Abstract
Although tissue engineering using human-induced pluripotent stem cells is a promising approach for treatment of cardiovascular diseases, some limiting factors include the survival, electrical integration, maturity, scalability, and immune response of three-dimensional (3D) engineered tissues. Here we discuss these important roadblocks facing the tissue engineering field and suggest potential approaches to overcome these challenges.
In this Comment, Ngan Huang et al. discuss recent advances in cardiovascular tissue engineering and some of the main challenges that remain in translating these advances to the clinic. The authors propose future direction for the field to focus research efforts.
Introduction
Cardiovascular diseases are the leading cause of heart failure and mortality in the United States, and heart transplant remains the most viable and effective option for treatment1. However, a major drawback for heart transplantation is the chronic shortage of donor organs and tissues. Furthermore, heart transplant recipients face serious challenges in long-term survival in the form of adverse effects of immunosuppression and chronic immune rejection2. Accordingly, there is a compelling need for alternative strategies to improve the management of heart failure. Tissue engineering—a multi-disciplinary approach that combines life sciences and engineering to manufacture functional tissue equivalents, such as engineered myocardial tissue—is emerging as a promising alternative to organ replacement or mechanical support3.
Owing to the generally non-proliferative nature of contractile cardiomyocytes (CM) in the myocardium, the efficient generation of CMs from human-induced pluripotent stem cells (hiPSCs)4 has been a major advancement in cardiovascular tissue engineering5. Although some reports have demonstrated the efficacy of hiPSC-CM-derived engineered myocardial tissue in small and large preclinical animal models of heart failure6–10, challenges exist that hinder the successful clinical application of hiPSC-CM-derived engineered myocardial tissue. These include the survival, electrical integration, and immune response of scalable three-dimensional (3D) engineered tissues, as well as issues concerning the maturity and function of hiPSC-CMs (Fig. 1). Below we discuss some of the important roadblocks facing this field, and the potential approaches to overcome these challenges.
Fig. 1.
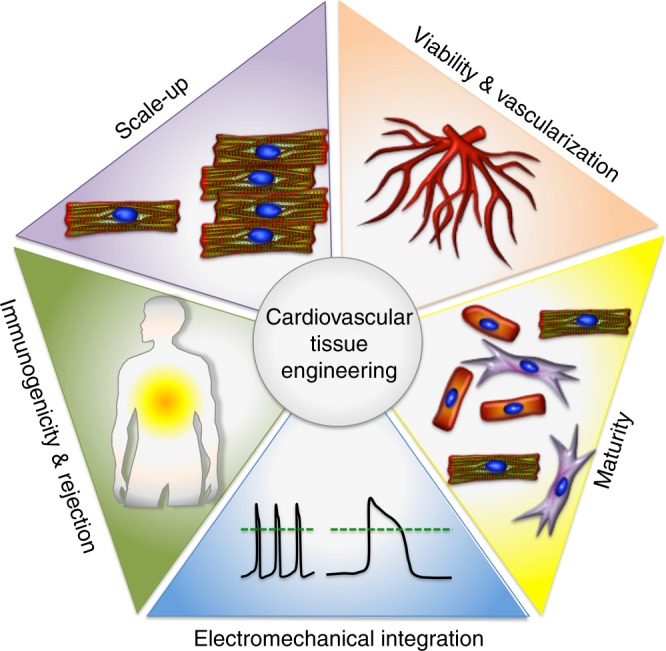
Bottlenecks in cardiovascular tissue engineering. These challenges include the survival, electrical integration, maturity, scalability, and immune response of three-dimensional engineered tissues
How can we generate clinically relevant numbers of hiPSC-CMs for engineering myocardial tissue?
The human myocardium consists of ~109 cells, among which CMs comprise about one-third of the total cells. The ability to generate such a large number of hiPSC-CMs for tissue engineering remains a challenge. Although highly efficient differentiation protocols can now produce on the order of ~107 cells in a single dish, scaling up to 109 cells would require nearly 100 dishes. Nevertheless, recent reports using 10-layer 1.2 L culture flasks demonstrate the feasibility of generating a clinically relevant number of 109 cells with >60% purity of hiPSC-CMs11. An alternative approach for scaling up the number of hiPSC-CMs in a more space-efficient and cost-effective manner is 3D suspension differentiation platforms. One example is microcarriers, which are materials that remain suspended in cell culture medium in a culture vessel and support cellular attachment. Owing to their large surface area per volume, microcarriers can facilitate the attachment and differentiation of hiPSCs12. Another example is the use of 3D aggregates of hiPSCs, which can be differentiated in suspension culture, achieving ~109 hiPSC-CMs in a 1 L spinner flask13. The next step toward generating clinically relevant numbers of CMs would be to engineer myocardial tissues (>10 cm × 10 cm) with a physiologically relevant cell density (~108/cm3)14 that consist of hiPSC-CMs in co-culture with support cells that comprise the remaining two-thirds of the myocardium (i.e., endothelial cells, pericytes, or fibroblasts) to promote intercellular interactions capable of sustaining the function and phenotype of hiPSC-CMs15. Future steps will also include the development of efficient suspension differentiation protocols for specific subtypes of hiPSC-CMs (i.e., atrial, ventricular, nodal, and Purkinje), because most differentiation protocols have been optimized to predominantly produce ventricular hiPSC-CMs16–18. Further development may make microcarriers more amenable to generating clinically relevant numbers of hiPSC-CMs in co-culture with vascular support cells.
How can we maintain the viability of 3D engineered myocardial tissues?
A major hurdle for the survival of 3D engineered tissues is poor perfusion of nutrients19. Whereas the typical inter-capillary distance in the myocardium is ~20 μm20, the thickness of 3D engineered myocardial tissue spans mm-to-cm thicknesses. Without a reliable method to transport nutrients and oxygen throughout the engineered tissue, the cells embedded in the tissue construct do not remain viable over time. Consequently, perfusion of the engineered myocardial tissue is critical for long-term tissue survival21. Although bioreactors can maintain the viability of engineered myocardial tissues in vitro by active perfusion22, in the absence of a pre-existing in vitro vascular network to integrate the engineered tissue with the host vasculature upon transplantation, cell viability is not sustainable in vivo. Vascularization of engineered myocardial tissue can be achieved by the induction of angiogenic molecules, cell–cell interactions, or mechanical factors23. Co-culture of hiPSC-CMs with endothelial cells or endothelial progenitor cells can form primitive vessel-like structures with the potential for in vivo anastomosis10. However, for greater control of the vessel architecture, techniques such as 3D bioprinting24, micropatterning25, and microfluidic systems26 have been shown to be beneficial for anastomosis and tissue integration. Among these approaches, 3D bioprinting has been particularly promising, but it is currently limited by inadequate bioinks and multi-material bioprinting modalities needed for creation of cell-laden, 3D vascular constructs that maintain tissue-mimetic stiffness, cell density, and function24,27. The next important step will be the creation of vascularized 3D engineered myocardial constructs that are perfusable both in vitro and in vivo, and supportive of cardiac muscle maturity and global contractile function28. An ongoing competition from the National Aeronautics and Space Administration (NASA) and the New Organ Alliance seeks to overcome the vascularization challenge by awarding a $500K prize to teams that successfully engineer functionally vascularized tissues29.
How can we achieve functional integration between engineered cardiovascular tissue and host myocardium?
In addition to the low engraftment rate being one of the first major roadblocks, another important hurdle with engineered myocardial tissue therapy is the electromechanical integration between the transplanted engineered myocardial tissue and the host myocardium. Because engineered myocardial tissue may possess greater heterogeneity in cellular organization than native tissues, reentry arrhythmia/block is a significant concern30. When the electrical wave fronts transit from the native myocardium to the 3D engineered myocardial tissue, passing through a fibrotic interface or vice-versa, a block of the wave front can be potentially life-threatening. Such a block may result from the heterogeneity of electrophysiological parameters, such as action potential duration or excitability. To minimize the risk of arrhythmia, recent advances in conductive scaffolds could help improve electrical communication between the engineered myocardial tissue implants and the host myocardium31–34. Furthermore, as epicardial patches are physically separated from the host myocardium, which hinders electrical coupling, approaches to recruit epicardial cells to the engineered myocardial tissue using bioactive peptides35 is a promising approach. These strategies will help provide engineered myocardial tissue with electromechanical characteristics equivalent to that of the naive cardiac myocardium.
How can we improve the maturity and function of engineered myocardial tissue composed of hiPSC-CMs?
Although highly efficient protocols for hiPSC-CM generation have greatly accelerated the pace of cardiovascular tissue engineering discoveries36–38, these protocols yield largely an immature cell population with variability in functions and structures. For instance, whereas primary adult CMs are morphologically rectangular in shape with distinctive electrical and mechanical properties, hiPSC-CMs generally are more amorphous in shape, with electrical and mechanical properties more resembling those of embryonic CMs. To engineer adult-like mature and functional engineered myocardial tissue, the heterogeneity and immaturity of hiPSC-CMs must be addressed. Mechanical factors have been shown to improve the maturity and function of hiPSC-CMs. For example, spatially patterned substrates with physiological stiffness (6–10 kPa) that impart a 7:1 aspect ratio in cell shape have been shown to increase hiPSC-CM contractility and enhance calcium handling and electrophysiology, thereby producing more mature and aligned sarcomere organization39,40. Mechanical strain stimulation of early-stage hiPSC-CMs with increasing intensity over time can also impact adult-like gene expression, sarcomeric length, and ultrastructure41. These studies underscore the importance of mechanical factors in enhancing hiPSC-CM maturity and function. Because these findings have been reported only in relative small engineered myocardial tissues, the next step will be to translate these approaches using larger 3D engineered myocardial tissues in large animal disease models.
How can we overcome rejection of engineered myocardial tissue after transplantation in vivo?
A major roadblock to the application of hiPSC-based therapies is immune rejection by the host42. Despite controversies surrounding the immunogenicity of hiPSC derivatives, almost all studies that involve transplantation of hiPSC-CMs induce immunosuppression in their animal models9,43. As a solution, there is a compelling need to advance the hiPSC technology using off-the-shelf sources of cardiovascular cells and development of tissue sources. Although human leukocyte antigen (HLA)-matched hiPSC tissue banks could be a valuable source of tissues for personalized therapeutics and an effective way to deliver cell therapy to a large number of patients44, a lack of basic understanding in the complexities of ethnic diversity is a challenge. Probabilistic models show that a bank of hiPSCs generated from 100 of the most prevalent HLA types would be a haplotype match for 78% of Europeans, 63% of Asians, and 45% of African Americans45, suggesting that the development of an allogeneic cell bank may be potentially feasible for relatively ethnically homogenous countries, but challenging for diverse ones. Moreover, such an endeavor would require a concerted effort by international groups to create a sufficient tissue repository45. Finally, even HLA-matched tissues are theoretically capable of triggering an immune rejection that would still require immunosuppression. Consequently, recent efforts aim to genetically engineer so-called master hiPSC lines that give rise to immune-tolerant hiPSC derivatives46. These universal off-the-shelf hiPSC derivatives can be generated by introducing multiple modalities that include immune evasion (by deleting HLA) and immune suppression (by overexpressing immunosuppressive proteins). Importantly, these HLA-null master hiPSCs could eventually be used to engineer a hypo-immunogenic cardiac patch as an off-the-shelf product that can be used universally for cardiac repair. Alternatively, engineering approaches may one day create allogeneic hiPSC derivatives that escape immune rejection. Allogenic hiPSCs could have far-reaching applications such as generating ready-to-use engineered myocardial tissue for therapeutic transplantation.
Future outlook
To date, the engineering of myocardial tissue for regenerative medicine has been greatly advanced by the use of hiPSCs, biocompatible materials, and the control of mechanical properties. However, besides these five bottlenecks, other important challenges that need to be addressed include cryopreservation of 3D engineered myocardium, attainment of functional cardiovascular tissue in vitro, the recapitulation of native cell–cell interactions between hiPSC-CMs and support cells within the engineered tissues, and development of cost-effective manufacturing processes for scaling up.
In the future, we anticipate increased use of microphysiological systems for high-throughput optimization of cellular composition, geometry, and paracrine factors to maximize the survival and function of engineered myocardial tissue. The aim of this microscale approach is to minimize the number of cells and reagents needed to determine optimal properties in engineered myocardial tissues. To accelerate clinical translation, engineered myocardial tissues derived from HLA-null lines will be further developed to be amenable to cryopreservation, enabling a true off-the-shelf product. With the goal of reducing the costs and time associated with regulatory approval, countries like Japan have recently adopted policies that conditionally approve hiPSC-based experimental therapies in humans based on limited clinical safety data, and allowing to up to 7 years for researchers to provide further evidence of safety and efficacy. Such policies enable clinical testing to be performed more expeditiously without the need for comprehensive data analysis before clinical testing47. As the generation of hiPSCs becomes routine using safe reprogramming approaches that prevent unintended genomic integration, hiPSC derivatives will gain further traction for clinical translation.
We envision a future in which patients who are diagnosed with heart failure will simply be prescribed a cryopreserved, immune-tolerant engineered myocardium composed of hiPSC-CMs and other support cells that comprise the myocardium. With the rapid progress in new technologies and continuing refinement of protocols being worked on by a large international community of active researchers in this field, this future is well within our reach and will benefit millions of heart disease patients.
Acknowledgements
This Comment is a product of discussions of the Progenitor Cell Biology Consortium Workshop, held at Stanford Cardiovascular Institute in April 2017. We acknowledge D. Buxton (National Institutes of Health) and M. Terrin (University of Maryland) for their leadership in organizing the workshop, as well as to other participants of the workshop. We also gratefully acknowledge support of the symposium by the National Institutes of Health Progenitor Cell Biology Consortium (HL099997).
Author contributions
N.F.H., V.S., V.B.M., N.S., G.P., K.H.N. and O.J.A. wrote the manuscript. B.L.P., S.M.W., Y.Y., J.Z. and J.C.W. provided scientific input and critically reviewed the manuscript.
Competing interests
J.C.W. has financial interest in Khoris Biosciences. All the remaining authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjamin EJ, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018 doi: 10.1161/cir.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Tonsho M., Michel S., Ahmed Z., Alessandrini A., Madsen J. C. Heart Transplantation: Challenges Facing the Field. Cold Spring Harbor Perspectives in Medicine. 2014;4(5):a015636–a015636. doi: 10.1101/cshperspect.a015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Lian X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye L, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miki K, et al. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl. Med. 2012;1:430–437. doi: 10.5966/sctm.2011-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamura M, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 10.Nakane T, et al. Impact of cell composition and geometry on human induced pluripotent stem cells-derived engineered cardiac tissue. Sci. Rep. 2017;7:45641. doi: 10.1038/srep45641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tohyama S, et al. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Rep. 2017;9:1406–1414. doi: 10.1016/j.stemcr.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, et al. Scalable and physiologically relevant microenvironments for human pluripotent stem cell expansion and differentiation. Biofabrication. 2018;10:025006. doi: 10.1088/1758-5090/aaa6b5. [DOI] [PubMed] [Google Scholar]
- 13.Chen VC, et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015;15:365–375. doi: 10.1016/j.scr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberger F, Mannhardt I, Eschenhagen T. Engineering cardiac muscle tissue: a maturating field of research. Circ. Res. 2017;120:1487–1500. doi: 10.1161/CIRCRESAHA.117.310738. [DOI] [PubMed] [Google Scholar]
- 15.Masumoto H, et al. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci. Rep. 2016;6:29933. doi: 10.1038/srep29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Protze SI, et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017;35:56–68. doi: 10.1038/nbt.3745. [DOI] [PubMed] [Google Scholar]
- 17.Argenziano M, et al. Electrophysiologic characterization of calcium handling in human induced pluripotent stem cell-derived atrial cardiomyocytes. Stem Cell Rep. 2018;10:1867–1878. doi: 10.1016/j.stemcr.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maass K, et al. Isolation and characterization of embryonic stem cell-derived cardiac Purkinje cells. Stem Cells. 2015;33:1102–1112. doi: 10.1002/stem.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang, W. G. & Niklason, L. E. A short discourse on vascular tissue engineering. Npj Regen. Med. 2, 10.1038/s41536-017-0011-6 (2017). [DOI] [PMC free article] [PubMed]
- 20.Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.CIR.86.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Iyer RK, Chiu LL, Reis LA, Radisic M. Engineered cardiac tissues. Curr. Opin. Biotechnol. 2011;22:706–714. doi: 10.1016/j.copbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radisic M, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H507–516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 23.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 24.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl Acad. Sci. USA. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng. Part A. 2010;16:2255–2263. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettinger CJ, et al. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv. Mater. 2005;18:165–169. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogle BM, et al. Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med. 2016;8:342ps313. doi: 10.1126/scitranslmed.aad2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Aeronautics and Space Administration. STMD: Centennial Challenges, https://www.nasa.gov/directorates/spacetech/centennial_challenges/vascular_tissue/about.html (2018).
- 30.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem. Biophys. Res. Commun. 2007;361:847–853. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dvir T, et al. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011;6:720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mawad D, et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016;2:e1601007. doi: 10.1126/sciadv.1601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conant G, Ahadian S, Zhao Y, Radisic M. Kinase inhibitor screening using artificial neural networks and engineered cardiac biowires. Sci. Rep. 2017;7:11807. doi: 10.1038/s41598-017-12048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharaziha M, et al. Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials. 2014;35:7346–7354. doi: 10.1016/j.biomaterials.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei K, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burridge PW, et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian X, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA. 2012;109:E1848–1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonoudi H, et al. A universal and robust integrated platform for the scalable production of human cardiomyocytes from pluripotent stem cells. Stem Cells Transl. Med. 2015;4:1482–1494. doi: 10.5966/sctm.2014-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro AJ, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro AJS, et al. Multi-imaging method to assay the contractile mechanical output of micropatterned human iPSC-derived cardiac myocytes. Circ. Res. 2017;120:1572–1583. doi: 10.1161/CIRCRESAHA.116.310363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronaldson-Bouchard K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Almeida PE, Ransohoff JD, Nahid A, Wu JC. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013;112:549–561. doi: 10.1161/CIRCRESAHA.111.249243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong JJH, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- 45.Gourraud PA, Gilson L, Girard M, Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells. 2012;30:180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- 46.Riolobos L, et al. HLA engineering of human pluripotent stem cells. Mol. Ther. 2013;21:1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azuma K, Yamanaka S. Recent policies that support clinical application of induced pluripotent stem cell-based regenerative therapies. Regen. Ther. 2016;4:36–47. doi: 10.1016/j.reth.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


