Summary
FMRP is an RNA-binding protein that is known to localize in the cytoplasm and in the nucleus. Here, we have identified an interaction of FMRP with a specific set of C/D box snoRNAs in the nucleus. C/D box snoRNAs guide 2’O methylations of ribosomal RNA (rRNA) on defined sites, and this modification regulates rRNA folding and assembly of ribosomes. 2’O methylation of rRNA is partial on several sites in human embryonic stem cells, which results in ribosomes with differential methylation patterns. FMRP-snoRNA interaction affects rRNA methylation on several of these sites, and in the absence of FMRP, differential methylation pattern of rRNA is significantly altered. We found that FMRP recognizes ribosomes carrying specific methylation patterns on rRNA and the recognition of methylation pattern by FMRP may potentially determine the translation status of its target mRNAs. Thus, FMRP integrates its function in the nucleus and in the cytoplasm.
Subject Areas: Molecular Interaction, Stem Cells Research, Omics
Graphical Abstract
Highlights
-
•
FMRP binds to C/D Box snoRNAs in the nucleus
-
•
Differential 2’O-methylation on rRNA contributes to ribosome heterogeneity in a cell
-
•
2’O-Methylation pattern on ribosomal RNA is altered in the absence of FMRP
-
•
FMRP recognizes 2’O-methylation on rRNA, which may determine interaction with ribosomes
Molecular Interaction; Stem Cells Research; Omics
Introduction
Fragile X mental retardation protein (FMRP) is an RNA-binding protein, loss of which leads to fragile X syndrome (Santoro et al., 2012). FMRP is recognized as a translation modulator, and its role in metabotropic glutamate receptor (mGluR) signaling is extensively studied. Dysregulated synaptic translation in the absence of FMRP is thought to be responsible for majority of the phenotypes in fragile X syndrome (Bassell and Warren, 2008, Muddashetty et al., 2007, Muddashetty et al., 2011, Richter et al., 2015). FMRP is also shown to have an important role in neuronal development particularly during neuronal differentiation. The absence of FMRP is reported to affect the maintenance of pluripotency, cell fate choice and rate of progression to the neuronal lineages both in animal and human stem cell models (Li and Zhao, 2014, Luo et al., 2010, Telias et al., 2013). Thus, FMRP-mediated regulation of translation is likely to be more global than confined to the synapse. However, the mechanism of FMRP-mediated translation regulation beyond the synapse is not understood.
FMRP is present in the cytosol as well as in the nucleus and localizes to the distal compartments in neurons. A significant amount of FMRP has been reported to be present in the nuclear and nucleolar compartments in neurons and other cell types (Eberhart et al., 1996, Feng et al., 1997b, Fridell et al., 1996, Kim et al., 2009, Taha et al., 2014). However, the function of FMRP in nucleus still remains unexplained. Since it has both nuclear localization and nuclear export signals, FMRP is proposed to be involved in the shuttling of its target mRNAs between the nucleus and cytosol (Kim et al., 2009). A mutation in the nuclear localization signal was also reported to cause fragile X-like syndrome (Collins et al., 2010). In addition, there is an intriguing possibility that FMRP might interact with a distinct class of RNA in the nuclear/nucleolar compartments and play a crucial role in global translation regulation.
Translational regulation by FMRP has been reported through its direct interaction with the 60S subunit of the ribosome (Chen et al., 2014, Khandjian et al., 1996). A mutation affecting FMRP's interaction with ribosomes leads to a severe form of mental retardation (Feng et al., 1997a, Myrick et al., 2014), whereas its interaction with mRNAs is not affected by this mutation. According to these evidences, we postulate that FMRP has an important function in the nucleus, which could modulate its function in the cytoplasm. Here, we report an interesting interaction of FMRP with a subset of small nucleolar RNAs (snoRNAs) in the nuclear compartment of human embryonic stem cells (hESCs) and neuronal precursor cells (hNPCs) derived from these hESCs. FMRP's interaction with snoRNAs contributes to differential methylation of rRNA, generating ribosome heterogeneity. The absence of FMRP results in an alteration of this methylation pattern. On the other hand, FMRP also recognizes specific methylation patterns on rRNA and hence marks a subset of ribosomes. Our results identify a nuclear function of FMRP and imply that it can integrate translation regulation between the nucleus and cytoplasm.
Results and Discussion
FMRP Interacts with a Selected Subset of snoRNAs
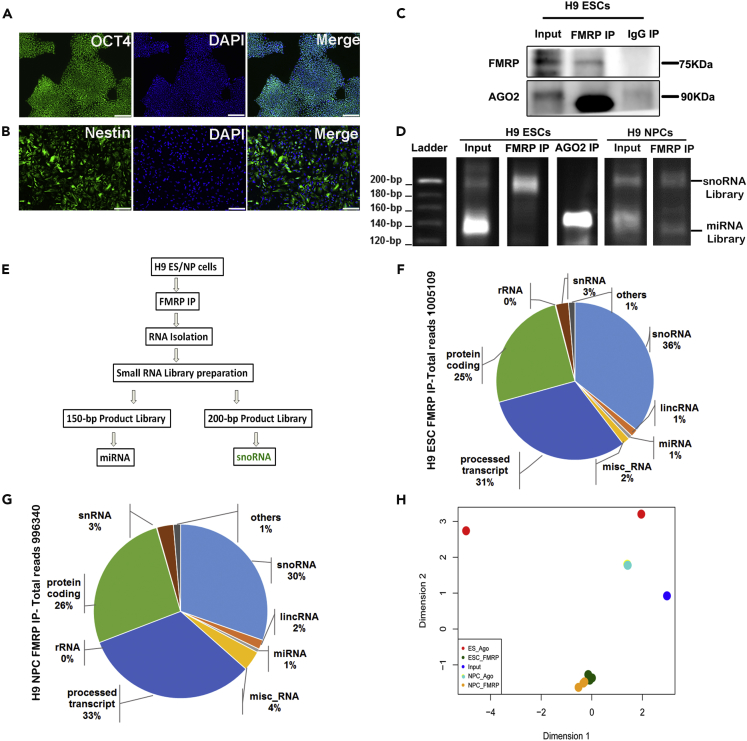
To investigate the small RNAs that interact with FMRP during neuronal development, we used human H9 hESCs and H9 neuronal precursor cells (hNPCs) as our model system. H9 hESCs were characterized for pluripotency by immunocytochemistry with the marker OCT4 (Figure 1A), and similarly, hNPCs were characterized for the expression of Nestin (Figure 1B). In H9 hESCs, we show that FMRP interacts with AGO2, a primary component of the miRNA induced silencing complex (miRISC) (Figure 1C) indicating its interaction with microRNA machinery. In our initial observations, other than microRNA, we found another class of RNA (80–100nt) predominantly present in the FMRP immunoprecipitates. To identify this unknown class of small RNAs, we performed an FMRP immunoprecipitation (IP) with H9 hESCs and hNPCs followed by small RNA library preparation from both the microRNA band (30–50 nt) and the higher-molecular-weight band (80–100 nt). An AGO2 IP was used as a positive control to profile the microRNAs (Figure 1D). The libraries were separated on a 6% polyacrylamide gel and showed a prominent band in AGO2 IP at a size of 140 bp representing the population of microRNAs (Figure 1D).
Figure 1.
FMRP Interacts with Selected Set of C/D Box snoRNAs in Human ESCs and NPCs
(A) Characterization of H9 hESCs with pluripotency marker OCT4 and nuclear marker DAPI (scale bar, 50 μm).
(B) Characterization of H9 hNPCs with differentiation marker Nestin and nuclear marker DAPI (scale bar, 50 μm).
(C) Immunoblot for FMRP and AGO2 from H9 hESC lysate after FMRP and IgG immunoprecipitation.
(D) Polyacrylamide gels showing mobility of cDNA libraries prepared from RNA extracted after immunoprecipitation with FMRP and AGO2 from H9 hESC and hNPC lysate.
(E) Schematic showing the experimental workflow to identify FMRP-associated small RNAs.
(F) Pie chart showing the distribution of different classes of small RNAs from the sequence obtained from the 200-bp band of the library from H9 hESCs, n = 3.
(G) Pie chart showing the distribution of different classes of small RNAs from the sequence obtained from the 200-bp band of the library from H9 hNPCs, n = 3.
(H) Principal component analysis (PCA) chart indicating clustering of snoRNA libraries in H9 hESCs and H9 hNPCs, hESC FMRP IP n = 3, hNPC FMRP IP n = 3, hESC Input n = 1, and hESC AGO2 IP n = 2.
Surprisingly, the prominent band in FMRP IP was identified at a much higher size (around 200 bp) (Figures 1D and S1A). We isolated the bands corresponding to both 140 and 200 bp from the FMRP IP samples and sequenced them separately (Figure 1E). We have included a mouse IgG control in this experiment, and since there was insignificant RNA precipitated with this (Figures 1C and S1B) compared to FMRP IP, it was not further considered for preparation of cDNA library. The band corresponding to 140 bp in FMRP IP majorly contained microRNAs (data not shown) similar to that in the AGO2 IP. Interestingly, the major component of the 200-bp band (corresponding to 80–100 nt RNAs) from the library was C/D box snoRNAs (Figures 1F and 1G). In contrast, AGO2 IP from hESCs and hNPCs showed negligible amount of snoRNA (Figures S1D and S1E) compared with the input (Figure S1C). The library profile of the 200-bp band (corresponding to snoRNA) derived from FMRP IP was similar in both hESCs and hNPCs as depicted by the principal-component analysis plot (Figure 1H). Top snoRNAs associated with FMRP in hESCs are listed in Table S1, and all these snoRNAs are predicted to target and methylate specific sites on 18S or 28S ribosomal RNA.
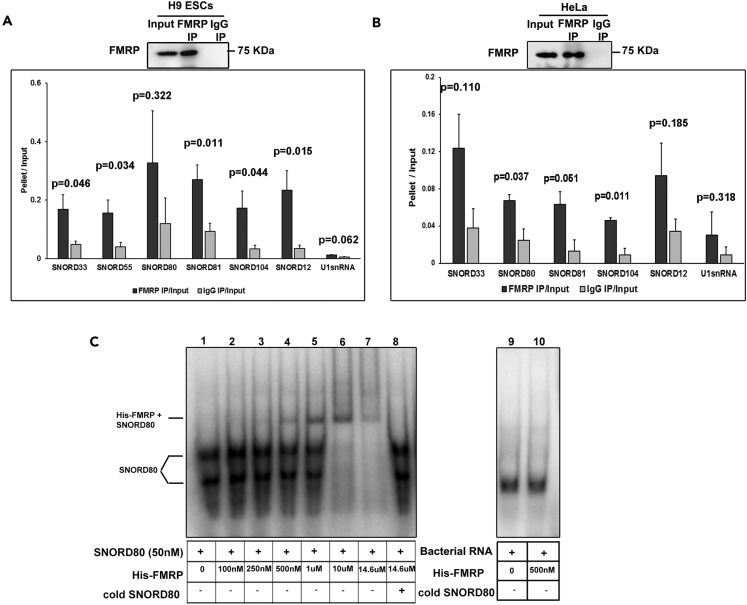
We validated the interaction of FMRP with snoRNA by performing qPCR for the snoRNAs that were highly enriched in the FMRP IP from H9 hESC lysate (Figure 2A). Our qPCR primers were designed such that an entire snoRNA was amplified from the FMRP IP, suggesting that FMRP interacts with the mature snoRNA (as determined by the sequencing data) rather than the products derived from their further processing (Brameier et al., 2011, Taft et al., 2009). All the selected snoRNA candidates showed a significant enrichment in FMRP IP (pellet/input ratio), compared with the corresponding IgG control (Figure 2A). In contrast, none of the snoRNAs were enriched in the AGO2 pellet (Figure S2B). The small fraction of snoRNA (5%) found in the AGO2 pellet (Figure S1D) could be small RNA derived from further snoRNA processing. The mature full-length snoRNA we tested for were absent in this sample. To confirm that FMRP's interaction with these snoRNAs is specific, we performed IP with an antibody against a different epitope of FMRP (Figure S2A). We further validated FMRP's interaction with target snoRNAs in a different human ESC line (Shef4) (Figure S2C) and in HeLa cells (Figure 2B) as well. Our results indicate that the interaction of FMRP with snoRNAs is a common feature among all the cell types we tested.
Figure 2.
Validation of FMRP Interaction with C/D Box snoRNA
(A) Validation of FMRP-interacting snoRNA in human H9 hESCs by qPCR with representative immunoblot for FMRP IP (n = 6, unpaired Student's t test, mean ± SEM).
(B) Validation of FMRP-interacting snoRNA in HeLa cells by qPCR with representative immunoblot for FMRP IP (n = 4, unpaired Student's t test, mean ± SEM). Also refer Tables S1 and S2.
(C) Electrophoretic mobility shift assay showing shift in mobility of radiolabeled SNORD80 by increasing concentration of His-FMRP. Lane 8 shows a complete abolishment of shift with molar excess of unlabeled (cold) SNORD80 RNA. Lanes 9 and 10 indicated no change in the mobility of His-FMRP with radiolabeled non-specific bacterial RNA. Samples in lanes 9 and 10 were run on a separate gel.
FMRP Directly Interacts with C/D Box snoRNA
Our next objective was to test whether FMRP directly interacts with C/D box snoRNAs. To study this, we performed an electrophoretic mobility shift assay with purified full-length human FMRP (Figure S2D). For this we chose SNORD80, one of the top snoRNA candidates which we found to be associated with FMRP in our high-throughput analysis (Tables S1 and S2). SNORD80 was radiolabeled with γ-P-ATP and was incubated with increasing concentrations of FMRP (from 100 nM to 14.6 μM). We observed a shift in SNORD80 mobility even with an FMRP concentration of 500 nM (Figure 2C), which was further enhanced in a concentration-dependent manner, indicating a direct interaction of SNORD80 with FMRP. The shift in SNORD80 mobility was completely reversed by incubation with molar excess of unlabeled SNORD80 (Figure 2C-lane 8). This competition was seen only with unlabeled SNORD80 and not with nonspecific bacterial RNA (lane 9 and 10) along with yeast tRNA, which was used in all other lanes thus confirming the specific interaction of SNORD80 with FMRP. Although we demonstrate the direct interaction of FMRP with one of the candidate snoRNAs, we cannot rule out the possibility that FMRP's interaction with C/D box snoRNAs may also be mediated through other snoRNP components or other nuclear FMRP interacting proteins such as NUFIP1 (Bardoni et al., 2003).
Among the two major classes of snoRNAs involved in rRNA modifications (C/D box snoRNAs that mediate 2-O'methylation and H/ACA box snoRNAs that mediate pseudo-uridylation) (Falaleeva et al., 2017, Henras et al., 2015), we detected only C/D box snoRNAs in the FMRP pellet. We did see a weak higher-molecular-weight band in the cDNA library prepared from the FMRP pellet (Figure S1A); however, we did not find any H/ACA box snoRNA or precursor snoRNA when we sequenced this band (data not shown). Thus, FMRP appears to interact only with C/D box snoRNA, although we cannot completely rule out an interaction with H/ACA box snoRNA in the FMRP pellet. Since H/ACA box snoRNAs possess complex secondary structures, there is a possible bias against them in the cDNA library preparation.
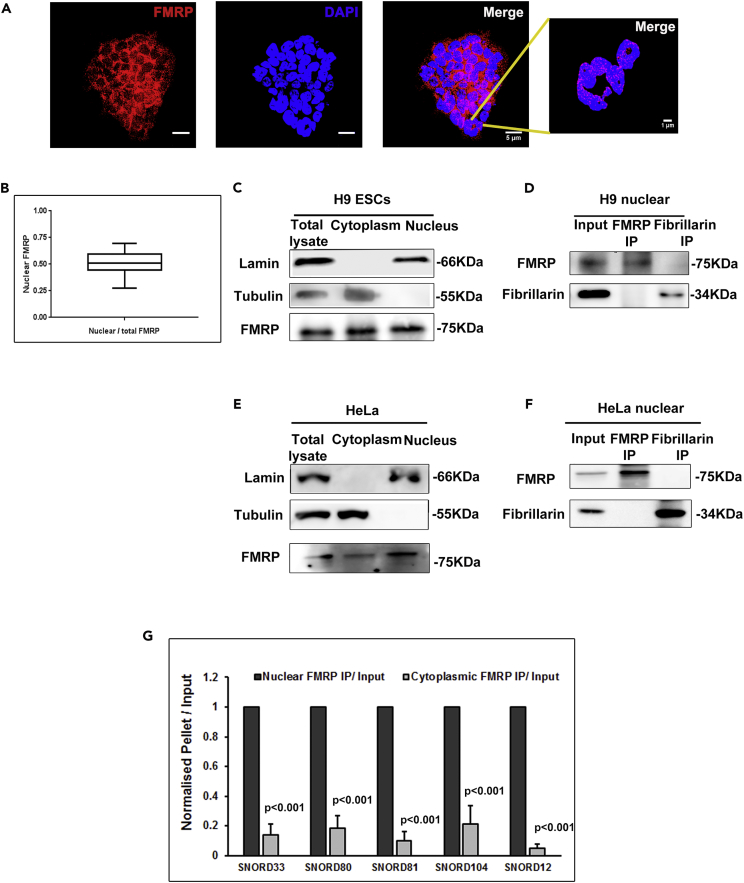
The specific interaction with C/D box snoRNAs indicates that FMRP seems to have an important role in rRNA methylation. These C/D box snoRNAs primarily guide the 2’O methylation on selected ribose sugars on rRNA, which is important for the folding of the rRNA and the assembly of ribosomal proteins (Falaleeva et al., 2017, Sharma and Lafontaine, 2015). Some snoRNAs are also reported to generate smaller RNAs, including microRNAs (Brameier et al., 2011), found in the cytoplasm. Here, we show that FMRP's interaction with snoRNAs is only confined to the nuclear fraction (Figure 3G). Also, it interacts with full-length snoRNAs and not the processed small RNA products of snoRNAs.
Figure 3.
Interaction of Nuclear FMRP with C/D Box snoRNA
(A) Immunostaining of H9 hESCs (blue-DAPI, red-FMRP, and scale bar, 5 μm) followed by segmented images showing nuclear distribution of FMRP (scale bar, 1 μm).
(B) Quantification of nuclear FMRP in H9 hESCs, n = 29 cells.
(C) Immunoblots showing the distribution of FMRP in H9 hESC nuclear and cytoplasmic fractions, Lamin as nuclear marker and Tubulin as cytoplasmic marker.
(D) Immunoblots for FMRP and Fibrillarin followed by FMRP or Fibrillarin immunoprecipitation from nuclear fractions of H9 hESCs.
(E) Immunoblots showing the distribution of FMRP in HeLa nuclear and cytoplasmic fractions with Lamin as nuclear marker and Tubulin as cytoplasmic marker.
(F) Immunoblots for FMRP and Fibrillarin followed by FMRP or Fibrillarin immunoprecipitation from nuclear fractions of HeLa cells.
(G) qPCR for selected snoRNAs after immunoprecipitation with FMRP from nuclear and cytoplasmic lysates of H9 hESCs. Values are the ratio of pellet/input of the cytoplasmic fraction normalized to the pellet/input ratio of the nuclear fraction (n = 3, unpaired Student's t test, mean ± SEM).
A group of snoRNAs without specific targets on rRNA (called orphan snoRNAs) are also known to have a role in mRNA splicing (Kishore and Stamm, 2006). All top snoRNA candidates precipitated with FMRP have predicted target sites on rRNA, and we did not find any orphan snoRNA enriched in the pellet (Table S1). These results clearly suggest that the interaction of FMRP with snoRNA is likely to have a role in rRNA methylation.
Interaction with snoRNA Is a Function of Nuclear FMRP
Although FMRP is often studied as a cytoplasmic protein, it has a nuclear localization signal, and its localization to the nucleus is reported by several studies (Kim et al., 2009, Taha et al., 2014). Here we show a localization of FMRP in the nucleus of H9 hESCs (Figure 3A) by FMRP immunostaining overlaid with DAPI, a nuclear marker. Similarly, we observed FMRP's localization in the nucleus of Neuro2A (Figure S3A), HeLa (Figure S3B), rat cortical neurons (Figure S3C), and rat astrocytes (Figure S3D) confirming that FMRP is present both in the nucleus and cytoplasm of the all the cell types that we studied. We quantified the percentage of FMRP in the nucleus based on DAPI staining (Figure 3B) and found that nearly 50% of FMRP localized in the nucleus of hESCs. Since the nucleus of ESCs is relatively large, we expect the ratio of nuclear to cytoplasmic FMRP to be lower in other cell types (Figures S3A–S3D). We also confirmed that FMRP's localization in the nucleus is specific through immunostaining with an antibody raised against a different epitope of FMRP (Figure S3E). To establish the compartment-specific localization of FMRP, we used nuclear and cytoplasmic fractions from H9 hESCs (Bensaddek et al., 2016) and probed for the presence of FMRP. The purity of the fractionation was validated by LaminB1 and α-Tubulin as nuclear and cytoplasmic markers respectively (Figure 3C). FMRP was found to be present in both nuclear and cytoplasmic fractions (Figure 3C).
The sequence between the C and D′ box (or C′ and D) is complementary to a specific region on rRNA, and this complementarity determines the site to be methylated (Cavaille et al., 1996). RNA-binding proteins forming the snoRNP complex are recruited to these sites by C/D box snoRNAs, and 2’O-methylation is carried out by a nucleolar-specific methyltransferase, Fibrillarin (Shubina et al., 2016). Since FMRP has a putative nucleolar localization signal and has been shown to localize to the nucleolus (Taha et al., 2014), we tested whether it interacts with Fibrillarin. For this, we probed for the presence of Fibrillarin in FMRP immunoprecipitates from the nuclear fraction of H9 hESCs. We did not see a co-precipitation of Fibrillarin with FMRP (Figure 3D). Similarly, we did not see FMRP in the Fibrillarin immunoprecipitate (Figure 3D). We tested for this interaction in nuclear extracts of HeLa (Figures 3E and 3F) and Neuro2A (Figure S3F) and did not find FMRP co-precipitating with Fibrillarin in any of these experiments. These results strongly suggest that FMRP and Fibrillarin are likely to be present in separate RNPs, although both interact with snoRNA. Finally, to confirm that the interaction of FMRP with snoRNA is in the nucleus, we performed an FMRP IP from H9 hESC nuclear and cytoplasmic fractions followed by qPCR for selected target snoRNAs. We found that these snoRNAs are significantly enriched in immunoprecipitates from nuclear fractions compared with cytoplasmic fractions (Figures 3G and S3G). Thus, FMRP-snoRNA interaction is primarily in the nuclear compartment and seems to be distinct from Fibrillarin-containing snoRNPs. FMRP may sequester specific snoRNAs and control their availability to rRNA methylation machinery and regulate the extent of methylation. Similarly, other RNA-binding proteins might be involved in regulating rRNA methylation through their interaction with snoRNAs cumulatively leading to the differential rRNA methylation.
Differential 2'O-Methylation of rRNA in Human ESCs
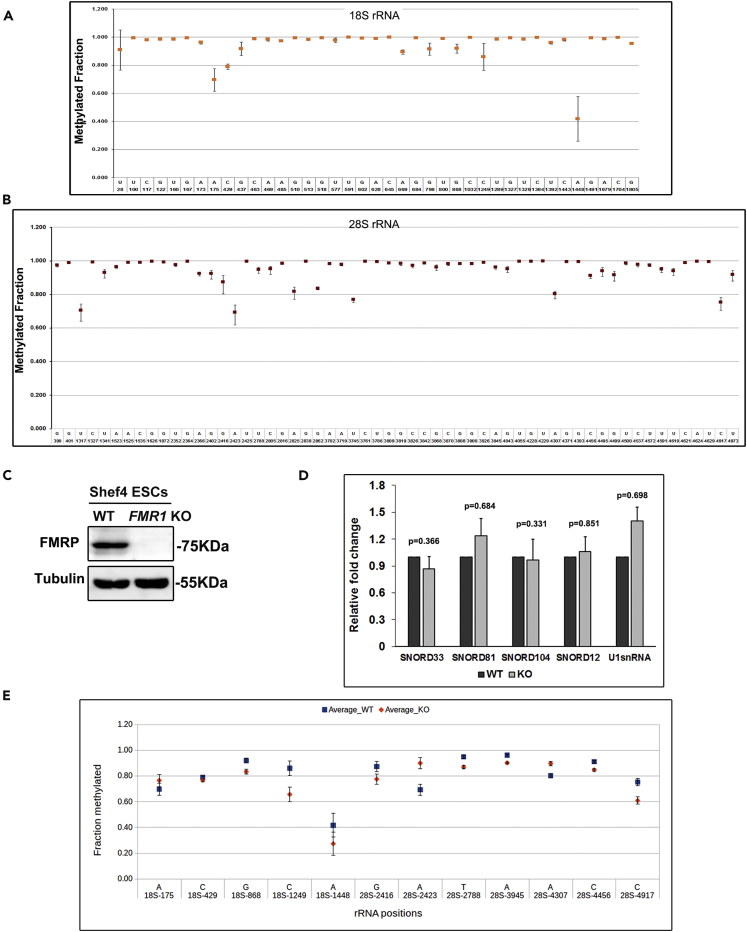
So far 106 methylation sites have been identified on human rRNA (Falaleeva et al., 2017, Incarnato et al., 2017, Machnicka et al., 2013). Recently it was reported that the differential methylation of selected sites on rRNA can give rise to ribosome heterogeneity as demonstrated in HeLa cells (Krogh et al., 2016). To establish the differential rRNA methylation in human ESCs, we estimated the extent of rRNA methylation of known sites in Shef4 hESCs using the recently developed high-throughput RiboMeth sequencing method (Marchand et al., 2017). In this method, ribosomal RNA extracted from hESCs was subjected to partial alkaline hydrolysis, followed by library preparation and sequencing (See Transparent Methods section). The sequenced data were analyzed to estimate the extent of methylation using a previously described (Krogh et al., 2016) bioinformatics pipeline (Figure S4A). In our assay we detected 97 sites in Shef4 hESC rRNA. A majority of the sites are completely methylated (methylation index close to 1); however, 9 sites on 18S rRNA (Figure 4A) and 15 sites on 28S rRNA were only partially methylated (methylation index significantly lower than 1) (Figure 4B) as reflected in Table S3. The extent of methylation on these sites varied from a methylation index of 0.6 to 0.9 (Figures 4A and 4B), which denotes that these sites are methylated in 60%–90% of the ribosomes, whereas they are unmethylated in the remaining 10%–40% of the ribosomes. The heterogeneity of rRNA methylation we observed in hESCs is distinct from that of HeLa cells as reported previously (Krogh et al., 2016).
Figure 4.
Ribosomal RNA 2'O-Methylation Pattern in Shef4 and Shef4 FMR1 KO hESCs
(A) Methylation index of the sites on 18S rRNA in Shef4 hESCs. The x axis represents the respective methylation position on 18S rRNA, and y axis represents the fraction methylated, n = 3.
(B) Methylation index of the sites on 28S rRNA in Shef4 hESCs. The x axis represents the respective methylation position on 28S rRNA, and y axis represents the fraction methylated, n = 3.
(C) Immunoblots showing absence of FMRP in Shef4 FMR1 KO hESCs with Tubulin as the control.
(D) Change in levels of top snoRNA candidates in Shef4 WT and Shef4 FMR1 KO hESCs by qPCR (n = 5, unpaired Student's t test, mean ± SEM).
(E) Sites in 18S and 28S rRNA that show 5% or more difference in the methylation index between Shef4 hESCs and Shef4 FMR1 KO hESCs (n = 3, mean ± SEM).
Absence of FMRP Alters the rRNA 2'O-Methylation Pattern in Human ESCs
We observe that FMRP interacts with a specific subset of C/D box snoRNAs (Table S1), and we wanted to test if the absence of FMRP will have any effect on the level of its target snoRNAs at steady state. For this we generated Shef4 FMR1 knockout (KO) hESCs using the CRISPR-Cas9 system (Figures 4C and S4B–S4E) and performed a qPCR for these candidate snoRNAs. Surprisingly, at steady state, we did not see any significant change in the level of FMRP target snoRNAs in FMR1 KO cells (Figure 4D). Since most of the snoRNAs we found associated with FMRP were C/D box snoRNA, we studied the impact of this interaction (FMRP-snoRNA) by looking at 2’O methylation in Shef4 wild-type (WT) and Shef4 FMR1 KO hESCs (Figures 4E, S4F, and S4G). We performed a high-throughput RiboMethSeq from WT and FMR1 KO hESCs as described previously. On comparing the methylation profile between WT and KO cells, we found that the sites that were fully methylated in WT (methylation index 1) were unaffected in the absence of FMRP (Figures S4F and S4G). Interestingly, many sites in 18S and 28S rRNA that were partially methylated in WT hESCs were significantly altered in the absence of FMRP (Figure 4E and Table S3).
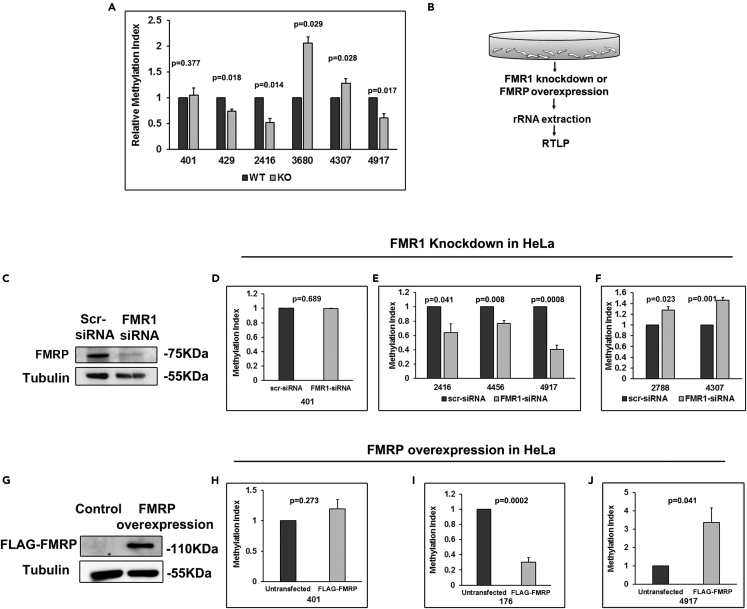
We independently validated the change in methylation of rRNA in the absence of FMRP for selected sites (Figure 5A) by a qPCR-based method called RTL-P (Dong et al., 2012) (Figure S5A). RTL-P has been previously used to identify the differential methylation of rRNA (Dong et al., 2012), and we have further tested the validity of this assay (Figures S5A–S5G). This method involves the amplification of PCR products under high and low dNTP concentrations for each primer set. There are two forward primers, one positioned upstream (P1) and other downstream (P2) of the methylation site. Amplification was done using a reverse primer (P3) downstream of the methylation site (Figure S5A). For RTL-P we have considered the smaller PCR product (from P2 and P3) as our control in both high and low dNTP conditions (since it is unaffected by methylation). Then we have quantified the larger PCR product (from P1 and P3) at similar conditions and normalized it to its respective smaller PCR product (Figures S5B and S5C). Example of RTL-P analysis for a given site has been provided in Transparent Methods. We have validated two selected sites 401 (complete methylation) and 4917 (partial methylation) through RTL-P. The PCR products were run on a gel, and the bands corresponding to the upstream product (*) were selectively cut out and cloned into a bacterial vector (Figures S5B and S5C) for Sanger sequencing (Figures S5F and S5G). Furthermore, we validated site 4917 in WT and in FMR1 knockdown HeLa cells. We observe that the intensity of the amplicon generated for the knockdown samples under low dNTPs is lesser compared to that of the WT (Figures S5D and S5E). Here, we clearly show that under low and high dNTP conditions, we observe the PCR reaction stopping downstream and upstream of the methylation site as expected (Figures S5F and S5G).
Figure 5.
Validation of Change in rRNA 2'O-Methylation Pattern by FMRP
(A) RTL-P for selected sites on 18S and 28S rRNA to show differences in methylation in Shef4 WT and Shef4 FMR1 KO hESC lysate (n = 3, unpaired Student's t test, mean ± SEM).
(B) Schematic for reverse transcription followed by PCR (RTL-P) on FMR1 knockdown or FMRP overexpression in HeLa cells.
(C) Representative immunoblots for FMRP showing its knockdown in HeLa cells with Tubulin as a control.
(D–F) RTL-P for selected sites showing the change in 2’O-methylation on FMR1 knockdown in HeLa cells (n = 3, unpaired Student's t test, mean ± SEM).
(G) Representative immunoblots for FMRP showing overexpression of FLAG-FMRP in HeLa cells with Tubulin as a control.
(H–J) RTL-P for selected sites showing the change in 2’O-methylation on overexpression of FLAG-FMRP in HeLa cells (n = 3, unpaired Student's t test, mean ± SEM).
We chose sites for RTL-P validation based on our high-throughput sequencing as well as sites that were targeted by our top snoRNA candidates. Our RTL-P results were similar to that of the RiboMethSeq. Sites 429, 2416 and 4917 are significantly hypo-methylated in FMR1 KO compared to WT. Site 4307 was significantly hyper-methylated in FMR1 KO, whereas the methylation on site 401 was unaltered (Figure 5A). Apart from these, we also show that site 3680 (targeted by FMRP associated SNORD88A) is significantly hyper-methylated in the FMR1 KO, although there was no difference in RiboMethSeq (Figure 5A). These results confirm that absence of FMRP has a significant impact on the methylation status of specific rRNA sites.
Heterogeneity among ribosomes is an emerging concept and provides a new dimension for translation regulation (Lafontaine, 2015, Shi et al., 2017). Modifications on rRNA, including snoRNA-guided methylation, are proposed to play an important role in contributing to heterogeneity (Falaleeva et al., 2017). However, the mechanism by which differential modification of rRNA occurs is not explored. Our work for the first time defines an interaction of an RNA-binding protein, FMRP, with a specific subset of C/D box snoRNAs. This interaction may have an influence in regulating rRNA methylation in humans. In the absence of FMRP, methylation was altered for 12 (5 on 18S rRNA and 7 on 28S rRNA) of 28 differentially methylated sites as identified by RiboMethSeq. Interestingly, sites that show an altered methylation in the absence of FMRP are the ones that also show partial methylation in the control (WT) hESCs (methylation index less than 1). This implies that FMRP has an important role in differential methylation of rRNA and has a significant contribution in generating ribosome heterogeneity.
Acute Knockdown or Overexpression of FMRP Alters rRNA 2'O-Methylation Pattern
To verify that the change in 2’O-methylation observed is FMRP specific, we acutely knocked down FMRP (48 hr with siRNA, Figures 5B, 5C, and S5H) and overexpressed FLAG-FMRP (24 hr Figures 5H and S5I) in HeLa cells. Following FMRP knockdown or overexpression, we estimated the methylation of rRNA on selected sites through RTL-P (Figures 5D–5F and 5H–5J). On acute knockdown of FMRP, we observed hypo-methylation on the sites 2416, 4456, and 4917 (Figure 5E). The hypo-methylation of these sites on FMRP knockdown is the same as we have identified in FMR1 KO hESCs both by high-throughput RiboMethSeq (Figure 4E) and RTL-P-based analysis from the same cells (Figure 5A). Sites 2788 and 4307, which showed hyper-methylation in FMR1 KO hESCs, also showed hyper-methylation on acute knockdown of FMRP in HeLa cells (Figure 5F). These results confirm that the change in methylation in FMR1 KO hESCs could be reproduced by acute knockdown of FMRP. In contrast, the effect of acute overexpression of FMRP was more complex. One of the sites (176), which showed hyper-methylation in FMR1 KO hESCs (Figure 4E), showed a hypo-methylation on FMRP overexpression (Figure 5I) in HeLa cells. Similarly, a site (4917) that showed hypo-methylation in FMR1 KO hESCs showed hyper-methylation on FMRP overexpression (Figure 5J), clearly indicating that the presence or absence of FMRP is the direct cause of change in methylation. Site 401, which was unaffected in FMR1 KO hESCs, remained unchanged (with respect to its methylation) on FMRP knockdown or overexpression in HeLa cells (Figures 5D and 5H). Although acute knockdown of FMRP reproduces the results of FMR1 KO, overexpression of FMRP has inconsistent effects. Overexpression may have limited effects owing to saturating concentration of endogenous FMRP.
Overall, our results indicate that absence of FMRP can lead to either hypo- or hyper-methylation on distinct sites. FMRP may sequester specific snoRNAs and control their availability to rRNA methylation machinery and regulate the extent of 2'O-methylation. However, the role of FMRP in controlling status of methylation is unclear; identifying the other components (both protein and RNA) involved is needed to address this issue.
FMRP Recognizes Differential 2'O-Methylation Pattern
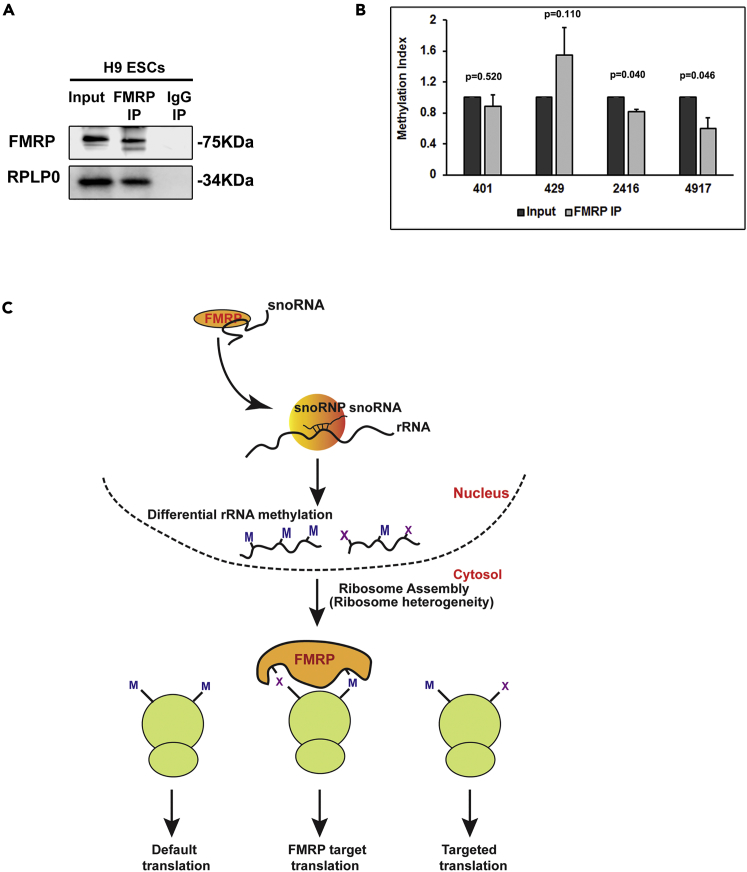
We have demonstrated that FMRP interacts with a subset of snoRNAs in the nuclear compartment. In the absence of FMRP, rRNA 2'O-methylation is significantly altered and this affects ribosome heterogeneity. We also show that FMRP interacts with ribosomal proteins (Figure 6A) as also previously reported (Chen et al., 2014) (Simsek et al., 2017). Based on these results we wanted to investigate if FMRP can recognize the 2'O-methylation pattern on ribosomes. For this we performed FMRP immunoprecipitation and isolated rRNA, which was pulled down with FMRP. We quantified the extent of 2'O-methylation of selected sites on rRNA by RTL-P and compared it with the extent of methylation to ribosomes in the input (Figure 6B). We chose the sites whose methylation is altered in the absence of FMRP. Among them, three sites showed a significant difference in the FMRP pellet compared to the input. Site 429 (18S rRNA) showed a trend of hyper-methylation in the FMRP pellet, whereas sites 2416 and 4917 (28S rRNA) were hypo-methylated compared to the input. Site 401 remained unchanged (Figure 6B). There was no difference in the methylation pattern for these sites in the control IP with IgG (Figure S6B). These results indicate that FMRP binds to the ribosome preferentially when site 429 is methylated in 18S rRNA and 2416 and 4917 are unmethylated in 28S rRNA. Although we show that FMRP interacts with ribosomal proteins in the nuclear fraction (Figure 6A), currently we do not know whether this recognition of rRNA methylation pattern by FMRP happens during the assembly of ribosomes or later. Our initial experiments indicate that FMRP shows a better interaction with rRNA when it is from cell lysate rather than with purified rRNA (Figure S6A).
Figure 6.
Recognition of rRNA 2'O-Methylation by FMRP
(A) Immunoblots showing the presence of ribosomal protein RPLP0 in FMRP immunoprecipitate from H9 hESC nuclear fractions.
(B) Relative methylation index of H9 hESC rRNA from FMRP IP normalized to input by RTL-P (n = 3–4, unpaired Student’s t test, mean ± SEM).
(C) Model illustrating the role of FMRP in regulating translation through differential rRNA methylation.
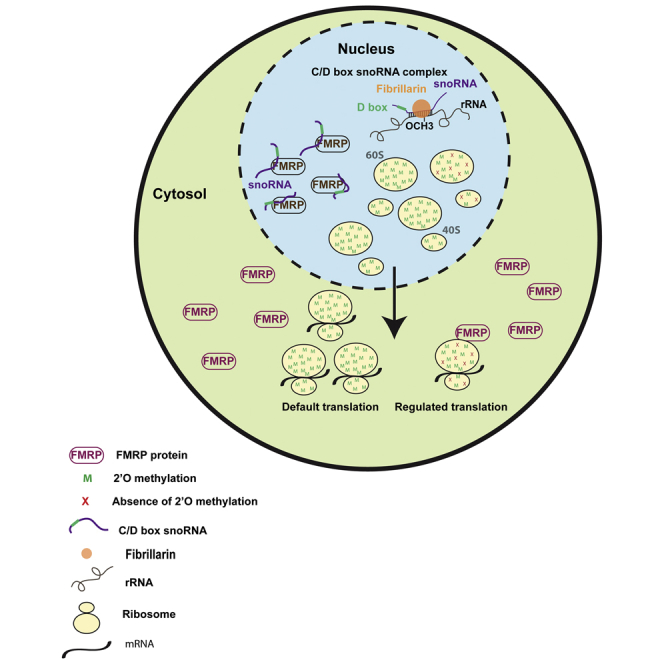
An interesting possibility is that the heterogeneity influenced by FMRP (in the form of differential rRNA methylation) is recognized by FMRP itself and thus recruits a subset of ribosomes for the translation of its own target mRNAs (Figure 6C-model). Our results indicate that FMRP recognizes differential 2'O-methylation pattern on selected sites of rRNA. Interestingly, there are many G-quartet structures (an RNA motif recognized by FMRP) that lie in close proximity to these rRNA methylation sites, which might be regulated by FMRP (Figure S6C). An altered methylation on these sites could influence the G-quartet structures, which interact with FMRP. We propose this to be the mechanism by which FMRP recognizes ribosomes based on differential rRNA methylation on specific sites (Figure 6C-model).
Limitations of the Study
This study focused on the interaction of FMRP with C/D box snoRNA in the nucleus. Our data suggests that the interaction of FMRP with specific C/D Box snoRNA might affect 2’O-methylation on rRNA. At the mechanistic level, our data can correlate only the levels of FMRP and the consequent change in methylation of specific sites. We show that FMRP and Fibrillarin (rRNA-specific methyltransferase) are in separate complexes, and we do not know the exact molecular mechanism involving the FMRP-snoRNA complex and how it regulates changes in rRNA post-transcriptional modifications in a cell.
We know from our data that FMRP can bind to the ribosome, especially through the RNA component. We propose a putative model describing how the rRNA methylation pattern can be recognized by FMRP based on a bio-informatics prediction, but we currently do not have any experimental evidence to prove that. Our data demonstrates the link between FMRP's interaction with a subset of C/D box snoRNAs and rRNA methylation. Although we have evidence regarding the recognition of specific 2’O-methylation patterns by RNA binding proteins (RBPs) like FMRP, we are yet to understand the correlation between the role of FMRP in rRNA methylation, FMRP's recognition of specific methylation patterns, and translation regulation of specific subset of mRNAs, which is beyond the scope of our manuscript.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The work has been funded by the NeuroStem grant (BT/IN/Denmark/07/RSM/2015-2016), RNAi grant from the Department of Biotechnology (BT/PR8723/AGR/36/776/2013), Centre for Brain Development and Repair (CBDR) grant (BT/PR11434/MED/30/1389/2014), India. R.S. acknowledges his DBT-RA fellowship for the same. We would like to thank the Stem Cell Facility and Central Imaging & Flow Cytometry Facility, InStem. We would like to thank all Muddashetty laboratory members for their suggestions and advice during the course of this work.
Author Contributions
Conceptualization - M.N.D., N.K.C.G., V.T., and R.S.M.; Validation - M.N.D., N.K.C.G., V.T., and A.R.; Formal Analysis - R.O.B., P.A., M.N.D., N.K.C.G., V.T., D.P., and R.S.M.; Resources - B.S., R.S., R. P., A.G., S. Chandran, S. Chattarji, and R.S.M; Writing, Review & Editing, M.N.D., N.K.C.G., V.T., and R.S.M.; Funding, R.S.M.; Supervision, R.S.M.
Declaration of Interests
The authors declare no competing interests.
Published: November 30, 2018
Footnotes
Supplemental Information includes Transparent Methods, six figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.11.007.
Supplemental Information
References
- Bardoni B., Willemsen R., Weiler I.J., Schenck A., Severijnen L.A., Hindelang C., Lalli E., Mandel J.L. NUFIP1 (nuclear FMRP interacting protein 1) is a nucleocytoplasmic shuttling protein associated with active synaptoneurosomes. Exp. Cell Res. 2003;289:95–107. doi: 10.1016/s0014-4827(03)00222-2. [DOI] [PubMed] [Google Scholar]
- Bassell G.J., Warren S.T. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaddek D., Nicolas A., Lamond A.I. Quantitative proteomic analysis of the human nucleolus. Methods Mol. Biol. 2016;1455:249–262. doi: 10.1007/978-1-4939-3792-9_20. [DOI] [PubMed] [Google Scholar]
- Brameier M., Herwig A., Reinhardt R., Walter L., Gruber J. Human box C/D snoRNAs with miRNA like functions: expanding the range of regulatory RNAs. Nucleic Acids Res. 2011;39:675–686. doi: 10.1093/nar/gkq776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaille J., Nicoloso M., Bachellerie J.P. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature. 1996;383:732–735. doi: 10.1038/383732a0. [DOI] [PubMed] [Google Scholar]
- Chen E., Sharma M.R., Shi X., Agrawal R.K., Joseph S. Fragile X mental retardation protein regulates translation by binding directly to the ribosome. Mol. Cell. 2014;54:407–417. doi: 10.1016/j.molcel.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S.C., Bray S.M., Suhl J.A., Cutler D.J., Coffee B., Zwick M.E., Warren S.T. Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males. Am. J. Med. Genet. A. 2010;152A:2512–2520. doi: 10.1002/ajmg.a.33626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z.W., Shao P., Diao L.T., Zhou H., Yu C.H., Qu L.H. RTL-P: a sensitive approach for detecting sites of 2'-O-methylation in RNA molecules. Nucleic Acids Res. 2012;40:e157. doi: 10.1093/nar/gks698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart D.E., Malter H.E., Feng Y., Warren S.T. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum. Mol. Genet. 1996;5:1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- Falaleeva M., Welden J.R., Duncan M.J., Stamm S. C/D-box snoRNAs form methylating and non-methylating ribonucleoprotein complexes: old dogs show new tricks. Bioessays. 2017;39 doi: 10.1002/bies.201600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Absher D., Eberhart D.E., Brown V., Malter H.E., Warren S.T. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell. 1997;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- Feng Y., Gutekunst C.A., Eberhart D.E., Yi H., Warren S.T., Hersch S.M. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J. Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell R.A., Benson R.E., Hua J., Bogerd H.P., Cullen B.R. A nuclear role for the Fragile X mental retardation protein. EMBO J. 1996;15:5408–5414. [PMC free article] [PubMed] [Google Scholar]
- Henras A.K., Plisson-Chastang C., O'Donohue M.F., Chakraborty A., Gleizes P.E. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip. Rev. RNA. 2015;6:225–242. doi: 10.1002/wrna.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incarnato D., Anselmi F., Morandi E., Neri F., Maldotti M., Rapelli S., Parlato C., Basile G., Oliviero S. High-throughput single-base resolution mapping of RNA 2-O-methylated residues. Nucleic Acids Res. 2017;45:1433–1441. doi: 10.1093/nar/gkw810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E.W., Corbin F., Woerly S., Rousseau F. The fragile X mental retardation protein is associated with ribosomes. Nat. Genet. 1996;12:91–93. doi: 10.1038/ng0196-91. [DOI] [PubMed] [Google Scholar]
- Kim M., Bellini M., Ceman S. Fragile X mental retardation protein FMRP binds mRNAs in the nucleus. Mol. Cell. Biol. 2009;29:214–228. doi: 10.1128/MCB.01377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S., Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- Krogh N., Jansson M.D., Häfner S.J., Tehler D., Birkedal U., Christensen-Dalsgaard M., Lund A.H., Nielsen H. Profiling of 2′-O-Me in human rRNA reveals a subset of fractionally modified positions and provides evidence for ribosome heterogeneity. Nucleic Acids Res. 2016;44:7884–7895. doi: 10.1093/nar/gkw482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine D.L.J. Noncoding RNAs in eukaryotic ribosome biogenesis and function. Nat. Struct. Mol. Biol. 2015;22:11–19. doi: 10.1038/nsmb.2939. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhao X. Concise review: fragile X proteins in stem cell maintenance and differentiation. Stem Cells. 2014;32:1724–1733. doi: 10.1002/stem.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Shan G., Guo W., Smrt R.D., Johnson E.B., Li X., Pfeiffer R.L., Szulwach K.E., Duan R., Barkho B.Z. Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand V., Ayadi L., El Hajj A., Blanloeil-Oillo F., Helm M., Motorin Y. High-throughput mapping of 2'-O-Me residues in RNA using next-generation sequencing (illumina RiboMethSeq protocol) Methods Mol. Biol. 2017;1562:171–187. doi: 10.1007/978-1-4939-6807-7_12. [DOI] [PubMed] [Google Scholar]
- Muddashetty R.S., Kelic S., Gross C., Xu M., Bassell G.J. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty R.S., Nalavadi V.C., Gross C., Yao X., Xing L., Laur O., Warren S.T., Bassell G.J. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell. 2011;42:673–688. doi: 10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick L.K., Nakamoto-Kinoshita M., Lindor N.M., Kirmani S., Cheng X., Warren S.T. Fragile X syndrome due to a missense mutation. Eur. J. Hum. Genet. 2014;22:1185–1189. doi: 10.1038/ejhg.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J.D., Bassell G.J., Klann E. Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nat. Rev. Neurosci. 2015;16:595–605. doi: 10.1038/nrn4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M.R., Bray S.M., Warren S.T. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu. Rev. Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Sharma S., Lafontaine D.L. 'View from a Bridge': a new perspective on eukaryotic rRNA base modification. Trends Biochem. Sci. 2015;40:560–575. doi: 10.1016/j.tibs.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Shi Z., Fujii K., Kovary K.M., Genuth N.R., Rost H.L., Teruel M.N., Barna M. Heterogeneous ribosomes preferentially translate distinct subpools of mRNAs genome-wide. Mol. Cell. 2017;67:71–83.e7. doi: 10.1016/j.molcel.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubina M.Y., Musinova Y.R., Sheval E.V. Nucleolar methyltransferase fibrillarin: evolution of structure and functions. Biochemistry (Mosc) 2016;81:941–950. doi: 10.1134/S0006297916090030. [DOI] [PubMed] [Google Scholar]
- Simsek D., Tiu G.C., Flynn R.A., Byeon G.W., Leppek K., Xu A.F., Chang H.Y., Barna M. The mammalian Ribo-interactome reveals ribosome functional diversity and heterogeneity. Cell. 2017;169:1051–1065.e18. doi: 10.1016/j.cell.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft R.J., Glazov E.A., Lassmann T., Hayashizaki Y., Carninci P., Mattick J.S. Small RNAs derived from snoRNAs. RNA. 2009;15:1233–1240. doi: 10.1261/rna.1528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M.S., Nouri K., Milroy L.G., Moll J.M., Herrmann C., Brunsveld L., Piekorz R.P., Ahmadian M.R. Subcellular fractionation and localization studies reveal a direct interaction of the fragile X mental retardation protein (FMRP) with nucleolin. PLoS One. 2014;9:e91465. doi: 10.1371/journal.pone.0091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telias M., Segal M., Ben-Yosef D. Neural differentiation of fragile X human embryonic stem cells reveals abnormal patterns of development despite successful neurogenesis. Dev. Biol. 2013;374:32–45. doi: 10.1016/j.ydbio.2012.11.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.