Abstract
Introduction
Invasive meningococcal disease (IMD, septicaemia and/or meningitis) has a severe acute and long-term burden: 5–10% of patients die within 48 h, and long-term sequelae have been reported in 10–20% of survivors. Health-related quality of life (HRQoL) is increasingly but inconsistently assessed.
Methods
A systematic literature review on Neisseria meningitidis IMD sequelae and HRQoL in survivors of all ages and their caregivers, including family, was conducted for high-income countries from 2001 to 2016 (in Medline and Embase, following Cochrane and PRISMA guidelines).
Results
A total of 31 studies, mostly of childhood IMD cases, were included. A broad range of physical, neurological and psychological IMD sequelae were identified. The literature has evolved, with more types of sequelae reported in more recent studies; however, meningococcal disease-specific and sequelae-specific HRQoL data are lacking, and existing studies used a wide variety of instruments. Physical sequelae included: amputations (up to 8% of children, 3% adolescents/adults) and skin scars (up to 55% of children, 18% adolescents, 2% adults). Neurologic sequelae included: hearing loss (up to 19% of infants, 13% children, 12% adolescents, 8% adults). Psychological sequelae included: anxiety, learning difficulties, emotional and behavioural difficulties. IMD negatively affects HRQoL in patients and also in their family and close caregiver network, both in the short- and long-term. Even IMD survivors without sequelae experienced an adverse impact on HRQoL after many years, affecting self-esteem, physical, mental and psychosocial health, and HRQoL was worse in those with cognitive and behavioural sequelae.
Conclusion
A high proportion of IMD survivors are affected by a broad range of sequelae and reduced HRQoL that persists years after infection. Childhood IMD survivors had more sequelae and more severe sequelae compared with adult survivors. HRQoL was affected in patients and also in their families, caregivers and surrounding network over the long term. More research is needed to resolve data gaps and to standardise HRQoL assessment.
Funding
GlaxoSmithKline Biologicals SA (Rixensart, Belgium).
Electronic supplementary material
The online version of this article (10.1007/s40121-018-0213-2) contains supplementary material, which is available to authorized users.
Keywords: Health-related quality of life, Invasive meningococcal disease, Neisseria meningitidis, Sequelae, Systematic literature review
Introduction
Neisseria meningitidis (N. meningitidis) is a Gram-negative bacterium present in the nasopharynx of 4.5–23.7% of the population and is transmitted through respiratory secretions and saliva [1]. Invasive meningococcal disease (IMD) most commonly causes life-threatening meningitis or septicaemia [2–4]. Even with therapy, 5–10% of patients die within 24–48 h of developing symptoms [4]. Severe permanent sequelae in septicaemia survivors include severe skin necrosis and scarring requiring skin grafts and amputation of limbs. Previous studies have reported severe long-term sequelae in 10–20% of survivors [4, 5]. Neisseria meningitidis serotypes A, B, C, W and Y cause the majority of IMD globally [4], while in the US and Europe, serotypes B and C (and Y in the US) are dominant [6, 7]. The main burden of disease is in children, with a second lower peak in 15–24 year olds. In 2015, the European Centre for Disease Prevention and Control (ECDC) reported 10.0 confirmed IMD cases per 100,000 infants and 2.8 per 100,000 children aged 1–4 years. Clinical presentation was known for 47% of all IMD cases, of which 36% were meningitis cases, 36% septicaemia cases, 18% combined meningitis and septicaemia cases, and 10% defined as ‘other’ IMD cases. Most cases (61%) were caused by serogroup B, with a case-fatality rate of 8%. Following the introduction of broad meningococcal C vaccination programs, serotype C now only accounts for 14% of IMD in Europe [8].
The infant meningococcal B vaccine 4CMenB (Bexsero; GSK) was approved in Europe in 2013 and is currently licensed in 39 countries for use in infants from the age of 2 months [9]. Since its introduction in September 2015 in the UK’s routine childhood immunization program, vaccination coverage (in children 18 months old between August and December 2017 in England) of 95.0, 92.9 and 87.4% have been attained for the first, second and booster dose, respectively, reflecting high public acceptance of this vaccine [10]. Another meningococcal B vaccine (Trumenba; Pfizer) was approved in Europe in 2017 for adolescents and adults from the age of 10 years [11].
IMD is a severe disease with a high negative impact on health-related quality of life (HRQoL) which needs to be comprehensively evaluated to detect the severity of symptoms both in the acute phase and long term. HRQoL outcomes are increasingly used to assess the impact of disease on physical, emotional, psychological, social, and behavioural components, both in IMD patients and their caregivers (i.e. carers and wider family/friends support network) [12]. Attributes such as self-perception, self-esteem, the quality of friendships with family or friends, and well-being in school are also relevant to children and adolescents [13, 14]. A recent systematic literature review (SLR) identified a lack of standardisation in HRQoL instruments used in IMD, and in the quantification of HRQoL, making it difficult to capture the comprehensive burden of disease caused by IMD [15].
The objectives of this study were to systematically identify IMD sequelae and the impact of acute and long-term IMD on HRQoL in survivors and their caregivers, supplementing findings from an existing [16] SLR.
Methods
An SLR was conducted following Cochrane and PRISMA guidelines [17, 18] and registered in the PROSPERO database (Registration number: CRD42016039987) [19]. The objective was to update and extend an existing SLR conducted by Strifler et al. [16] on N. meningitidis IMD HRQoL and related sequelae (including published literature up to May 2013). This updated SLR includes publications from 2001 to 2016 on survivors of IMD (independent from presentation) conducted in high-income countries as defined by the Organisation for Economic Co-operation and Development (OECD), additional languages to the previous SLR (i.e. English plus German, Dutch and Spanish), and extended criteria to include outcomes for HRQoL in caregivers including family/friends support networks of IMD patients.
Medline and Embase databases were searched from 1 August 2001 to 1 August 2016, using MeSH terms and keywords for the disease combined with quality of life or sequelae terms (Supplementary Table 1). Titles and abstracts were screened independently by two researchers using pre-defined inclusion and exclusion criteria (see below). References of full text articles were searched for additional publications of interest. Full text articles of included studies were critically appraised for risk of bias using the SIGN (Scottish Intercollegiate Guidelines Network) framework. Level of evidence was assessed ranging from ‘1 ++’ (high-quality meta-analysis, systematic review of clinical trials with low risk of bias) to 4 (expert opinion) [20]. Data were extracted into a pre-defined template [21] (i.e. author, year, country, study design, population, outcomes, time of assessment and results), and, grouped by HRQoL instrument and by type of sequelae. Long-term sequelae were assessed in IMD survivors and stratified by clinical presentation of meningitis, septicaemia, septic shock or meningococcemia, regardless of pathogen if a significant proportion was associated with N. meningitidis. The findings were stratified by age as reported in each study; where age ranges were not reported, they were defined according to WHO guidelines as follows; infants (less than 1 year), children (1–9 years), adolescents (10–19 years) and adults (20 and over) [22]. Outcomes included IMD sequelae and HRQoL in patients and caregivers. Comparative and non-comparative studies were included. In terms of study design, primary studies were included (e.g. prospective cohort and case–control studies, retrospective chart reviews). SLRs, case reports, medication or vaccination studies and epidemiologic or economic studies were excluded. The countries of interest were the high-income OECD countries defined by the World Bank [23].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
A total of 31 studies published in 32 articles were included for qualitative analysis (Fig. 1); most studies primarily included childhood/infant IMD cases. Most studies reported overall IMD, while four studies specifically assessed IMD due to serogroup B or C. A total of 26 studies described sequelae and included a mix of cohort and case–control studies as well as retrospective chart reviews (from single-centre studies to national surveillance databases). According to the SIGN risk of bias framework, studies were rated from 3 (non-analytical studies such as case reports and case series) to 2++ (high-quality systematic reviews of cohort or case and control studies; cohort or case and control studies with very low risk of bias and high probability of establishing a causal relationship). Overall, 14 studies reported physical sequelae, 21 neurologic sequelae (6 months–17 years follow-up) and 12 psychological and behavioural sequelae (6 months–17 years follow-up). Most HRQoL studies were case–control and cohort studies, the majority rated 2+ (well-conducted cohort or case and control studies with low risk of bias and moderate probability of establishing a causal relationship) to 2++. There were 13 HRQoL studies; 10 on HRQoL in survivors and 6 on HRQoL in caregivers (both 4 months–12 years follow-up). All HRQoL instruments used assessed physical and emotional attributes, while some also assessed impact on mental/cognition, pain, social, motor function, general health, behaviour, parental impact, autonomy and family attributes. Several studies reported on multiple outcomes (i.e. HRQoL in survivors, caregivers and/or IMD sequelae).
Fig. 1.
Systematic literature review flow diagram. n number. *includes 20 cost or cost-effectiveness studies, 10 epidemiological studies, and 9 reviews/SR. **indicates studies conducted in low-income countries
IMD Sequelae
Three distinct categories of IMD sequelae were identified: physical, neurological and psychological (i.e. mental health and behavioural problems). Table 1 highlights the broad range of IMD sequelae identified across studies (Table 1), while the following sections discuss the most frequently reported sequelae.
Table 1.
Categories of IMD sequelae identified
| Physical | Neurological | Psychological/behavioural | ||
|---|---|---|---|---|
|
Dermatological conditions Skin Scarring Skin graft Skin necrosis Eczema Psoriasis |
Cardio/vascular conditions Symptoms consistent with Raynaud Phenomenon Venous thrombosis Vasculitis |
Sensory system deficits Blindness Cranial nerve palsies Optic disc swelling Esotropia Hearing loss (mild, moderate, severe, profound) Tinnitus Numbness Paresthesia/reduced sensitivity Sensitivity to light |
Intellectual disability Mental retardation (IQ < 70) Mild IQ loss (IQ 70–85) Learning disabilities Cognitive deficits |
Anxiety disorders Generalized anxiety Separation anxiety Social anxiety disorder Specific phobias |
|
Musculoskeletal deficiencies (bone, muscle, joint) Arthritis Limb deficiency/deformities Amputation Arthralgia |
Other physical conditions Anemia Pulmonary condition Autoimmune disease Fatigue Adrenal insufficiency Cardiorespiratory failure |
Motor deficits Paralysis Cerebral palsies Muscle weakness Monoparesis, hemiparesis Movement coordination Spasticity Mobility problems Severe neuromotor-impairment Balance impairment |
Abnormal brain activity Seizures (epileptic and non-epileptic) Chronic headaches Migraine Vegetative state Vertigo |
Behavioural disorders Oppositional defiant disorder Conduct disorder |
|
Renal conditions Renal failure Urinary retention Renal insufficiency |
Communication disorders Aphasia Stuttering General speech, language and communication difficulties |
Other severe neurological disorders Brain nerve damage Hydrocephalus Severe brain damage Febrile convulsions Multi-cerebral infarct Radiculopathy Subdural empyema Development delay |
Other psychological/emotional/behavioral disorders Depression Attention deficits ADHD Post-traumatic stress disorder Autistic spectrum disorder Eating disorder |
|
|
Other non-severe neurological disorders Sleep disturbances Lethargy | ||||
IQ intelligence quotient, ADHD attention-deficit/hyperactivity disorder
An analysis of studies that presented numbers of cases with sequelae revealed an increase in the types of sequelae identified over time. Most (7/11) studies published since 2010 reported sequelae from all three identified categories (i.e. physical, neurological and psychological/behavioural) compared with 3/15 studies before 2010. In addition, the numbers of reported sequelae within these categories increased (Fig. 2). Al-Janabi et al. [54] conducted a large survey of family members of survivors in order to assess the health status impact of IMD beyond the patient. Although this was a HRQoL study, the survey also asked family members to report the presence of any sequelae observed in survivors; however, only summary findings on sequelae were presented in the publication. Therefore, these summary data on the most commonly observed sequelae are also included in Fig. 2.
Fig. 2.
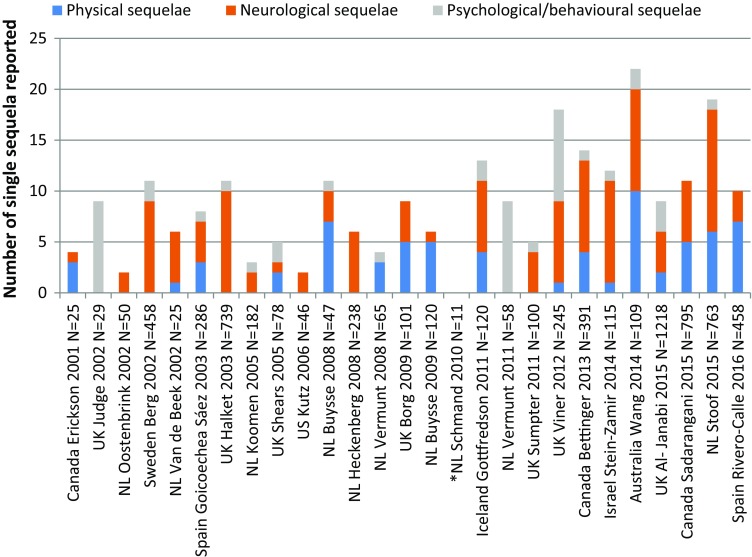
Number of IMD sequelae reported in observational studies from 2000 to 2016. The numbers on the y axis represent the numbers of each type of sequelae reported; however, it is possible that more than one type of sequela occurred in a subject. *Schmand is a follow-up study of Van de Beek; N total number of subjects in the study
The following sections present the findings of IMD sequelae studies (see Supplementary file 2 for study details). The sequelae identified most frequently in studies are presented, separated by type and age at infection (childhood including infancy, adolescent, adult).
Physical Sequelae Related to IMD
The most commonly reported physical sequelae were amputations and skin scarring, both more frequently reported in septicaemia survivors than in meningitis survivors. Other physical sequelae identified included limb deficiencies/deformity, other skin diseases and renal conditions.
Studies in Children Including Infants
Two studies in children with severe disease (meningococcal septic shock, MSS) treated in the paediatric intensive care unit (PICU) reported the highest rates of amputations (4–8.3% of cases) and skin scarring (48–55% of cases) [24, 25]. All other studies in children also reported amputations; the highest was 3.3% (n = 60, 77% treated in PICU, 42% had meningitis and septicaemia) [26], followed by 2.2% in a large review of 36 Spanish hospitals over 6 years [of which 4.1% (7/10) occurred in septicaemia cases] [27], followed by around 1.7–1.9% (27–49% septicaemia cases, 28–31% meningitis with septicaemia cases) [28–30]. One study reported 1.3% with amputations (29% in PICU, 63% with septicaemia, 18% with meningitis and septicaemia) [31], and the last study reported 0.7% with amputations (60% in ICU, 46% with meningitis and bacteraemia or septicaemia) [32]. Skin scarring/grafting was reported in four studies in children; 10.1% had scars in a study in Australia including 49% septicaemia cases [29, 30], while 2.4% had skin lesions (n = 6 serogroup B and n = 1 serogroup C) in a study in Spain which included 46% meningitis plus bacteraemia or septicaemia cases [32]. Skin grafting occurred in 5% (3/60) children in a UK study (77% treated in PICU) [26]. A retrospective cohort study in Spain identified 2.6% overall with skin grafting [5.9% (10/12) among septicaemia patients] [27].
Studies in Children and Adults
A Canadian study reported 29/868 amputations (3.4% overall), with a higher rate in children than adults (7.6 vs. 3.1%), and 46/868 skin scars (5.4% overall), also higher in children than adults (4.3 vs. 2.4%) [33]. The higher occurrence of scars among septicaemia versus meningitis patients was also reported in a Dutch study. Among septicaemia cases, scars (requiring treatment or not) occurred in 8.5 and 8.3% of children and adults, respectively. Scars (requiring treatment or not) rarely occurred in meningitis cases (0.68% in children and 1% in adults) [34]. One Canadian study in serogroup B cases reported 15/391 amputations (3.8% overall), of which 14 were in septic shock patients and 10 in children aged 0–4 years [35]. Skin scarring occurred in 25/391 cases (6.4% overall) of which 24 had septic shock [35]. A study in Iceland reported 1/70 serogroup B (mainly in children) and 1/50 serogroup C (mainly in adolescents) cases with skin scarring [36].
Studies in Adolescents and Adults
Among adolescents with IMD (54% ICU, 40% meningitis plus septicaemia, 27% septicaemia), 3% had amputations and 18% skin scarring in a UK study [37]. A retrospective analysis and follow-up of young adults who acquired IMD in college (n = 25 survivors) found that three survivors had amputations and one had extensive skin scarring [38].
Neurologic Sequelae Related to IMD
In the identified studies, the most frequently reported neurologic sequelae were hearing loss, seizures, cognitive problems with consequences for academic achievement, motor deficits, and visual impairment. Many neurological problems were reported more frequently in meningitis versus septicaemia cases.
Hearing Problems in Studies in Children Including Infants
Most of the identified studies reported ‘any hearing loss or impairment’ in childhood cases; higher rates were reported in long-term follow-up studies and infant meningitis studies (i.e. 11% at 7.4 years follow-up in study of bacterial meningitis cases (78% due to N. meningitidis) [39], 11.7% (n = 5 serogroup B, n = 9 serogroup C) at 16.6 years follow-up [36], and 19% at 8 years follow-up in a study of infant meningitis survivors (mean age 8 months [40]). A national follow-up survey (after 5–10 years) in Sweden of childhood meningitis survivors reported 12.7% (58/458) sensorineural hearing loss (SNHL) cases due to meningitis, and found that auditory impairment was significantly more frequent among cases infected after the age of 12 months (p < 0.01) [41]. A US study reported 11% (5/46) of children with N. meningitidis meningitis having severe hearing loss and 13% (6/46) with mild/moderate hearing loss at discharge [42]. A retrospective review of childhood IMD survivors in Israel (68% with meningitis with or without septicaemia, 78% serogroup B) reported 7% (8/115) cases with hearing loss including 4/8 with severe hearing loss [28]. A follow-up of a case–control study of serogroup B meningococcal disease survivors (32% with meningitis with or without septicaemia) in the UK found SNHL in 15 out of 232 cases of which 2% (6) had profound bilateral SNHL and 5% (11) had moderately severe bilateral SNHL [31]. In a retrospective database analysis in Spain, the overall rate of deafness was 2.6% (12/458); however, most cases (7/12) were in meningitis cases, resulting in a rate of 6.1% among meningitis survivors [27] compared to 1% of the general paediatric population [28, 31]. Another Spanish study among children in Valencia found sensorineural deafness in 2.1% (6/286) cases overall, all occurring in meningitis cases [32]. Lower rates were reported at short-term follow-up periods [i.e. 2% at 6.7 months follow-up in a study of bacterial meningitis (50% N. meningitidis)] [43], and 3.7% at 23 months follow-up in a study with 52% meningitis with or without septicaemia cases [29, 30]).
Hearing Problems in Studies in Children and Adults
In a retrospective analysis of national IMD cases between 2002 and 2011 in Canada (58% with meningitis with or without septicaemia), 46 hearing loss events were reported out of 868 cases, of which 7.4% (31) occurred in children and 3.3% (15) in adults [33]. A retrospective analysis of national serogroup B IMD in Canada found 7.2% (28/391) had deafness at discharge, of which 23/28 were meningitis cases without septic shock, 18/28 were children aged 0–4 years, and 7/28 were infected as adults (mean age 41.3 years) [35]. A retrospective analysis of Dutch IMD hospital cases (1999–2011) reported 5.6% (43/763) hearing loss events, of which 2.5% (19) were among children 0–4 years old, 1.3% (10) among 5- to 19-year-olds and 1.7% (13) among adults 20–64 years old [34].
Hearing Problems in Studies in Adolescents and Adults
Among survivors of adolescent IMD (73% meningitis with or without septicaemia), 11.9% had hearing problems at 21 months follow-up [37], compared with 4% (1/25) college students [38]. Among adults with meningococcal meningitis (68% serogroup B), 13% had neurologic deficits of which 8% had hearing loss at discharge [44].
Seizures in Studies in Children Including Infants
The studies identified on children reported varying rates of epilepsy/seizures: the highest (9.2%, 6/65) was among children with N. meningitidis meningitis [42], followed by 5% among infant meningitis cases (34% with N. meningitidis) at an 8-year follow-up [40], and 4.6% among a mixed IMD population (24% meningitis, 28% meningitis plus septicaemia) at 23 months follow-up, of which four out of five cases were due to serogroup B infection [29, 30]. Another study (14% meningitis, 18% meningitis plus septicaemia) reported seizures in 2% of cases versus 1% of controls at 3.8 years follow-up [31], and the last study (37% meningitis, 31% meningitis plus septicaemia) reported seizures in 1.7% of IMD cases at a mean 5.8 years follow-up [28].
Seizures in Studies in Children and Adults
Seizures in cases with serogroup B IMD (56% meningitis, 13% meningitis plus septicaemia) in Canada were reported in 10 out of 391 cases, of which 2.1% (8) were infants, 0.5% (2) were 1–4 years old and none were adults [35]. A study in Iceland also found 6% (4/70) of serogroup B cases (mainly children) with seizures but no seizures among serogroup C cases (mainly adolescents) [36].
Seizures in Studies in Adolescents and Adults
Among adolescents (35% meningitis, 40% meningitis plus septicaemia), 2% reported having seizures [37].
Cognitive Sequelae in Studies in Children Including Infants
At 5.8 years after IMD, 23% of cases in Israel had learning or academic difficulties, which compares with 12.8% of children overall in Israel with any disability, of which 52.5% have behavioral and learning–academic difficulties [28]. In a UK study, 16% had a learning disability 8 years after infant IMD, 80% were in mainstream education, 13% required support and 7% were in special education [40]. On average 12 years after IMD in infancy, another UK study found 85% with complicated meningitis and 90% with uncomplicated meningitis were in mainstream education (compared with 98% of controls). Significantly more cases required help at school (p < 0.005) or attended a special school (p < 0.001) versus controls [45]. A Dutch study in MSS patients (n = 58) found no difference in education levels achieved at a median 13 years after disease compared with the Dutch population [46]. Most of these studies did not differentiate between meningitis and septicaemia cases; the one study that did specify meningitis cases showed an adverse effect of the disease on education [45], whereas the one study that specified MSS cases showed no adverse education effects [46].
Four years after IMD, intelligence, memory and executive function scores were all significantly lower in the IMD group versus controls (p < 0.0001) in a UK study [31]. A median 13 years after MSS, several cognitive function domains assessed remained lower in IMD cases versus controls (verbal comprehension, number and word fluency p < 0.05 or p < 0.01) although total IQ was comparable to the Dutch population [46].
Cognitive Sequelae in Studies in Adults Including Adolescents
A study in England found that at a mean of 21 months after IMD, nearly half of IMD subjects’ academic achievements were affected, and 19% failed the previous year’s exams (vs. 8% of controls, p = 0.04) [37]. Intelligence, memory, attention and executive function scores in adult cases with IMD discharged with no sequelae were comparable to controls, 9 years after IMD [47, 48].
Psychological and Behavioural Sequelae Related to IMD
Psychological and behavioural sequelae were identified in both septicaemia and meningitis survivors. There were no studies in adolescents or adults with IMD.
Studies in Children Including Infants
In a study of children with severe IMD admitted to the PICU (mean 5.7 years old), post-traumatic stress symptoms were observed in 62% and post-traumatic stress disorder in 10% (3/29) cases, while 3 out of 8 children under 4 years and 3 out of 21 children aged 4–16 years were assessed as high-risk psychiatric children, 8.9 months after discharge [49]. Another study also found that 4 out of 26 PICU subjects had post-traumatic stress disorder, while none of the general paediatric ward subjects did [26]. A case of autism and of post-traumatic stress disorder (each 0.9%, 1/109) were reported in an Australian mean 23-month follow-up study of childhood IMD survivors [29, 30]. A median 3.8 years after IMD, significantly more survivors of serogroup B meningococcal disease in the UK had ≥ 50% probability of having any mental health disorder (22 vs. 8% of controls, p < 0.0001), and 15% versus 3% had a probability ≥ 70 % (p < 0.0001). Significantly more cases than controls had ≥ 50% probability of having anxiety disorders, behavioural problems or attention-deficit/hyperactivity disorder [31]. At a mean of 16.6 years follow-up, a study of IMD survivors in Iceland reported cases (n = 23/120) of children with mental health problems (20% of serogroup B and 18% of serogroup C cases overall), depression and/or anxiety. Mean anxiety scores were significantly worse than general population controls on the DASS-Anxiety scale (p < 0.05) [36]. A long-term follow-up (4–16 years) of MSS cases treated in the PICU found worse outcomes on social acceptance, close friendships and global self-worth in teenagers compared to Dutch matched general population controls. For MSS children with scars (n = 17), a significant negative correlation was found between their scar evaluations and SPP-C social acceptance scores. Further, MSS adolescents with scars (n = 18) reported a significantly lower global self-worth compared with adolescents without scars. [50].
A study in Israel reported 14.8% of childhood IMD survivors had behavioural and emotional problems [28]. Parents of 60 children with meningococcal disease (46 admitted to PICU and 14 to general paediatric units) reported significant increases in Strength and Difficulties Questionnaire (SDQ) total emotional, hyperactivity and conduct problem sub-scores and impact scores in children admitted to PICU versus general paediatric units, at a mean 4.1-month follow-up. In six children with severe physical sequelae and their parents, there was a nonsignificant trend towards more psychological symptoms at follow-up [26]. A survey follow-up (mean 8 years after IMD) of parents and teachers of meningitis survivors (median age at infection 8 months, 34% with N. meningitidis, 38% admitted to PICU, 36% with acute complications) found clinically significant behavioural difficulties on the SDQ, reported by 32% of parents and 19% of teachers. Parents also reported significantly lower psychosocial health (emotional, school and social) and fatigue scores on the Paediatric Quality of Life Inventory (PedsQL) compared to UK and US normative data. Acute disease complications were associated with sequelae [40]. At 12 years after infection, a follow-up survey of parents and teachers of survivors of infantile (< 1 year) meningitis reported significantly more cases with behavioural problems versus controls on the SDQ total deviance score (46% of complicated cases, 38% of uncomplicated cases, 21% of controls), and more cases versus controls with a negative impact (on home and social life) score (35% complicated, 25% uncomplicated cases, 10% controls). More complicated cases had behavioural problems and negative impact scores than uncomplicated cases. Both teachers and parents also reported fewer survivors than controls with prosocial scores (normal positive social attributes): 85% of cases versus 91% of controls [45]. A median 13 years after MSS treated in the PICU, male survivors aged 19–23 were significantly more likely than controls to report behavioural/emotional problems [46]. A case–control study of serogroup B meningococcal disease survivors (median 1.6 years of age at infection, including 29% admitted to PICU and 18% with meningitis and septicaemia) found that 23% of survivors had ‘abnormal’ SDQ total difficulties scores versus 7% of controls (p < 0.0001) at a median 3.8 years follow-up [31].
HRQoL Due to Acute IMD and Its Sequelae
The following sections describe the impact of IMD and its sequelae on HRQoL in survivors and health status (utility) findings in survivors (see Supplementary file 3 for study details).
HRQoL Assessment in IMD Cases Without Severe Sequelae
A case–control study of childhood meningitis survivors (mean age at infection of 2.4 years) discharged with no severe sequelae was conducted in the Netherlands a mean 7.4 years after discharge. Compared to the general Dutch population, survivors scored significantly lower on self-esteem, general health perceptions and physical summary scores of the Child Health Questionnaire-Parent Form (CHQ-PF). The HRQoL of survivors with academic and/or behavioural limitations was significantly lower on all CHQ scales (role functioning (emotional), physical pain, general behaviour, mental health, self-esteem, psychosocial summary score, and emotional impact on parents) compared with survivors without these limitations [39].
Dutch adults (16–65 years) with meningitis discharged with good physical and mental recovery were assessed a median of 14 months (n = 25) and a mean of 9.3 years (n = 11) after discharge using the Short Form Health Survey (SF-36). Vitality scores were significantly lower than age-matched Dutch population controls 14 months after discharge. However, all scores were comparable to controls 9.3 years after discharge [47, 48].
HRQoL Assessment in IMD Cases with Sequelae
There were no HRQoL studies specific to meningitis patients with sequelae or to neurological sequelae of IMD such as hearing loss, seizures or cognitive deficits. The following studies assessed HRQoL in septicaemia cases without a focus on specific physical sequelae. The timeframe of studies was 14 months to 10 years, highlighting the physical and emotional impact of septicaemia and its sequelae in the short and long term.
A short-term (14-month) follow-up study of 45 MSS children admitted to the PICU found significant reductions in physical ability, general health perception, and emotional impact on parents, using the Infant and Toddler Quality of Life Questionnaire (IT-QOL) for toddlers (0–3 years), despite a significant improvement in health since discharge, compared to Dutch age-matched population norms. Among 1- to 17-year-olds (median age at follow-up was 4.8 years), there was a significant reduction in general health perception, physical functioning and total physical scores on the CHQ-PF, and 21 out of 26 patients reported chronic complaints (e.g. pain and behavioural/emotional problems) [24]. After a median 10-year follow-up of 145 MSS PICU survivors (admitted between 1988 and 2001), those aged 12–17 years reported a negative outcome on physical domains but a positive outcome on psychosocial domains (general behaviour and family activity) of the CHQ-CF87 versus the Dutch reference population. Parents of 4- to 17-year-old survivors reported significant reductions in other domains [physical functioning, self-esteem, role functioning (emotional/behaviour) and total physical scores] on the CHQ-CF50. Both children and parents rated general health perception in children as significantly lower than controls. Adult cases in this study rated their own HRQoL using the SF-36, and found that vitality and physical summary scores were significantly lower, while role limitation (due to emotional problems) and psychosocial scores were significantly better than Dutch population norms, suggesting young adult survivors may enjoy life more (study authors’ interpretation) [51].
In a study among children aged 8–11 years with chronic conditions, significantly more children with severe IMD (n = 38) were at risk of HRQoL problems on motor functioning and autonomy, using the TACQOL (TNO-AZL Child Quality Of Life), than Dutch controls [52].
SDQ assesses the presence of behavioural problems (i.e. as a screening tool); however, the impact on HRQoL of IMD behavioural sequelae was not assessed in any study. One HRQoL study in adolescents with IMD reported mental health problems that persisted after 21 months, using SF-36. This follow-up of a prospective population-based matched cohort study of adolescents with IMD (age at infection 15–19 years, 54% admitted to ICU, 40% with meningitis and septicaemia) reported lower mean mental summary scores on SF-36 [(46.6 standard deviation (SD) 30.2] versus 53.5 (SD 23.9, p = 0.07) after a median 21-month follow-up. Cases reported no improvement in HRQoL since discharge [37].
Health Status (Utility Values) of Septicaemia and Meningitis Survivors
In one Dutch study, survivors of childhood MSS (83% with meningitis, treated in the PICU) had significantly lower scores on all Health Utilities Index-3 (HUI-3) attributes (vision, speech, ambulation, dexterity, emotion, cognition and pain) except hearing, compared with Dutch age-matched population norms. The mean multi-attribute utility value on Health Utilities Index-3 (HUI-3) for MSS survivors, a median 9.8 years after MSS, was 0.82 (SD 0.25) versus 0.93 (SD 0.12) for controls (p < 0.01) [53]. MSS survivors also scored significantly lower than controls on all Health Utilities Index-2 (HUI-2) attributes (sensation, mobility, emotion, cognitive and pain) except self-care. Thus, MSS survivors had a mean multi-attribute utility score on HUI-2 of 0.88 (SD 0.16) versus 0.94 (SD 0.09) for population controls (p < 0.01) [53].
These outcomes on the HUI-2 are consistent with another study comparing meningitis subjects with or without academic and/or behavioural problems 7.4 years after being discharged without severe sequelae. In this study, survivors with problems had significantly lower scores on emotion, cognitive and pain attributes than survivors without problems, resulting in a mean multi-attribute utility score of 0.84 (SD 0.14) in problem cases versus 0.93 (SD 0.09) in survivors with good recovery (p < 0.001) [39]. A follow-up study of family members of meningitis survivors with (n = 1053) and without (n = 517) long-term sequelae used the EuroQOL (EQ-5D) to assess health status after 12 years. The most common sequelae were behavioural/emotional problems (28%), mild/moderate learning disabilities (16%), scarring or tissue damage (14%), balance problems (13%) and speech/language problems (11%). Family-reported health status of survivors with sequelae was significantly lower than for those without sequelae on all attributes of the EQ-5D (mobility problems 24 vs. 1%, self-care problems 19 vs. 1%, usual activity problems 37 vs. 3%, pain/discomfort 38 vs. 4%, anxiety/depression 46 vs. 9%). The overall utility value for survivors with sequelae on the EQ-5D was 0.78 compared with 0.97 for survivors with no sequelae (p < 0.001) [54].
Beyond the Patient: Impact on HRQoL in Caregivers and Family/Support Network
The following sections describe the larger HRQoL impact IMD has on the support network (family, friends, caregivers) of the survivor (see Supplementary file 3 for study details).
Short-Term Impact on HRQoL
Mothers and fathers of IMD survivors (mean age at infection 6.8 years) were found to have mental distress scores indicative of psychiatric disorder (> 5 on General Health Questionnaire GHQ) in 59 and 42% at admission, respectively, decreasing to 43 and 24% at mean 4.1 months follow-up. The risk of post-traumatic stress disorder (38% of mothers and 19% of fathers overall) was significantly higher in mothers of PICU-treated children [Impact of Event Scale (IES) median score of 33 (quartiles 22, 43) vs. 12.5 (8.5, 33), p = 0.009] compared with other mothers [26]. A study (n = 27) found that a large proportion of mothers of childhood IMD survivors (mean 5.7 years at infection) treated in the PICU were at high psychiatric risk (42% with scores ≥ 4 on GHQ, 48% on IES), with 29% seeking help for these problems at a mean of 8.9 months after discharge [49]. At 14 months post-discharge, parents of childhood MSS survivors (median 3.7 years at infection) treated in the PICU were not significantly different to Dutch age-matched population references on most SF-36 items (e.g. physical, social, mental health), except for role limitation (physical) and pain, where parents scored significantly better than controls. This may be due to parents comparing their own physical HRQoL with their child’s, according to the study authors. The child’s age at PICU admission was the only significant predictor of emotional problems in parents [24].
Long-Term Impact on HRQoL
In one study, psychological distress was assessed using the GHQ at five time points post-discharge (3, 6, 12, 24 and 36 or more months), in five cross-sectional groups of mothers and fathers of a retrospective cohort of child survivors (with severe meningococcal disease treated in the PICU). All mean scores were above the cut-off (> 5), indicating high and prolonged psychological distress for parents at all time points, except in mothers at 36 or more months after infection. Differences in scores between time points were not significant [55].
Another long-term follow-up of parents of a retrospective cohort of MSS children treated in the PICU found that parents’ HRQoL, assessed on the SF-36, had significantly improved on most subscales (e.g. pain, social functioning and mental health rated higher than Dutch population norms), after a median 10 years since discharge. The physical functioning attribute was lower (p < 0.01) than population normative data. The authors suggest that these data might show a positive impact on HRQoL after the child survived a life-threatening illness, possibly with no sequelae, or could indicate that denial and overcompensation influenced parents’ responses [51].
One study assessed the health status of a broad network of family members of meningitis survivors with and without long-term sequelae, using the EQ-5D (mean of 12 years after discharge). Sequelae were associated with lower health status for family members (utility value of family members with sequelae was 0.87 vs. 0.91 for without). Among families whose children were affected by sequelae, significant annual utility decrements of 0.18 for the closest family members and 0.11 for the second closest family members were noted [54].
Discussion
This SLR provides an overview of IMD sequelae and the disease impact on HRQoL in survivors and their families over many years. More recent literature (since 2010) captured a broader range of sequelae and described psychological/behavioural sequelae more frequently. Another important finding was that most identified data were on childhood IMD cases, where more sequelae and more severe sequelae were reported compared with adolescent and adult IMD cases.
The findings on widely known and studied physical and neurological long-term sequelae suggest differences in observed frequency by age and disease, with amputations reported in around 4–8% of severe septic shock cases especially in young children, compared with 3% of adolescents [24, 25, 37]. Furthermore, high scarring rates were reported, again with lower rates reported in adults versus children and meningitis versus septicaemia cases [24, 25, 37, 38]. As for neurologic sequelae, hearing loss in meningitis cases was reported in around 19% of infant (< 1 year) meningitis [40], 11–13% of childhood meningitis [36, 39, 41, 42], 12% of adolescent meningitis [37], and 8% of adult meningitis cases [44]. Seizures were reported in 2–9% of children with IMD [29, 31, 40, 42], with more cases occurring among serogroup B disease than serogroup C [36]. Academic achievement and cognitive function, primarily investigated in childhood IMD, seemed affected up to 12 years after IMD [45].
More importantly, findings on the occurrence of sequelae also revealed that, in more recent studies [28, 29, 31, 34–36, 40, 46, 54], psychological and behavioural long-term sequelae were studied and observed more often. Post-traumatic stress symptoms were frequently observed in PICU cases [26, 49]. Childhood IMD survivors had a significantly higher risk of long-term mental health problems versus controls (e.g. 11 vs. 2% for ADHD and 7 vs. < 1% for separation anxiety [31], and around 20% had depression at 17 years follow-up [36]), as well as emotional and behavioural problems affecting school, home and social life [26, 28, 31, 40, 45]. Thus, childhood IMD survivors also experience long-term psychological and behavioural sequelae besides the widely known physical and neurological sequelae.
No psychological sequelae studies were identified in adolescents or adults with IMD, and there were fewer studies reporting physical and neurological sequelae in this age group. There is, therefore, a need to include adolescents and adult survivors of IMD more frequently in observational studies, in particular to assess long-term psychological and behavioural sequelae.
Many of the sequelae studies assessed outcomes, particularly cognitive and psychological sequelae, after a long follow-up (up to 17 years), highlighting the persistence of IMD sequelae in survivors.
Although serogroup B is now responsible for the majority of IMD cases in the US and Europe, only two recent studies focussed solely on serogroup B IMD cases (i.e. in Canada [35] and the UK [31]), while two studies presented results separately for serogroup B (i.e. in Iceland [36] and Spain [32]). These studies reported the full range of sequelae types due to serogroup B IMD. In Iceland, serogroup B IMD was more prevalent in children and serogroup C in adolescents, with more seizures observed in serogroup B versus C cases (6 vs. 0%) [36]. Studies on developing countries were excluded from this systematic literature review, even though most cases are reported outside of developed countries. Included studies focussed on Australia, Canada, Europe, Iceland, Israel and the US, and thus the burden presented in this study might be limited in the representation of the comprehensive burden of disease.
IMD was found to have an adverse effect on HRQoL, not only in patients but also in their family, caregiver and support network, both in the short and long term. Even among childhood meningitis survivors without sequelae, the disease had an adverse impact on HRQoL after 7 years, affecting self-esteem, physical, mental and psychosocial health, and HRQoL was worse in those with cognitive and behavioural sequelae [39]. At a 10-year follow-up of MSS PICU survivors, physical problems still had a negative impact on HRQoL in both children and adult survivors [51]. HRQoL mental health scores had not improved from discharge to 21 months follow-up in IMD survivors [37]. There is a need for more long-term follow-up data, particularly on the impact of neurological, behavioural and psychological sequelae on HRQoL. The long-term negative impact of both septicaemia and meningitis was reflected by findings of several health utility studies, whereby a significantly lower health status was found for survivors after 7.4–12 years, with worse outcomes for survivors with cognitive/behavioural or other long-term sequelae [39, 53, 54]. The impact of IMD was not limited to the impact on survivors’ HRQoL. Studies assessing the HRQoL impact beyond the patient found that parents of children admitted with IMD had high and persistent psychological distress (especially PICU-treated young cases) after 36 months follow-up [55]. Family members of survivors with sequelae had a lower health status than families of survivors without sequelae at 12 years follow-up [54]. The severe and long-term burden of the disease to patients and their close network highlights the importance of considering the impact on survivors and also the surrounding network when evaluating IMD interventions and prevention strategies.
Several gaps were identified in the literature. Sequelae definitions (e.g. hearing loss categories) were lacking or inconsistent. In general, meningococcal disease-specific and sequelae-specific HRQoL data were lacking (e.g. lack of data on HRQoL in septicaemia survivors without any sequelae, in meningitis survivors with sequelae, in IMD survivors with psychological sequelae and in serogroup B cases). Available HRQoL estimates were from a range of different instruments and did not elaborate on the HRQoL impact of specific IMD sequelae. Only three health state utility values estimating overall, i.e. non-sequelae-specific, HrQoL loss related to IMD were reported; however, the corresponding studies differed by study population, setting and type of questionnaires and instrument used. The available instruments did not adequately consider the broad range of sequelae, as well as applied instruments such as EQ-5D questionnaires, being limited in the assessment of HrQoL loss related to the sequelae hearing loss and cognitive deficits [56], thus limiting an analysis to provide pooled estimates of HrQoL loss associated with IMD. More research is needed around the identification of IMD sequelae (e.g. lack of psychological sequelae studies in adolescents and adults), but this is challenging due to the low numbers of IMD cases. Sequelae with a late onset (e.g. behavioural and psychological problems) require sufficiently long duration follow-up periods to be identified, and may therefore have been underestimated in some studies.
A limitation of this review was that comparisons and a proper summary of results across studies were challenging due to the above-mentioned gaps, variations in study design, disease manifestation, age of subjects, study population investigated, time point and length of follow-up, as well as definitions and types of assessment used for sequelae. We decided not to present a pooled estimate per sequelae, i.e. an overall % and SD, since heterogeneity is too high across studies. To support the derivation of a reliable estimate in future studies, consensus regarding (1) study type, (2) definitions of sequelae within group of sequelae; (3) ways of sequelae assessment, (4) follow-up times within same age-groups, and (5) documentation of serogroups causing IMD should exist. Due to the low incidence of IMD, high-level evidence study designs, such as case–control studies, are limited in their applicability, in particular for the observation of long-term sequelae. Within this challenging context, and despite the heterogeneity across studies, the current SLR considered most available evidence to allow for the most comprehensive view of long-term sequelae and their impact on HRQoL in survivors of IMD. Only the most frequent sequelae reported have been discussed in detail, while Table 1 presents the full range of sequelae identified.
Conclusion
IMD survivors are affected by a broad range of sequelae, including psychological and behavioural sequelae in addition to physical and neurological sequelae, and a reduction in HRQoL that persists many years after infection. Suffering from IMD during infancy and childhood is likely to result in more sequelae and more severe sequelae than during adolescence or adulthood. Age and severity of disease at infection, and the development of permanent or long-term sequelae, affect subsequent HRQoL in both patients and their families.
IMD is a uncommon disease, that, however, causes one of the highest burdens among < 15 year olds in Europe [57, 58]. Hence, the broad range of potential outcomes, such as diverse types of long-term sequelae or HRQoL impact, is difficult to observe in one single study. Thus, limited evidence and available information might impede public health decision making. This SLR, therefore, provides several important insights for public health decision making: (1) besides the acute phase burden, IMD can cause long-term sequelae; (2) the burden in infants and children appears to be higher than in adolescents and adults; (3) the literature showed that, in addition to the widely-acknowledged physical (e.g. amputations) and neurological (e.g. hearing loss) sequelae, psychological (e.g. depression, anxiety) and behavioural sequelae are also reported, affecting both patients and their family members; (4) focussing on IMD survivors and their family/support network is crucial as IMD had a prolonged psychological impact on family members and resulted in lower health status among family members of survivors with long-term sequelae; (5) therefore, considering the individual case should also include the burden to the surrounding network; (6) Physical sequelae are more frequently reported in IMD cases presented as septicaemia, and neurological sequelae are more frequently reported in IMD cases presented as meningitis, whereas psychological sequelae are reported in both IMD presentations, septicaemia and meningitis, respectively; (7) the results of this review may support a universal immunization recommendation against serogroup B IMD, especially in young children as they experience more, and more severe, IMD sequelae; (8) more research is needed, especially in adolescents/adults and on HRQoL; and (9) research may benefit from the development of a disease-specific HRQoL instrument.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Bexsero is a trade mark owned by the GSK group of companies. Trumenba is a trade mark of Pfizer. The authors would like to thank Robert Welte, (GSK, Germany) for his help during the conduct of this study. They would also like to thank Bernhard Ultsch and Magdalena Schwarz (GSK, Germany) for their support during manuscript development.
Funding
GlaxoSmithKline Biologicals SA (Rixensart, Belgium) was involved in all stages of study conduct, including analysis of the data, and funded the development and publication of this manuscript.
Medical Writing and Editorial Assistance
The authors thank Business & Decision Life Sciences platform for editorial assistance and publications coordination, on behalf of GSK. Stephanie Garcia coordinated publications development and editorial support. The authors also thank Kavi Littlewood (Littlewood Writing Solutions, on behalf of GSK) for providing medical writing support. All medical writing and editorial assistance was funded by GlaxoSmithKline Biologicals SA (Rixensart, Belgium).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Sarah Schumacher is an employee of the GSK group of companies. Ekkehard Beck is an employee of the GSK group of companies. Kinga Meszaros is an employee of the GSK group of companies and also holds shares in the GSK group of companies. At the time of the study, Florian Koerber was an employee of and holds shares in the GSK group of companies. His current affiliation is Institute of Health Economics and Health Care Management, Helmholtz Center Munich, German Research Center for Environmental Health (GmbH), Neuherberg, Germany. During the conduct of this study, Kerstin J Olbrich was performing an internship and reports having therefore received personal fees from the GSK group of companies. Dirk Müller has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7054223.
References
- 1.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–861. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 2.Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–245. doi: 10.2147/CLEP.S28410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotter C, Ramsay M, Harrison L. Introduction and epidemiology of meningococcal disease. In: Feavers IPA, Sadarangani M, editors. Handbook of meningococcal disease management. New York: Springer; 2016. [Google Scholar]
- 4.World Health Organization. Meningococcal meningitis fact sheet #141 2015. 2015. http://www.who.int/mediacentre/factsheets/fs141/en/. Accessed 11 Apr 2017.
- 5.Pelton S, Sadarangani M, Glennie L, Levin M. Clinical aspects of meningococcal disease. In: Feavers IPA, Sadarangani M, editors. Handbook of meningococcal disease management. New York: Springer; 2016. [Google Scholar]
- 6.European Centre for Disease Prevention and Control (ECDC). Annual epidemiological report 2016—invasive meningococcal disease 2016 (2014 data). 2017. https://ecdc.europa.eu/en/publications-data/invasive-meningococcal-disease-annual-epidemiological-report-2016-2014-data Accessed 20 Mar 2017.
- 7.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi: 10.1186/1478-7954-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control (ECDC). Annual epidemiological report for 2015—invasive meningococcal disease. 2015. https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-meningococcal-disease.pdf. Accessed 15 Mar 2018.
- 9.Toneatto D, Pizza M, Masignani V, Rappuoli R. Emerging experience with meningococcal serogroup B protein vaccines. Expert Rev Vaccines. 2017;16(5):433–451. doi: 10.1080/14760584.2017.1308828. [DOI] [PubMed] [Google Scholar]
- 10.Public Health England. Preliminary vaccine coverage estimates for the meningococcal b (MenB) immunisation programme for England, update from August to December 2017. Health protection report volume 12 number 3. 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/677275/hpr0318_menb.pdf. Accessed 04 Apr 2018.
- 11.European Medicines Agency. Trumenba summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004051/WC500228995.pdf. Accessed 04 Apr 2018.
- 12.Ständige Impfkommission (STIKO). Methoden zur durchführung und berücksichtigung von modellierungen zur vorhersage epidemiologischer und gesundheits-ökonomischer effekte von impfungen für die ständige impfkommission. 2017. https://www.rki.de/DE/Content/Kommissionen/STIKO/Aufgaben_Methoden/Methoden_Modellierung.pdf?__blob=publicationFile. Accessed 11 Apr 2017.
- 13.Ellert U, Brettschneider AK, Ravens-Sieberer U. Health-related quality of life in children and adolescents in Germany: results of the KiGGS study: first follow-up (KiGGS wave 1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014;57(7):798–806. doi: 10.1007/s00103-014-1978-4. [DOI] [PubMed] [Google Scholar]
- 14.Rajmil L, Herdman M, Fernandez de Sanmamed MJ, Detmar S, Bruil J, et al. Generic health-related quality of life instruments in children and adolescents: a qualitative analysis of content. J Adolesc Health. 2004;34(1):37–45. doi: 10.1016/S1054-139X(03)00249-0. [DOI] [PubMed] [Google Scholar]
- 15.Herdman M, Hoyle CK, Coles V, Carroll S, Devlin N. Assessing patient-reported outcomes in pediatric populations with vaccine-preventable infectious diseases: a systematic review of the literature (the PROCHID study) Value Health. 2016;19(1):109–119. doi: 10.1016/j.jval.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Strifler L, Morris SK, Dang V, Tu HA, Minhas RS, et al. The health burden of invasive meningococcal disease: a systematic review. J Pediatric Infect Dis Soc. 2016;5(4):417–430. doi: 10.1093/jpids/piv065. [DOI] [PubMed] [Google Scholar]
- 17.Bollschweiler ESS. Systematischer review, metaanalyse und Cochrane collaboration. In: Lauterbach KLM, Schrappe M, editors. Gesunheitsökonomie, management und evidence-based medicine. Stuttgart: Schattauer; 2010. [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Centre for Reviews and Dissemination. PROSPERO—international prospective register of systematic reviews university of York 2016. 2017. http://www.crd.york.ac.uk/PROSPERO/. Accessed 20 Mar 2017.
- 20.Scottish Intercollegiate Guidelines Network (SIGN) A guideline developer’s handbook. Edinburgh: SIGN; 2015. [Google Scholar]
- 21.Cochrane Consumers and Communication Group. Resources for authors—data extraction template 2016. 2017. http://cccrg.cochrane.org/author-resources. Accessed 20-03-2017.
- 22.World Health Organization (2013) Definition of key terms—age groups and populations. http://www.who.int/hiv/pub/guidelines/arv2013/intro/keyterms/en/. Accessed 30 May 2018.
- 23.The World Bank (2017) High income 2016. http://data.worldbank.org/income-level/high-income. Accessed 20 Mar 2017.
- 24.Buysse CM, Raat H, Hazelzet JA, Hop WC, Maliepaard M, et al. Surviving meningococcal septic shock: health consequences and quality of life in children and their parents up to 2 years after pediatric intensive care unit discharge. Crit Care Med. 2008;36(2):596–602. doi: 10.1097/01.CCM.0000299740.65484.CA. [DOI] [PubMed] [Google Scholar]
- 25.Buysse CM, Oranje AP, Zuidema E, Hazelzet JA, Hop WC, et al. Long-term skin scarring and orthopaedic sequelae in survivors of meningococcal septic shock. Arch Dis Child. 2009;94(5):381–386. doi: 10.1136/adc.2007.131862. [DOI] [PubMed] [Google Scholar]
- 26.Shears D, Nadel S, Gledhill J, Garralda ME. Short-term psychiatric adjustment of children and their parents following meningococcal disease. Pediatr Crit Care Med. 2005;6(1):39–43. doi: 10.1097/01.PCC.0000144705.81825.EE. [DOI] [PubMed] [Google Scholar]
- 27.Rivero-Calle I, Vilanova-Trillo L, Pardo-Seco J, Salvado LB, Quinteiro LI, et al. The burden of pediatric invasive meningococcal disease in Spain (2008–2013) Pediatr Infect Dis J. 2016;35(4):407–413. doi: 10.1097/INF.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 28.Stein-Zamir C, Shoob H, Sokolov I, Kunbar A, Abramson N, et al. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dis J. 2014;33(7):777–779. doi: 10.1097/INF.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Clarke M, Thomas N, Howell S, Afzali HH, et al. The clinical burden and predictors of sequelae following invasive meningococcal disease in Australian children. Pediatr Infect Dis J. 2014;33(3):316–318. doi: 10.1097/INF.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Haji Ali AH, Marshall H. The inpatient costs and hospital service use associated with invasive meningococcal disease in South Australian children. Vaccine. 2014;32(37):4791–4798. doi: 10.1016/j.vaccine.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 31.Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, et al. Outcomes of invasive meningococcal serogroup b disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11(9):774–783. doi: 10.1016/S1474-4422(12)70180-1. [DOI] [PubMed] [Google Scholar]
- 32.Goicoechea SM, Fullana Montoro AM, Momparler CP, Redondo Gallego MJ, Brines SJ, et al. Evolution of meningococcal infection among infant population in the autonomous community of Valencia (1996–2000). Effectiveness of A+C meningococcal vaccination. Rev Esp Salud Publ. 2003;77(1):125–142. [PubMed] [Google Scholar]
- 33.Sadarangani M, Scheifele DW, Halperin SA, Vaudry W, Le SN, et al. Outcomes of invasive meningococcal disease in adults and children in Canada between 2002 and 2011: a prospective cohort study. Clin Infect Dis. 2015;60(8):e27–e35. doi: 10.1093/cid/civ028. [DOI] [PubMed] [Google Scholar]
- 34.Stoof SP, Rodenburg GD, Knol MJ, Rumke LW, Bovenkerk S, et al. Disease burden of invasive meningococcal disease in the Netherlands between June 1999 and June 2011: a subjective role for serogroup and clonal complex. Clin Infect Dis. 2015;61(8):1281–1292. doi: 10.1093/cid/civ506. [DOI] [PubMed] [Google Scholar]
- 35.Bettinger JA, Scheifele DW, Le SN, Halperin SA, Vaudry W, et al. The disease burden of invasive meningococcal serogroup B disease in Canada. Pediatr Infect Dis J. 2013;32(1):e20–e25. doi: 10.1097/INF.0b013e3182706b89. [DOI] [PubMed] [Google Scholar]
- 36.Gottfredsson M, Reynisson IK, Ingvarsson RF, Kristjansdottir H, Nardini MV, et al. Comparative long-term adverse effects elicited by invasive group B and C meningococcal infections. Clin Infect Dis. 2011;53(9):e117–e124. doi: 10.1093/cid/cir500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched-cohort study. Pediatrics. 2009;123(3):e502–e509. doi: 10.1542/peds.2008-0581. [DOI] [PubMed] [Google Scholar]
- 38.Erickson LJ, De WP, McMahon J, Heim S. Complications of meningococcal disease in college students. Clin Infect Dis. 2001;33(5):737–739. doi: 10.1086/322587. [DOI] [PubMed] [Google Scholar]
- 39.Koomen I, Raat H, Jennekens-Schinkel A, Grobbee DE, Roord JJ, et al. Academic and behavioral limitations and health-related quality of life in school-age survivors of bacterial meningitis. Qual Life Res. 2005;14(6):1563–1572. doi: 10.1007/s11136-004-7706-z. [DOI] [PubMed] [Google Scholar]
- 40.Sumpter R, Brunklaus A, McWilliam R, Dorris L. Health-related quality-of-life and behavioural outcome in survivors of childhood meningitis. Brain Inj. 2011;25(13–14):1288–1295. doi: 10.3109/02699052.2011.613090. [DOI] [PubMed] [Google Scholar]
- 41.Berg S, Trollfors B, Hugosson S, Fernell E, Svensson E. Long-term follow-up of children with bacterial meningitis with emphasis on behavioural characteristics. Eur J Pediatr. 2002;161(6):330–336. doi: 10.1007/s00431-002-0957-1. [DOI] [PubMed] [Google Scholar]
- 42.Kutz JW, Simon LM, Chennupati SK, Giannoni CM, Manolidis S. Clinical predictors for hearing loss in children with bacterial meningitis. Arch Otolaryngol Head Neck Surg. 2006;132(9):941–945. doi: 10.1001/archotol.132.9.941. [DOI] [PubMed] [Google Scholar]
- 43.Oostenbrink R, Maas M, Moons KG, Moll HA. Sequelae after bacterial meningitis in childhood. Scand J Infect Dis. 2002;34(5):379–382. doi: 10.1080/00365540110080179. [DOI] [PubMed] [Google Scholar]
- 44.Heckenberg SG, de Gans J, Brouwer MC, Weisfelt M, Piet JR, et al. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Med (Baltim) 2008;87(4):185–192. doi: 10.1097/MD.0b013e318180a6b4. [DOI] [PubMed] [Google Scholar]
- 45.Halket S, de Louvois J, Holt DE, Harvey D. Long term follow up after meningitis in infancy: behaviour of teenagers. Arch Dis Child. 2003;88(5):395–398. doi: 10.1136/adc.88.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermunt LC, Buysse CM, Joosten KF, Duivenvoorden HJ, Hazelzet JA, et al. Survivors of septic shock caused by Neisseria meningitidis in childhood: psychosocial outcomes in young adulthood. Pediatr Crit Care Med. 2011;12(6):e302–e309. doi: 10.1097/PCC.0b013e3182192d7f. [DOI] [PubMed] [Google Scholar]
- 47.van de Beek D, Schmand B, de Gans J, Weisfelt M, Vaessen H, et al. Cognitive impairment in adults with good recovery after bacterial meningitis. J Infect Dis. 2002;186(7):1047–1052. doi: 10.1086/344229. [DOI] [PubMed] [Google Scholar]
- 48.Schmand B, de Bruin E, de Gans J, van de Beek D. Cognitive functioning and quality of life nine years after bacterial meningitis. J Infect. 2010;61(4):330–334. doi: 10.1016/j.jinf.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Judge D, Nadel S, Vergnaud S, Garralda ME. Psychiatric adjustment following meningococcal disease treated on a PICU. Intensive Care Med. 2002;28(5):648–650. doi: 10.1007/s00134-002-1237-2. [DOI] [PubMed] [Google Scholar]
- 50.Vermunt LC, Buysse CM, Joosten KF, Oranje AP, Hazelzet JA, et al. Self-esteem in children and adolescents after septic shock caused by Neisseria meningitidis: scars do matter. J Adolesc Health. 2008;42(4):386–393. doi: 10.1016/j.jadohealth.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Buysse CM, Raat H, Hazelzet JA, Vermunt LC, Utens EM, et al. Long-term health-related quality of life in survivors of meningococcal septic shock in childhood and their parents. Qual Life Res. 2007;16(10):1567–1576. doi: 10.1007/s11136-007-9271-8. [DOI] [PubMed] [Google Scholar]
- 52.Grootenhuis MA, Koopman HM, Verrips EG, Vogels AG, Last BF. Health-related quality of life problems of children aged 8–11 years with a chronic disease. Dev Neurorehabil. 2007;10(1):27–33. doi: 10.1080/13682820600691017. [DOI] [PubMed] [Google Scholar]
- 53.Buysse CM, Raat H, Hazelzet JA, Hulst JM, Cransberg K, et al. Long-term health status in childhood survivors of meningococcal septic shock. Arch Pediatr Adolesc Med. 2008;162(11):1036–1041. doi: 10.1001/archpedi.162.11.1036. [DOI] [PubMed] [Google Scholar]
- 54.Al-Janabi H, Van EJ, Brouwer W, Trotter C, Glennie L, et al. Measuring health spillovers for economic evaluation: a case study in meningitis. Health Econ. 2016;25(12):1529–1544. doi: 10.1002/hec.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrlich TR, Von Rosenstiel IA, Grootenhuis MA, Gerrits AI, Bos AP. Long-term psychological distress in parents of child survivors of severe meningococcal disease. Pediatr Rehabil. 2005;8(3):220–224. doi: 10.1080/13638490400022246. [DOI] [PubMed] [Google Scholar]
- 56.Joint Committee on Vaccination and Immunisation. Minutes of the JCVI meningoccocal subcommittee. 2014. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_128724.pdf. Accessed 20 Aug 2018.
- 57.World Health Organization. Priority medicines for Europe and the World 2013 update—rare diseases. 2013. http://www.who.int/medicines/areas/priority_medicines/Ch6_19Rare.pdf. Accessed 30 May 2018.
- 58.Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the burden of communicable diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23:16. doi: 10.2807/1560-7917.ES.2018.23.16.17-00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.



