Abstract
Aim: We aimed to clarify post-prandial accumulation of remnant-like particles (RLP) in patients with sitosterolemia.
Methods: Oral fat tolerance test cream (Jomo Shokuhin, Takasaki, Japan) 50 g was given per body surface area (m2); blood sampling was performed at 2 h intervals up to 6 h. Plasma lipoprotein fractions and RLP fractions were determined in four sitosterolemic subjects with double mutations in ATP-binding cassette (ABC) sub-family G member 5 or member 8 (ABCG5 or ABCG8) gene (mean age = 18 yr, median low-density lipoprotein cholesterol [LDL-C] = 154 mg/dL), six heterozygous carriers (mean age = 31 yr, median LDL-C = 105 mg/dL), and five subjects with heterozygous familial hypercholesterolemia (FH, mean age = 32 yr, median LDL-C = 221 mg/dL). The incremental area under curve (iAUC) of lipids, including LDL-C, apolipoprotein B-48 (apoB48), RLP cholesterol (RLP-C), and RLP triglyceride (RLP-TG) were evaluated.
Results: After oral fat load, there was no significant difference of the iAUC of LDL-C between sitosterolemia and heterozygous FH, whereas the iAUC of apoB48 was significantly larger in the sitosterolemic subjects compared with that of heterozygous FH (2.9 µg/mL × h vs. 1.3 µg/mL × h, p < 0.05). Under these conditions, the iAUCs of RLP-C and RLP-TG levels were significantly larger in the sitosterolemic subject compared with those of heterozygous FH (9.5 mg/dL × h vs. 5.7 mg/dL × h, p < 0.05; 149 mg/dL × h vs. 40 mg/dL × h, p < 0.05, respectively), whereas those of heterozygous carriers were comparable with those with heterozygous FH.
Conclusions: Post-prandial lipoprotein metabolism in sitosterolemia appeared to be impaired, leading to their elevation in serum sterol levels. (UMIN Clinical Trials Registry number, UMIN000020330)
Keywords: Sitosterolemia, Remnant, Familial hypercholesterolemia, Remnant-like-particles, OFTT
See editorial vol. 25: 1183–1184
Introduction
Familial hypercholesterolemia (FH) is a common inherited disorder of plasma lipoprotein metabolism, characterized by an elevated level of low-density lipoprotein cholesterol (LDL-C), tendon xanthomas, and premature coronary artery disease1). Monogenic causes of FH involve gene mutations such as LDL receptor, apolipoprotein B-100 (apoB100), and proprotein convertase subtilisin/kexin type 9 (PCSK9)2). Post-prandial accumulation of lipoprotein remnants has been shown to be related with elevated cardiovascular risk3). Under these conditions, it has been shown that postprandial lipoprotein metabolism is severely impaired in a dominant form of FH4), whereas we have shown that such lipoprotein metabolism is preserved in a recessive form of FH called autosomal recessive hypercholesterolemia caused by mutations in LDL receptor adaptor protein 1 (LDLRAP1) gene5). Investigating the extreme cases harboring mutations in a specific gene provides an opportunity to directly observe the role of certain molecules in lipoprotein metabolism.
Sitosterolemia (OMIM #210250) is a rare, inherited, autosomal recessive disorder of lipid metabolism characterized by increased absorption and decreased biliary excretion of plant sterols and cholesterol, resulting in prominently elevated serum concentrations of plant sterols such as sitosterol and campesterol1). This disease is caused by mutations in either of the two genes, named ATP-binding cassette (ABC) sub-family G member 5 and member 8 (ABCG5 and ABCG8). Subjects suffering from sitosterolemia present primarily with tendinous and tuberous xanthomas, premature coronary atherosclerosis such as FH6–8). LDL-C levels are more variable in sitosterolemia than in other genetic hyperlipidemias, but can be extremely elevated in some patients, especially in breastfed infantile patients9). This report indicates that patients with sitosterolemia might be vulnerable to post-prandial hyperlipidemia. Remnant-like particles (RLP) are known as a good marker to evaluate lipid metabolism after diet. Also, increased RLP levels are associated with high LDL-C, endothelium dysfunction, and coronary artery disease (CAD). Thus, this post-prandial condition may play an important role of plasma high LDL-C levels in sitosterolemic patients. In this study, we examined post-prandial lipoprotein metabolism in sitosterolemia to determine their vulnerability to oral fat load for the first time10).
Materials and Methods
Study Design
This study is a single-arm, non-randomized, open-label, uncontrolled trial. Patients with homozygous sitosterolemia with double mutations, heterozygous carriers with single mutation, and heterozygous FH are enrolled in this study. They receive an oral fat tolerance test (OFTT) cream when they met the inclusion criteria. We then compare post-prandial plasma RLP cholesterol (RLP-C) levels between the patients with sitosterolemia with double mutations in ABCG5 or ABCG8 gene, heterozygous mutation carriers with single mutation, and heterozygous FH. This study has been registered at the University Hospital Medical Information Network (UMIN) (UMIN ID: 000020330)10).
Study Subjects
Four patients with sitosterolemia harboring double mutations in ABCG5 or ABCG8 gene, six relatives with a heterozygous mutation, and five unrelated subjects with heterozygous FH were enrolled in this study. None of the subjects had taken medication known to affect plasma lipids for at least 4 weeks before this study was conducted. None had smoking habits and excessive alcohol intake (> ethanol 60 g/day).
Oral Fat Tolerance Test
OFTT cream of 50 g was given per body surface area (squared meter) as described elsewhere (Jomo Shokuhin, Takasaki, Japan)11). The cream consisted of fat 33%, cholesterol 74 mg, and 341 kcal per 100 g and is rich in palmitic and oleic acids. Blood sampling was performed at 2 h intervals up to 6 h.
Lipoprotein Analysis and ApoE Phenotype
LDL-C values were measured directly using QUALIGENT® (Sekisui Medical, Tokyo, Japan). RLP is estimated as the unbound fraction of plasma after incubation with immunoaffinity gel of apoB100 monoclonal antibody and apolipoprotein A-I monoclonal antibody as described12). ApoE phenotype was separated by isoelectric focusing and detected by western blot with apoE polyclonal antibody (phenotyping apoE IEF system, JOKOH, Tokyo, Japan). Apolipoprotein B-48 (apoB48) levels were determined by ELISA method using monoclonal antibody against C-terminal decapeptide of apoB4813). Serum levels of sterols, including sitosterol, lathosterol, and campesterol, were determined using gas-liquid chromatography-mass spectrometry as previously described9).
Statistical Analysis
Values are expressed as medians (interquartile [IQR]) unless otherwise stated. Area under curve (AUC) for triglyceride (TG), LDL-C, apoB48, sitosterol, RLP-C, and RLP triglyceride (RLP-TG) at baseline and after fat load were calculated using the trapezoid rule. The differences in the incremental AUC (iAUC) of the plasma variables between the three groups were examined using Kruskal–Wallis test. Post hoc analyses using Scheffé's method were performed when the main effect was significant. All tests of statistical significance were assumed at a level of p < 0.05.
Ethical Considerations
This study was approved by the Ethics Committee of Kanazawa University and carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1975, as revised in 2008. Informed consents were obtained from all subjects for being included in the study.
Results
The baseline characteristics of the three study groups are shown in Table 1. Genetic backgrounds of the study subjects are shown in Supplemental Table 1. These groups of patients were comparable in terms of age, gender, and body mass index (BMI). Interestingly, the baseline LDL-C level of sitosterolemic patients with double mutations was significantly higher than that of single mutation, whereas baseline LDL-C level of heterozygous FH was significantly higher than that in sitosterolemic subjects. On the other hand, serum levels of sitosterol and campesterol in sitosterolemic patients with double mutations were significantly higher than those with a single mutation as well as those in heterozygous FH. In addition, apoB48, TG, RLP-C, and RLP-TG levels were significantly higher in sitosterolemic subjects than those in heterozygous FH (Table 1).
Table 1. Characteristics of the study subjects.
| Sitosterolemia | Single mutation carrier | Heterozygous FH | |
|---|---|---|---|
| (n = 4) | (n = 6) | (n = 5) | |
| Age | 18 ± 13 | 31 ± 7 | 32 ± 11 |
| Sex (male/female) | 2/2 | 3/3 | 2/3 |
| BMI (kg/m2) | 23.1 ± 4.1 | 23.9 ± 6.5 | 24.5 ± 4.0 |
| TC (mg/dl) | 241 [226–275] | 223 [189–233] | 289 [284–305] |
| TG (mg/dl) | 201 [132–241] | 99 [91–138] | 101 [94–146] |
| HDL-C (mg/dl) | 50 [47–60] | 57 [50–58] | 49 [46–55] |
| LDL-C (mg/dl) | 154 [150–156] | 105 [89–164] | 221 [220–246] |
| apoB48 (µg/ml) | 5.7 [3.6–7.9] | 2.8 [1.9–3.4] | 1.4 [1.4–2.2] |
| Lathosterol (µg/ml) | 3.0 [2.3–3.6] | 1.5 [1.1–1.6] | 3.8 [2.6–3.8] |
| Campesterol (µg/ml) | 57 [49–70] | 14.9 [13.9–15.5] | 4.6 [3.3–4.9] |
| Sitosterol (µg/ml) | 88.5 [73.6–113.2] | 6.5 [5.1–8.4] | 2.5 [1.9–2.9] |
| RLP-C (mg/dl) | 14.4 [11.8–16.5] | 3.6 [3.7–8.4] | 9.1 [7.2–10.8] |
| RLP-TG (mg/dl) | 41.5 [37.0–82.6] | 14.2 [11.4–40.0] | 12.7 [12.5–54.2] |
FH: familial hypercholesterolemia, BMI: body mass index, TC: total cholesterol, TG: triglyceride, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, apoB48: apolipoprotein B-48, RLP-C: remnant-like particle cholesterol, RLP-TG: remnant-like particle triglyceride
Supplemental Table 1. Genetic backgrounds of the subjects.
| Sitosterolremia | Heterozygous carrier | Heterozygous FH |
|---|---|---|
| (n = 4) | (n = 6) | (n = 5) |
| c.1256G > A/c.1763-1G > A | c.1256G > A | c.1702C > G |
| (ABCG5) | (ABCG5) | (LDLR) |
| c.454C > T/c.1403_1404delTC | c.1763-1G > A | c.1845 + 2T > C |
| (ABCG8) | (ABCG5) | (LDLR) |
| c.1306G > A/c.1813_1817delCTTTT | c.1306G > A | c.2054C > T |
| (ABCG5) | (ABCG5) | (LDLR) |
| c.130T > G/c.1306G > A | c.1813_1817delCTTTT | c.2431A > T |
| (ABCG5) | (ABCG5) | (LDLR) |
| c.1306G > A | c.94G > A | |
| (ABCG5) | (PCSK9) | |
| c.130T > G | ||
| (ABCG5) |
FH: familial hypercholesterolemia, ABCG5: ATP-binding cassette (ABC) sub-family G member 5, ABCG8: ATP-binding cassette (ABC) sub-family G member 8, LDLR: low-density lipoprotein receptor, PCSK9: proprotein convertase subtilisin/kexin type 9
After oral fat load, lipoproteins, other than LDL-C, increased through 2 to 4 h (Figs. 1 and 2). There was no significant difference in the iAUC of LDL-C between sitosterolemia and heterozygous FH, whereas the iAUC of TG was significantly larger in sitosterolemic subjects than that in heterozygous FH (154 mg/dL × h vs. 50 mg/dL × h, p < 0.05, Fig. 3).
Fig. 1.
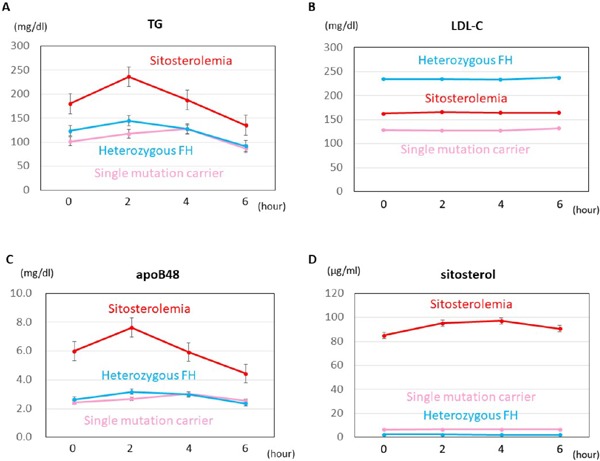
Line charts of lipoproteins
Bars indicate standard errors.
Red: Sitosterolemia
Pink: Single mutation carrier
Light blue: Heterozygous FH
Fig. 2.
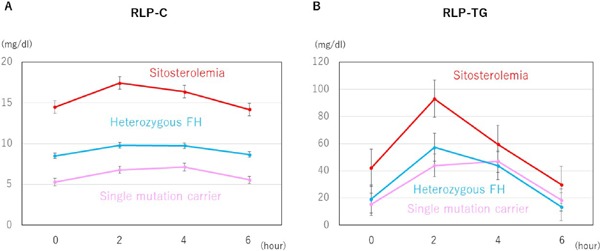
Line charts of RLP fractions
Bars indicate standard errors.
Red: Sitosterolemia
Pink: Single mutation carrier
Light blue: Heterozygous FH
Fig. 3.
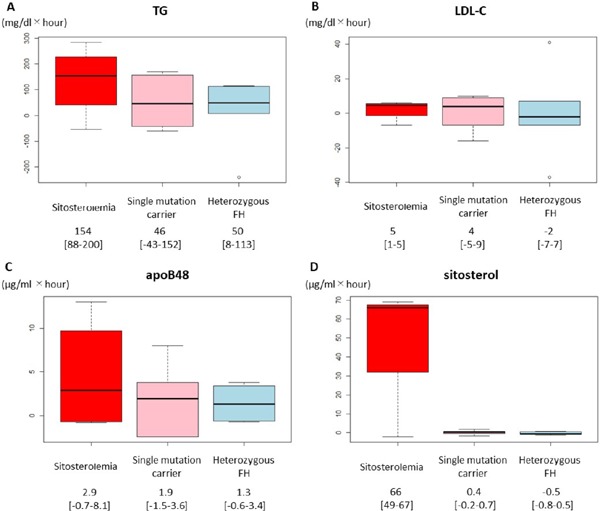
The iAUC of lipoproteins
Boxplots illustrating the iAUC of (A) TG, (B) LDL-C, (C) apoB48, and (D) sitosterol in three groups.
Red: Sitosterolemia
Pink: Single mutation carrier
Light blue: Heterozygous FH
Interestingly, the iAUC of apoB48 was significantly larger in the sitosterolemic subjects compared with that of heterozygous FH (2.9 µg/mL × h vs. 1.3 µg/mL × h, p < 0.05, Fig. 3). In addition to those results, the iAUC of sitosterol in the sitosterolemic subjects was significantly larger than that of heterozygous FH as expected (66 µg/mL × h vs. −0.5 µg/mL × h, p < 0.05). Under these conditions, the iAUCs of RLP-C and RLP-TG levels were significantly larger in the sitosterolemic subjects compared with those of heterozygous FH (9.5 mg/dL × h vs. 5.7 mg/dL × h, p < 0.05; 149 mg/dL × h vs. 40 mg/dL × h, p < 0.05, respectively, Fig. 4), whereas those of single mutation carrier in ABCG5 or ABCG8 gene were comparable with those with heterozygous FH.
Fig. 4.
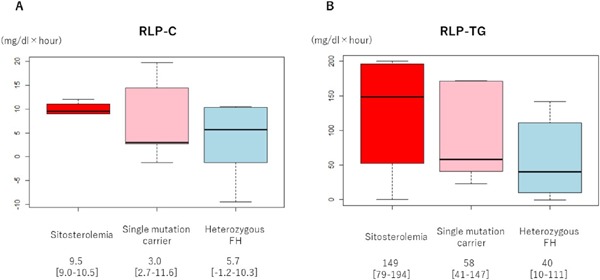
The iAUC of RLP fractions
Boxplots illustrating the iAUC of (A) RLP-C and (B) RLP-TG in three groups.
Red: Sitosterolemia
Pink: Single mutation carrier
Light blue: Heterozygous FH
Discussion
The main finding of the present study is that the clearance of post-prandial RLP fraction was impaired in sitosterolemia compared with heterozygous FH. This is the first study to demonstrate the impaired postprandial RLP metabolism in sitosterolemia.
Impaired TG-rich lipoprotein metabolism under the condition of disturbance of ABCG5/ABCG8 have been implicated by the observational and interventional studies of sitosterolemic subjects14, 15) as well as by the experimental study using ABCG5/ABCG8 knockout mice (sitosterolemic mice)16). Previously, we demonstrated that breastfed infantile cases with sitosterolemia harboring double mutations in ABCG5 gene exhibit transient extreme hyper-LDL cholesterolemia9). In addition, there have been great diversities in the LDL-C levels among the subjects with sitosterolemia described so far17). Such observations as well as the facts that ABCG5/ABCG8 are playing an important role in excretion of sterols in the intestine could lead us to investigate if the patients with sitosterolemia harboring ABCG5/ABCG8 mutations are vulnerable to diet-induced hyperlipidemia. We confirmed that post-prandial RLP metabolism was disturbed in the patients with sitosterolemia for the first time. Moreover, apoB48 level, which has been shown to be associated with atherosclerotic cardiovascular diseases18), was significantly elevated in sitosterolemic patients both in fasting state, as well as in postprandial state. In addition, we observed that the peaks of the post-prandial lipoproteins were earlier in sitosterolemic patients than those in heterozygous FH. Moreover, sitosterol level in sitosterolemic patients increased after fat load, although such trends were not observed in heterozygous FH nor in single mutation carriers. Thus, dietary counseling for the patients with sitosterolemia should be one of the reasonable approaches for their risk management.
In this study, we compared post-prandial lipoprotein metabolism in sitosterolemia with that in heterozygous FH, not with normal controls. It is well known that the patients with sitosterolemia exhibit dyslipidemia, including elevation of LDL-C. And RLP-C has been shown to be correlated with LDL-C, especially in cases with elevated LDL-C19). Accordingly, it is rather fair to compare sitosterolemia and heterozygous FH under the condition of elevated LDL-C in both sides.
This study has several limitations. First, only a small number of subjects were included in this study. It is difficult to enroll more sitosterolemic patients because of the rarity of this disorder. However, our subjects were comparatively uniform in terms of factors potentially affecting the post-prandial lipoprotein metabolism—age, sex, and BMI—among study subjects. Second, we used OFTT cream without plant sterols making it difficult to see the effect on such sterols in this study. However, loading sitosterol on sitosterolemia are considered ethically unacceptable, although the increased plant sterols themselves may not relate with atherosclerosis20, 21). Third, TG level was significantly higher in sitosterolemia than in other two groups. In this regard, it has been shown that fasting TG level was significantly associated with post-prandial lipemia22). Accordingly, our results may not be reflected by actual “post-prandial” lipemia itself. Despite those limitations, we believe that this study provides new insights into the roles of ABCG5/ABCG8 in the postprandial lipoprotein metabolism.
Acknowledgements and Notice of Grant Support
We express our special thanks to Kazuko Honda and Sachio Yamamoto (staff of Kanazawa University) for their outstanding technical assistance. We also express our special thanks to Drs. Takuya Nakahashi, Yoshihiro Tanaka, Taro Ichise, Takashi Kobayashi, Azusa Ohbatake, and Akari Wada for their assistance in data collection. We have received the research grants from Sakakibara Memorial Research Grant from the Japan Research Promotion Society for Cardiovascular Diseases and Astellas Foundation for Research on Metabolic Disorders.
COI
Hayato Tada has received a research grant from Sanofi K.K. Atsushi Nohara and Hiroshi Mabuchi have received research grants from MSD K.K., Sanofi K.K., Shionogi & Co., Ltd., Kowa Co., Ltd., Astellas Pharma Inc., AstraZeneca K.K., Keiai-Kai Medical Corp., and Biopharm of Japan Co. Masakazu Yamagishi has received research grants from MSD K.K., Astellas Pharma Inc., Daiichi-Sankyo Co., Ltd., and Otsuka Pharmaceutical Co., Ltd., and he has received payments for lectures from Astellas Pharma Inc., Daiichi-Sankyo Co., Ltd., Shionogi & Co., Ltd., and Kowa Co., Ltd. Masa-aki Kawashiri has received payments for lectures from Amgen Astellas Biopharma K.K. and Astellas Pharma Inc.
References
- 1). Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In The Metabolic and Molecular Bases of Inherited Diseases. 8th edition Scriver CR, Beaudet AL, Sly WS, Valle D. editors. McGraw-Hill, New York: 2001; 2863-2913 [Google Scholar]
- 2). Soutar AK, Naoumova RP. Mechanisms of Disease: genetic causes of familial hypercholesterolemia. Nat Pract Cardiovasc Med. 2007; 4: 214-225 [DOI] [PubMed] [Google Scholar]
- 3). Karpe F, Steiner G, Uffelmann K, Olivecrona T, Hamsten A: Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis. 1994; 106: 83-97 [DOI] [PubMed] [Google Scholar]
- 4). de Sauvage Nolting PR, Twickler MB, Dallinga-Thie GM, Buirma RJ, Hutten BA, Kastelein JJ, Examination of Probands and Relatives in Statin Studies with Familial Hypercholesterolemia (ExPRESS) Study Group Elevated remnant-like particles in heterozygous familial hypercholesterolemia and response to statin therapy. Circulation. 2002; 106: 788-792 [DOI] [PubMed] [Google Scholar]
- 5). Tada H, Kawashiri MA, Tanaka A, Nakano T, Nakajima K, Inoue T, Noguchi T, Nakanishi C, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M. Post-prandial remnant lipoprotein metabolism in autosomal recessive hypercholesterolaemia. Eur J Clin Invest. 2012; 42: 1094-1099 [DOI] [PubMed] [Google Scholar]
- 6). Tada H, Kawashiri MA, Okada H, Endo S, Toyoshima Y, Konno T, Nohara A, Inazu A, Takao A, Mabuchi H, Yamagishi M, Hayashi K. A Rare Coincidence of Sitosterolemia and Familial Mediterranean Fever Identified by Whole Exome Sequencing. J Atheroscler Thromb. 2016; 23: 884-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Sakuma N, Tada H, Mabuchi H, Hibino T, Kasuga H. Lipoprotein Apheresis for Sitosterolemia. Ann Intern Med. 2017; 167: 896-899 [DOI] [PubMed] [Google Scholar]
- 8). Kawamura R, Saiki H, Tada H, Hata A. Acute myocardial infarction in a 25-year-old woman with sitosterolemia. J Clin Lipidol. 2018; 12: 246-249 [DOI] [PubMed] [Google Scholar]
- 9). Tada H, Kawashiri MA, Takata M, Matsunami K, Imamura A, Matsuyama M, Sawada H, Nunoi H, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M. Infantile Cases of Sitosterolaemia with Novel Mutations in the ABCG5 Gene: Extreme Hypercholesterolaemia is Exacerbated by Breastfeeding. JIMD Rep. 2015; 21: 115-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Nomura A, Tada H, Nohara A, Kawashiri MA, Yamagishi M. Oral Fat Tolerance Test for Sitosterolemia and Familial Hypercholesterolemia: A Study Protocol. J Atheroscler Thromb. 2018; 25: 741-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Inazu A, Nakajima K, Nakano T, Niimi M, Kawashiri MA, Nohara A, Kobayashi J, Mabuchi H. Decreased postprandial triglyceride response and diminished remnant lipoprotein formation in cholesteryl ester transfer protein (CETP) deficiency. Atherosclerosis. 2008; 196: 953-957 [DOI] [PubMed] [Google Scholar]
- 12). Nakajima K, Saito T, Tamura A, Suzuki M, Nakano T, Adachi M, Tanaka A, Tada N, Nakamura H, Campos E, Havel RJ. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta. 1993; 223: 53-71 [DOI] [PubMed] [Google Scholar]
- 13). Kinoshita M, Kojima M, Matsushima T, Teramoto T. Determination of apolipoprotein B-48 in serum by a sandwich ELISA. Clin Chim Acta. 2005; 351: 115-120 [DOI] [PubMed] [Google Scholar]
- 14). Salen G, von Bergmann K, Lütjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B, Multicenter Sitosterolemia Study Group Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004; 109: 966-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Hidaka H, Nakamura T, Aoki T, Kojima H, Nakajima Y, Kosugi K, Hatanaka I, Harada M, Kobayashi M, Tamura A, Fujii Tatsuzo, Shigeta Yukio. Increased plasma plant sterol levels in heterozygotes with sitosterolemia and xanthomatosi. J Lipid Res. 1990; 31: 881-888 [PubMed] [Google Scholar]
- 16). Wang J, Joy T, Mymin D, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. J Lipid Res. 2004; 45: 2361-2367 [DOI] [PubMed] [Google Scholar]
- 17). Méndez-González J, Julve J, Rotllan N, Llaverias G, Blanco-Vaca F, Escolà-Gil JC. ATP-binding cassette G5/G8 deficiency causes hypertriglyceridemia by affecting multiple metabolic pathways. Biochim Biophys Acta. 2011; 1811: 1186-1193 [DOI] [PubMed] [Google Scholar]
- 18). Masuda D, Yamashita S. Postprandial Hyperlipidemia and Remnant Lipoproteins. J Atheroscler Thromb. 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Nakajima K, Nakajima Y, Takeichi S, Fujita MQ. Plasma remnant-like lipoprotein particles or LDL-C as major pathologic factors in sudden cardiac death cases. Atherosclerosis. 2008; 198: 237-246 [DOI] [PubMed] [Google Scholar]
- 20). Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W, Jones PJ, Lütjohann D, Maerz W, Masana L, Silbernagel G, Staels B, Borén J, Catapano AL, De Backer G, Deanfield J, Descamps OS, Kovanen PT, Riccardi G, Tokgözoglu L, Chapman MJ, European Atherosclerosis Society Consensus Panel on Phytosterols Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. 2014; 232: 346-360 [DOI] [PubMed] [Google Scholar]
- 21). Silbernagel G, Chapman MJ, Genser B, Kleber ME, Fauler G, Scharnagl H, Grammer TB, Boehm BO, Mäkelä KM, Kähönen M, Carmena R, Rietzschel ER, Bruckert E, Deanfield JE, Miettinen TA, Raitakari OT, Lehtimäki T, März W. High intestinal cholesterol absorption is associated with cardiovascular disease and risk alleles in ABCG8 and ABO: evidence from the LURIC and YFS cohorts and from a meta-analysis. J Am Coll Cardiol. 2013; 62: 291-299 [DOI] [PubMed] [Google Scholar]
- 22). Masuda D, Sakai N, Sugimoto T, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Yuasa-Kawase M, Tsubakio-Yamamoto K, Ohama T, Nakagawa-Toyama Y, Nishida M, Ishigami M, Masuda Y, Matsuyama A, Komuro I, Yamashita S. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb. 2011; 18: 1062-1070 [DOI] [PubMed] [Google Scholar]


