Abstract
Aim: Shift workers have a high risk of cardiovascular disease (CVD). Systemic inflammation measured has been associated with the risk of CVD onset, in addition to classical risk factors. However, the association between work schedule and inflammatory cytokine levels remains unclear. The purpose of this study was to examine the association between work schedule and interleukin-6 (IL-6)/high-sensitivity C-reactive protein (hs-CRP) levels among Japanese workers.
Methods: The present cross-sectional study was a part of the Japanese Study of Health, Occupation and Psychosocial Factors Related Equity (J-HOPE). A total of 5259 persons who measured inflammatory cytokine were analyzed in this study. One-way analysis of variance was used to test log-transformed IL-6/hs-CRP differences by work schedule. Multiple regression analysis was used to examine the difference adjusted for other possible CVD risk factors.
Results: There were 3660 participants who had a regular work schedule; the remaining schedules were shift work without night work for 181 participants, shift work with night work for 1276 participants, and only night work for 142 participants. The unadjusted model showed that only night workers were significantly related to high levels of IL-6 compared with regular workers. Even in the multiple regression analysis, the higher level of IL-6 among only night workers remained significant (β = 0.058, P = 0.01). On the contrary, hs-CRP was not.
Conclusion: The present study revealed that only night shift work is significantly associated with high levels of IL-6 in Japanese workers. These observations help us understand the mechanism for the association between work schedule and CVD onset.
Keywords: Interleukin-6, Work schedule, Night work, CVD, J-HOPE
Introduction
Shift workers represent a high-risk group for cardiovascular disease (CVD) and metabolic syndrome; however, the underlying reasons for this are still not completely understood. Shift work is a work schedule that involves irregular or unusual hours compared with those of a normal daytime work schedule. Several types of work schedules can be described as shift work, including night work and rotating shift work1). In recent studies, significant associations have been found between shift work, especially night shift work, and the risk of CVD, metabolic syndrome, and cancer1, 2).
In an analysis of systematic review and metaanalysis, Vyas et al. showed that shift work was associated with myocardial infarction (risk ratio [RR], 1.23; 95% confidence interval [CI], 1.15–1.31) and ischemic stroke (RR, 1.05; 95% CI, 1.01–1.09)3). They also indicated that night shift work was associated with the greatest increase in the risk for coronary events (RR, 1.41; 95% CI, 1.13–1.76). Moreover, Vetter et al. suggested that over a 24-year observation period, longer duration of rotating night shift work was associated with a statistically significant increase in CHD risk2). Some studies have indicated that shift work is associated with not only CVD but also neoplasm. Hansen and Lassen recently reported an increased risk of breast cancer among female military employees working night shifts4).
Systemic inflammation, measured using high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6), has been associated with the risk of CVD and stroke, in addition to classical risk factors such as hypertension (HT), diabetes mellitus (DM), obesity, dyslipidemia (DL), and smoking5). Inflammatory cytokines such as hs-CRP and IL-6 are being investigated not only as inflammatory markers but also as prognostic markers for CVD, stroke, and mortality5, 6). In unstable coronary artery disease, increased levels of systemic markers of inflammation, such as CRP and IL-6, have been reported7–9). Large cohort studies have shown an association between elevated levels of circulating CRP and IL-6 and increased risk for cardiac events or death9–11). Therefore, examining the relationship between work schedule and measured systemic inflammation may help clarify one of the reasons why shift work leads to increased risk of CVD.
In addition, some observational studies have showed that higher levels of work engagement and socioeconomic status (SES) are associated with lower inflammatory cytokines levels12, 13). However, the association between work schedule and inflammatory cytokine levels remains unclear. Thus, the purpose of this study was to examine the association between work schedule and IL-6 and hs-CRP levels in a cohort of Japanese workers.
Methods
Data Source
The present cross-sectional study is part of the Japanese Study of Health, Occupation and Psychosocial Factors Related Equity (J-HOPE), which was conducted to develop and expand research to elucidate mechanisms underlying social disparities in health and establishment of control measures. The J-HOPE was based on a baseline survey of our occupational cohort study on social class and health, supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan. A total of 14,534 individuals from 13 independent cohorts were enrolled in the study. Data were collected using a self-administered questionnaire that included work schedule and other individual-level characteristics. Physical measurements and blood samples were also obtained from workers. Workers were informed before completing the baseline questionnaire that participation was strictly voluntary and that all information provided would remain confidential. Written informed consent was obtained from all participants. The protocol and documents explaining our study were approved by the ethics committee of the Graduate School of Medicine and Faculty of Medicine at the University of Tokyo (No. 2772), Kitasato University School of Medicine Hospital (B12-103) and the University of Occupational and Environmental Health, Japan (10-004).
Measurements
Data on age, sex, height, weight, fasting plasma glucose (FPG) level, HbA1c in value according to the National Glycohemoglobin Standardization Program, serum lipid levels (triglyceride [TG], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C]), and levels of hs-CRP and IL-6 were collected at health checkups for all participants. Body mass index (BMI) was calculated as weight (in kg) divided by the square of height (in meters).
Work Schedule
Work schedule was classified as “regular work,” “shift work without night work,” “shift work with night work,” or “only night work.”
Lifestyle Risks
The following three lifestyle risks were included in the analysis: smoking status, frequency of alcohol consumption, and physical exercise. Smoking status was classified as “never smoked,” “former smoker,” or “current smoker.” Frequency of alcohol drinking was divided into “does not drink,” “drinks but not every day,” and “drinks every day.” Physical exercise was classified as either “no exercise,” “light exercise more than once a week,” “heavy exercise once or twice a week,” or “heavy exercise more than three times a week.”
Socioeconomic Status
We used completed education level, working hours per week, and annual household income as indicators of SES. Education level was categorized into two groups: “≤ 12 years” or “> 12 years” of formal education. Weekly working hours were divided into three groups: “≤ 40 h,” “41–59 h,” and “≥ 60 h.” Annual household income was considered the sum of income of each family member, and the number of family members was assessed based on a self-administered questionnaire. Each participant was asked to indicate to which of six income levels their household income belonged: 1) < 3.0 million JPY/year; 2) 3.0–4.99 million JPY/year; 3) 5.0–7.99 million JPY/year; 4) 8.0–9.99 million JPY/year; 5) 10.0–15.0 million JPY/year; 6) > 15.0 million JPY/year. For the analysis, three categories of annual household income were used: less than 2,999,000, 3,000,000–7,999,000, and 8,000,000 JPY or more.
Disease Definitions
The following four disease definitions were used in the analysis: DL, DM, and obesity. DL was defined as (1) LDL-C ≥ 140 mg/dL, (2) HDL-C < 40 mg/dL, or/and (3) TG ≥ 150 or (4) taking anticholesterol medication14). DM was defined as either (1) FPG ≥ 126 mg/dL and/or (2) HbA1c value (National Glycohemoglobin Standardization Program) ≥ 6.5%15). BMI was calculated as described above, and obesity was defined as BMI ≥ 25 kg/m2.
Inflammatory Markers
Blood samples were collected at baseline during yearly health checkups. An authorized external professional institution conducted the health checkups. Most participants had a scheduled health check during working hours. Serum IL-6 (pg/mL) was measured using high-sensitivity enzyme-linked immunosorbent assay, according to manufacturer guidelines (R&D Systems, Minneapolis, MN, USA).
Serum hs-CRP levels were measured using nephelometry, a latex particle-enhanced immunoassay (N-latex CRP II, Behring Nephelometer II, Tokyo, Japan) at a laboratory of SRL, Inc. (Tokyo, Japan), a commercial clinical laboratory testing company. The system of internal quality control and external quality assessment for the laboratory was certified by ISO15189 and College of American Pathologists. The detection limit was 0.05 mg/L. An hs-CRP level of less than 0.05 mg/L was treated as 0.05 mg/L.
Statistical Analysis
A total 13,806 workers who underwent blood sample testing were enrolled in this study. We excluded those who had a history of myocardial infarction, stroke, malignant tumor, depression, or those who did not measure IL-6 and hs-CRP. Finally, 5259 persons were analyzed in this study. When IL-6 and hs-CRP levels were used as continuous variables, values were log-transformed to normalize the distributions. Differences in independent variables were expressed as mean and standard deviation, or as median with 25th and 75th percentiles, or percentages. The chi-square test was used for categorical variables and one-way analysis of variance (ANOVA) was used for continuous variables. Bonferroni correction was used to investigate the differences in IL-6/hs-CRP levels for each work schedule. Multiple regression analysis was used to calculate the regression coefficient (β) by log-transformed IL-6/hs-CRP differences after adjusting for age, sex, BMI, working form, smoking status, frequency of alcohol consumption, physical exercise, years of education, working hours per week, annual household income, laboratory data (LDL-C, HbA1c), and job stress in various models.
In addition, the following two analyses were performed as sensitivity analysis; the working form was divided into only night shift and other, and without excluding those who had a history of myocardial infarction, stroke, malignant tumor, and depression, which are expected to be related to IL-6.
Differences with P < 0.05 were considered to be statistically significant. All statistical analyses were performed using EZR (Version 1.33, Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R Commander designed to add statistical functions frequently used in biostatistics16).
Results
Characteristics of study participants are shown in Table 1. There were 3660 participants (69.6%) who worked regular work schedules, 181 (3.4%) worked shifts without night work, 1276 (24.3%) worked shifts with night work, and 142 (2.7%) had schedules with only night work. Night workers were older. Years of education < 12 years, hours worked per week < 40 h, and household income < 2.99 million JPY/year were much more prevalent among night workers.
Table 1. Characteristics of the study participants by work schedule.
| Regular work | Shift work without | Shift work with | Only night | P value | |
|---|---|---|---|---|---|
| (N = 3660) | night work | night work | work | ||
| (N = 181) | (N = 1276) | (N = 142) | |||
| Age (years) | 41.9 (10.4) | 37.4 (11.3) | 35.4 (12.1) | 43.2 (8.8) | < 0.001 |
| Sex (male, %) | 69.1 | 87.8 | 92.0 | 39.4 | < 0.001 |
| Height (cm) | 167.1 (8.2) | 169.5 (7.5) | 170.0 (6.7) | 162.6 (7.3) | < 0.001 |
| Weight (kg) | 64.74 (12.7) | 66. (11.8) | 66.72 (11.9) | 59. (12.1) | < 0.001 |
| BMI (Kg/m2) | 23.1 (3.6) | 23.2 (3.5) | 23.0 (3.6) | 22.6 (4.0) | 0.446 |
| Lifestyle | |||||
| Smoking status | |||||
| Never (%) | 60.4 | 53.3 | 48.4 | 50.7 | < 0.001 |
| Former (%) | 11.2 | 9.4 | 12.8 | 11.3 | |
| Current (%) | 28.4 | 37.2 | 38.9 0 | 38.0 | |
| Frequency of alcohol drinking | |||||
| None (%) | 37.7 | 41.1 | 40.2 | 52.1 | < 0.001 |
| Sometimes (%) | 33.7 | 34.4 | 38.7 | 31.7 | |
| Every day (%) | 28.6 | 24.4 | 21.1 | 16.2 | |
| Physical exercise (%) | |||||
| None (%) | 64.7 | 66.3 | 68.9 | 71.9 | 0.207 |
| Light exercise more than once a week (%) | 21.5 | 21.3 | 18.2 | 17.3 | |
| Heavy exercise once or twice a week (%) | 10.9 | 10.1 | 10.5 | 7.2 | |
| Heavy exercise more than three times a week (%) | 2.9 | 2.2 | 2.4 | 3.6 | |
| Socioeconomic status | |||||
| Years of education 12 or less (%) | 48.9 | 63.4 | 71.3 | 81.6 | < 0.001 |
| Hours worked per week | |||||
| Less than 40 (%) | 43.2 | 35.5 | 43.5 | 91.6 | < 0.001 |
| 41–60 (%) | 50.8 | 57.4 | 9.3 | 7.0 | |
| 60 or more (%) | 6.0 | 13.1 | 47.2 | 1.4 | |
| Household income (million JPY/year) | |||||
| Less than 2.99 (%) | 9.3 | 7.9 | 9.8 | 38.3 | < 0.001 |
| 3.00–7.99 (%) | 55.1 | 179.7 | 69.7 | 45.4 | |
| More than 8.00 (%) | 35.6 | 12.4 | 20.6 | 16.3 | |
| Illness history | |||||
| Dyslipidemia (%) | 41.4 | 37.6 | 39.9 | 36.6 | 0.41 |
| Diabetes mellitus (%) | 8.6 | 4.4 | 8.1 | 4.9 | 0.107 |
| Hypertension (%) | 15.9 | 12.7 | 14.3 | 12.7 | 0.298 |
| Laboratory data | |||||
| TG (mg/dL) | 99.0 [66.0, 157.0] | 102.0 [68.0, 152.0] | 105.0 [67.0, 163.0] | 91.5 [64.3, 126.5] | 0.119 |
| LDL-C (mg/dL) | 117.5 (30.5) | 112.3 (28.4) | 112.3 (30.6) | 117.4 (30.5) | < 0.001 |
| HDL-C (mg/dL) | 62.6 (16.1) | 61.4 (16.5) | 59.6 (15.0) | 65.4 (15.6) | < 0.001 |
| HbA1c (%, NGSP) | 5.0 [4.8, 5.2] | 4.9 [4.8, 5.1] | 5.0 [4.8, 5.2] | 5.0 [4.8, 5.2] | < 0.001 |
| Hs-CRP (mg/L) | 0.26 [0.12, 0.62] | 0.22 [0.11, 0.45] | 0.26 [0.12, 0.60] | 0.22 [0.11, 0.48] | 0.13 |
| IL-6 (pg/mL) | 1.40 [0.90, 2.10] | 1.60 [1.00, 2.10] | 1.50 [0.90, 2.10] | 1.70 [1.20, 2.50] | < 0.001 |
| Job stress | |||||
| Effort–Reward Balance | 0.44 (0.15) | 0.50 (0.16) | 0.44 (0.14) | 0.41 (0.15) | < 0.001 |
Note: Data are expressed as mean and standard deviation, or as median with 25th and 75th percentiles, or percentages.
Abbreviations: BMI, body mass index; Hs-CRP, high sensibility C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Log (Hs-CRP), log-transformed Hs-CRP; Log (IL-6), log-transformed IL-6; NGSP, National Glycohemoglobin Standardization Program; TG, triglyceride.
The unadjusted associations between log-transformed IL-6/hs-CRP and work schedule are shown in Table 2 and Fig. 1. The participant group with only night work was significantly related to higher levels of log-transformed IL-6 compared with the group of regular work. On the contrary, hs-CRP was not.
Table 2. Value of log-transformed IL-6 and hs-CRP by work schedule.
| Regular work | Shift work | Shift work | Only | ANOVA | |
|---|---|---|---|---|---|
| without night work | with night work | night work | P value | ||
| Log (IL-6) | 0.16 (0.26) | 0.17 (0.22) | 0.16 (0.26) | 0.24 (0.23) | 0.006 |
| Log (hs-CRP) | −0.52 (0.53) | −0.59 (0.48) | −0.53 (0.53) | −0.60 (0.54) | 0.165 |
Note: Data are expressed as mean and standard deviation,
Abbreviations: ANOVA, one-way analysis of variance; Log (hs-CRP), log-transformed high sensibility C-reactive protein; Log (IL-6), log-transformed IL-6.
Fig. 1.
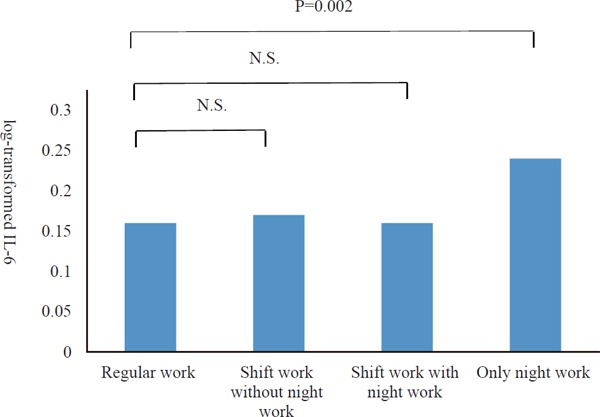
Differences in log-transformed IL-6 levels in participants by work schedule.
The participant group with only night work was significantly related to higher levels of logtransformed IL-6 compared with the group of regular work. Abbreviations: N.S., not significant.
Table 3 shows multivariate regression analysis log-transformed IL-6 and log-transformed hs-CRP for age, sex, years of education, hours worked per week, annual household income, BMI, laboratory data (LDL-C, HbA1c), and job stress. As shown in Model 1, participants with only night work had significantly higher levels of log-transformed IL-6 than those with regular work schedules (β = 0.078, standard error [SE] = 0.023, P < 0.001). The results remained statistically significant after adjusting for age, sex (Model 2; β = 0.087, SE = 0.008, P = 0.001), years of education, hours worked per week, annual household income, BMI, laboratory data (LDL-C, HbA1c), and job stress (Model 3; β = 0.058, SE = 0.023, P = 0.01). Similar results were also observed in the sensitivity analysis (Table 4). However, there was no significant association between work schedule and hs-CRP level.
Table 3. Standardized regression coefficients for log-transformed IL-6 and hs-CRP by multiple regression analysis by work schedule (N = 5259).
| Log-transformed IL-6 |
Log-transformed hs-CRP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 1 |
Model 2 |
Model 3 |
|||||||
| Work schedule | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value |
| Regular work | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Shift work | 0.005 | 0.79 | 0.013 | 0.50 | 0.004 | 0.82 | −0.075 | 0.06 | −0.073 | 0.06 | −0.064 | 0.09 |
| without night work | (0.020) | (0.019) | (0.020) | (0.040) | (0.039) | (0.039) | ||||||
| Shift work | 0.002 | 0.83 | 0.015 | 0.08 | 0.002 | 0.83 | −0.018 | 0.29 | −0.008 | 0.64 | −0.008 | 0.67 |
| with night work | (0.008) | (0.009) | (0.009) | (0.017) | (0.017) | (0.018) | ||||||
| Only night work | 0.078 | < 0.001 | 0.087 | 0.001 | 0.058 | 0.01 | −0.069 | 0.14 | −0.022 | 0.63 | −0.025 | 0.58 |
| (0.023) | (0.008) | (0.023) | (0.047) | (0.047) | (0.045) | |||||||
Model 1: unadjusted
Model 2: includes age, sex
Model 3: includes Model 2, years of education, hours worked per week, annual household income, BMI, laboratory data (LDL-C, HbA1c), job stress
Abbreviations: β, regression coefficients; hs-CRP, high-sensibility C-reactive protein; LDL-C, low-density lipoprotein cholesterol; ref, reference; SE, standard error.
Table 4. Standardized regression coefficient for log-transformed IL-6 by multiple regression analysis (1) grouped by those who worked only night shift and other groups (N = 5259), and (2) including those with a history of myocardial infarction, stroke, depression, and malignant tumor (N = 6407).
| (1) |
(2) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 1 |
Model 2 |
Model 3 |
|||||||
| Work schedule | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value |
| Regular work | Ref | Ref | Ref | |||||||||
| Shift work | 0.005 | 0.79 | −0.013 | 0.50 | 0.002 | 0.07 | ||||||
| without night work | (0.019) | (0.019) | (0.020) | |||||||||
| Shift work | 0.017 | 0.83 | 0.015 | 0.08 | 0.001 | 0.81 | ||||||
| with night work | (0.008) | (0.008) | (0.009) | |||||||||
| Works other than | Ref | Ref | Ref | |||||||||
| only night shift | ||||||||||||
| Only night work | 0.077 | < 0.001 | 0.086 | < 0.001 | 0.061 | 0.005 | −0.078 | < 0.001 | 0.089 | < 0.001 | 0.058 | 0.007 |
| (0.022) | (0.022) | (0.021) | (0.022) | (0.022) | (0.022) | |||||||
Model 1: unadjusted
Model 2: includes age, sex
Model 3: includes Model 2, years of education, hours worked per week, annual household income, BMI, laboratory data (LDL-C, HbA1c), job stress
Abbreviations: β, regression coefficients; LDL-C, low-density lipoprotein cholesterol; ref, reference; SE, standard error.
Discussion
The purpose of this study was to examine the association between work schedule and inflammatory cytokine levels in a cohort of Japanese workers. We found that participants who worked only night work had significantly higher IL-6 levels than those who had regular work schedules. Our findings were interesting in the following points. Working only night work was associated with higher levels of IL-6, but shift work alone was not. We conducted two types of sensitivity analyses to confirm this finding. In the first, the work schedule was divided into two groups: only night work and all other shifts. The result indicated that participants who worked only night work had higher levels of IL-6 than those who worked other shifts. In the second analysis, we did not exclude participants with a history of myocardial infarction, stroke, malignant tumor, and depression, which are expected to be related to IL-6. We analyzed the association between work schedule and IL-6 and obtained a similar result.
There are several reasons why only night work might influence IL-6 levels. The first possible reason is that insufficient sleep and circadian rhythm disturbance, which generate chronic stress, may be linked to increased IL-6 levels. Insufficient sleep and circadian rhythm disturbance are much more common among only night workers than other workers. A recent study found increased 24-hour urinary norepinephrine, a stress hormone, in people with both sleep restriction and circadian rhythm disturbances17). Lutgendorf et al. suggested that chronic stressors in older women are associated with significant increases in IL-6 over and above those associated with normal aging18). In another study, Maes et al. found that psychological stress significantly increased the stimulated production of tumor necrosis factor-α (TNF-α), IL-6, IL-1 receptor antagonist, interferon gamma, and IL-1019). However, in our study, even after adjusting for job stress, there was an association between only night shift workers and higher Il-6 level. It is expected that chronic stress from insufficient sleep and circadian rhythm disturbance may be related to a higher IL-6 level in people who only night work.
A second possible reason for the effect of only night work is that lower SES may be linked to increased IL-6 levels. Recently, researchers have focused on the association between lifestyle, SES, and inflammatory cytokines. Higher levels of IL-6 are strongly related to aging, sex, obesity, high rates of smoking20), alcohol consumption21), and physical inactivity20). In some large cohort studies, factors associated with low SES, such as education and income, were associated with high IL-6 levels13, 22). The present study corroborated these previous findings (Supplementary Table 1). Our study showed that lower education and household income levels were related to higher IL-6 levels. Thus, night workers, who tended to have lower SES, may have had higher IL-6 levels.
Supplementary Table 1. Standardized regression coefficients for log-transformed IL-6 by multiple regression analysis for each covariate.
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| β (SE) | P-value | β (SE) | P-value | |
| Age (per 10 years) | 0.004 | < 0.001 | 0.038 | < 0.001 |
| (0.001) | (0.004) | |||
| Sex (female/male) | −0.064 | < 0.001 | −0.042 | 0.003 |
| (0.008) | (0.010) | |||
| BMI (per 1 kg/m2) | 0.017 | < 0.001 | 0.013 | < 0.001 |
| (0.001) | (0.001) | |||
| Work schedule | ||||
| Regular work | Ref | Ref | ||
| Shift work without night work | 0.005 | 0.79 | 0.004 | 0.82 |
| (0.020) | (0.020) | |||
| Shift work with night work | 0.002 | 0.83 | 0.002 | 0.83 |
| (0.008) | (0.009) | |||
| Only night work | 0.078 | < 0.001 | 0.058 | 0.01 |
| (0.023) | (0.023) | |||
| Lifestyle | ||||
| Smoking status | ||||
| Never | Ref | Ref | ||
| Former | 0.013 | 0.27 | −0.005 | 0.69 |
| (0.011) | (0.012) | |||
| Current | 0.052 | < 0.001 | 0.041 | < 0.001 |
| (0.008) | (0.008) | |||
| Frequency of alcohol drinking | ||||
| None | Ref | Ref | ||
| Sometimes | −0.012 | 0.12 | −0.012 | 0.15 |
| (0.008) | (0.008) | |||
| Daily | −0.011 | 0.21 | −0.046 | < 0.001 |
| (0.009) | (0.010) | |||
| Physical exercise | ||||
| None | Ref | Ref | ||
| Light exercise more than once a week | −0.008 | 0.39 | −0.021 | 0.02 |
| (0.009) | (0.009) | |||
| Heavy exercise once or twice a week | −0.045 | 0.002 | −0.039 | < 0.001 |
| (0.012) | (0.012) | |||
| Heavy exercise more than three times a week | −0.081 | 0.002 | −0.074 | 0.001 |
| (0.022) | (0.023) | |||
| Socioeconomic status | ||||
| Years of education (12 or less/ more than 12) | 0.039 | < 0.001 | 0.005 | 0.47 |
| (0.007) | (0.008) | |||
| Hours worked per week | ||||
| Less than 40 | Ref | Ref | ||
| 41–60 | −0.007 | 0.34 | −0.009 | 0.25 |
| (0.007) | (0.008) | |||
| 61 or more | 0.006 | 0.72 | 0.012 | 0.47 |
| (0.016) | (0.016) | |||
| Annual household income (million JPY/year) | ||||
| More than 800 | Ref | Ref | ||
| 300–799 | 0.004 | 0.61 | 0.047 | < 0.001 |
| (0.007) | (0.008) | |||
| Less than 299 | −0.016 | 0.21 | 0.057 | < 0.001 |
| (0.012) | (0.014) | |||
| Laboratory data | ||||
| LDL-C (per 10mg/dl) | 0.010 | < 0.001 | 0.002 | 0.20 |
| (0.001) | (0.001) | |||
| HbA1c (per 1%, NGSP) | 0.070 | < 0.001 | 0.019 | 0.005 |
| (0.006) | (0.007) | |||
| Job stress | ||||
| Effort-Reward Balance | −0.001 | 0.96 | −0.015 | 0.48 |
| (0.019) | (0.022) | |||
Model 1: unadjusted
Model 2: includes age, sex, years of education, hours worked per week, annual household income, BMI, Laboratory data (LDL-C, HbA1c), job stress
Abbreviations: β, regression coefficients; LDL-C, low-density lipoprotein cholesterol; ref, reference; SE, standard error
Added to the sensitivity analyses in this study, we believe our findings are valid for helping to explain one of the underlying mechanisms of CVD onset in people who only work night shifts.
The other important results were as follows. There was no significant association between hs-CRP and work schedule. A previous study showed that elevated stress was not significantly associated with lower CRP levels23). Because stress may not be related to hs-CRP, it is possible that this study did not show a significant relationship.
Because night workers are groups at high risk for CVD onset who tend to have higher IL-6 levels, more unhealthy lifestyles, and lower SES, various intervention programs need to be implemented in these populations. Screening programs for modifiable risk factors including DL, smoking, glucose intolerance, and HT among night workers should be carried out. Night workers should be educated about CVD and the risks of developing CVD to forestall or avert the earliest clinical manifestations of disease. Night workers should be supported in efforts to lower their IL-6 levels by encouraging exercise and smoking cessation, which can lead to preventing CVD.
There were several limitations to our study. First, our study was a cross-sectional design and causal inferences could not be made because of the inability to determine temporal sequence. Prospective study designs should be considered in further research on these relationships to provide more insight on the question of the causal direction. Second, we used IL-6 and hs-CRP as the sole indicators of inflammatory cytokines. Future studies should examine the relationship between work schedules and other inflammatory cytokines such as TNF-α and fibrinogen. Third, we did not consider about timing or days of measuring IL-6. Although we did not know the detailed time when Il-6 was measured, blood samples were collected at baseline during yearly health checkups and most participants had a scheduled health check during working hours. A meta-analysis confirmed that IL-6 varied across the day, the most conspicuous effect being a trough in the morning24). Future study should consider diurnal fluctuation of IL-6. Fourth, both the quality and quantity of sleeping of participants were not completely understood, because the method used in J-HOPE did not include information about sleep deprivation. In addition, we did not know how long they had worked under night work. Future studies should evaluate the association between IL-6 and sleep deprivation in night shift workers. Fifth, we did not investigate the association between classical symptoms (i.e., chronic fatigue, stomach or duodenal ulcer) and inflammatory cytokines. Because the three classical symptoms were not investigated in the cohort study, this is an important area for future research. Sixth, we could not find the relationship between night shift work and CRP, as the reason might be attributable to under-detection of CRP levels. Lastly, the direct biological causal relationship between night shift work and IL-6 level remains unclear and warrants further investigation.
Conclusion
The present study revealed that working only night shifts was significantly related to high levels of IL-6 among Japanese workers. These observations can help us to understand the mechanisms underlying the relationship between work schedule and CVD onset. Further studies are needed to assess the association between temporal variations in shift work and IL-6; additionally, interventions such as lifestyle changes should be considered.
Acknowledgments
This study was conducted as part of the Japanese Study of Health, Occupation and Psychosocial Factors Related Equity (J-HOPE). The funding organizations had no role in the conception and design of study, the collection and analysis of data, interpretation of the findings, or writing the manuscript.
Notice of grant Support
This study was supported by a Grant-in-Aid for Scientific Research (C) (No. 16K09073) from the Japan Society for the Promotion of Science, Japan.
Conflict of Interest
We do not have conflicts of interest to disclose.
References
- 1). Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC: Shift work and chronic disease: the epidemiological evidence. Occup Med, 2011; 61: 78-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES: Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA, 2016; 315: 1726-1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG: Shift work and vascular events: systematic review and meta-analysis. BMJ, 2012; 345: e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Hansen J, Lassen CF: Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med, 2012; 69: 551-556 [DOI] [PubMed] [Google Scholar]
- 5). Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R: Associations of elevated Interleukin-6 and C-Reactive protein levels with mortality in the elderly. Am J Med, 1999; 106: 506-512 [DOI] [PubMed] [Google Scholar]
- 6). Makita S, Nakamura M, Satoh K, Tanaka F, Onoda T, Kawamura K, Ohsawa M, Tanno K, Itai K, Sakata K, Okayama A, Terayama Y, Yoshida Y, Ogawa A: Serum C-reactive protein levels can be used to predict future ischemic stroke and mortality in Japanese men from the general population. Atherosclerosis, 2009; 204: 234-238 [DOI] [PubMed] [Google Scholar]
- 7). Toss H, Lindahl B, Siegbahn A, Wallentin L: for theFRISC study group Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. Circulation, 1997; 96: 4204-4210 [DOI] [PubMed] [Google Scholar]
- 8). Berk BC, Weintraub WS, Alexander RW: Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol, 1990; 65: 168-172 [DOI] [PubMed] [Google Scholar]
- 9). Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, Rebuzzi AG, Ciliberto G, Maseri A: Elevated levels of interleukin-6 in unstable angina. Circulation, 1996; 94: 874-877 [DOI] [PubMed] [Google Scholar]
- 10). Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A: Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation, 1999; 99: 2079-2084 [DOI] [PubMed] [Google Scholar]
- 11). Lindmark E, Diderholm E, Wallentin L, Siegbahn A: Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA, 2001; 286: 2107-2013 [DOI] [PubMed] [Google Scholar]
- 12). Eguchi H, Shimazu A, Kawakami N, Inoue A, Nakata A, Tsutsumi A: Work engagement and high-sensitivity C-reactive protein levels among Japanese workers: a 1-year prospective cohort study. Int Arch Occup Environ Health, 2015; 88: 651-658 [DOI] [PubMed] [Google Scholar]
- 13). Friedman EM, Herd P: Income, education, and inflammation: differential associations in a national probability sample (The MIDUS study). Psychosom Med, 2010; 72: 290-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases. J Atheroscler Thromb, 2007: 5-57 [PubMed] [Google Scholar]
- 15). Tajima N, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Fujimoto Kei, Sakamoto M, Haneda M: Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int, 2015; 6: 151-187 [Google Scholar]
- 16). Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation, 2013; 48: 452-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Grimaldi D, Carter JR, Van Cauter E, Leproult R: Adverse Impact of Sleep Restriction and Circadian Misalignment on Autonomic Function in Healthy Young Adults. Hypertension, 2016; 68: 243-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM: Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci, 1999; 54: 434-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpé S, Smith RS: The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine, 1998; 10: 313-318 [DOI] [PubMed] [Google Scholar]
- 20). Fraga S, Marques-Vidal P, Vollenweider P, Waeber G, Guessous I, Paccaud F, Barros H, Stringhini S: Association of socioeconomic status with inflammatory markers: a two cohort comparison. Prev Med, 2015; 71: 12-19 [DOI] [PubMed] [Google Scholar]
- 21). Bell S, Mehta G, Moore K, Britton A: Ten-year alcohol consumption typologies and trajectories of C-reactive protein, interleukin-6 and interleukin-1 receptor antagonist over the following 12 years: a prospective cohort study. J Intern Med, 2017; 281: 75-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Hamer M, Sabia S, Batty GD, Shipley MJ, Tabák AG, Singh-Manoux A, Kivimaki M: Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation, 2012; 126: 928-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Shimanoe C, Otsuka Y, Hara M, Nanri H, Nishida Y, Nakamura K, Higaki Y, Imaizumi T, Taguchi N, Sakamoto T, Horita M, Shinchi K, Tanaka K: Gender-specific associations of perceived stress and coping strategies with C-reactive protein in middle-aged and older men and women. Int J Behav Med, 2014; 21: 821-832 [DOI] [PubMed] [Google Scholar]
- 24). Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M: Diurnal Variation of Circulating Interleukin-6 in Humans: A Meta-Analysis. PLoS One, 2016; 11: e0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]


