Abstract
Sulfolobus islandicus is a model microorganism in the TACK superphylum of the Archaea, a key lineage in the evolutionary history of cells. Here we report a genome-wide identification of the repertoire of genes essential to S. islandicus growth in culture. We confirm previous targeted gene knockouts, uncover the non-essentiality of functions assumed to be essential to the Sulfolobus cell, including the proteinaceous S-layer, and highlight essential genes whose functions are yet to be determined. Phyletic distributions illustrate the potential transitions that may have occurred during the evolution of this archaeal microorganism, and highlight sets of genes that may have been associated with each transition. We use this comparative context as a lens to focus future research on archaea-specific uncharacterized essential genes that may provide valuable insights into the evolutionary history of cells.
Sulfolobus islandicus is a model organism within the TACK superphylum of the Archaea. Here, the authors perform a genome-wide analysis of essential genes in this organism, show that the proteinaceous S-layer is not essential, and explore potential stages of evolution of the essential gene repertoire in Archaea.
Introduction
Forty-one years ago, Woese and Fox identified the Archaea as a novel microbial lineage distinct from Bacteria1. The same year, Woese and Fox proposed a model of cellular evolution in which early cellular life diverged in two directions, one to the Bacteria and the other to LEACA, the Last Eukaryotic and Archaeal Common Ancestor, which subsequently split forming the Archaea and Eukaryota domains2–4. Increases in genome and metagenome sequence data continue to refine this picture, providing reinforcement for many of its key aspects, improving phylogenetic sampling, and providing additional details5–12. With the addition of new lineages, the tree of life itself has evolved with great controversy13–17. Some of these new analyses suggest that the Thaumarcheota, Aigarchaeota, Crenarchaeota, and Korarchaeota (TACK) lineages of Archaea have the closest relationship to the Eukaryota with cultured representatives5,6,13,14,18,19.
Today, the tree of life provides a framework for studying the evolution of cellular complexity. Genomics and metagenomics provide data on the distribution of genes across this tree and in doing so provide an understanding of the origins and evolutionary dynamics of gene sequences. However, phyletic distributions fall short of establishing the functional evolutionary history of the cell since gene presence does not link directly to function. Mapping evolution of today’s complex cells involves a comparative approach in which functional cellular systems and the interactions of their constituent components are examined at a molecular level in organisms representing key evolutionary lineages across the tree of life.
As a step in that direction, we take here a genome-wide functional approach to define 441 genes essential to the growth of Sulfolobus islandicus. Sulfolobus, a thermoacidophilic genus from geothermal hot springs, is one of the few organisms within the TACK archaea that can be cultured and is genetically tractable20,21, and it is the most developed model for studying the biology of cells in this lineage. We find that the S-layer protein, which is present in most cells in the archaeal domain22, is not essential in this model organism. Furthermore, we explore potential stages of evolution of the essential gene repertoire of the archaeal cell.
Results and Discussion
Identifying essential genes in the genome of S. islandicus
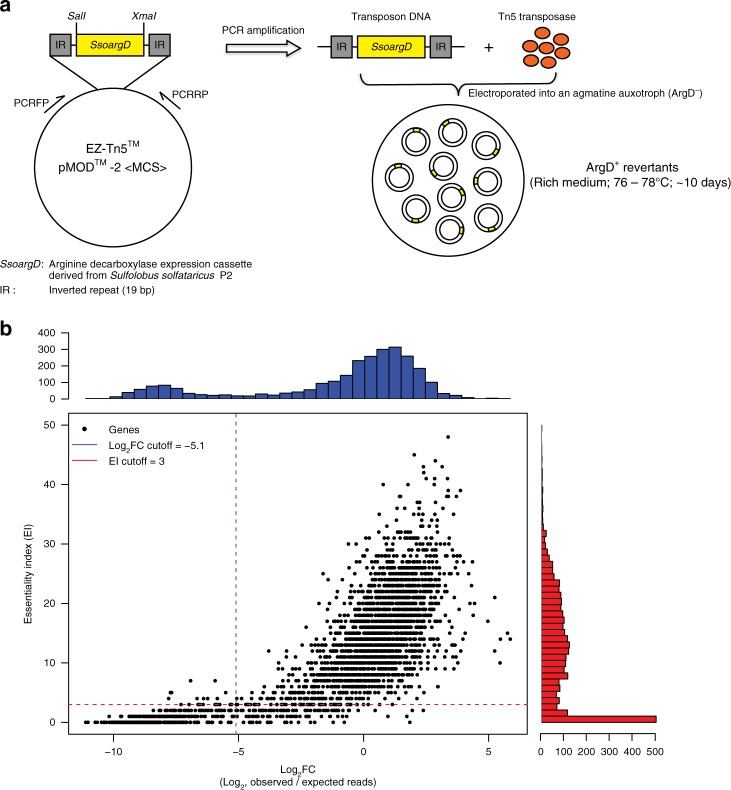
We established three independent genome-wide disruption libraries in an agmatine-auxotrophic strain of S. islandicus M.16.4 by using a modified in vitro transposon mutagenesis system derived from Tn5 (Epicentre, USA). The transposable element was comprised of a nutritional marker cassette, SsoargD (arginine decarboxylase derived from Sulfolobus solfataricus P2), flanked by two 19-base pair (bp) inverted repeats (Fig. 1a). After electroporation-mediated transformation of ArgD− cells with the EZ-Tn5 transposome, cells were allowed 10 days of growth on rich media. While valuable information about metabolic and regulatory genes could have been gained by comparing results from different media conditions, we restricted this study to one rich medium to focus on central cellular rather than metabolic functions. Insertion locations were determined via genome tagging and fragmentation (“tagmentation”) on colony pools, followed by amplification and sequencing of the junction sites, which were then mapped onto the genome. In all, 89,758 unique insertion events with at least 3 reads each were identified across all three libraries, corresponding to an average of 1 insertion every 29 bp and an average expected 29 insertions in each annotated protein-coding gene (see Methods; Supplementary Table 1 contains colony, insertion, and read counts for each library while all insertion locations can be found in Supplementary Data 1).
Fig. 1.
Defining the essential genes in S. islandicus M.16.4. a Schematic overview of the genome-wide transposon mutagenesis strategy. b Evaluation of gene essentiality by two computational programs: ESSENTIALS23 and Tn-Seq-Explorer24. Points indicate individual genes plotted according to the scores returned by each program. Histograms indicate the number of genes of a particular score, and the dotted lines indicate the recommended cutoffs returned by each program as the local minimum between the essential and non-essential score distributions. Essential genes meet both criteria (lower-left quadrant). The protein-coding genes that only met the ESSENTIALS or Tn-Seq-Explorer criteria were deemed as “unassigned candidates” leaving the rest as likely non-essential to S. islandicus M.16.4 growth under these conditions. A complete list of the log2FC and EI for the S. islandicus M.16.4 genes from the combined mutant libraries are provided in Supplementary Data 2
Essential genes were predicted to be significantly under-represented in the insertion locations extracted from the transposon mutagenesis and sequencing data (Tn-seq). It is important to note that this may make them indistinguishable from genes that are not strictly essential for growth but instead cause a severe growth defect, and thus our definition of “essential” extends to these genes too. To determine the statistical separation between essential and non-essential genes, we used a combination of two programs: ESSENTIALS23 and Tn-Seq Explorer24. Both methods report essential gene candidates by separating essential and non-essential genes into a bimodal distribution of scores. ESSENTIALS does so by calculating a log ratio of observed and expected reads in each gene (log2FC), while Tn-Seq Explorer uses a sliding window approach to examine the absolute number of insertions in and around genes and calculates an Essentiality Index (EI) for each. The former tends to underestimate the number of essential genes, while the latter tends to overestimate24. Four hundred and forty-five genes lie within the suggested range for both methods (log2FC ≤ -5.1 and EI < 4), leaving 175 genes within only one range or “unassigned” as essential or non-essential. Fourteen protein-encoding genes could not be assigned a log2FC score due to the presence of identical sequence elsewhere in the genome (score “NA” in Supplementary Data 2). The remaining 2100 protein-coding genes are likely non-essential for growth under these conditions (Fig. 1b and Supplementary Data 2). Three genes identified as essential through automated methods were additionally removed because misplaced multiply mapped reads falsely reduced read count (M164_0862, M164_1012, and M164_1867; see Supplementary Table 2). Assignments of all genes to categories with their scores for each method are listed in Supplementary Data 2.
Genetic confirmation of essential gene criteria
To support our informatic essentiality/non-essentiality criteria, 129 genes were compared with gene knockout studies performed in our model S. islandicus M.16.4 and another two genetically tractable S. islandicus strains: RYE15A and LAL 14/1 (Supplementary Table 3). We were unable to acquire knockouts for 42 of the 45 predicted essential genes in this set. Two exceptions, topR2 (M164_1245) and apt (M164_0158), were identified to have significant growth defects on plates once they were knocked out (Supplementary Fig. 1c and 2a and ref. 25), likely resulting in their under-representation in our transposon library. The third, cdvB3 (M164_1510), a paralog of cdvB, may be incorrectly called essential in our Tn-seq analysis. We can readily obtain cdvB3 disruption mutants (Supplementary Fig. 3b) and the growth of a cdvB3 mutant strain is indistinguishable from the wild-type strain (data not shown), thus this gene was removed from the essential gene list. An explanation of why this gene is mischaracterized would require further investigation, but it is possible that, because the score distributions for essential and non-essential genes overlap, this gene was simply not hit enough times to achieve significance. This could be true for a small number of other genes as well and is a fundamental limitation of Tn-seq.
To further investigate our automated assignments, we screened eight “unassigned” genes in S. islandicus M.16.4 that were called essential by one method or the other but not both. We were unable to obtain mutants for six of them. Of these, five genes, i.e., lig (M164_1953), priL (M164_1568), priX (M164_1652), rnhII (M164_0197), and tfs2 (M164_1524), were called essential via EI but not log2FC, while thrS1 (M164_0290) was called essential based on log2FC but not EI. In contrast, knockouts of the two “unassigned” genes called essential by EI but not log2FC, udg4 (M164_0085), encoding uracil-DNA glycosylase family 4, and rpo8 (M164_1872), encoding a subunit of RNA polymerase, were obtained after an extended 14 days incubation of transformation plates, again consistent with a severe growth defect (Supplementary Fig. 2b, c and 3b). This suggests the presence of false negatives and a stronger bias to underestimate than overestimate the true number of essential genes. Because not all genes in the unassigned categories were genetically tested, we conservatively excluded all unassigned genes from the essential gene list. By contrast, knockouts for all 76 non-essential genes tested were successfully obtained and verified by PCR analysis (Supplementary Table 3 and Supplementary Fig. 3a and 3b). These include hjm/hel308a (M164_0269), cdvB1 (M164_1700), topR1 (M164_1732), and three DExD/H-box family helicase genes (M164_0809, M164_2103, and M164_2020), the homologs of which were previously thought to be essential in a related strain S. islandicus Rey15A26–29 (Supplementary Table 3 and Supplementary Fig. 3b). Taken together, these experimental results supported the overall validity of our computational approaches for conservatively classifying putative gene essentiality.
Essential gene repertoire
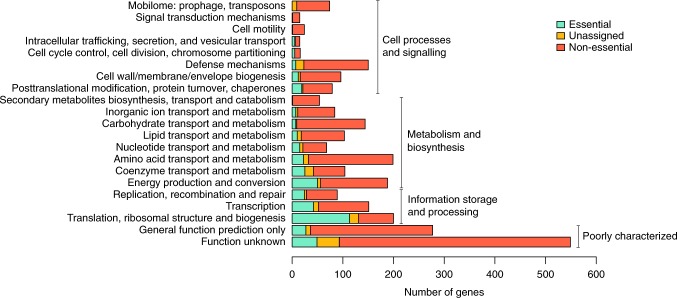
The functional repertoire of the predicted essential, unassigned, and non-essential genes of S. islandicus is shown in Fig. 2. With the above adjustments, the size of this essential genome (441 genes) is close in size to that observed for other bacteria and archaea30. For example, ~526 genes are required for growth in Methanococcus maripaludis S231 and 473 genes within the engineered Mycoplasma mycoides JCVI Syn3.0 minimal bacterial cell32. The proportion of different functional categories represented in this set (as defined by archaeal clusters of orthologous genes12 (arCOGs)) are also similar to that observed in other studies31,32 (Fig. 2), with the largest fraction of genes (178, ~40%) representing information processing (translation, transcription, and DNA replication/recombination/repair) and 76 (~17%) either classified as “function unknown” or “general functional prediction only.” The latter two categories are hereby collectively referred to as “poorly characterized.” Descriptions of the specific essential components found in central information processing and the cell cycle, as well as central carbon metabolism, are detailed in Supplementary Notes 1-5. We highlight only a few interesting and novel observations below.
Fig. 2.
arCOG category and essentiality criteria for protein-coding genes in S. islandicus M.16.4. Functional distribution of essential, non-essential, and unassigned genes via arCOG category. Essentiality criteria based on cutoffs in Fig. 1b
S-layer is non-essential in S. islandicus
Our essential gene predictions include several surprising findings. First, SlaA (M164_1763) and SlaB (M164_1762), the two known components of the surface layer (S-layer) of Sulfolobus cells33 were shown to be non-essential. SlaA is the dominant component of the S-layer that forms a quasi-crystalline matrix outside the cell membrane34. Current models suggest a “stalk-and-cap” structure in which the C-terminal-transmembrane-helix-domain-containing SlaB projects from the cell membrane and anchors SlaA to the cell membrane22,35,36. The cellular function of the Sulfolobus S-layer is unknown but is believed to provide resistance to osmotic stress and contribute to cell morphology33. S-layer-deficient mutants have never been successfully cultivated before in any archaeal species, therefore it was assumed to be essential.
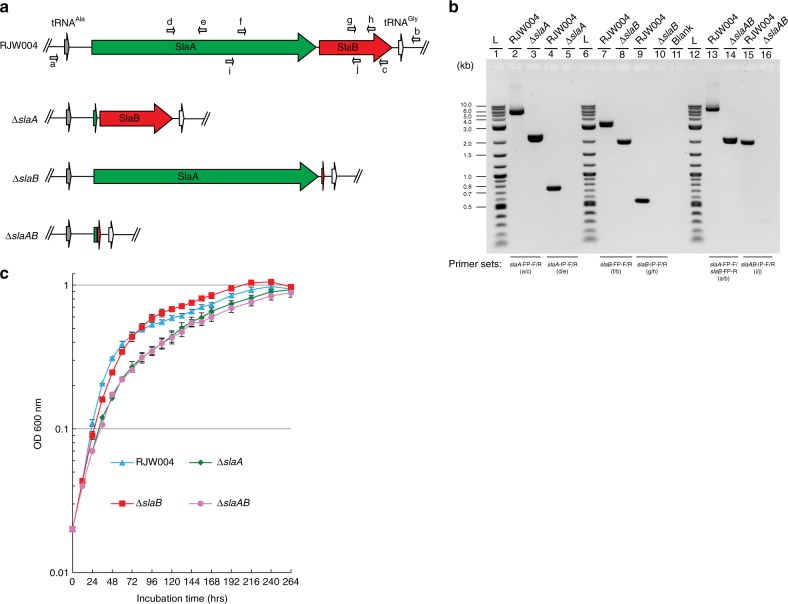
To confirm the non-essentiality of the S-layer genes, we constructed in-frame deletion mutants of slaA, slaB, and slaAB via a MID (marker insertion and unmarked target gene deletion) recombination strategy37. PCR amplification with two primer sets, which bind the flanking and internal region of S-layer genes, respectively (Fig. 3a), confirmed the successful deletion of slaA, slaB, and slaAB from the chromosome of the genetic host RJW004 (wild type) (Fig. 3b). We next tested for absence of the S-layer proteins in growing cells. Isolation of a white precipitant, described as the S-layer previously38, was possible only in the wild type and to a much lesser extent in the ΔslaB mutant strain (Supplementary Fig. 4a and 4b). Transmission electron microscopy (TEM) analysis confirmed this extracted protein precipitate from both wild type and ΔslaB mutant strains formed crystalline lattice structures (Supplementary Fig. 4c). Finally, we tested the mutant phenotypes by comparing their growth profiles with wild type in a standard laboratory condition (pH 3.3, 76 °C). As shown in Fig. 3c, cells lacking the S-layer protein lattice SlaA (including slaA and slaAB mutants) are viable but have a measurable growth defect. This confirms the non-essentiality of the S-layer lattice in S. islandicus. The deletion of slaB alone had no significant impact on the growth rate in comparison with that of wild type (Fig. 3c). For a complete knockout of all potential S-layer components, we successfully created a viable triple knockout of slaA, slaB, and a paralog of SlaB encoded by M164_1049 (42% coverage, 53% amino acid identity via BLAST), demonstrating non-essentiality of all S-layer components together in S. islandicus (Supplementary Fig. 5).
Fig. 3.
S-layer genes are not essential for the S. islandicus cell survival. a Genomic context of S-layer genes in the genetic host and mutant strains. Relative positions of primers used to confirm S-layer gene deletions are labelled with small arrows. b PCR verification of ΔslaA, ΔslaB, and ΔslaAB mutants with two primer sets, which bind the flanking and internal regions of S-layer genes, respectively. Expected sizes of amplicons can be found in Supplementary Table 8. L (lanes 1, 6, and 12) indicates the 2-Log DNA Ladder (NEB, USA). Blank (lane 11) denotes that no sample was loaded in the well. c Growth profiles of RJW004 (wild type), ΔslaA, ΔslaB, and ΔslaAB mutant strains. Wild-type and S-layer gene knockout strains were cultivated at pH 3.3, 76 °C for 11 days in DY liquid medium supplemented with uracil and agmatine without shaking. Cell culture growth was monitored by optical density measurements at 600 nm every 12 or 24 h. Error bars represented standard deviations from three independent experiments
We performed thin-section TEM analyses of the RJW004 (wild type) and S-layer gene knockout strains. The thin-section micrographs of wild-type cells clearly revealed that the S-layer was separated from the cytoplasmic membrane by a quasi-periplasmic space (Fig. 4a, e), in agreement with previous studies in Sulfolobus acidocaldarius39 and Sulfolobus shibatae34. The S-layer in the wild type was observed as a distinct dark band on the outermost edge of the cell, and the quasi-periplasmic space was seen as a light gray band between the outermost band and the cell membrane (Fig. 4a, e). However, the dark, outermost layer surrounding the cell was not observed in the ΔslaA or ΔslaAB mutant cells (Fig. 4b, d, f, h), confirming that SlaA contributes to the formation of the outermost layer. Additionally, the cell surface appeared diffuse in the ΔslaA mutant cell, which was attributed to the periodic extensions of membrane proteins, likely including the SlaB protein, and/or their extensive N-glycosylation22. In the ΔslaB mutant cell, a smooth outermost layer similar to the SlaA layer in wild-type cells was observed; however, it appears to be discontinuous around the cell membrane (Fig. 4c, g). The partial lattice of SlaA in the ΔslaB mutant may be anchored by other membrane proteins even in the absence of SlaB, including the aforementioned M164_1049. Together these images suggest that additional components may contribute to the non-essential Sulfolobus S-layer.
Fig. 4.
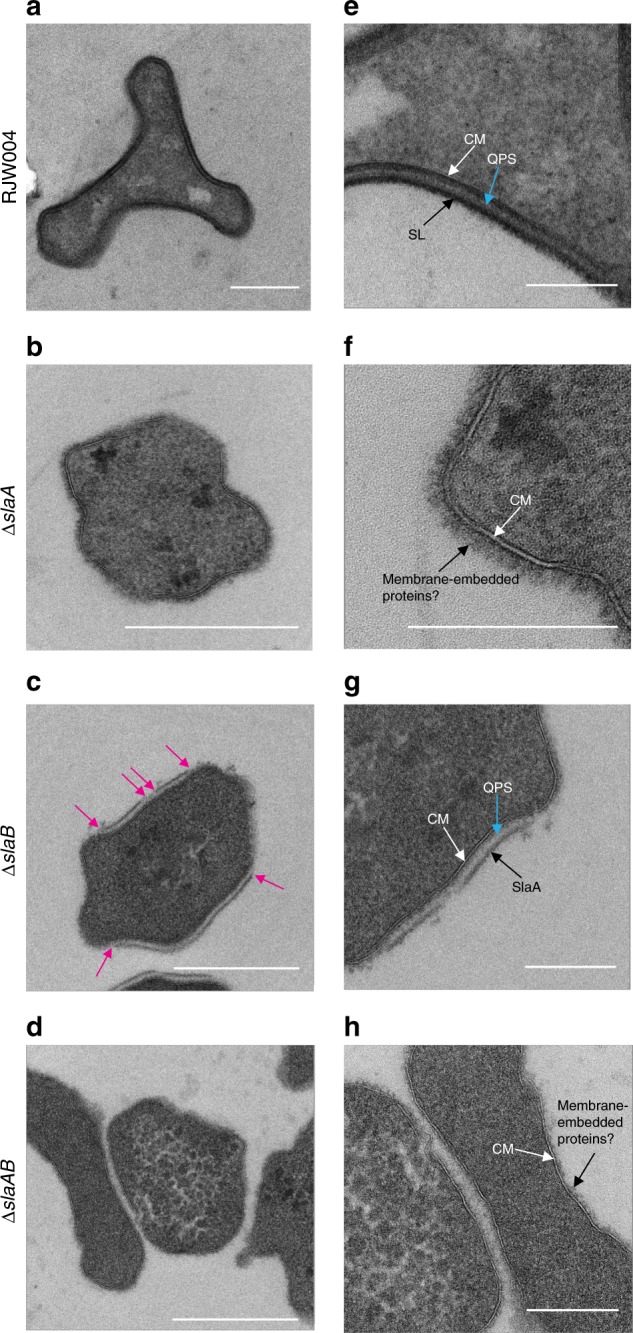
Thin-section TEM analysis of the wild-type and S-layer gene knockout strains. a–d Representative TEM micrographs of thin-sectioned cells of the wild-type, ΔslaA, ΔslaB, and ΔslaAB mutant strains, respectively. Images e–h are closeups of images a–d, respectively. Magenta arrows indicate the breaking points of S-layer. CM cytoplasmic membrane, SL surface layer, QPS quasi-periplasmic space, SlaA surface layer protein A. Scale bars, 500 nm (a–d) and 200 nm (e–h)
Incomplete complementarity of reverse gyrase
As an additional surprise from our genome-wide essential gene identification, we found incomplete complementarity between two copies of the reverse gyrase in S. islandicus M.16.4. Unlike Euryarchaota and most extremely thermophilic bacteria, Crenarchaeota possess two copies of reverse gyrase40,41, both believed to be essential for growth26,42. Tn-seq analysis indicated that the topR1 (M164_1732) was non-essential, which was confirmed by a successful disruption (Supplementary Fig. 1a and 1b). Interestingly, as mentioned above, topR2 (M164_1245) was called essential but we could obtain topR2 disruption mutants (Supplementary Fig. 1a and 1c) if we prolonged the incubation time (up to 14–20 days) of transformation plates in gene knockout experiments. These observations suggest that topR2 plays a more important role than topR1 in Sulfolobus cell survival at optimal temperature.
Lethal deletion mutants
Tn-seq also uncovered genes that may not be essential to growth but instead are toxic when disrupted. Among them, arCOG analysis predicts that M164_0131, M164_0217, M164_0268, M164_2076, M164_1728, and M164_1060 are antitoxin-encoding genes. We reason that inactivation of these antitoxin genes might cause overproduction of toxins and then trigger cell death. This finding suggests that associated toxins are constitutively expressed in our laboratory conditions. Interestingly, unlike most of the family II (VapBC) and family HEPN-NT toxin/antitoxin gene pairs in S. islandicus M.16.4 (Supplementary Data 3), partners (toxin genes) adjacent to these predicted antitoxin genes (with the exception of M164_1060; see Supplementary Fig. 6) were not observed. This indicates that VapB-VapC or HEPN-NT do not always correspond to their neighbors and some gene pairs might have exchanged counterparts. The Tn-seq-based analyses also classified cas5 (M164_0911), a part of the Cascade (CRISPR-associated complex for antiviral defense) complex43, as essential. Consistent with this assignment, disruption of cas5 by replacing it with the StoargD marker cassette via homologous recombination failed after repeated attempts. However, the entire Type-IA module of CRISPR-Cas system, consisting of eight genes with cas5 included, could be deleted from the S. islandicus M.16.4 chromosome with no detectable effect on cell growth (data not shown). One possible explanation is that, in the absence of cas5, the Cascade complex becomes misfolded and thus toxic for the cells, but future studies are needed to confirm this interpretation.
Shared essential genes
To establish how this essential gene set compares with those found in other organisms, we retrieved sets of essential genes from the database of essential genes30,44 in eight model organisms that span the tree of life31,45–49, including the minimal genes set in the JCVI Syn 3.0 Mycoplasma mycoides genome32 (Fig. 5, Supplementary Data 4). We find that 242 S. islandicus essential genes are essential in at least 1 other organism we surveyed, while 199 essential genes are uniquely essential in S. islandicus. Eighty-nine genes are essential in representatives of all 3 domains, 78 of which are also essential in Syn 3.0 (Supplementary Data 4). As shown in Fig. 5, comparisons of shared essential genes support the shared cellular systems between the archaeal and eukaryotic domains. More total S. islandicus essential gene orthologs are shared with archaea and eukaryotes (gray in Fig. 5), and more of these shared orthologs are essential (colors), than are shared between S. islandicus and the bacteria we use for comparison. The highest number of shared essential genes (187) is between S. islandicus and M. maripaludis S231, an organism from the euryarchaeal lineage of the archaeal domain (Table 1). The large size of the essential gene set shared between Sulfolobus and Methanococcus, in spite of their widely different habitats and life styles, reinforces the fundamental nature of Archaea as a distinct cell type50.
Fig. 5.
Shared essential genes across the three domains of life. Heatmap shows the presence of essential (colored) or non-essential (gray) shared NOGs compared with the S. islandicus M.16.4 essential genome. Single-letter codes for functional categories are as follows: J, translation, ribosomal structure, and biogenesis; K, transcription; L, DNA replication, recombination, and repair; C, energy production and conversion; E, amino acid transport and metabolism; H, coenzyme transport and metabolism; F, nucleotide transport and metabolism; G, carbohydrate transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolite biosynthesis, transport, and catabolism; O, posttranslational modification, protein turnover, chaperone functions; M, cell wall/membrane/envelope biogenesis; U, intracellular trafficking, secretion, and vesicular transport; D, cell cycle control and mitosis; N, cell motility; T, signal transduction; S, function unknown. CPS, cellular processes and signaling
Table 1.
Number of S. islandicus essential genes shared with seven model organisms
| Phyletic categorya | ||||||||
|---|---|---|---|---|---|---|---|---|
| Universal | EA | Archaea | TACK | Sulfolobales | Other | |||
| Archaea | Methanococcus maripaludis | Shared | 128 | 77 | 42 | 2 | 2 | 42 |
| Essential | 93 | 55 | 20 | 2 | 0 | 17 | ||
| Eukarya | Saccharomyces cerevisiae | Shared | 134 | 77 | 2 | 0 | 1 | 27 |
| Essential | 68 | 41 | 1 | 0 | 0 | 4 | ||
| Schizosaccharomyces pombe | Shared | 134 | 78 | 2 | 0 | 1 | 26 | |
| Essential | 82 | 43 | 1 | 0 | 0 | 10 | ||
| Bacteria | Bacteroides fragilis | Shared | 124 | 10 | 0 | 0 | 2 | 40 |
| Essential | 70 | 1 | 0 | 0 | 0 | 12 | ||
| Bacillus subtilis | Shared | 131 | 7 | 5 | 1 | 4 | 49 | |
| Essential | 72 | 0 | 0 | 0 | 0 | 11 | ||
| Escherichia coli | Shared | 136 | 7 | 6 | 0 | 8 | 64 | |
| Essential | 73 | 0 | 1 | 0 | 0 | 12 | ||
| JCVI Syn 3.0 | Shared | 76 | 2 | 0 | 0 | 0 | 10 | |
| Essential | 76 | 2 | 0 | 0 | 0 | 10 | ||
aGenes are put into a category if they are present in ≥50% of the organisms in each group, i.e., universal is in ≥50% of each of the Bacteria, Archaea, and Eukarya groups. "Other" refers to genes that do not meet these criteria
Phyletic distributions of essential genes
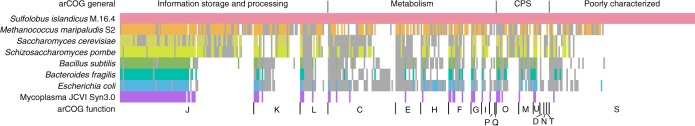
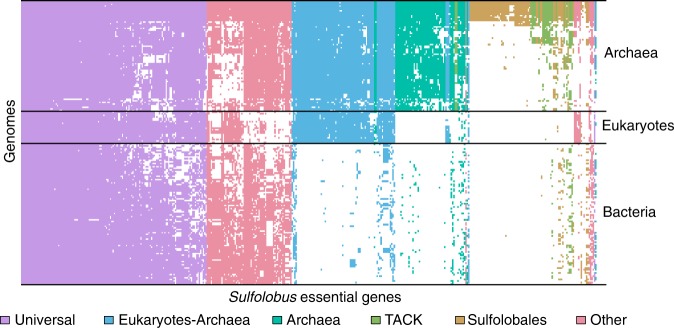
To investigate the broader phyletic distributions of S. islandicus essential genes, we used assignments from the evolutionary genealogy of genes: Non-supervised Orthologous Groups (eggNOG) database51 (see Methods) to map the presence and absence of putative essential gene orthologs from 168 complete genomes representing major clades in all 3 domains based on a previously published analysis6 (Supplementary Data 5 and Supplementary Data 6). Figure 6 graphically shows the S. islandicus essential genes shared in other genomes in a set of hierarchical clusters based on Euclidean distance. From this Fig. 4, primary transitions emerge in the evolution of the contemporary S. islandicus essential genome. The number of genes in phyletic groups (Table 2) is significantly different from random sampling among phyletic categories (Supplementary Table 4). Similarly ranked distributions are seen in two additional datasets: (1) all genomes in the eggNOG database subsampled to have equal representation in each domain and (2) all genomes in the eggNOG database for which assignments are available. These data are supported by parsimony analysis with bootstrap support for the grouping of each of the three major domains (Supplementary Figs. 7 and 8). Together these data support four primary stages in the evolution of the contemporary S. islandicus cells and allow us to assign specific essential genes to these potential transitions in the evolution of the cell.
Fig. 6.
Presence/absence of genes shows phyletic patterns. Heatmap of shared NOG/arNOGs according to annotations in the eggNOG database corresponding to the essential gene set of S. islandicus M.16.4 across the three domains of life. Each row is 1 of the 177 taxa including the set of 169 used for other distribution analyses, 1 from the candidate phylum Bathyarchaeota, and 7 Asgardarchaeota genomes (Supplementary Data 5 and 6). Each column is 1 of the 441 essential genes discovered in this study. A white box indicates that no matching NOG or arNOG was found, while a colored box indicates presence. Colors indicate categories defined in Tables 1 and 2
Table 2.
Number of S. islandicus essential genes shared with 168 full genome sequences spanning the tree of life
| Phyletic categorya | ||||||
|---|---|---|---|---|---|---|
| Universal | EA | Archaea | TACK | Sulfolobales | Other | |
| Shared genes | 141 | 80 | 55 | 18 | 73 | 74 |
| Poorly characterizedb | 5 | 1 | 14 | 7 | 46 | 3 |
aGenes are put into a category if they are present in ≥50% of the organisms in each group, i.e., universal is in ≥50% of each of the Bacteria, Archaea, and Eukarya groups. “Other” refers to genes that do not meet these criteria
bNOG categories “Function unknown” or “General functional prediction only”. Full list shown in Supplementary Table 6
The highest number of essential genes are shared broadly across the tree of life (Universal in Table 2), supporting the early evolution of the majority of essential gene functions in the contemporary archaeal cell. Most of these have putative functional assignments in information processing, particularly translation and transcription (Supplementary Data 7). Many previous studies have reported the evolutionary conservation of information processing components going back to the Last Universal Common Ancestor (LUCA) using computational methods7–9,11,52. We find that in all studies the majority of conserved orthologous gene sets that we could interrogate in this system are essential (Supplementary Table 5 and Supplementary Data 8). Of the 200 metabolic COGs identified in the S. islandicus genome from a recent estimate of the LUCA gene set10, only 19 were found to be essential (Supplementary Data 8). This is expected, due to our use of rich medium. The first phase of the cell contains the universal set of genes with conserved cellular components that are likely to have evolved early in evolutionary history remain essential components of the contemporary S. islandicus genome today.
The next largest category of essential genes is found between Sulfolobus and other organisms in the Eukarya/Archaea (EA) domains (Table 2). These genes are largely involved in core information processing functions and support the shared evolutionary ancestry of the Archaea and Eukarya after their divergence from Bacteria. Only one gene in this category is poorly characterized: M164_0237, a homolog to eukaryotic zpr1. zpr1 is a gene essential for transcription and cell cycle progression in fungal and mammalian cells53–56 and has recently been reported as a regulator of circadian rhythm in plants57. Though it has been noted that this gene is exclusively shared in EA56, it remains uncharacterized in the Archaea outside of our results recognizing its essentiality in Sulfolobus (Supplementary Table 6).
Fifty-five essential genes belong to NOGs that are shared by organisms in the archaeal domain (Table 2). Functional assignments of the archaeal-specific genes represent a diversity of functions split between core functions (translation, transcription, and replication) and peripheral functions, such as transport, defense (including all the above-mentioned predicted antitoxin genes), and metabolism. Archaea-specific DNA replication/recombination/repair genes are nurA and gins15, while genes in arCOG category “transcription (K)” are largely transcription factors and do not represent core RNA polymerase functionality like the EA genes mentioned above. Fourteen of the archaeal-specific genes are poorly characterized (Table 2), 9 of which are also essential in M. maripaludis S2 (Supplementary Data 4). In an evolutionary context, this set of poorly characterized, but essential, archaea-specific genes are key targets for future molecular characterization since they likely highlight the unique biology of archaeal cells. We also show that the majority of S. islandicus genes are conserved in evolutionary history through the archaeal domain.
The final set of essential genes are specific or largely specific to the Sulfolobales, most of which have uncharacterized functions (Table 2). The essentiality of these genes and whether they fit into central cellular functions as non-orthologous gene replacements or peripheral ones are important subjects of future work. This set of genes, unique to this lineage, may represent environmental adaptations. The fact that they are poorly characterized attests to the need for further study even in this model archaeon.
The key next steps toward comparative cell biology will be understanding the functional interactions among essential genes so that new gene inventions, non-orthologous gene transfers, and/or loss of specific functions can be identified. From the unique perspective of the TACK archaea, this work provides a list of genes whose future molecular and systems characterization are likely to provide further understanding for evolutionary steps in the Archaea.
In conclusion, this is the first comprehensive genome-wide study of essential gene content in a model crenarchaeon. Our profile of S. islandicus essential genes uncovers several surprising findings, most notably the non-essentiality of the Sulfolobus S-layer. Comparative phyletic patterns provide a perspective on the stages of evolution of the contemporary S. islandicus, its shared ancestry with the eukaryotes, and the key components that define its uniqueness as an archaeal cell.
Methods
Strains and culture conditions
The complete list of strains and plasmids used in this study is shown in Supplementary Table 7. All S. islandicus strains were routinely grown aerobically at 76–78 °C and pH 3.3 without shaking in basal salt medium37 containing 0.2% [wt/vol] dextrin (Sigma-Aldrich, USA) and 0.1% [wt/vol] tryptone (BD Biosciences, USA) (the medium is hereafter named as DY). When required, agmatine, uracil, and 5-FOA were added to a final concentration of 50, 20, and 50 μg/ml respectively. For solid plates, 2 × DY medium was supplemented with 20 mM MgSO4 and 7 mM CaCl2·2H2O and mixed with 1.4% gelrite (Sigma-Aldrich, USA) with a ratio of 1:1 [vol/vol]. Plates were put into sealed bags and generally incubated for 10–14 days at 76–78 °C. Cell culture growth was monitored by optical density measurements at 600 nm using a portable cell density meter (CO8000, WPA, Cambridge, UK).
Construction of S. islandicus transposon mutant library
The 755-bp argD gene cassette (SsoargD) was PCR-amplified from the genomic DNA of S. solfataricus P2 using the primer set SsoargD-F1/R1, introducing the SalI and XmalI sites, respectively. The resultant PCR products were digested with SalI/Xmal and then cloned into the EZ-Tn5TM pMODTM-2 < MCS > Transposon Construction Vector (Epicentre, USA) in the corresponding sites, generating pT-SsoargD. The Tn5 < SsoargD > transposon DNA was prepared by PCR amplification from linearized pT-SsoargD with 5’phosphorylated primers PCRFP/PCRRP. The PCR products, consisting of a nutritional marker flanked by a 19-bp inverted repeat (Mosaic Ends, ME), were purified and highly concentrated using the DNA Clean & ConcentratorTM-5 Kit (Zymo Research, USA). Preparation of transposomes was made in a 10 μl reaction system as follows: 2.2 μg of transposon, 1 μl of EZ-Tn5 transposase (Epicentre, USA), and 2.5 μl of 100% glycerol. The reaction was incubated at room temperature for 30 min and then switched to 4 °C for another 72 h. In all, 1–2 μl of transposomes were transformed into S. islandicus RJW008 (ΔargD) via electroporation as described in ref. 37. Cell transformation assays were repeated dozens of times in order to collect a sufficient number of transformants to achieve saturation mutagenesis. The theoretical number of transposon insertion colonies was calculated using a derivative of Poisson’s law: N = ln(1 − P)/ln(1 − f), f = average gene size (900.64 bp)/genome size (2,586,647 bp). To make sure the transposon insertions cover approximately 99.99% (P = 0.9999) of the genome, around 26,448 colonies per library are required. The transformed cells were plated on DY plates either by glass beads or over-lay58. After 10 days of incubation, the ArgD+ revertants were harvested from plates either by manually picking or with sterile spreaders and then pooled into three independent transposon mutant libraries (CYZ-TL1, CYZ-TL2, and CYZ-TL3), with approximately 100,000 colonies in total (Supplementary Table 1). We routinely obtained an average of ca. 103 colonies/μg transposon, and approximately 105 colonies/μg DNA using a replicative plasmid pSeSd-SsoargD. The pSeSd-SsoargD was constructed by cloning the SsoargD marker cassette, amplified from S. solfataricus P2 genomic DNA with primer set SsoargD-F2/R2, into the XmalI site of a Sulfolobus-E.coli shutter vector pSeSd59. Thus, the estimated frequency of transposition is ~10−2 per cell.
DNA library preparation and high-throughput DNA sequencing
Genomic DNA from each mutant pool was extracted as described in ref. 60 and then quantified with Qubit® 2.0 Fluorometer (Invitrogen, USA). DNA libraries were prepared using the Nextera XT DNA Library Prep Kit (Illumina, USA) with proper modifications. Briefly, 2 ng of input genomic DNA in total was simultaneously fragmented and tagged with sequencing adapters in a single enzymatic reaction tube. Afterwards, a primer mixture of Tn-seq-F (Supplementary Data 9) and N705 (a randomly selected primer from the Nextera XT DNA Library Prep Kit) was added in the same tube to enrich the transposon–chromosome junction regions via PCR. The PCR conditions were as follows: 72 °C for 3 min, 95 °C for 30 s, and 22 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. A final extension was performed at 72 °C for 5 min. The resultant library DNA was cleaned up with AMPure XP beads for three times, eluted in 45-μl EB buffer (QIAprep Spin Miniprep Kit, USA), and then quantified with Qubit® 2.0 Fluorometer. The final DNA library was quantitated on High-Sensitivity Qubit (Life Technologies) and fragment size was evaluated using the Agilent 2100 Bioanalyzer on a DNA7500 chip (Agilent Technologies), then further quantitated by quantitative PCR on a BioRad CFX Connect Real-Time System (Bio-Rad Laboratories, Inc. CA) to ensure accuracy of quantitation of the library containing properly adapted fragments. The final pool was loaded onto 2 lanes (CYZ_TL1) and 1 lane each (CYZ_TL2 and CYZ_TL3) of a HiSeq 2500 Rapid flowcell for cluster formation and sequencing on an Illumina HiSeq 2500 with Rapid SBS sequencing reagents version 2. Sequencing by synthesis was performed from one end of the molecules for a total read length of 160 nt. The 100 μM of custom Read 1 sequencing primer, specific for the Tn-seq-F sequence (Supplementary Data 9), was spiked into the standard Read1 HP10 primer tube (position 18) for sequencing. The run generated.bcl files, which were converted into demultiplexed compressed fastq files using the bcl2fastq v1.8.4 Conversion Software (Illumina, CA) at the W. M. Keck Center for Comparative and Functional Genomics at the University of Illinois at Urbana-Champaign.
Tn-seq data processing and analysis
Illumina FASTQ reads from all three libraries that were <50 bp in length, had a quality score <30, and did not contain the 23-bp transposon sequence were removed. The remaining reads were stripped of transposon and adapter sequence and aligned to the S. islandicus M.16.4 genome (NC_012726) using the Burrows–Wheeler Bowtie 2 alignment tool61. Reads that mapped to multiple locations in the genome or to ambiguous sites were set aside, as were those with an alignment length <11 bp. Using the in-house software, the resulting .sam alignment files were converted to lists that included unique insertion locations, the strand to which they aligned, and the number of reads associated with that event (Supplementary Data 1). Insertions that occurred in the same location but on different strands or in separate libraries were considered independent events. Tn5 transposase has been shown to prefer certain insertion sites over others62, so each reported site was extracted and nucleotide frequency was measured 20 bases upstream and downstream as compared to an equal number of random sites in the genome. Random sampling via the Python numpy.random.choice function (with replacement) yielded sites with overall frequencies matching the known G+C content of the genome (35%), but a pronounced and palindromic pattern was observed at insertion sites even when normalizing for this bias (Supplementary Fig. 9). Overall Tn5 appears to prefer a G–C bp flanked by an AT-rich region, which is consistent with other studies62,63. However, when normalized to the overall G+C content, no single biased site was more than two-fold enriched in a certain base compared to the rest of the genome, meaning that there was considerable variation in the sites themselves and thus the chance that the bias would significantly affect our results is reduced. Gene essentiality was then evaluated using the software previously designed and published for this purpose: Tn-Seq Explorer24 and ESSENTIALS23. The ESSENTIALS software was run with mostly default settings with a list of insertion locations and associated reads as the input. The locations for each of the three libraries were submitted as separate files and the total library size specified as 105,968 (Supplementary Table 1). Repeat filtering was enabled to avoid calling repeated regions as essential. The LOESS smoothing feature normally meant to compensate for the over-representation of bacterial origins of replication (caused by multiple simultaneous replication rounds) was disabled because Sulfolobus only undergoes one round of replication per cell cycle64. Because of the lack of observed sequence specificity, the insertion site was specified as “random.” The program uses “log2FC” as its measure of essentiality, which is proportional to log2 (reads observed/reads expected) for each gene and sets a cutoff automatically as the local minimum between essential and non-essential distributions in a density plot of the scores. The program suggested a putative maximum log2FC of −5.1 for essential genes.
For the Tn-Seq Explorer software, insertion sites of all three libraries were combined and insertion sites with <4 reads were excluded for analysis due to their vast over-representation in the insertion sites and the uncertainty of their source (Supplementary Table 1). The program uses a sliding window approach and returns an EI based on the number, location, and spatial concentration of insertion sites within each individual gene. It also allows for the adjustment of the stated start and end points of the gene. As is default, insertions in the first 5% and last 20% of genes were excluded to compensate for misannotated start codons and proteins for which C-terminal deletions are tolerated, respectively. The program suggested an EI maximum of 3 (Fig. 1b).
Construction of S. islandicus mutants
Except where otherwise stated, disruption of the chromosomal genes was achieved by replacing their coding regions (57–100% of the length of the gene was deleted) with the argD expression cassette (StoargD) derived from Sulfolobus tokodaii via a microhomology-mediated gene inactivation approach we recently developed65. Briefly, a functional argD gene was PCR-amplified from a linearized Sulfolobus–E.coli shuttle vector pSesD-StoargD with 35–40 bp homology of the targeted gene introduced, yielding the gene disruption cassettes. The resultant PCR products were purified and electroporated into the argD auxotrophic strain S. islandicus RJW008, selecting ArgD+ transformants on the plates lacking agmatine. S-layer genes slaA, slaB, and slaAB were deleted from the chromosome of the genetic host S. islandicus RJW004 via an improved MID strategy37,66 with knockout plasmids pMID-slaA, pMID-slaB, and pMID-slaAB, respectively. The resulting ΔslaA and ΔslaB mutants harbored an in-frame deletion of the coding region from nucleotides +52 to +3687 relative to the start codon of slaA (3690 bp in length), and +13 to +1185 relative to the start codon of slaB (1194 bp in length), respectively. The ΔslaAB mutant was constructed similarly leaving 51 bp of the slaA (nt 1–51 relative to the start codon of slaA), 6 bp of restriction enzyme (MluI) site, and 9 bp of slaB (nt 1186–1194 relative to the start codon of slaB) in the chromosome of S. islandicus RJW004. Verification of each gene replacement or deletion mutant was determined through PCR diagnosis with both flanking primers (bind outside of the targeted region) and internal primers (bind inside of targeted region), which examined the genotype and purity of mutants, respectively. The primers used to generate and confirm gene disruptions or deletions were described in Supplementary Data 9, and the expected sizes of amplicons generated from the genetic host (wt) and mutant strains were provided in Supplementary Table 8.
Transmission electron microscopy
Proteinaceous S-layer was extracted from S. islandicus cell cultures as described in ref. 38. To prepare the samples that were used for TEM, glow-discharged, carbon-stabilized Formvar-coated 200-mesh copper grids (Carbon Type-B, cat. no. 01811, Ted Pella, Inc., USA) were placed on 8–20-μl droplets of each sample for 3 min, rinsed with degassed water, and negative-stained with 2% uranyl acetate for 15–60 s. Thin-sectioned S. islandicus cells were prepared essentially as described in ref. 67, with minor modifications as follows: after microwave fixation with the primary fixative, cells were washed in Sorenson’s Phosphate buffer with no further additives. All samples were observed using a Philips CM200 transmission electron microscope at 120 kV. Images were taken at various magnifications using a TVIPS (Tietz Video and Image Processing Systems GmbH; Germany) 2k × 2k Peltier-cooled CCD camera. Scale bars were added with the ImageJ software.
Homology search
Homologs for the 441 essential genes found in Supplementary Data 2 were found across the 168 genomes listed in Supplementary Data 6 via the European Molecular Biology Laboratory eggNOG database51. Genomes were downloaded from the National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov). The genomes to survey are based on the set6 in Raymann et al. (2015) with the following additions: M. maripaludis S2 was added to compare essential gene content; the genome of Toxoplasma gondii ME49 was added because its essential genome became available during the course of this analysis68; Schizosaccharomyces pombe69 was added to compare with Saccharomyces cerevisiae S288C; additional Sulfolobales genomes were added for intra-order comparison of essential gene content (listed in Supplementary Data 6). While not included in the phyletic distribution analysis, the sequences for Lokiarchaeum sp. GC14_7513 and Thorarchaeota70 SMTZ-45, SMTZ1-45, and SMTZ1-83 were retrieved and analyzed; presence/absence data can be found in Supplementary Data 5. Several bacterial genomes were added to include additional model systems (e.g., E. coli str. K-12 substr. MG1655 and Bacillus subtilis subsp. subtilis str. 168). The complete list is found in Supplementary Data 6. Owing to their incomplete or highly reduced nature, we excluded DPANN and Asgard lineages, as well as Bacteria from the candidate phyla radiation71 and the minimal Mycoplasma Syn 3.032; however, presence/absence of information for selected genomes are provided in Supplementary Data 5. For organisms not in the eggNOG database, the amino acid sequences of protein-coding genes were uploaded to the eggNOG mapper tool (http://eggnogdb.embl.de/#/app/emapper) and run with default settings. These data were translated into a presence/absence matrix and evaluated with custom Python and Zsh scripts to assess the phyletic distribution of essential gene candidates. Finally, for each S. islandicus M.16.4 essential gene candidate, the amino acid sequences of all bidirectional best BLAST hits (BBBH) of that gene across all 168 genomes (BLASTP; minimum 70% sequence coverage, 20% identity, 40% positively scoring sites, and maximum E-value of 0.001) were used to scan genomes in which no homologs were found using tBLASTn, and the results were filtered according to the same cutoff criteria as the bidirectional best BLAST hits. The tBLASTn hits that overlapped with annotated genes by >50 bp were discarded. This was to fill in gaps left by annotation mistakes, where the protein may still be in the genome but was not published as such; however, the approach introduced many false-positives and both bidirecitonal best hits and tBLASTn gene matches often were in disagreement with the eggNOG assignments (data not shown). Due to the better quality of eggNOG matches, neither set of BLAST results were included in presence/absence matrices (data not shown). However, BBBH within selected Sulfolobales can be found in Supplementary Data 2.
Parsimony analysis
Presence/absence matrices were converted to NEXUS format files with a custom Python script and used in the Phylogenetic Analysis Using Parsimony (and other methods) (PAUP*) tool72. The main tree was found with the heuristic search function with a maximum of 1000 trees in memory, default settings. The first tree was saved as an unrooted NEXUS format tree with branch lengths. Bootstrapping was run with default settings for 1000 iterations with 100 maximum trees in memory. The resulting tree was saved with support values as node labels. A custom python script using the Phylo package within the Biopython73 suite was used to transfer the support values from the bootstrap consensus tree to the corresponding nodes on the heuristic search tree. Trees were visualized in the interactive tree of life74 interface.
Phyletic distribution analysis
The presence/absence matrices were also cross-referenced with phylogenetic data via NCBI taxonomy information to determine how widespread each gene was in different orders spanning the tree of life. Simulated random distributions of genes were created by counting how many organisms in which they were found and assigning that many random organisms to each gene (without replacement) for each gene 100 times using the numpy.random.choice function. P values were generated by counting the number of simulated observations above or below the true observation and dividing by 100. To determine the proportion of COGs or arCOGs that have essential members in S. islandicus, we removed unique clusters with >1 gene in S. islandicus that showed no essentiality from the total due to possible redundancy in functional orthologs from the same cluster. To test for bias in the phyletic sampling of the set of 169 genomes, we assembled 100 organism sets with genomes randomly sampled from the eggNOG database to equal proportions of TACK archaea, euryarchaeota, eukaryotes, and bacteria as to that in the 169 genome set. Organisms were chosen at random without replacement using the numpy.random.choice function again. In all data involving the eggNOG database, organisms missing from the NOG member file were excluded.
Code availability
The custom Python and Zsh scripts used for analyses in this study are available from the corresponding author upon request.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
We thank Chris L. Wright and Alvaro G. Hernandez from W.M. Keck Center for Comparative and Functional Genomics, University of Illinois at Urbana-Champaign (UIUC) for advice with primer design, DNA library construction, and sequencing. We also thank Whitney E. England, Angelo Blancaf, and Ted Kim for assistance in Tn-seq data processing; Carlos A. Vega, Elizabeth H. Marr, and Melinda E. Baughman for providing technical assistance in collecting transposon insertion colonies. We thank Isaac K.O. Cann and Scott C. Dawson for fruitful discussions. We are thankful to Kira S. Makarova for technical assistance with data retrieval from the arCOG database and for helpful suggestions. We thank Marleen van Wolferen for providing the S-layer extraction protocol. We acknowledge Yuan Li and Emily N. Hallett for providing S-layer extraction assistance. We would also like to thank Scott J. Robinson from Beckman Institute for Advance Science and Technology, UIUC for technical assistance with TEM imaging and sample preparation. We thank Lou A. Miller from Frederick Seitz Materials Research Laboratory Central Research Facilities, UIUC for preparing the thin-sectioned S. islandicus cells. Funding for this work was mainly provided by the National Aeronautics and Space Administration (NASA) through the NASA Astrobiology Institute under cooperative agreement no. NNA13AA91A, issued through the Science Mission Directorate. This work was also partially supported by Division of Environmental Biology (DEB: 1355171 to R.J.W.), US National Science Foundation, the Department of Microbiology Alice Helm Graduate Research Excellence Fellowship, UIUC (to A.P.R.P.), the Carl R. Woese Institute for Genomic Biology Undergraduate Research Scholar program, and the Office of Undergraduate Research, UIUC (to R.L.W).
Author contributions
C.Z., A.P.R.P. and R.J.W. conceived and designed the research; C.Z. and R.L.W. carried out experimental work; A.P.R.P., C.Z., G.J.O., R.L.W. and R.J.W. analyzed the data; R.J.W. and G.J.O contributed new reagents/analytic tools; and C.Z., A.P.R.P., R.L.W. and R.J.W. wrote the paper. All authors edited the manuscript.
Data availability
The raw Tn-seq data of three independent transposon insertion libraries CYZ-TL1, CYZ-TL2, and CYZ-TL3 have been deposited at NCBI under BioSample accession numbers SAMN08628694, SAMN08628695, and SAMN08628696, respectively; Bioproject accession number PRJNA436600; and Sequence Read Archive (SRA) accession number SRP133799. Analyzed data showing the insertion locations across three independent transposon libraries can be found in Supplementary Data 1. All other data that support the findings of this work are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Changyi Zhang, Alex P. R. Phillips.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07379-4.
References
- 1.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl. Acad. Sci. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woese CR, Fox GE. The concept of cellular evolution. J. Mol. Evol. 1977;10:1–6. doi: 10.1007/BF01796132. [DOI] [PubMed] [Google Scholar]
- 3.Iwabe N, Kuma K, Hasegawa M, Osawa S, Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc. Natl. Acad. Sci. 1989;86:9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy L, Ettema TJG. The archaeal ‘TACK’ superphylum and the origin of eukaryotes. Trends Microbiol. 2011;19:580–587. doi: 10.1016/j.tim.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Raymann K, Brochier-Armanet C, Gribaldo S. The two-domain tree of life is linked to a new root for the Archaea. Proc. Natl. Acad. Sci. 2015;112:6670–6675. doi: 10.1073/pnas.1420858112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puigbò P, Wolf YI, Koonin EV. Search for a ‘tree of life’ in the thicket of the phylogenetic forest. J. Biol. 2009;8:59. doi: 10.1186/jbiol159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JK, Kelley ST, Spiegelman GB, Pace NR. The genetic core of the universal ancestor. Genome Res. 2003;13:407–412. doi: 10.1101/gr.652803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil R, et al. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc. Natl. Acad. Sci. 2003;100:9388–9393. doi: 10.1073/pnas.1533499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss MC, et al. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016;1:16116. doi: 10.1038/nmicrobiol.2016.116. [DOI] [PubMed] [Google Scholar]
- 11.Mirkin BG, Fenner TI, Galperin MY, Koonin EV. Algorithms for computing parsimonious evolutionary scenarios for genome evolution, the last universal common ancestor and dominance of horizontal gene transfer in the evolution of prokaryotes. BMC Evol. Biol. 2003;3:2. doi: 10.1186/1471-2148-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarova K, Wolf Y, Koonin E. Archaeal clusters of orthologous genes (arCOGs): an update and application for analysis of shared features between Thermococcales, Methanococcales, and Methanobacteriales. Life. 2015;5:818–840. doi: 10.3390/life5010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spang A, et al. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature. 2015;521:173–179. doi: 10.1038/nature14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaremba-Niedzwiedzka K, et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature. 2017;541:353–358. doi: 10.1038/nature21031. [DOI] [PubMed] [Google Scholar]
- 15.Cunha VD, Gaia M, Gadelle D, Nasir A, Forterre P. Lokiarchaea are close relatives of Euryarchaeota, not bridging the gap between prokaryotes and eukaryotes. PLoS Genet. 2017;13:e1006810. doi: 10.1371/journal.pgen.1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spang A, et al. Asgard archaea are the closest prokaryotic relatives of eukaryotes. PLoS Genet. 2018;14:e1007080. doi: 10.1371/journal.pgen.1007080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha VD, Gaia M, Nasir A, Forterre P. Asgard archaea do not close the debate about the universal tree of life topology. PLoS Genet. 2018;14:e1007215. doi: 10.1371/journal.pgen.1007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera MC, Lake JA. The ring of life provides evidence for a genome fusion origin of eukaryotes. Nature. 2004;431:152. doi: 10.1038/nature02848. [DOI] [PubMed] [Google Scholar]
- 19.Eme L, Spang A, Lombard J, Stairs CW, Ettema TJG. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017;15:133. doi: 10.1038/nrmicro.2017.133. [DOI] [PubMed] [Google Scholar]
- 20.Leigh JA, Albers SV, Atomi H, Allers T. Model organisms for genetics in the domain Archaea: methanogens, halophiles, Thermococcales and Sulfolobales. FEMS Microbiol. Rev. 2011;35:577–608. doi: 10.1111/j.1574-6976.2011.00265.x. [DOI] [PubMed] [Google Scholar]
- 21.Peng N, Han W, Li Y, Liang Y, She Q. Genetic technologies for extremely thermophilic microorganisms of Sulfolobus, the only genetically tractable genus of crenarchaea. Sci. China Life Sci. 2017;60:370–385. doi: 10.1007/s11427-016-0355-8. [DOI] [PubMed] [Google Scholar]
- 22.Albers SV, Meyer BH. The archaeal cell envelope. Nat. Rev. Microbiol. 2011;9:414–426. doi: 10.1038/nrmicro2576. [DOI] [PubMed] [Google Scholar]
- 23.Zomer A, Burghout P, Bootsma HJ, Hermans PWM, van Hijum SAFT. ESSENTIALS: software for rapid analysis of high throughput transposon insertion sequencing data. PLoS ONE. 2012;7:e43012. doi: 10.1371/journal.pone.0043012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solaimanpour S, Sarmiento F, Mrázek J. Tn-Seq explorer: a tool for analysis of high-throughput sequencing data of transposon mutant libraries. PLoS ONE. 2015;10:e0126070. doi: 10.1371/journal.pone.0126070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, She Q, Bi H, Whitaker RJ. The apt/6-methylpurine counterselection system and its applications in genetic studies of the hyperthermophilic archaeon Sulfolobus islandicus. Appl. Environ. Microbiol. 2016;82:3070–3081. doi: 10.1128/AEM.00455-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, et al. Genetic manipulation in Sulfolobus islandicus and functional analysis of DNA repair genes. Biochem. Soc. Trans. 2013;41:405–410. doi: 10.1042/BST20120285. [DOI] [PubMed] [Google Scholar]
- 27.Song X, Huang Q, Ni J, Yu Y, Shen Y. Knockout and functional analysis of two DExD/H-box family helicase genes in Sulfolobus islandicus REY15A. Extremophiles. 2016;20:537–546. doi: 10.1007/s00792-016-0847-5. [DOI] [PubMed] [Google Scholar]
- 28.Liu, J. et al. Functional assignment of multiple ESCRT-III homologs in cell division and budding in Sulfolobus islandicus. Mol. Microbiol. 105, 540–553 (2017). [DOI] [PubMed]
- 29.Hong Y, et al. Dissection of the functional domains of an archaeal Holliday junction helicase. DNA Repair (Amst.) 2012;11:102–111. doi: 10.1016/j.dnarep.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Luo H, Lin Y, Gao F, Zhang CT, Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014;42:D574–D580. doi: 10.1093/nar/gkt1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarmiento F, Mrazek J, Whitman WB. Genome-scale analysis of gene function in the hydrogenotrophic methanogenic archaeon Methanococcus maripaludis. Proc. Natl. Acad. Sci. 2013;110:4726–4731. doi: 10.1073/pnas.1220225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchison CA, et al. Design and synthesis of a minimal bacterial genome. Science. 2016;351:aad6253. doi: 10.1126/science.aad6253. [DOI] [PubMed] [Google Scholar]
- 33.Taylor KA, Deatherage JF, Amos LA. Structure of the S-layer of Sulfolobus acidocaldarius. Nature. 1982;299:840. doi: 10.1038/299840a0. [DOI] [Google Scholar]
- 34.Baumeister W, Wildhaber I, Phipps BM. Principles of organization in eubacterial and archaebacterial surface proteins. Can. J. Microbiol. 1989;35:215–227. doi: 10.1139/m89-034. [DOI] [PubMed] [Google Scholar]
- 35.Veith A, et al. Acidianus, Sulfolobus and Metallosphaera surface layers: structure, composition and gene expression. Mol. Microbiol. 2009;73:58–72. doi: 10.1111/j.1365-2958.2009.06746.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues-Oliveira, T., Belmok, A., Vasconcellos, D., Schuster, B. & Kyaw, C. M. Archaeal S-layers: overview and current state of the art. Front. Microbiol. 8, 2597 (2017). [DOI] [PMC free article] [PubMed]
- 37.Zhang C, Cooper TE, Krause DJ, Whitaker RJ. Augmenting the genetic toolbox for Sulfolobus islandicus with a stringent positive selectable marker for agmatine prototrophy. Appl. Environ. Microbiol. 2013;79:5539–5549. doi: 10.1128/AEM.01608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyfoon, E. et al. The S-layer glycoprotein of the Crenarchaeote Sulfolobus acidocaldarius is glycosylated at multiple sites with chitobiose-linked N-glycans. Archaea2010, 754101 (2010). [DOI] [PMC free article] [PubMed]
- 39.Reitz T, et al. Spectroscopic study on uranyl carboxylate complexes formed at the surface layer of Sulfolobus acidocaldarius. Dalton Trans. 2015;44:2684–2692. doi: 10.1039/C4DT02555E. [DOI] [PubMed] [Google Scholar]
- 40.Forterre P. A hot story from comparative genomics: reverse gyrase is the only hyperthermophile-specific protein. Trends Genet. 2002;18:236–237. doi: 10.1016/S0168-9525(02)02650-1. [DOI] [PubMed] [Google Scholar]
- 41.Brochier-Armanet C, Forterre P. Widespread distribution of archaeal reverse gyrase in thermophilic bacteria suggests a complex history of vertical inheritance and lateral gene transfers. Archaea. 2006;2:83–93. doi: 10.1155/2006/582916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han W, Feng X, She Q. Reverse gyrase functions in genome integrity maintenance by protecting DNA breaks in vivo. Int J. Mol. Sci. 2017;18:1340. doi: 10.3390/ijms18071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lintner NG, et al. Structural and functional characterization of an archaeal clustered regularly interspaced short palindromic repeat (CRISPR)-associated complex for antiviral defense (CASCADE) J. Biol. Chem. 2011;286:21643–21656. doi: 10.1074/jbc.M111.238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R, Lin Y. DEG 5.0, a database of essential genes in both prokaryotes and eukaryotes. Nucleic Acids Res. 2009;37:D455–D458. doi: 10.1093/nar/gkn858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giaever G, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 46.Kim DU, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 2010;28:617–623. doi: 10.1038/nbt.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veeranagouda Y, Husain F, Tenorio EL, Wexler HM. Identification of genes required for the survival of B. fragilis using massive parallel sequencing of a saturated transposon mutant library. BMC Genomics. 2014;15:429. doi: 10.1186/1471-2164-15-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi K, et al. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Commichau M, F., Pietack N, Stülke J. Essential genes in Bacillus subtilis: a re-evaluation after ten years. Mol. Biosyst. 2013;9:1068–1075. doi: 10.1039/c3mb25595f. [DOI] [PubMed] [Google Scholar]
- 50.Woese CR. On the evolution of cells. Proc. Natl. Acad. Sci. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huerta-Cepas J, et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016;44:D286–D293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciccarelli FD, et al. Toward automatic reconstruction of a highly resolved tree of life. Science. 2006;311:1283. doi: 10.1126/science.1123061. [DOI] [PubMed] [Google Scholar]
- 53.Galcheva-Gargova Z, Konstantinov KN, Wu IH, Klier FG. Binding of zinc finger protein ZPR1 to the epidermal growth factor receptor. Science. 1996;272:1797. doi: 10.1126/science.272.5269.1797. [DOI] [PubMed] [Google Scholar]
- 54.Gangwani L, Mikrut M, Galcheva-Gargova Z, Davis RJ. Interaction of ZPR1 with translation elongation factor-1α in proliferating cells. J. Cell Biol. 1998;143:1471–1484. doi: 10.1083/jcb.143.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gangwani L. Deficiency of the zinc finger protein ZPR1 causes defects in transcription and cell cycle progression. J. Biol. Chem. 2006;281:40330–40340. doi: 10.1074/jbc.M608165200. [DOI] [PubMed] [Google Scholar]
- 56.Mishra AK, Gangwani L, Davis RJ, Lambright DG. Structural insights into the interaction of the evolutionarily conserved ZPR1 domain tandem with eukaryotic EF1A, receptors, and SMN complexes. Proc. Natl. Acad. Sci. 2007;104:13930–13935. doi: 10.1073/pnas.0704915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiełbowicz-Matuk A, Czarnecka J, Banachowicz E, Rey P, Rorat T. Solanum tuberosum ZPR1 encodes a light-regulated nuclear DNA-binding protein adjusting the circadian expression of StBBX24 to light cycle. Plant Cell Environ. 2017;40:424–440. doi: 10.1111/pce.12875. [DOI] [PubMed] [Google Scholar]
- 58.Deng L, Zhu H, Chen Z, Liang YX, She Q. Unmarked gene deletion and host–vector system for the hyperthermophilic crenarchaeon Sulfolobus islandicus. Extremophiles. 2009;13:735. doi: 10.1007/s00792-009-0254-2. [DOI] [PubMed] [Google Scholar]
- 59.Peng N, et al. A synthetic arabinose-inducible promoter confers high levels of recombinant protein expression in hyperthermophilic archaeon Sulfolobus islandicus. Appl. Environ. Microbiol. 2012;78:5630–5637. doi: 10.1128/AEM.00855-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reno ML, Held NL, Fields CJ, Burke PV, Whitaker RJ. Biogeography of the Sulfolobus islandicus pan-genome. Proc. Natl. Acad. Sci. 2009;106:8605–8610. doi: 10.1073/pnas.0808945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green B, Bouchier C, Fairhead C, Craig NL, Cormack BP. Insertion site preference of Mu, Tn5, and Tn7 transposons. Mob. DNA. 2012;3:3. doi: 10.1186/1759-8753-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reznikoff WS. The Tn5 transposon. Annu Rev. Microbiol. 1993;47:945–964. doi: 10.1146/annurev.mi.47.100193.004501. [DOI] [PubMed] [Google Scholar]
- 64.Bernander R, Poplawski A. Cell cycle characteristics of thermophilic archaea. J. Bacteriol. 1997;179:4963–4969. doi: 10.1128/jb.179.16.4963-4969.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C, Whitaker RJ. Microhomology-mediated high-throughput gene inactivation strategy for the hyperthermophilic crenarchaeon Sulfolobus islandicus. Appl. Environ. Microbiol. 2018;84:e02167–17. doi: 10.1128/AEM.02167-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang C, et al. Revealing the essentiality of multiple archaeal PCNA genes using a mutant propagation assay based on an improved knockout method. Microbiology. 2010;156:3386–3397. doi: 10.1099/mic.0.042523-0. [DOI] [PubMed] [Google Scholar]
- 67.Bautista MA, Zhang C, Whitaker RJ. Virus-induced dormancy in the archaeon Sulfolobus islandicus. mBio. 2015;6:e02565-14. doi: 10.1128/mBio.02565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sidik SM, et al. A genome-wide CRISPR screen in toxoplasma identifies essential apicomplexan genes. Cell. 2016;166:1423.e12–1435.e12. doi: 10.1016/j.cell.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood V, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- 70.Seitz Kiley W, Lazar Cassandre S, Hinrichs Kai-Uwe, Teske Andreas P, Baker Brett J. Genomic reconstruction of a novel, deeply branched sediment archaeal phylum with pathways for acetogenesis and sulfur reduction. The ISME Journal. 2016;10(7):1696–1705. doi: 10.1038/ismej.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hug, L. A. et al. A new view of the tree of life. Nat. Microbiol. 1, 16048 (2016). [DOI] [PubMed]
- 72.Swofford, D. L. PAUP*. Phylogenetic Analysis using Parsimony (*and Other Methods). Version 4 (Sinauer Associates, Sunderland, MA, 2003).
- 73.Cock PJA, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Letunic Ivica, Bork Peer. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research. 2016;44(W1):W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The raw Tn-seq data of three independent transposon insertion libraries CYZ-TL1, CYZ-TL2, and CYZ-TL3 have been deposited at NCBI under BioSample accession numbers SAMN08628694, SAMN08628695, and SAMN08628696, respectively; Bioproject accession number PRJNA436600; and Sequence Read Archive (SRA) accession number SRP133799. Analyzed data showing the insertion locations across three independent transposon libraries can be found in Supplementary Data 1. All other data that support the findings of this work are available from the corresponding author upon request.