than the bimodal listeners tested earlier. This indicates that the limiting factor in ITD sensitivity is not the acuity of acoustic hearing per se. On the contrary: perhaps the increased spread of excitation in the severely hearing impaired ear of the bimodal listeners was in some cases beneficial for ITD sensitivity. However, care should be taken interpreting these results due to limited sample sizes and procedural differences between studies. Our CI SSD listeners were on average less sensitive to ITD than bilateral CI listeners, suggesting that the combination of electric and acoustic hearing might be detrimental to ITD sensitivity, on top of the generally already poor performance with bilateral CIs.
While most of our subjects were sensitive to ITD, performance was much worse than for normal-hearing listeners. The median JND in ITD was 438 μs, while for normal-hearing listeners, a threshold of around 10–50 μs would be expected with similar stimuli. Considering envelope ITD perception with normal hearing, Bernstein and Trahiotis (2002) found a threshold of around 100 μs for both pure tones and transposed tones; however, in their procedure, the subjects had to detect a difference between an ITD of 0 μs and the target ITD, while in our study, the bimodal listeners had to detect the difference between + and − target ITD, essentially doubling the available cue. Similar to our previous studies with bimodal listeners (Francart et al. 2009; Francart et al. 2011; Lenssen et al. 2011; Francart and McDermott 2013), sensitivity to ITD and salience of the ITD cue were consistent with envelope ITD perception in normal-hearing listeners.
Our subjects have not had exposure to ITD since acquiring their single-sided deafness, even with their CI (some reasons are outlined below), and therefore, ITD sensitivity may have declined over time. Indeed, most subjects needed some practice before being able to detect ITDs in our stimuli. It is currently unknown if and to what extent chronic electrical stimulation by a CI with a stimulation strategy and fitting that optimally preserve envelope ITDs would prevent this decline.
Binaural Fusion—Place Matching
Binaural fusion ranges differed widely across subjects (cf Fig. 3). They were generally much wider than NH subjects (Nuetzel and Hafter 1981) and showed similar patterns to subjects with bilateral CIs (e.g., Kan et al. 2013).
It has been proposed to use ITD sensitivity to match places of stimulation across the cochleae (e.g., Francart et al. 2009; Hu and Dietz 2015; Poon et al. 2009; Bernstein et al. 2018), assuming that for place-matched stimulation, ITD sensitivity is best (e.g., Goupell et al. 2013; Kan et al. 2013; Long et al. 2003; van Hoesel and Clark 1997). Our current results indicate that this is indeed possible, at least for subjects who are sensitive to ITD. However, the matching is not precise: sometimes adjacent octave-bands yield similar sensitivity, and the current procedures are not suitable for clinical application due to their complexity and duration. The upside of the imprecision of the match is that perhaps the matching does not need to be precise for good fusion and ITD sensitivity, which would make individual fitting easier. A caveat relating to our procedures is that it is theoretically possible that an electrode-frequency combination that did not pass or was not tested in the fusion screening would have yielded ITD sensitivity. However, this seems unlikely because all reasonable electrode-frequency combinations were included in the screening, and our final results are plausible compared to the literature (see below). Overall, our procedures can be viewed as matching based on fusion, followed by a refinement and validation based on ITD sensitivity.
Our results are consistent with those of Bernstein et al. (2018), who also concluded that it may be feasible to match the place of excitation based on ITD sensitivity. The main difference with our study is that they used a faster procedure, which may be more feasible to implement clinically, but which led to some trade-offs in terms of accuracy, as discussed in the paper: the reference was not centered, loudness balancing was relatively rough, and the ITD range was larger than physiologically plausible, leading to some doubt whether the task was actually binaural. In our methods, these concerns were addressed, allowing to precisely measure ITD JNDs, however, with the trade-off of not being able to do so across the entire acoustic frequency range due to time constraints (hence the fusion screening).
In Fig. 5, the default clinical frequency-to-electrode allocation of the sound processor used by the subjects is shown by red dots. While there are large individual differences in the difference between clinical allocation and place for best ITD sensitivity, the clinical frequency allocation tends to be lower than the one based on ITD matching, by 0.5 to 1 octaves. This is consistent with (Landsberger et al. 2015), who found a typical mismatch between actual cochlear place and mapped frequency of around one octave.
Note that the lower-frequency acoustical stimuli might not have generated the intended modulations at auditory-nerve level, as the harmonics at 300–500 Hz would be resolved in a normal-hearing ear. This might have led to underestimation of the ITD sensitivity at those frequencies and could have resulted in a bias in place matching towards higher frequencies. In future studies, this could be addressed by lowering the fundamental frequency.
Synchronization
To compensate for the acoustic traveling-wave delay, the CI processing needs to be delayed. Lower frequencies are detected more apically on the basilar membrane, and have a longer traveling-wave delay. In our earlier study with bimodal stimulation (Francart et al. 2009), we were unable to measure this frequency dependence and found that an average compensation delay of electrical stimulation of 1.5 ms was sufficient for bimodal listeners with a severe hearing loss (assuming that the processing delays of the CI processor and the hearing aid are equalized). Our inability to measure the frequency dependence was probably due to the nature of their hearing loss, as cochlear filter times decrease with increasing hearing loss (Don et al. 1998). In the current study, we did find this frequency dependence, and the obtained delays are plausible according to the literature (e.g., Ruggero and Temchin 2007; Strelcyk and Dau 2009), and consistent with the measured differences in wave V delay from ABR and eABR measurements. Note that our estimates of the delay depend on a number of steps in the experimental procedures, in particular, the determination of the ILD, so small errors can have accumulated.
While there is some evidence that the neural system can adapt to small systematic asymmetric interaural delays (Trapeau and Schönwiesner 2015), it is unlikely that the system would be able to adapt to delays that are an order of magnitude larger than those that could occur naturally by a growing head. Therefore, to enable ITD perception with auditory prostheses, difference in processing delays between the two ears should be set according to either individual delay difference measurements or population average values. However, there are a number of practical problems: (1) if the non-implanted ear is (near) normal hearing, no hearing aid is required, so no delay is possible in this ear, to compensate for the processing delay of the CI, which can for some sound processors and frequencies be larger than the acoustic traveling-wave delay, (2) with open fit in the non-implanted ear, no delay can be imposed for the low frequencies, (3) the delay is not only frequency-dependent, but also level-dependent (Neely et al. 1988), which would be hard to implement in practice, and (4) while it may be possible to sufficiently correct the delay, ITD sensitivity will probably remain poor due to other factors (see below). However, if binaural unmasking, i.e., improved sound perception due to the combination of information across the ears, is possible with bimodal stimulation, which is currently unknown, it may benefit from equalized delays, as it is robust to a small offset in delay (Bernstein and Trahiotis 2015; Bernstein and Trahiotis 2016).
Relation with Hearing Abilities
Four out of our 11 subjects were not sensitive to ITD at all, and there was much variability across the other seven individuals and even across electrodes within each subject. It is unclear what causes this variation, but as there was no reason to expect much differences originating from the acoustically stimulated ear, and the large variation in ITD sensitivity between electrodes within subjects, we find it most likely that the differences originate from local variation at the interface of the CI, i.e., differences in neural survival, possibly related to etiology of deafness and electrode location relative to the stimulated neurons.
We were not able to find any correlations between audiometric data and ITD sensitivity, but our study may have been underpowered to find such correlations.
Implications for Sound Coding Strategies
With current fitting and sound coding strategies, it is unlikely that CI users with contralateral normal hearing (or impaired hearing) would be able to use ITD cues in practice because of the following: (1) the required temporal information is poorly coded by most CI sound processing strategies (Francart et al. 2014), (2) many CI sound processors have a processing delay longer than the required delay of electrical stimulation for synchronization with the acoustic signal, and for broadband signals, the delay of the sound processor does not vary in the same way with frequency as the delay of the traveling wave in the acoustically stimulated ear, (3) with the standard frequency-to-electrode allocation of the sound processor, the place of stimulation in the two cochleae is not optimally matched for ITD perception, for most subjects (4) for the human head size, the maximal physically plausible ITD is around 690 μs (Feddersen 1957). If the smallest ITD that can be detected is in the best case 200 μs, and in many cases, it is unlikely to yield much practical use.
If a certain subject is sufficiently sensitive to ITD in controlled laboratory conditions, the following changes to sound coding and fitting would be required to allow the use of ITDs for sound source localization:
On the CI side that faithfully encode the timing information in the temporal envelope of the incoming acoustical signal, in a way compatible with the temporal envelopes available in the acoustically stimulated ear (Francart et al. 2014). While we did not explicitly investigate sensitivity to temporal fine-structure ITDs in SSD CI listeners, we previously found no sensitivity to temporal fine-structure ITDs in bimodal listeners (Lenssen et al. 2011).
Match the place of stimulation between the cochleae based on ITD sensitivity, by changing the frequency-to-electrode allocation of the CI sound processor,
Equalize the processing delay of CI and HA, and introduce frequency-dependent delays in the CI processing according to either individual delay measurements (using an ITD-based lateralization procedure or ABR-eABR wave V latency), or population averages.
If a subject is not sufficiently sensitive to ITD, matching the place of stimulation across cochleae might still be beneficial for speech intelligibility or binaural unmasking (Ma et al. 2016; Wess et al. 2017).
Conclusions
SSD CI listeners show poorer ITD sensitivity than bilateral CI listeners and than bimodal listeners, so the hearing impaired acoustically stimulated ear does not seem to be the limiting factor for ITD sensitivity.
Our lateralization procedure can be used to measure the frequency-dependent delay required to synchronize the acoustic and electric stimulus and yields results consistent with differences in wave V latency between ABR and eABR.
Acoustic frequency ranges can be matched to electrodes based on fusion, refined and validated by ITD sensitivity, which may serve as a method to allocate acoustic frequency ranges to electrodes when fitting a CI to a subject with (near) normal hearing in the contralateral ear.
Acknowledgements
We are grateful to our test subjects for voluntarily spending time listening to beeps. We thank Bernhard Laback for providing the numerical bilateral CI data shown in Fig. 3.
Funding Information
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 637424, ERC starting Grant to Tom Francart). Financial support was provided by the KU Leuven Special Research Fund under grant OT/14/119 to Tom Francart and by the Freiburg association “Taube Kinder lernen Hören e.V.” to the cochlear-implant rehabilitation center in Freiburg.
Compliance with Ethical Standards
This study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Contributor Information
Tom Francart, Email: tom.francart@med.kuleuven.be.
Konstantin Wiebe, Email: konstantin.wiebe@uniklinik-freiburg.de.
Thomas Wesarg, Email: thomas.wesarg@uniklinik-freiburg.de.
References
- Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, Ihorst G, Wesarg T. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32(1):39–47. doi: 10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- Baumgärtel RM, Hu H, Kollmeier B, Dietz M. Extent of lateralization at large interaural time differences in simulated electric hearing and bilateral cochlear implant users. J Acoust Soc Am. 2017;141(4):2338–2352. doi: 10.1121/1.4979114. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli”. J Acoust Soc Am. 2002;112(3 Pt 1):1026–1036. doi: 10.1121/1.1497620. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Converging measures of binaural detection yield estimates of precision of coding of interaural temporal disparities. J Acoust Soc Am. 2015;138(5):EL474–EL479. doi: 10.1121/1.4935606. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Behavioral manifestations of audiometrically-defined “slight” or “hidden” hearing loss revealed by measures of binaural detection. J Acoust Soc Am. 2016;140(5):3540–3548. doi: 10.1121/1.4966113. [DOI] [PubMed] [Google Scholar]
- Bernstein JGW, Stakhovskaya OA, Schuchman GI, Jensen KK, Goupell MJ (2018) Interaural time-difference discrimination as a measure of place of stimulation for cochlear-implant users with single-sided deafness. Trends Hear 22:2331216518765514 [DOI] [PMC free article] [PubMed]
- Buechner A, Brendel M, Lesinski-Schiedat A, Wenzel G, Frohne-Buechner C, Jaeger B, Lenarz T. Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol Neurotol. 2010;31(9):1381–1385. doi: 10.1097/MAO.0b013e3181e3d353. [DOI] [PubMed] [Google Scholar]
- Buss E, Dillon MT, Rooth MA, King ER, Deres EJ, Buchman CA, Pillsbury HC, Brown KD (2018) Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends Hear 22:2331216518771173 [DOI] [PMC free article] [PubMed]
- Domnitz R. The interaural time JND as a simultaneous function of interaural time and interaural amplitude. J Acoust Soc Am. 1973;53(6):1549–1552. doi: 10.1121/1.1913500. [DOI] [PubMed] [Google Scholar]
- Don M, Ponton CW, Eggermont JJ, Kwong B. The effects of sensory hearing loss on cochlear filter times estimated from auditory brainstem response latencies. J Acoust Soc Am. 1998;104(4):2280–2289. doi: 10.1121/1.423741. [DOI] [PubMed] [Google Scholar]
- Egger K, Majdak P, Laback B. Channel interaction and current level affect across-electrode integration of interaural time differences in bilateral cochlear-implant listeners. J Assoc Res Otolaryngol. 2016;17(1):55–67. doi: 10.1007/s10162-015-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddersen WE. Localization of high-frequency tones. J Acoust Soc Am. 1957;29(9):988–991. doi: 10.1121/1.1909356. [DOI] [Google Scholar]
- Francart T, McDermott HJ. Psychophysics, fitting, and signal processing for combined hearing aid and cochlear implant stimulation. Ear Hear. 2013;34(6):685–700. doi: 10.1097/AUD.0b013e31829d14cb. [DOI] [PubMed] [Google Scholar]
- Francart T, Wouters J. Perception of across-frequency interaural level differences. J Acoust Soc Am. 2007;122(5):2826–2831. doi: 10.1121/1.2783130. [DOI] [PubMed] [Google Scholar]
- Francart T, van Wieringen A, Wouters J (2008) APEX 3: a multi-purpose test platform for auditory psychophysical experiments. J Neurosci Methods 172(2):283–293 [DOI] [PubMed]
- Francart T, Brokx J, Wouters J. Sensitivity to interaural time differences with combined cochlear implant and acoustic stimulation. J Assoc Res Otolaryngol. 2009;10(1):131–141. doi: 10.1007/s10162-008-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francart T, Lenssen A, Wouters J. Sensitivity of bimodal listeners to interaural time differences with modulated single- and multiple-channel stimuli. Audiol Neurootol. 2011;16(2):82–92. doi: 10.1159/000313329. [DOI] [PubMed] [Google Scholar]
- Francart T, Lenssen A, Wouters J. Modulation enhancement in the electrical signal improves perception of interaural time differences with bimodal stimulation. J Assoc Res Otolaryngol. 2014;15(4):633–647. doi: 10.1007/s10162-014-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ, Stoelb C, Kan A, Litovsky RY. Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. J Acoust Soc Am. 2013;133(4):2272–2287. doi: 10.1121/1.4792936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW (2007) New handbook of auditory evoked responses. Pearson
- Hansen MR, Gantz BJ, Dunn C. Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Ménière’s disease. Otol Neurotol. 2013;34(9):1681–1687. doi: 10.1097/MAO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann WM, Raked B. On the minimum audible angle–a decision theory approach. J Acoust Soc Am. 1989;85(5):2031–2041. doi: 10.1121/1.397855. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Sensitivity to binaural timing in bilateral cochlear implant users. J Acoust Soc Am. 2007;121(4):2192–2206. doi: 10.1121/1.2537300. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ, Clark GM. Psychophysical studies with two binaural cochlear implant subjects. J Acoust Soc Am. 1997;102(1):495–507. doi: 10.1121/1.419611. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ, Tong YC, Hollow RD, Clark GM. Psychophysical and speech perception studies: a case report on a binaural cochlear implant subject. J Acoust Soc Am. 1993;94(6):3178–3189. doi: 10.1121/1.407223. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJM, Jones GL, Litovsky RY. Interaural time-delay sensitivity in bilateral cochlear implant users: effects of pulse rate, modulation rate, and place of stimulation. J Assoc Res Otolaryngol. 2009;10(4):557–567. doi: 10.1007/s10162-009-0175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Dietz M. Comparison of interaural electrode pairing methods for bilateral cochlear implants. Trends Hear. 2015;19:233121651561714. doi: 10.1177/2331216515617143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Stelzig Y, Nopp P, Schleich P. Audiological results with cochlear implants for single-sided deafness. HNO. 2011;59(5):453–460. doi: 10.1007/s00106-011-2321-0. [DOI] [PubMed] [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, Goupell MJ. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J Acoust Soc Am. 2013;134(4):2923–2936. doi: 10.1121/1.4820889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitterick PT, Smith SN, Lucas L. Hearing instruments for unilateral severe-to-profound sensorineural hearing loss in adults: a systematic review and meta-analysis. Ear Hear. 2016;37(5):495–507. doi: 10.1097/AUD.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine Punte A, Vermeire K, Hofkens A, De Bodt M, De Ridder D, de Heyning P (2011) Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implant Int 12 Suppl 1:26–29 [DOI] [PubMed]
- Laback B, Majdak P, Baumgartner WD. Lateralization discrimination of interaural time delays in four-pulse sequences in electric and acoustic hearing. J Acoust Soc Am. 2007;121(4):2182–2191. doi: 10.1121/1.2642280. [DOI] [PubMed] [Google Scholar]
- Laback B, Egger K, Majdak P. Perception and coding of interaural time differences with bilateral cochlear implants. Hear Res. 2015;322:138–150. doi: 10.1016/j.heares.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Svrakic M, Roland JT, Svirsky M. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear. 2015;36(5):e207–e213. doi: 10.1097/AUD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenssen A, Francart T, Brokx J, Wouters J. Bimodal listeners are not sensitive to interaural time differences in unmodulated low-frequency stimuli (L) J Acoust Soc Am. 2011;129(6):3457–3460. doi: 10.1121/1.3557051. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Jones GL, Agrawal S, van Hoesel RJ. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J Acoust Soc Am. 2010;127(1):400–414. doi: 10.1121/1.3257546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Moua K, Godar S, Kan A, Misurelli SM, Lee DJ (2018) Restoration of spatial hearing in adult cochlear implant users with single-sided deafness. Hear Res [DOI] [PMC free article] [PubMed]
- Long CJ, Eddington DK, Colburn HS, Rabinowitz WM. Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user. J Acoust Soc Am. 2003;114(3):1565–1574. doi: 10.1121/1.1603765. [DOI] [PubMed] [Google Scholar]
- Ma N, Morris S, Kitterick PT. Benefits to speech perception in noise from the binaural integration of electric and acoustic signals in simulated unilateral deafness. Ear Hear. 2016;37(3):248–259. doi: 10.1097/AUD.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely ST, Norton SJ, Gorga MP, Jesteadt W. Latency of auditory brain-stem responses and otoacoustic emissions using tone-burst stimuli. J Acoust Soc Am. 1988;83(2):652–656. doi: 10.1121/1.396542. [DOI] [PubMed] [Google Scholar]
- NIDCD (2016) NIDCD fact sheet: who gets cochlear implants? Children and adults who are deaf or severely hard-of hearing can be fitted for cochlear implants
- NIH NIH consensus conference. Cochlear implants in adults and children. JAMA. 1995;274(24):1955–1961. doi: 10.1001/jama.1995.03530240065043. [DOI] [PubMed] [Google Scholar]
- Nuetzel J, Hafter ER. Discrimination of interaural delays in complex waveforms: spectral effects. J Acoust Soc Am. 1981;69(4):1112–1118. doi: 10.1121/1.385690. [DOI] [Google Scholar]
- Oh Y, Reiss LAJ. Binaural pitch fusion: pitch averaging and dominance in hearing-impaired listeners with broad fusion. J Acoust Soc Am. 2017;142(2):780–791. doi: 10.1121/1.4997190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon BB, Eddington DK, Noel V, Colburn HS. Sensitivity to interaural time difference with bilateral cochlear implants: development over time and effect of interaural electrode spacing. J Acoust Soc Am. 2009;126(2):806–815. doi: 10.1121/1.3158821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Eggleston JL, Walker EP, Oh Y. Two ears are not always better than one: mandatory vowel fusion across spectrally mismatched ears in hearing-impaired listeners. J Assoc Res Otolaryngol. 2016;17(4):341–356. doi: 10.1007/s10162-016-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Shayman CS, Walker EP, Bennett KO, Fowler JR, Hartling CL, Glickman B, Lasarev MR, Oh Y. Binaural pitch fusion: comparison of normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2017;141(3):1909–1920. doi: 10.1121/1.4978009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Fowler JR, Hartling CL, Oh Y. Binaural pitch fusion in bilateral cochlear implant users. Ear Hear. 2018;39(2):390–397. doi: 10.1097/AUD.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Temchin AN. Similarity of traveling-wave delays in the hearing organs of humans and other tetrapods. J Assoc Res Otolaryngol. 2007;8(2):153–166. doi: 10.1007/s10162-007-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallop JK, Beiter AL, Goin DW, Mischke RE. Electrically evoked auditory brain stem responses (EABR) and middle latency responses (EMLR) obtained from patients with the nucleus multichannel cochlear implant. Ear Hear. 1990;11(1):5–15. doi: 10.1097/00003446-199002000-00004. [DOI] [PubMed] [Google Scholar]
- Shepard NT, Colburn HS. Interaural time discrimination of clicks: dependence on interaural time and intensity differences. J Acoust Soc Am. 1976;59(S1):S23. doi: 10.1121/1.2002500. [DOI] [Google Scholar]
- Steel MM, Papsin BC, Gordon KA. Binaural fusion and listening effort in children who use bilateral cochlear implants: a psychoacoustic and pupillometric study. PLoS One. 2015;10(2):e0117611. doi: 10.1371/journal.pone.0117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelcyk O, Dau T. Estimation of cochlear response times using lateralization of frequency-mismatched tones. J Acoust Soc Am. 2009;126(3):1302–1311. doi: 10.1121/1.3192220. [DOI] [PubMed] [Google Scholar]
- Távora-Vieira D, Marino R, Acharya A, Rajan GP. Cochlear implantation in adults with unilateral deafness: a review of the assessment/evaluation protocols. Cochlear Implants Int. 2016;17(4):184–189. doi: 10.1080/14670100.2016.1176303. [DOI] [PubMed] [Google Scholar]
- Trapeau R, Schönwiesner M. Adaptation to shifted interaural time differences changes encoding of sound location in human auditory cortex. Neuroimage. 2015;118:26–38. doi: 10.1016/j.neuroimage.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117(9):645–652. doi: 10.1177/000348940811700903. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol. 2009;14(3):163–171. doi: 10.1159/000171478. [DOI] [PubMed] [Google Scholar]
- Wess JM, Brungart DS, Bernstein JGW. The effect of interaural mismatches on contralateral unmasking with single-sided vocoders. Ear Hear. 2017;38(3):374–386. doi: 10.1097/AUD.0000000000000374. [DOI] [PubMed] [Google Scholar]
- Zirn S, Arndt S, Aschendorff A, Wesarg T. Interaural stimulation timing in single sided deaf cochlear implant users. Hear Res. 2015;328:148–156. doi: 10.1016/j.heares.2015.08.010. [DOI] [PubMed] [Google Scholar]